Sparassis crispa Intake Improves the Reduced Lipopolysaccharide-Induced TNF-α Production That Occurs upon Exhaustive Exercise in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Protocol
2.2. Drugs
2.3. Exercise Protocol
2.4. Enzyme-Linked Immunosorbent Assay for TNF-α
2.5. Immunoblot Analysis
2.6. Statistical Analysis
3. Results
3.1. Animal Study
3.1.1. Animal Characteristics
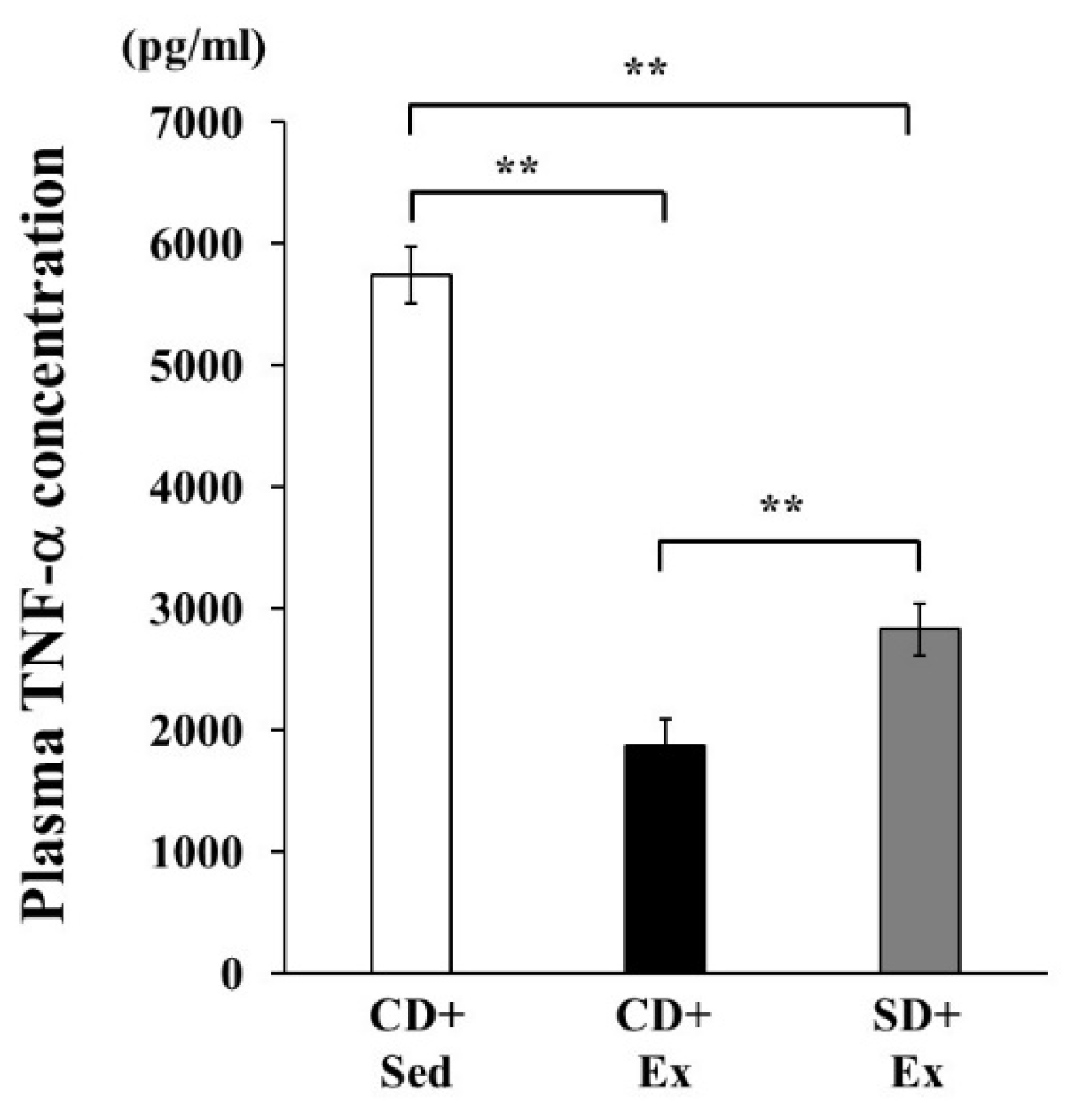
3.1.2. Plasma TNF-α Concentration in Response to LPS Stimulation
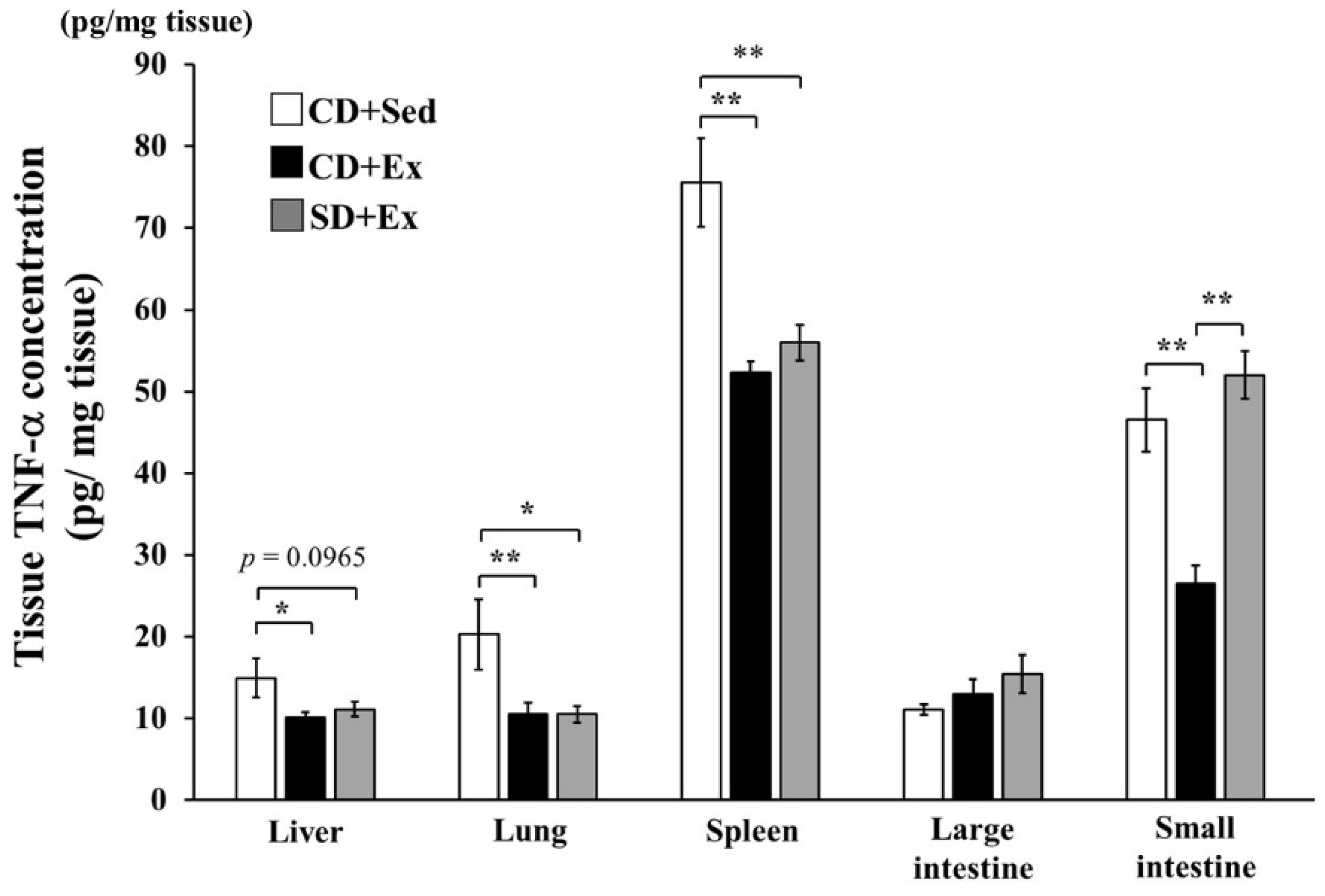
3.1.3. Effect of S. crispa Intake and Exhaustive Exercise on TNF-α Concentration in Mouse Tissues in Response to LPS
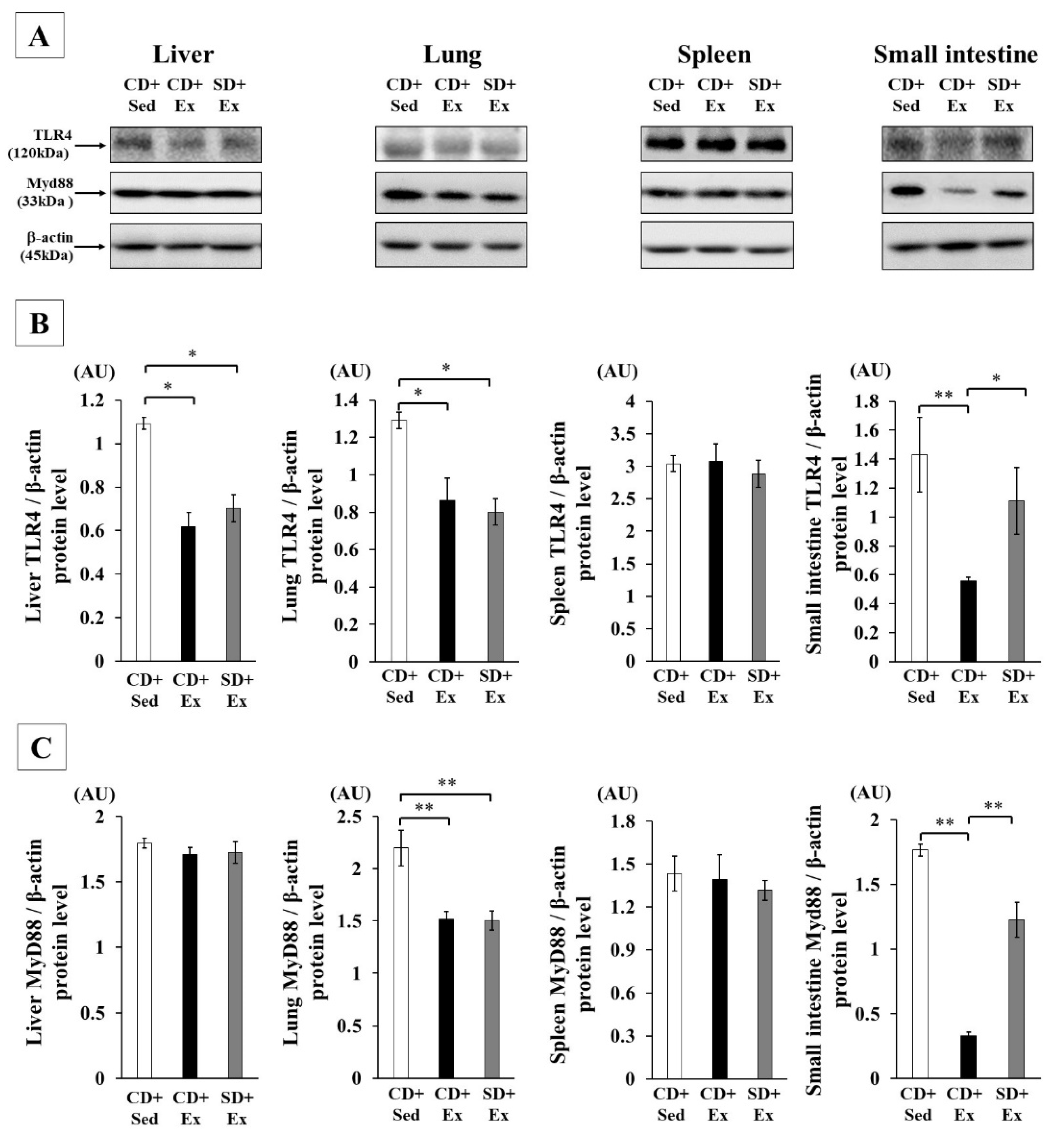
3.1.4. Effect of S. crispa Intake and Exhaustive Exercise on TLR4 Signaling in Mouse Tissues in Response to LPS
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nieman, D.C.; Nehlsen-Cannarella, S.L.; Markoff, P.A.; Balk-Lamberton, A.J.; Yang, H.; Chritton, D.B.; Lee, J.W.; Arabatzis, K. The effects of moderate exercise training on natural killer cells and acute upper respiratory tract infections. Int. J. Sports Med. 1990, 11, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Rohde, T.; Ostrowski, K. Recovery of the immune system after exercise. Acta Physiol. Scand. 1998, 162, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kawanishi, N.; Shiva, D.; Tsutsumi, N.; Uchida, M.; Kitamura, H.; Kato, Y.; Yano, H. Exhaustive exercise reduces tumor necrosis factor-alpha production in response to lipopolysaccharide in mice. Neuroimmunomodulation 2010, 17, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.M.; Xu, M.F.; Tang, P.L. Polysaccharide peptide (PSP) restores immunosuppression induced by cyclophosphamide in rats. Am. J. Chin. Med. 1997, 25, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Inoue, H.; Ohta, H.; Miyake, A.; Konishi, M.; Nanba, H. Oral administration of soluble β-glucans extracted from Grifola frondosa induces systemic antitumor immune response and decreases immunosuppression in tumor-bearing mice. Int. J. Cancer 2013, 133, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T. Natural Products and biological activity of the pharmacologically active cauliflower mushroom Sparassis crispa. BioMed Res. Int. 2013, 2013, 982317. [Google Scholar] [CrossRef] [PubMed]
- Shibata, A.; Hida, T.H.; Ishibashi, K.; Miura, N.N.; Adachi, Y.; Ohno, N. Disruption of actin cytoskeleton enhanced cytokine synthesis of splenocytes stimulated with beta-glucan from the cauliflower medicinal mushroom, Sparassis crispa Wulf.: Fr. (higher Basidiomycetes) in vitro. Int. J. Med. Mushrooms 2012, 14, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Ohno, N.; Miura, N.N.; Nakajima, M.; Yadomae, T. Antitumor 1,3-beta-glucan from cultured fruit body of Sparassis crispa. Biol. Pharm. Bull. 2000, 23, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Nameda, S.; Harada, T.; Miura, N.N.; Adachi, Y.; Yadomae, T.; Nakajima, M.; Ohno, N. Enhanced cytokine synthesis of leukocytes by a beta-glucan preparation, SCG, extracted from a medicinal mushroom. Immunopharmacol. Immunotoxicol. 2003, 25, 321–335. [Google Scholar] [CrossRef] [PubMed]
- Uchida, M.; Oyanagi, E.; Kawanishi, N.; Iemitsu, M.; Miyachi, M.; Kremenik, M.J.; Onodera, S.; Yano, H. Exhaustive exercise increases the TNF-α production in response to flagellin via the upregulation of toll-like receptor 5 in the large intestine in mice. Immunol. Lett. 2014, 158, 151–158. [Google Scholar] [CrossRef]
- Yano, H.; Uchida, M.; Nakai, R.; Ishida, K.; Kato, Y.; Kawanishi, N.; Shiva, D. Exhaustive exercise reduces TNF-α and IFN-α production in response to R-848 via toll-like receptor 7 in mice. Eur. J. Appl. Physiol. 2010, 110, 797–803. [Google Scholar] [CrossRef] [PubMed]
- Horii, N.; Uchida, M.; Hasegawa, N.; Fujie, S.; Oyanagi, E.; Yano, H.; Iemitsu, M. Resistance training prevents muscle fibrosis and atrophy via down-regulation of C1q-induced Wnt signaling in senescent mice. FASEB J. 2018, 32, 3547–3559. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, J.Y.; Ryu, H.S.; Park, H.G.; Kim, Y.O.; Kang, J.S.; Kim, H.M.; Hong, J.T.; Kim, Y.; Han, S.B. Induction of dendritic cell maturation by β-glucan isolated from Sparassis crispa. Int. Immunopharmacol. 2010, 10, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Volman, J.J.; Mensink, R.P.; Ramakers, J.D.; de Winther, M.P.; Carlsen, H.; Blomhoff, R.; Buurman, W.A.; Plat, J. Dietary (1→3), (1→4)-beta-D-glucans from oat activate nuclear factor-kappaB in intestinal leukocytes and enterocytes from mice. Nutr. Res. 2010, 30, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Ren, H.; Tomiyama-Miyaji, C.; Suga, Y.; Suga, T.; Kuwano, Y.; Iiai, T.; Hatakeyama, K.; Abo, T. Potentiation of intestinal immunity by micellary mushroom extracts. Biomed. Res. 2007, 28, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Schwellnus, M.; Soligard, T.; Alonso, J.M.; Bahr, R.; Clarsen, B.; Dijkstra, H.P.; Gabbett, T.J.; Gleeson, M.; Hägglund, M.; Hutchinson, M.R.; et al. How much is too much? (Part 2) International Olympic Committee consensus statement on load in sport and risk of illness. Br. J. Sports Med. 2016, 50, 1043–1052. [Google Scholar] [CrossRef]
- Walsh, N.P.; Gleeson, M.; Shephard, R.J.; Gleeson, M.; Woods, J.A.; Bishop, N.C.; Fleshner, M.; Green, C.; Pedersen, B.K.; Hoffman-Goetz, L.; et al. Position statement. Part one: Immune function and exercise. Exerc. Immunol. Rev. 2011, 17, 6–63. [Google Scholar]
- Peake, J.M.; Neubauer, O.; Walsh, N.P.; Simpson, R.J. Recovery of the immune system after exercise. J. Appl. Physiol. 2017, 122, 1077–1087. [Google Scholar] [CrossRef]
- Otsuki, T.; Shimizu, K.; Iemitsu, M.; Kono, I. Chlorella intake attenuates reduced salivary SIgA secretion in kendo training camp participants. Nutr. J. 2012, 11, 103. [Google Scholar] [CrossRef]
- O’Brien, G.C.; Wang, J.H.; Redmond, H.P. Bacterial lipoprotein induces resistance to Gram-negative sepsis in TLR4-deficient mice via enhanced bacterial clearance. J. Immunol. 2005, 174, 1020–1026. [Google Scholar] [CrossRef] [PubMed]
- Kubota, A.; Kobayashi, M.; Sarashina, S.; Takeno, R.; Okamoto, K.; Narumi, K.; Furugen, A.; Suzuki, Y.; Takahashi, N.; Iseki, K. Reishi mushroom Ganoderma lucidum Modulates IgA production and alpha-defensin expression in the rat small intestine. J. Ethnopharmacol. 2018, 214, 240–243. [Google Scholar] [CrossRef] [PubMed]
| CD + Sed (n = 8) | CD + Ex (n = 10) | SD + Ex (n = 10) | |
|---|---|---|---|
| Body weight (g) | 27.82 ± 0.66 | 28.10 ± 0.25 | 27.53 ± 0.36 |
| Heart mass (mg) | 106 ± 2 | 109 ± 3 | 110 ± 3 |
| Gastrocnemius muscle mass (mg) | 112 ± 4 | 115 ± 4 | 118 ± 5 |
| Soleus muscle mass (mg) | 6 ± 1 | 7 ± 1 | 6 ± 1 |
| Plantaris muscle mass (mg) | 5 ± 1 | 17 ± 1 | 14 ± 1 |
| Tibialis anterior muscle mass (mg) | 43 ± 1 | 44 ± 2 | 43 ± 1 |
| Food intake (g / day) | 3.19 ± 0.66 | 3.18 ± 0.28 | 3.18 ± 0.16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uchida, M.; Horii, N.; Hasegawa, N.; Oyanagi, E.; Yano, H.; Iemitsu, M. Sparassis crispa Intake Improves the Reduced Lipopolysaccharide-Induced TNF-α Production That Occurs upon Exhaustive Exercise in Mice. Nutrients 2019, 11, 2049. https://doi.org/10.3390/nu11092049
Uchida M, Horii N, Hasegawa N, Oyanagi E, Yano H, Iemitsu M. Sparassis crispa Intake Improves the Reduced Lipopolysaccharide-Induced TNF-α Production That Occurs upon Exhaustive Exercise in Mice. Nutrients. 2019; 11(9):2049. https://doi.org/10.3390/nu11092049
Chicago/Turabian StyleUchida, Masataka, Naoki Horii, Natsuki Hasegawa, Eri Oyanagi, Hiromi Yano, and Motoyuki Iemitsu. 2019. "Sparassis crispa Intake Improves the Reduced Lipopolysaccharide-Induced TNF-α Production That Occurs upon Exhaustive Exercise in Mice" Nutrients 11, no. 9: 2049. https://doi.org/10.3390/nu11092049
APA StyleUchida, M., Horii, N., Hasegawa, N., Oyanagi, E., Yano, H., & Iemitsu, M. (2019). Sparassis crispa Intake Improves the Reduced Lipopolysaccharide-Induced TNF-α Production That Occurs upon Exhaustive Exercise in Mice. Nutrients, 11(9), 2049. https://doi.org/10.3390/nu11092049





