Association of the Dietary Index Underpinning the Nutri-Score Label with Oral Health: Preliminary Evidence from a Large, Population-Based Sample
Abstract
1. Introduction
2. Materials and Methods
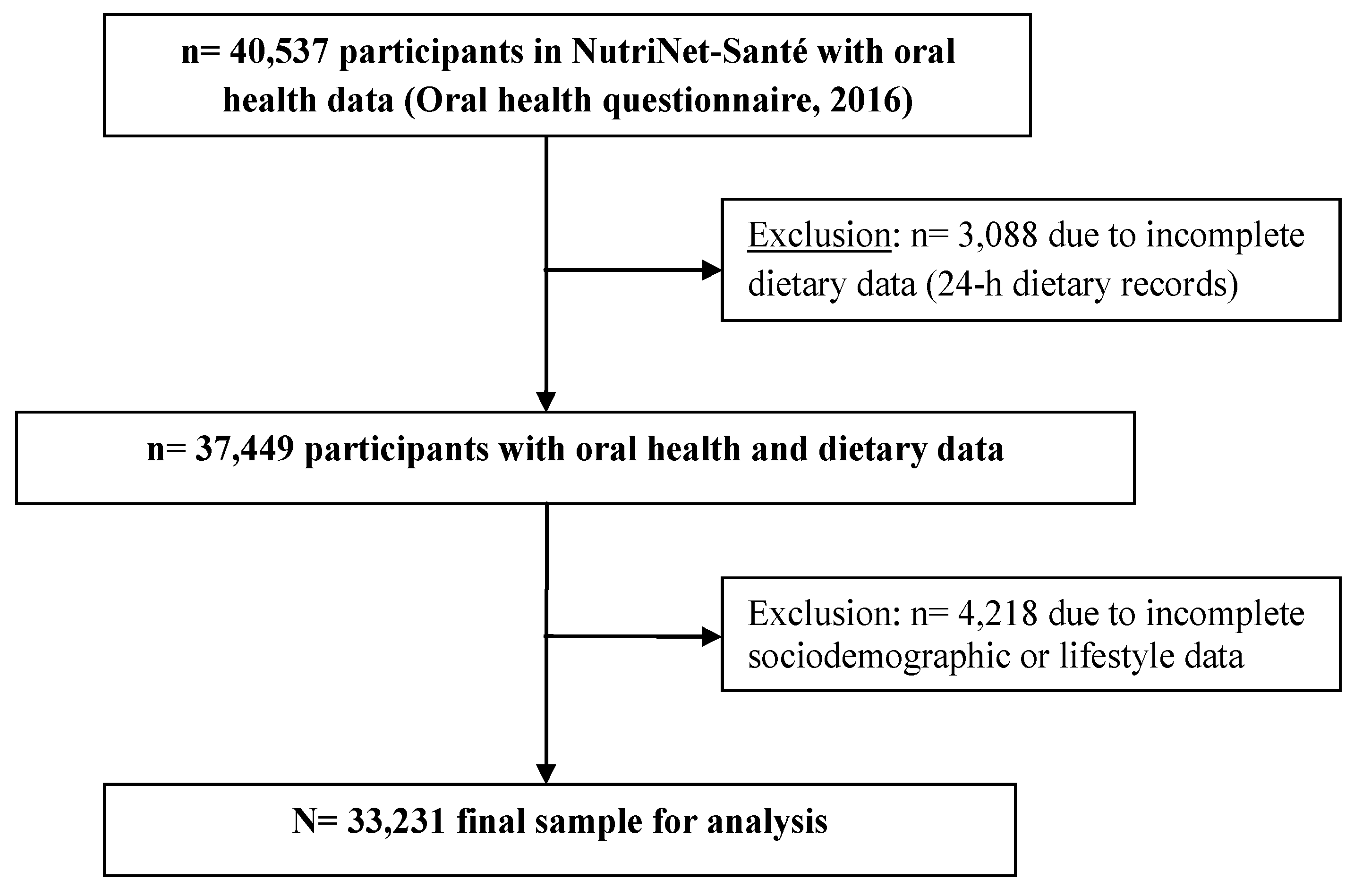
2.1. NutriNet-Santé e-Cohort
2.2. Oral Health Assessment
2.3. Dietary Index Calculation
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Sample Characteristics
3.2. Association between FSAm-NPS-DI and Oral Health
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- World Cancer Research Fund (WCRF) International. Building Momentum: Lessons on Implementing a Robust Front-of-Pack Food Label; WCRF International: London, UK, 2019. [Google Scholar]
- Post, R.E.; Mainous, A.G., III; Diaz, V.A.; Matheson, E.M.; Everett, C.J. Use of the nutrition facts label in chronic disease management: Results from the National Health and Nutrition Examination Survey. J. Am. Diet. Assoc. 2010, 110, 628–632. [Google Scholar] [CrossRef] [PubMed]
- Loureiro, M.L.; Yen, S.T.; Nayga, R.M., Jr. The effects of nutritional labels on obesity. Agric. Econ. 2012, 43, 333–342. [Google Scholar] [CrossRef]
- Ministère des Solidarités et de la Santé. Étiquetage Nutritionnel: Signature de l’Arrêté Recommandant l’Utilisation de “Nutri-Score”; Ministère des Solidarités et de la Santé: Paris, France, 2017.
- Rayner, M.; Scarborough, P.; Boxer, A.; Stockley, L. Nutrient Profiles: Development of Final Model. Final Report; British Heart Foundation Health Promotion Research Group, Department of Public Health, University of Oxford: Oxford, UK, 2005. [Google Scholar]
- Rayner, M.; Scarborough, P.; Lobstein, T. The UK OfCom Nutrient Profiling Model. Defining ‘Healthy’ and ‘Unhealthy’ Foods and Drinks for TV Advertising to Children; British Heart Foundation Health Promotion Research Group, Department of Public Health, University of Oxford: Oxford, UK, 2009. [Google Scholar]
- Haut Conseil de la Santé Publique. Avis Relatif à l’Information sur la Qualité Nutritionelle des Produits Alimentaires; Haut Conseil de la Santé Publique: Paris, France, 2015. [Google Scholar]
- Julia, C.; Ducrot, P.; Lassale, C.; Fezeu, L.; Mejean, C.; Peneau, S.; Touvier, M.; Hercberg, S.; Kesse-Guyot, E. Prospective associations between a dietary index based on the British Food Standard Agency nutrient profiling system and 13-year weight gain in the SU.VI.MAX cohort. Prev. Med. 2015, 81, 189–194. [Google Scholar] [CrossRef]
- Julia, C.; Fezeu, L.K.; Ducrot, P.; Méjean, C.; Peneau, S.; Touvier, M.; Hercberg, S.; Kesse-Guyot, E. The nutrient profile of foods consumed using the British Food Standards Agency Nutrient Profiling System is associated with metabolic syndrome in the SU.VI.MAX cohort. J. Nutr. 2015, 145, 2355–2361. [Google Scholar] [CrossRef] [PubMed]
- Donnenfeld, M.; Julia, C.; Kesse-Guyot, E.; Méjean, C.; Ducrot, P.; Peneau, S.; Deschasaux, M.; Latino-Martel, P.; Fezeu, L.; Hercberg, S.; et al. Prospective association between cancer risk and an individual dietary index based on the British Food Standards Agency Nutrient Profiling System. Br. J. Nutr. 2015, 114, 1702–1710. [Google Scholar] [CrossRef] [PubMed]
- Deschasaux, M.; Julia, C.; Kesse-Guyot, E.; Lécuyer, L.; Adriouch, S.; Méjean, C.; Ducrot, P.; Peneau, S.; Latino-Martel, P.; Fezeu, L.K.; et al. Are self-reported unhealthy food choices associated with an increased risk of breast cancer? Prospective cohort study using the British Food Standards Agency nutrient profiling system. BMJ Open 2017, 7, e013718. [Google Scholar] [CrossRef] [PubMed]
- Adriouch, S.; Julia, C.; Kesse-Guyot, E.; Méjean, C.; Ducrot, P.; Peneau, S.; Donnenfeld, M.; Deschasaux, M.; Menai, M.; Hercberg, S.; et al. Prospective association between a dietary quality index based on a nutrient profiling system and cardiovascular disease risk. Eur. J. Prev. Cardiol. 2016, 23, 1669–1676. [Google Scholar] [CrossRef] [PubMed]
- Adriouch, S.; Julia, C.; Kesse-Guyot, E.; Ducrot, P.; Peneau, S.; Méjean, C.; Assmann, K.E.; Deschasaux, M.; Hercberg, S.; Touvier, M.; et al. Association between a dietary quality index based on the food standard agency nutrient profiling system and cardiovascular disease risk among French adults. Int. J. Cardiol. 2017, 234, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Cinar, A.B.; Murtomaa, H. A holistic food labelling strategy for preventing obesity and dental caries. Obes. Rev. 2009, 10, 357–361. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Oral Health. Fact Sheet N°318; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Hercberg, S.; Castetbon, K.; Czernichow, S.; Malon, A.; Méjean, C.; Kesse, E.; Touvier, M.; Galan, P. The NutriNet-Santé Study: A web-based prospective study on the relationship between nutrition and health and determinants of dietary patterns and nutritional status. BMC Public Health. 2010, 10, 242. [Google Scholar] [CrossRef] [PubMed]
- Kesse-Guyot, E.; Andreeva, V.; Castetbon, K.; Vernay, M.; Touvier, M.; Méjean, C.; Julia, C.; Galan, P.; Hercberg, S. Participant profiles according to recruitment source in a large Web-based prospective study: Experience from the NutriNet-Santé study. J. Med. Internet Res. 2013, 15, e205. [Google Scholar] [CrossRef] [PubMed]
- Atchison, K.A.; Dolan, T.A. Development of the Geriatric Oral Health Assessment Index. J. Dent. Educ. 1990, 54, 680–687. [Google Scholar] [PubMed]
- Tubert-Jeannin, S.; Riordan, P.J.; Morel-Papernot, A.; Porcheray, S.; Saby-Collet, S. Validation of an oral health quality of life index (GOHAI) in France. Community Dent. Oral Epidemiol. 2003, 31, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Kishi, M.; Aizawa, F.; Matsui, M.; Yokoyama, Y.; Abe, A.; Minami, K.; Suzuki, R.; Miura, H.; Sakata, K.; Ogawa, A. Oral health-related quality of life and related factors among residents in a disaster area of the Great East Japan Earthquake and giant tsunami. Health Qual. Life Outcomes 2015, 13, 143. [Google Scholar] [CrossRef] [PubMed]
- Le Moullec, N.; Deheeger, M.; Preziosi, P.; Monteiro, P.; Valeix, P.; Rolland-Cachera, M.F.; Potier de Courcy, G.; Christides, J.P.; Cherouvrier, F.; Galan, P.; et al. Validation du manuel photos utilisé pour l’enquête alimentaire de l’étude SU.VI.MAX. Cahier de Nutrition et de Diététique 1996, 31, 158–164. [Google Scholar]
- Etude NutriNet-Santé. Table de Composition des Aliments; Économica: Paris, France, 2013. [Google Scholar]
- Julia, C.; Touvier, M.; Méjean, C.; Ducrot, P.; Peneau, S.; Hercberg, S.; Kesse-Guyot, E. Development and validation of an individual dietary index based on the British Food Standard Agency Nutrient Profiling System in a French context. J. Nutr. 2014, 144, 2009–2017. [Google Scholar] [CrossRef] [PubMed]
- IPAQ Group. IPAQ Scoring Protocol. Available online: www.ipaq.ki.se (accessed on 16 August 2019).
- Black, A.E. Critical evaluation of energy intake using the Goldberg cut-off for energy intake: Basal metabolic rate. A practical guide to its calculation, use and limitations. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Schofield, W.N. Predicting basal metabolic rate, new standards and review of previous work. Hum. Nutr. Clin. Nutr. 1985, 39 (Suppl. 1), 5–41. [Google Scholar]
- Andreeva, V.A.; Kesse-Guyot, E.; Galan, P.; Feron, G.; Hercberg, S.; Hennequin, M.; Sulmont-Rosse, C. Adherence to national dietary guidelines in association with oral health impact on quality of life. Nutrients 2018, 10, 527. [Google Scholar] [CrossRef]
- Ortiz-Barrios, L.B.; Granados-Garcia, V.; Cruz-Hervert, P.; Moreno-Tamayo, K.; Heredia-Ponce, E.; Sanchez-Garcia, S. The impact of poor oral health on the oral health-related quality of life (OHRQoL) in older adults: The oral health status through a latent class analysis. BMC Oral Health 2019, 19, 141. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, R.J.; Hugo, F.N.; Hilgert, J.B.; Padilha, D.M. Association between oral health status and nutritional status in south Brazilian independent-living older people. Nutrition 2008, 24, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Sheiham, A.; Steele, J.G.; Marcenes, W.; Finch, S.; Walls, A.W. The relationship between oral health status and Body Mass Index among older people: A national survey of older people in Great Britain. Br. Dent. J. 2002, 192, 703–706. [Google Scholar] [CrossRef] [PubMed]
- Joshipura, K.J.; Willett, W.C.; Douglass, C.W. The impact of edentulousness on food and nutrient intake. J. Am. Dent. Assoc. 1996, 127, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Sahyoun, N.R.; Lin, C.L.; Krall, E. Nutritional status of the older adult is associated with dentition status. J. Am. Diet. Assoc. 2003, 103, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Nowjack-Raymer, R.E.; Sheiham, A. Numbers of natural teeth, diet, and nutritional status in US adults. J. Dent. Res. 2007, 86, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Marcenes, W.; Steele, J.G.; Sheiham, A.; Walls, A.W. The relationship between dental status, food selection, nutrient intake, nutritional status, and body mass index in older people. Cad Saude Publica 2003, 19, 809–816. [Google Scholar] [CrossRef]
- Savoca, M.R.; Arcury, T.A.; Leng, X.; Chen, H.; Bell, R.A.; Anderson, A.M.; Kohrman, T.; Frazier, R.J.; Gilbert, G.H.; Quandt, S.A. Severe tooth loss in older adults as a key indicator of compromised dietary quality. Public Health Nutr. 2010, 13, 466–474. [Google Scholar] [CrossRef]
- Hung, H.C.; Colditz, G.; Joshipura, K.J. The association between tooth loss and the self-reported intake of selected CVD-related nutrients and foods among US women. Community Dent. Oral Epidemiol. 2005, 33, 167–173. [Google Scholar] [CrossRef]
- El Osta, N.; Hennequin, M.; Tubert-Jeannin, S.; Abboud Naaman, N.B.; El Osta, L.; Geahchan, N. The pertinence of oral health indicators in nutritional studies in the elderly. Clin. Nutr. 2014, 33, 316–321. [Google Scholar] [CrossRef]
- Deschasaux, M.; Huybrechts, I.; Murphy, N.; Julia, C.; Hercberg, S.; Srour, B.; Kesse-Guyot, E.; Latino-Martel, P.; Biessy, C.; Casagrande, C.; et al. Nutritional quality of food as represented by the FSAm-NPS nutrient profiling system underlying the Nutri-Score label and cancer risk in Europe: Results from the EPIC prospective cohort study. PLoS Med. 2018, 15, e1002651. [Google Scholar] [CrossRef] [PubMed]
- Julia, C.; Kesse-Guyot, E.; Touvier, M.; Méjean, C.; Fezeu, L.; Hercberg, S. Application of the British Food Standards Agency nutrient profiling system in a French food composition database. Br. J. Nutr. 2014, 112, 1699–1705. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.K.; Lichtenstein, A.H.; Kris-Etherton, P.M.; Carson, J.A.; Pappas, A.; Rupp, L.; Vafiadis, D.K. Enhanced and updated American Heart Association heart-check front-of-package symbol: Efforts to help consumers identify healthier food choices. J. Acad. Nutr. Diet. 2015, 115, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, A.H.; Carson, J.S.; Johnson, R.K.; Kris-Etherton, P.M.; Pappas, A.; Rupp, L.; Stitzel, K.F.; Vafiadis, D.K.; Fulgoni, V.L., 3rd. Food-intake patterns assessed by using front-of-pack labeling program criteria associated with better diet quality and lower cardiometabolic risk. Am. J. Clin. Nutr. 2014, 99, 454–462. [Google Scholar] [CrossRef] [PubMed]
- Touger-Decker, R.; Mobley, C. Academy of Nutrition & Dietetics. Position of the Academy of Nutrition and Dietetics: Oral health and nutrition. J. Acad. Nutr. Diet. 2013, 113, 693–701. [Google Scholar] [PubMed]
- Wu, L.L.; Cheung, K.Y.; Lam, P.Y.P.; Gao, X.L. Oral health indicators for risk of malnutrition in elders. J. Nutr. Health Aging 2018, 22, 254–261. [Google Scholar] [CrossRef]
- Kanehira, Y.; Arai, K.; Kanehira, T.; Nagahisa, K.; Baba, S. Oral health-related quality of life in patients with implant treatment. J. Adv. Prosthodont. 2017, 9, 476–481. [Google Scholar] [CrossRef]
- Campos, J.A.; Zucoloto, M.L.; Geremias, R.F.; Nogueira, S.S.; Maroco, J. Validation of the Geriatric Oral Health Assessment Index in complete denture wearers. J. Oral Rehabil. 2015, 42, 512–520. [Google Scholar] [CrossRef]
- Pegon-Machat, E.; Faulks, D.; Eaton, K.A.; Widstrom, E.; Hugues, P.; Tubert-Jeannin, S. The healthcare system and the provision of oral healthcare in EU Member States: France. Br. Dent. J. 2016, 220, 197–203. [Google Scholar] [CrossRef]
- Statista. Structure de la Population Selon qu’elle Dispose ou pas d’une Connexion Internet à Domicile en France en 2017, Selon la Catégorie Socio-Professionnelle (CSP); Statista: New York, NY, USA, 2019. [Google Scholar]
- Alt, K.W.; Rossbach, A. Nothing in nature is as consistent as change. Front. Oral Biol. 2009, 13, 190–196. [Google Scholar]
- Waijers, P.M.; Feskens, E.J.; Ocke, M.C. A critical review of predefined diet quality scores. Br. J. Nutr. 2007, 97, 219–231. [Google Scholar] [PubMed]
- Arambepola, C.; Scarborough, P.; Rayner, M. Validating a nutrient profile model. Public Health Nutr. 2008, 11, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Azais-Braesco, V.; Goffi, C.; Labouze, E. Nutrient profiling: Comparison and critical analysis of existing systems. Public Health Nutr. 2006, 9, 613–622. [Google Scholar] [PubMed]
- Julia, C.; Méjean, C.; Touvier, M.; Peneau, S.; Lassale, C.; Ducrot, P.; Hercberg, S.; Kesse-Guyot, E. Validation of the FSA nutrient profiling system dietary index in French adults-findings from SU.VI.MAX study. Eur. J. Nutr. 2016, 55, 1901–1910. [Google Scholar] [CrossRef] [PubMed]
- Féart, C.; Jutand, M.A.; Larrieu, S.; Letenneur, L.; Delcourt, C.; Combe, N.; Barberger-Gateau, P. Energy, macronutrient and fatty acid intake of French elderly community dwellers and association with socio-demographic characteristics: Data from the Bordeaux sample of the Three-City Study. Br. J. Nutr. 2007, 98, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- LaMonte, M.J.; Genco, R.J.; Hovey, K.M.; Wallace, R.B.; Freudenheim, J.L.; Michaud, D.S.; Mai, X.; Tinker, L.F.; Salazar, C.R.; Andrews, C.A.; et al. History of periodontitis diagnosis and edentulism as predictors of cardiovascular disease, stroke, and mortality in postmenopausal women. J. Am. Heart Assoc. 2017, 6, e004518. [Google Scholar] [CrossRef] [PubMed]
| Age 18–59 years (n = 24,243) | Age ≥60 years (n = 8988) | P1 | |
|---|---|---|---|
| Sex | <0.0001 | ||
| Male | 4754 (19.61) | 3626 (40.34) | |
| Female | 19,489 (80.39) | 5362 (59.66) | |
| FSAm-NPS-DI, mean (SD) 2,3 | 6.29 (2.25) | 5.26 (1.92) | <0.0001 |
| Dietary energy, Kcal/day, mean (SD) 3 | 1836.10 (475.70) | 1833.00 (490.30) | 0.60 |
| Oral health, mean (SD) 4 | 54.03 (5.30) | 53.03 (5.85) | <0.0001 |
| Poor oral health, score ≤50 | 5100 (21.04) | 2416 (26.88) | |
| Average oral health, score 51–56 | 9377 (38.68) | 3578 (39.81) | |
| Good oral health, score 57–60 | 9766 (40.28) | 2994 (33.31) | |
| Age, years, mean (SD) | 42.84 (11.30) | 64.59 (4.22) | <0.0001 |
| Educational level | <0.0001 | ||
| Less than high school | 3091 (12.75) | 2340 (26.03) | |
| High school diploma or equivalent | 4261 (17.58) | 2087 (23.22) | |
| Some college, undergraduate degree | 7855 (32.40) | 2196 (24.43) | |
| Graduate degree | 9036 (37.27) | 2365 (26.31) | |
| Occupational status | <0.0001 | ||
| Homemaker/disabled/unemployed | 3058 (12.61) | 305 (3.39) | |
| Student/trainee | 1332 (5.49) | 0 (0.00) | |
| Manual/blue collar | 5417 (22.34) | 162 (1.80) | |
| Office work/administrative staff | 5451 (22.48) | 155 (1.72) | |
| Professional/executive staff | 7457 (30.76) | 457 (5.08) | |
| Retired | 1528 (6.30) | 7909 (88.00) | |
| Household income (monthly) | <0.0001 | ||
| <1200 € | 3591 (14.81) | 466 (5.19) | |
| 1200 €–1799 € | 5881 (24.26) | 1771 (19.70) | |
| 1800 €–2299 € | 3566 (14.71) | 1438 (16.00) | |
| 2300 €–2699 € | 2149 (8.86) | 1222 (13.60) | |
| 2700 €–3699 € | 3940 (16.25) | 1803 (20.06) | |
| ≥ 3700 € | 2629 (10.84) | 1494 (16.62) | |
| Not reported | 2487 (10.26) | 794 (8.83) | |
| Marital status | <0.0001 | ||
| Married/cohabiting | 17,862 (73.68) | 6830 (75.99) | |
| Living alone (single, divorced, widowed) | 6381 (26.32) | 2158 (24.01) | |
| Body Mass Index, kg/m2, mean (SD) | 23.71 (4.50) | 24.91 (4.13) | <0.0001 |
| Underweight (<18.5) | 1361 (5.61) | 211 (2.35) | |
| Normal weight (18.5–24.9) | 15,783 (65.10) | 4934 (54.90) | |
| Overweight (25.0–29.9) | 4996 (20.61) | 2909 (32.37) | |
| Obese (≥30.0) | 2103 (8.67) | 934 (10.39) | |
| Physical activity 5 | <0.0001 | ||
| Low | 5443 (24.49) | 1324 (15.54) | |
| Moderate | 9680 (43.56) | 3081 (36.16) | |
| Vigorous | 7099 (31.95) | 4115 (48.30) | |
| Smoking status | <0.0001 | ||
| Never | 12,949 (53.41) | 4007 (44.58) | |
| Former | 7999 (33.00) | 4418 (49.15) | |
| Current | 3295 (13.59) | 563 (6.26) | |
| Alcohol use, g ethanol/d, mean (SD) | 6.91 (10.42) | 10.97 (13.40) | <0.0001 |
| Age 18–59 years (n = 24,243) | Age ≥60 years (n = 8988) | |||||
|---|---|---|---|---|---|---|
| β | P(β) | F(model) | β | P(β) | F(model) | |
| Model 1 | −0.02 | 0.02 | 5.85 | 0.02 | 0.11 | 2.58 |
| Model 2 | 0.02 | 0.005 | 112.59 | 0.02 | 0.06 | 11.49 |
| Model 3 | 0.01 | 0.04 | 28.54 | 0.02 | 0.04 | 6.26 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreeva, V.A.; Egnell, M.; Galan, P.; Feron, G.; Hercberg, S.; Julia, C. Association of the Dietary Index Underpinning the Nutri-Score Label with Oral Health: Preliminary Evidence from a Large, Population-Based Sample. Nutrients 2019, 11, 1998. https://doi.org/10.3390/nu11091998
Andreeva VA, Egnell M, Galan P, Feron G, Hercberg S, Julia C. Association of the Dietary Index Underpinning the Nutri-Score Label with Oral Health: Preliminary Evidence from a Large, Population-Based Sample. Nutrients. 2019; 11(9):1998. https://doi.org/10.3390/nu11091998
Chicago/Turabian StyleAndreeva, Valentina A., Manon Egnell, Pilar Galan, Gilles Feron, Serge Hercberg, and Chantal Julia. 2019. "Association of the Dietary Index Underpinning the Nutri-Score Label with Oral Health: Preliminary Evidence from a Large, Population-Based Sample" Nutrients 11, no. 9: 1998. https://doi.org/10.3390/nu11091998
APA StyleAndreeva, V. A., Egnell, M., Galan, P., Feron, G., Hercberg, S., & Julia, C. (2019). Association of the Dietary Index Underpinning the Nutri-Score Label with Oral Health: Preliminary Evidence from a Large, Population-Based Sample. Nutrients, 11(9), 1998. https://doi.org/10.3390/nu11091998





