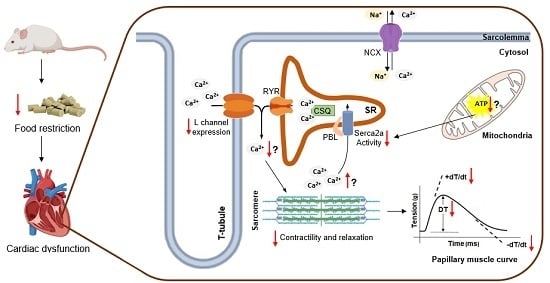
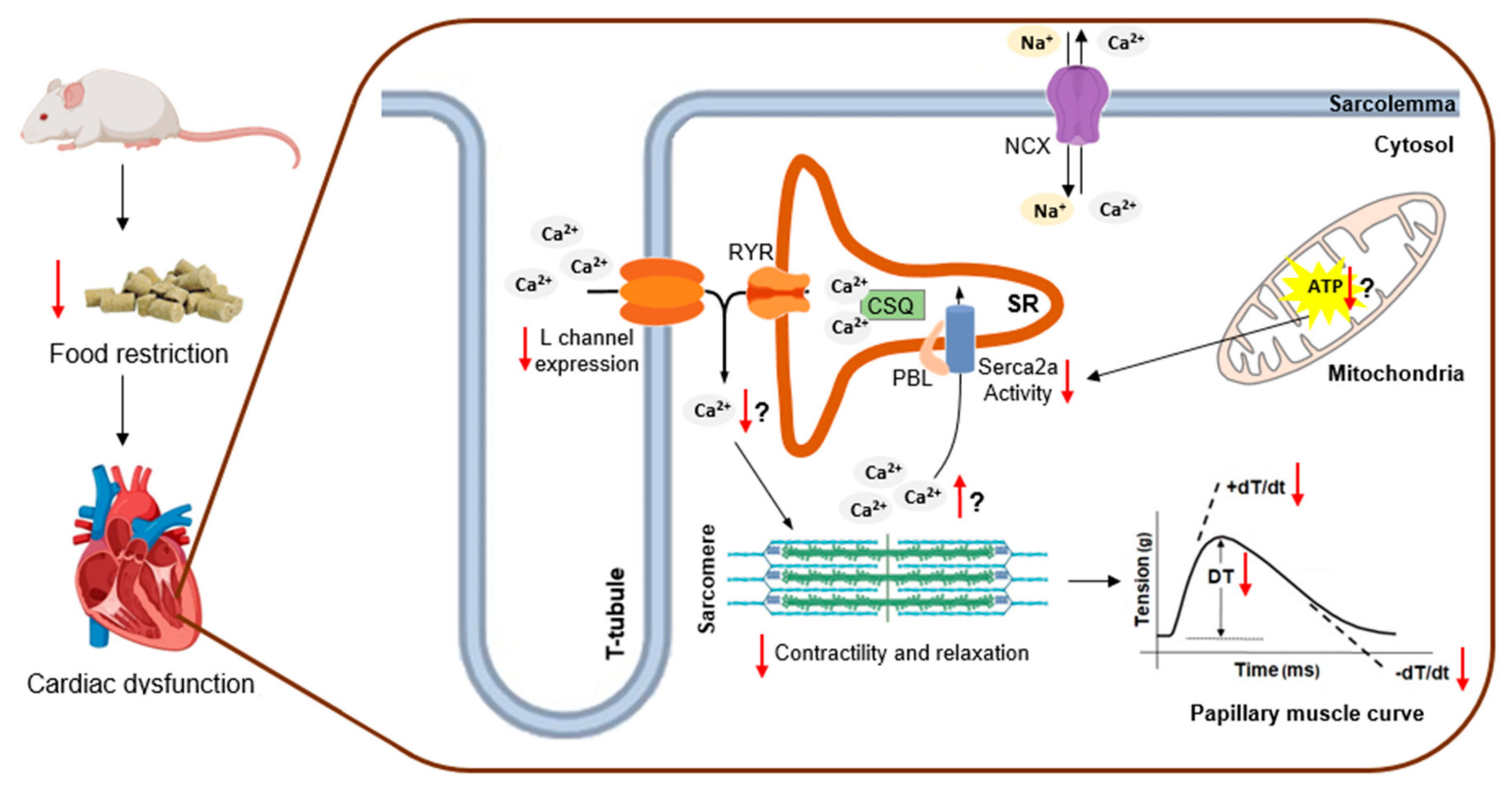
Myocardial Dysfunction after Severe Food Restriction Is Linked to Changes in the Calcium-Handling Properties in Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model and Experimental Protocol
2.2. General Characteristics
2.3. Cardiac Morphological Post Mortem Study
2.4. Myocardial Function
2.5. Expression of Calcium-Handling Protein
2.6. Statistical Analysis
3. Results
3.1. Physical Characteristics
3.2. Macroscopic Cardiac Morphology
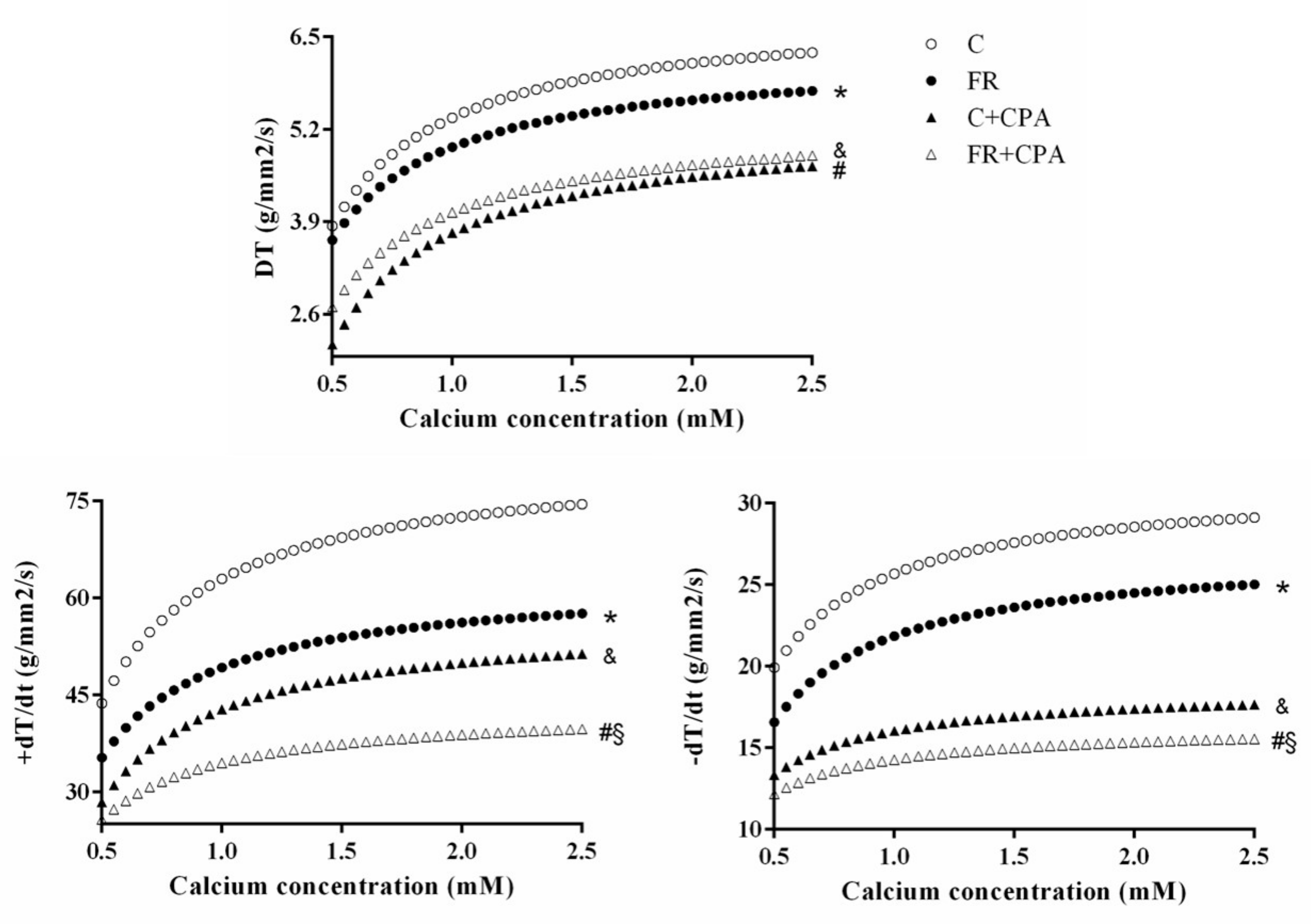
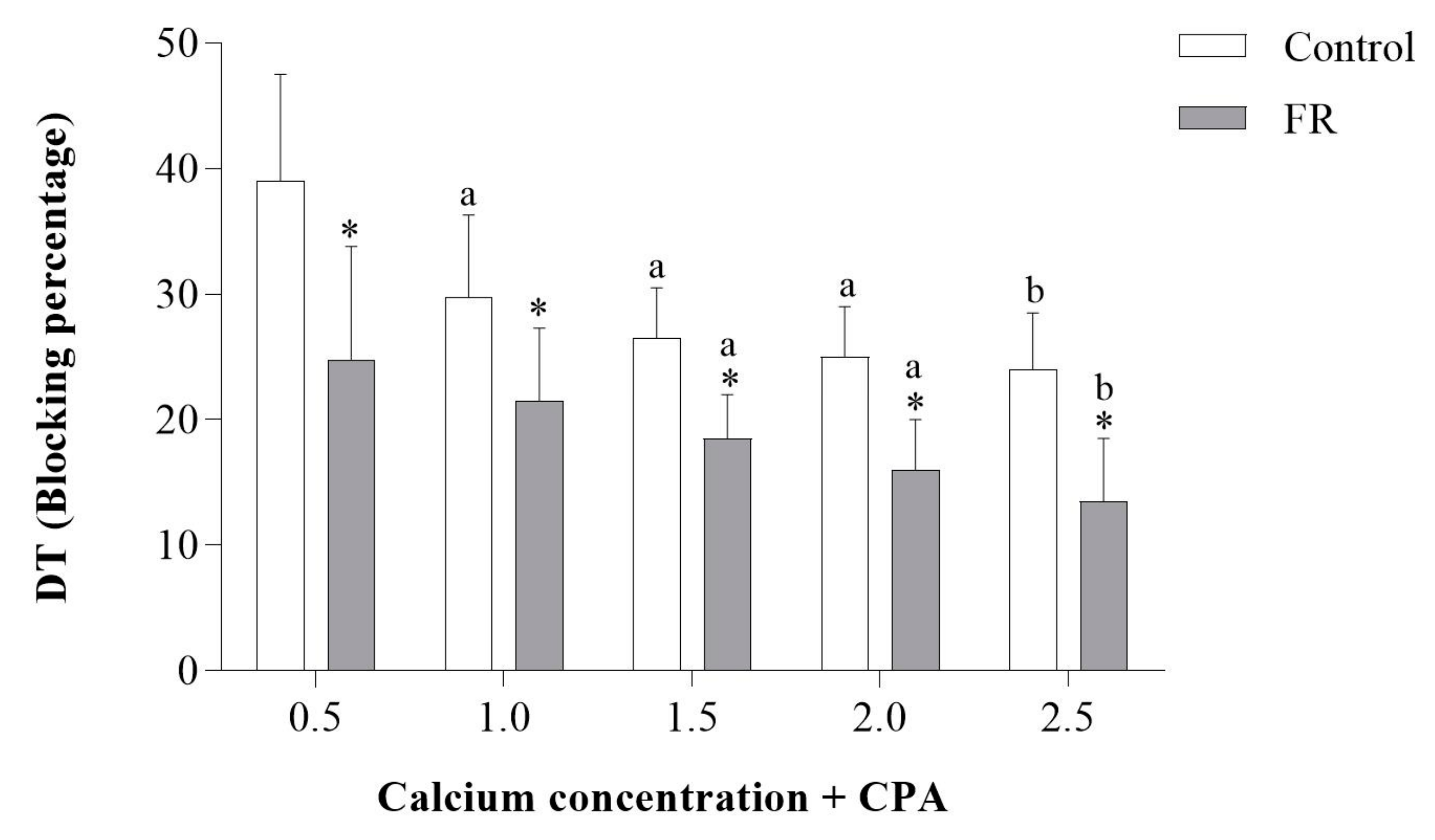
3.3. Assessment of Papillary Muscle Function
3.4. Expression of Calcium-Handling Protein
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Alugoju, P.; Narsimulu, D.; Bhanu, J.U.; Satyanarayana, N.; Periyasamy, L. Role of quercetin and caloric restriction on the biomolecular composition of aged rat cerebral cortex: An FTIR study. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 220, 117128. [Google Scholar] [CrossRef] [PubMed]
- Mattison, J.A.; Roth, G.S.; Beasley, T.M.; Tilmont, E.M.; Handy, A.M.; Herbert, R.L.; Longo, D.L.; Allison, D.B.; Young, J.E.; Bryant, M.; et al. Impact of caloric restriction on health and survival in rhesus monkeys from the NIA study. Nature 2012, 489, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Trepanowski, J.F.; Canale, E.R.; Marshall, E.K.; Kabir, M.M.; Bloomer, R.J. Impact of caloric and dietary restriction regimens on markers of health and longevity in humans and animals: A summary of available findings. Nutr. J. 2011, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, C.J. The genetics of ageing. Nature 2010, 464, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Rebrin, I.; Kamzalov, S.; Sohal, R.S. Effects of age and caloric restriction on glutathione redox state in mice. Free Radic. Biol. Med. 2003, 35, 626–635. [Google Scholar] [CrossRef]
- Weindruch, R.; Sohal, R.S. Caloric Intake and Aging. N. Engl. J. Med. 1997, 337, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Brent, B.; Obonyo, N.; Akech, S.; Shebbe, M.; Mpoya, A.; Mturi, N.; Berkley, J.A.; Tulloh, R.M.R.; Maitland, K. Assessment of Myocardial Function in Kenyan Children with Severe, Acute Malnutrition: The Cardiac Physiology in Malnutrition (CAPMAL) Study. JAMA Netw. Open 2019, 2, e191054. [Google Scholar] [CrossRef] [PubMed]
- Bebars, G.M.; Askalany, H.T. Assessment of left ventricular systolic and diastolic functions in severely malnourished children. Egypt. Pediatr. Assoc. Gaz. 2018. [Google Scholar] [CrossRef][Green Version]
- Silverman, J.A.; Chimalizeni, Y.; Hawes, S.E.; Wolf, E.R.; Batra, M.; Khofi, H.; Molyneux, E.M. The effects of malnutrition on cardiac function in African children. Arch. Dis. Child. 2016, 101, 166–171. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed, H.L.; Nassar, M.F.; Habib, N.M.; Elmasry, O.A.; Gomaa, S.M. Structural and functional affection of the heart in protein energy malnutrition patients on admission and after nutritional recovery. Eur. J. Clin. Nutr. 2006, 60, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Cicogna, A.C.; Padovani, C.R.; Okoshi, K.; Aragon, F.F.; Okoshi, M.P. Myocardial Function during Chronic Food Restriction in Isolated Hypertrophied Cardiac Muscle. Am. J. Med. Sci. 2000, 320, 244–248. [Google Scholar] [CrossRef]
- Okoshi, M.P.; Okoshi, K.; Pai, V.D.; Pai-Silva, M.D.; Matsubara, L.S.; Cicogna, A.C. Mechanical, biochemical, and morphological changes in the heart from chronic food-restricted rats. Can. J. Physiol. Pharmacol. 2001, 79, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Gut, A.L.; Okoshi, M.P.; Padovani, C.R.; Aragon, F.F.; Cicogna, A.C. Myocardial dysfunction induced by food restriction is related to calcium cycling and beta-adrenergic system changes. Nutr. Res. 2003, 23, 911–919. [Google Scholar] [CrossRef]
- Sugizaki, M.M.; Leopoldo, A.P.L.; Conde, S.J.; Campos, D.S.; Damato, R.; Leopoldo, A.S.; Nascimento, A.F.D.; Júnior, S.D.A.O.; Cicogna, A.C. Upregulation of mRNA myocardium calcium handling in rats submitted to exercise and food restriction. Arq. Bras. Cardiol. 2011, 97, 46–52. [Google Scholar] [CrossRef]
- De Tomasi, L.C.; Bruno, A.; Sugizaki, M.M.; Lima-Leopoldo, A.P.; Nascimento, A.F.; Júnior, S.A.; Pinotti, M.F.; Padovani, C.R.; Leopoldo, A.S.; Cicogna, A.C. Food restriction promotes downregulation of myocardial L-type Ca2+ channels. Can. J. Physiol. Pharmacol. 2009, 87, 426–431. [Google Scholar] [CrossRef] [PubMed]
- Gut, A.L.; Sugizaki, M.M.; Okoshi, M.P.; Carvalho, R.F.; Pai-Silva, M.D.; Aragon, F.F.; Padovani, C.R.; Okoshi, K.; Cicogna, A.C. Food restriction impairs myocardial inotropic response to calcium and beta-adrenergic stimulation in spontaneously hypertensive rats. Nutr. Res. 2008, 28, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Sugizaki, M.M.; Carvalho, R.F.; Aragon, F.F.; Padovani, C.R.; Okoshi, K.; Okoshi, M.P.; Zanati, S.G.; Pai-Silva, M.D.; Novelli, E.L.B.; Cicogna, A.C. Myocardial Dysfunction Induced by Food Restriction is Related to Morphological Damage in Normotensive Middle-Aged Rats. J. Biomed. Sci. 2005, 12, 641–649. [Google Scholar] [CrossRef] [PubMed]
- Okoshi, K.; Fioretto, J.; Okoshi, M.; Cicogna, A.; Aragon, F.; Matsubara, L.; Matsubara, B. Food restriction induces in vivo ventricular dysfunction in spontaneously hypertensive rats without impairment of in vitro myocardial contractility. Braz. J. Med. Biol. Res. 2004, 37, 607–613. [Google Scholar] [CrossRef]
- Sugizaki, M.M.; Leopoldo, A.S.; Okoshi, M.P.; Bruno, A.; Conde, S.J.; Lima-Leopoldo, A.P.; Padovani, C.R.; Carvalho, R.F.; Nascimento, A.F.D.; De Campos, D.H.S.; et al. Severe food restriction induces myocardial dysfunction related to SERCA2 activity. Can. J. Physiol. Pharmacol. 2009, 87, 666–673. [Google Scholar] [CrossRef]
- Sugizaki, M.M.; Pai-Silva, M.D.; Carvalho, R.F.; Padovani, C.R.; Bruno, A.; Nascimento, A.F.; Aragon, F.F.; Novelli, E.L.B.; Cicogna, A.C. Exercise training increases myocardial inotropic response in food restricted rats. Int. J. Cardiol. 2006, 112, 191–201. [Google Scholar] [CrossRef]
- Fioretto, J.R.; Querioz, S.S.; Padovani, C.R.; Matsubara, L.S.; Okoshi, K.; Matsubara, B.B. Ventricular remodeling and diastolic myocardial dysfunction in rats submitted to protein-calorie malnutrition. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H1327–H1333. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hilderman, T.; McKnight, K.; Dhalla, K.S.; Rupp, H.; Dhalla, N.S. Effects of long-term dietary restriction on cardiovascular function and plasma catecholamines in the rat. Cardiovasc. Drugs Ther. 1996, 10, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Katzeff, H.L.; Powell, S.R.; Ojamaa, K. Alterations in cardiac contractility and gene expression during low-T3 syndrome: Prevention with T3. Am. J. Physiol. Metab. 1997, 273, E951–E956. [Google Scholar] [CrossRef] [PubMed]
- Haddad, F.; Bodell, P.W.; McCue, S.A.; Herrick, R.E.; Baldwin, K.M. Food restriction-induced transformations in cardiac functional and biochemical properties in rats. J. Appl. Physiol. 1993, 74, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, A.; Savabi, F. Effect of food restriction on the phosphocreatine energy shuttle components in rat heart. J. Mol. Cell. Cardiol. 1992, 24, 821–830. [Google Scholar] [CrossRef]
- Okoshi, M.P.; Okoshi, K.; Matsubara, L.S.; Pai-Silva, M.D.; Gut, A.L.; Padovani, C.R.; Pai, V.D.; Cicogna, A.C. Myocardial remodeling and dysfunction are induced by chronic food restriction in spontaneously hypertensive rats. Nutr. Res. 2006, 26, 567–572. [Google Scholar] [CrossRef]
- Rossi, M.A.; Zucoloto, S. Ultrastructural changes in nutritional cardiomiopathy of protein-calorie malnourished rats. Br. J. Exp. Pathol. 1982, 63, 242–253. [Google Scholar] [PubMed]
- McKnight, K.A.; Rupp, H.; Beamish, R.E.; Dhalla, N.S. Modification of catecholamine-induced changes in heart function by food restriction in rats. Cardiovasc. Drugs Ther. 1996, 10, 239–246. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.J.; Shen, H.; Bissonette, D.; Jeejeebhoy, K.N. Effects of hypocaloric feeding and refeeding on myocardial Ca and ATP cycling in the rat. Mol. Cell. Biochem. 1995, 142, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Klebanov, S.; Herlihy, J.T.; Freeman, G.L. Effect of long-term food restriction on cardiac mechanics. Am. J. Physiol. Circ. Physiol. 1997, 273, H:2333–H:2342. [Google Scholar] [CrossRef]
- A Vizotto, V.; Carvalho, R.F.; Sugizaki, M.M.; Lima, A.P.; Aragon, F.F.; Padovani, C.R.; Castro, A.V.B.; Pai-Silva, M.D.; Nogueira, C.R.; Cicogna, A.C. Down-regulation of the cardiac sarcoplasmic reticulum ryanodine channel in severely food-restricted rats. Braz. J. Med. Biol. Res. 2007, 40, 27–31. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ghazalpour, A.; Bennett, B.; Petyuk, V.A.; Orozco, L.; Hagopian, R.; Mungrue, I.N.; Farber, C.R.; Sinsheimer, J.; Kang, H.M.; Furlotte, N.; et al. Comparative Analysis of Proteome and Transcriptome Variation in Mouse. PLoS Genet. 2011, 7, e1001393. [Google Scholar] [CrossRef] [PubMed]
- Foss, E.J.; Radulovic, D.; Shaffer, S.A.; Ruderfer, D.M.; Bedalov, A.; Goodlett, D.R.; Kruglyak, L. Genetic basis of proteome variation in yeast. Nat. Genet. 2007, 39, 1369–1375. [Google Scholar] [CrossRef] [PubMed]
- Leopoldo, A.S.; Lima-Leopoldo, A.P.; Sugizaki, M.M.; Nascimento, A.F.D.; De Campos, D.H.S.; Luvizotto, R.D.A.M.; Castardeli, É.; Alves, C.A.B.; Brum, P.C.; Cicogna, A.C.; et al. Involvement of L-type calcium channel and serca2a in myocardial dysfunction induced by obesity. J. Cell. Physiol. 2011, 226, 2934–2942. [Google Scholar] [CrossRef] [PubMed]
- Campos, D.H.S.; De Leopoldo, A.S.; Lima-Leopoldo, A.P.; Nascimento, A.F.; Do Oliveira-Junior, S.A.; De Silva, D.C.T.; Sugizaki, M.M.; Padovani, C.R.; Cicogna, A.C. Obesity Preserves Myocardial Function During Blockade of the Glycolytic Pathway. Arq. Bras. Cardiol. 2014, 103, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Maxwell, L.; Enwemeka, C.; Fernandes, G. Effects of exercise and food restriction on rat skeletal muscles. Tissue Cell 1992, 24, 491–498. [Google Scholar] [CrossRef]
- Katzeff, H.L.; Ojamaa, K.M.; Klein, I. The effects of long-term aerobic exercise and energy restriction on protein synthesis. Metabolism 1995, 44, 188–192. [Google Scholar] [CrossRef]
- Cicogna, A.C.; Padovani, C.R.; Okoshi, K.; Matsubara, L.S.; Aragon, F.F.; Okoshi, M.P. The influence of temporal food restriction on the performance of isolated cardiac muscle. Nutr. Res. 2001, 21, 639–648. [Google Scholar] [CrossRef]
- Hanft, L.M.; Korte, F.S.; McDonald, K.S. Cardiac function and modulation of sarcomeric function by length. Cardiovasc. Res. 2008, 77, 627–636. [Google Scholar] [CrossRef]
- Sequeira, V.; Nijenkamp, L.L.; Regan, J.A.; Van Der Velden, J. The physiological role of cardiac cytoskeleton and its alterations in heart failure. Biochim. Biophys. Acta (BBA) Biomembr. 2014, 1838, 700–722. [Google Scholar] [CrossRef] [PubMed]
- Gorski, P.A.; Ceholski, D.K.; Hajjar, R.J. Altered myocardial calcium cycling and energetics in heart failure —A rational approach for disease treatment. Cell Metab. 2015, 21, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Lou, Q.; Janardhan, A.; Efimov, I.R. Remodeling of Calcium Handling in Human Heart Failure. Pharm. Biotechnol. 2012, 740, 1145–1174. [Google Scholar]
- Fan, I.Q.; Chen, B.; Marsh, J.D. Transcriptional Regulation of L-type Calcium Channel Expression in Cardiac Myocytes. J. Mol. Cell. Cardiol. 2000, 32, 1841–1849. [Google Scholar] [CrossRef]
- Golden, K.L.; Ren, J.; Dean, A.; Marsh, J.D. Norepinephrine regulates the in vivo expression of the L-type calcium channel. Mol. Cell. Biochem. 2002, 236, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.T.; Wang, D.L.; Chen, W.P.; Hwang, J.J.; Hsieh, C.S.; Hsu, K.L.; Tseng, C.D.; Lai, L.P.; Tseng, Y.Z.; Chiang, F.T.; et al. Angiotensin II Increases Expression of α1C Subunit of L-Type Calcium Channel Through a Reactive Oxygen Species and cAMP Response Element–Binding Protein–Dependent Pathway in HL-1 Myocytes. Circ. Res. 2007, 100, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Schroder, E.; Magyar, J.; Burgess, D.; Andres, D.; Satin, J. Chronic verapamil treatment remodels ICa,L in mouse ventricle. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1906–H1916. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schroder, E.; Byse, M.; Satin, J. The L-Type Calcium Channel C-terminus Auto-regulates Transcription. Circ. Res. 2009, 104, 1373–1381. [Google Scholar] [CrossRef] [PubMed]
- Satin, J.; Schroder, E.A.; Crump, S.M. L-type Calcium Channel Auto-Regulation of Transcription. Cell Calcium 2011, 49, 306–313. [Google Scholar] [CrossRef]

| Groups | ||
|---|---|---|
| C (n = 14) | FR (n = 13) | |
| IBW (g) | 313 ± 41.2 | 301 ± 35.2 |
| FBW (g) | 445 ± 39.1 | 228 ± 19.1 * |
| Food intake (g/day) | 21.1 ± 2.2 | 10.6 ± 1.1 |
| Epididymal fat (g) | 9.60 ± 3.42 | 0.90 ± 0.56 * |
| Retroperitoneal fat (g) | 7.00 ± 2.80 | 0.19 ± 0.12 * |
| Visceral fat (g) | 5.24 ± 1.68 | 0.67 ± 0.33 * |
| Total body fat (g) | 21.8 ± 6.90 | 1.75 ± 0.70 * |
| Adiposity index | 4.86 ± 1.45 | 0.76 ± 0.27 * |
| Naso-anal length (cm) | 27.5 ± 0.70 | 24.6 ± 0.80 * |
| Soleus muscle (g) | 0.19 ± 0.03 | 0.10 ± 0.01 * |
| Lung (g) | 2.00 ± 0.41 | 1.19 ± 0.11 * |
| Tibia length (cm) | 4.38 ± 0.07 | 4.15 ± 0.03 * |
| IBW/FBW ratio (g/g) | 0.71 ± 0.10 | 1.33 ± 0.19 * |
| Groups | ||
|---|---|---|
| C (n = 14) | FR (n = 1 3) | |
| LV (g) | 0.84 ± 0.10 | 0.44 ± 0.05 * |
| RV(g) | 0.25 ± 0.04 | 0.12 ± 0.01 * |
| AT (g) | 0.10 ± 0.02 | 0.05 ± 0.01 * |
| Total heart(g) | 1.20 ± 0.15 | 0.62 ± 0.07 * |
| LV/FBW (mg/g) | 1.90 ± 0.22 | 1.93 ± 0.13 |
| RV/FBW (mg/g) | 0.56 ± 0.08 | 0.53 ± 0.03 |
| AT/FBW (mg/g) | 0.24 ± 0.04 | 0.24 ± 0.02 |
| Heart/FBW (mg/g) | 2.70 ± 0.32 | 2.70 ± 0.16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deus, A.F.d.; Silva, V.L.d.; de Souza, S.L.B.; Mota, G.A.F.; Sant’Ana, P.G.; Vileigas, D.F.; Lima-Leopoldo, A.P.; Leopoldo, A.S.; Campos, D.H.S.d.; de Tomasi, L.C.; et al. Myocardial Dysfunction after Severe Food Restriction Is Linked to Changes in the Calcium-Handling Properties in Rats. Nutrients 2019, 11, 1985. https://doi.org/10.3390/nu11091985
Deus AFd, Silva VLd, de Souza SLB, Mota GAF, Sant’Ana PG, Vileigas DF, Lima-Leopoldo AP, Leopoldo AS, Campos DHSd, de Tomasi LC, et al. Myocardial Dysfunction after Severe Food Restriction Is Linked to Changes in the Calcium-Handling Properties in Rats. Nutrients. 2019; 11(9):1985. https://doi.org/10.3390/nu11091985
Chicago/Turabian StyleDeus, Adriana Fernandes de, Vítor Loureiro da Silva, Sérgio Luiz Borges de Souza, Gustavo Augusto Ferreira Mota, Paula Grippa Sant’Ana, Danielle Fernandes Vileigas, Ana Paula Lima-Leopoldo, André Soares Leopoldo, Dijon Henrique Salomé de Campos, Loreta Casquel de Tomasi, and et al. 2019. "Myocardial Dysfunction after Severe Food Restriction Is Linked to Changes in the Calcium-Handling Properties in Rats" Nutrients 11, no. 9: 1985. https://doi.org/10.3390/nu11091985
APA StyleDeus, A. F. d., Silva, V. L. d., de Souza, S. L. B., Mota, G. A. F., Sant’Ana, P. G., Vileigas, D. F., Lima-Leopoldo, A. P., Leopoldo, A. S., Campos, D. H. S. d., de Tomasi, L. C., Padovani, C. R., Kolwicz, S. C., Jr., & Cicogna, A. C. (2019). Myocardial Dysfunction after Severe Food Restriction Is Linked to Changes in the Calcium-Handling Properties in Rats. Nutrients, 11(9), 1985. https://doi.org/10.3390/nu11091985