Neonatal Consumption of Oligosaccharides Greatly Increases L-Cell Density without Significant Consequence for Adult Eating Behavior
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animal Experiment
2.3. Tissue Collection
2.4. Eating Behavior
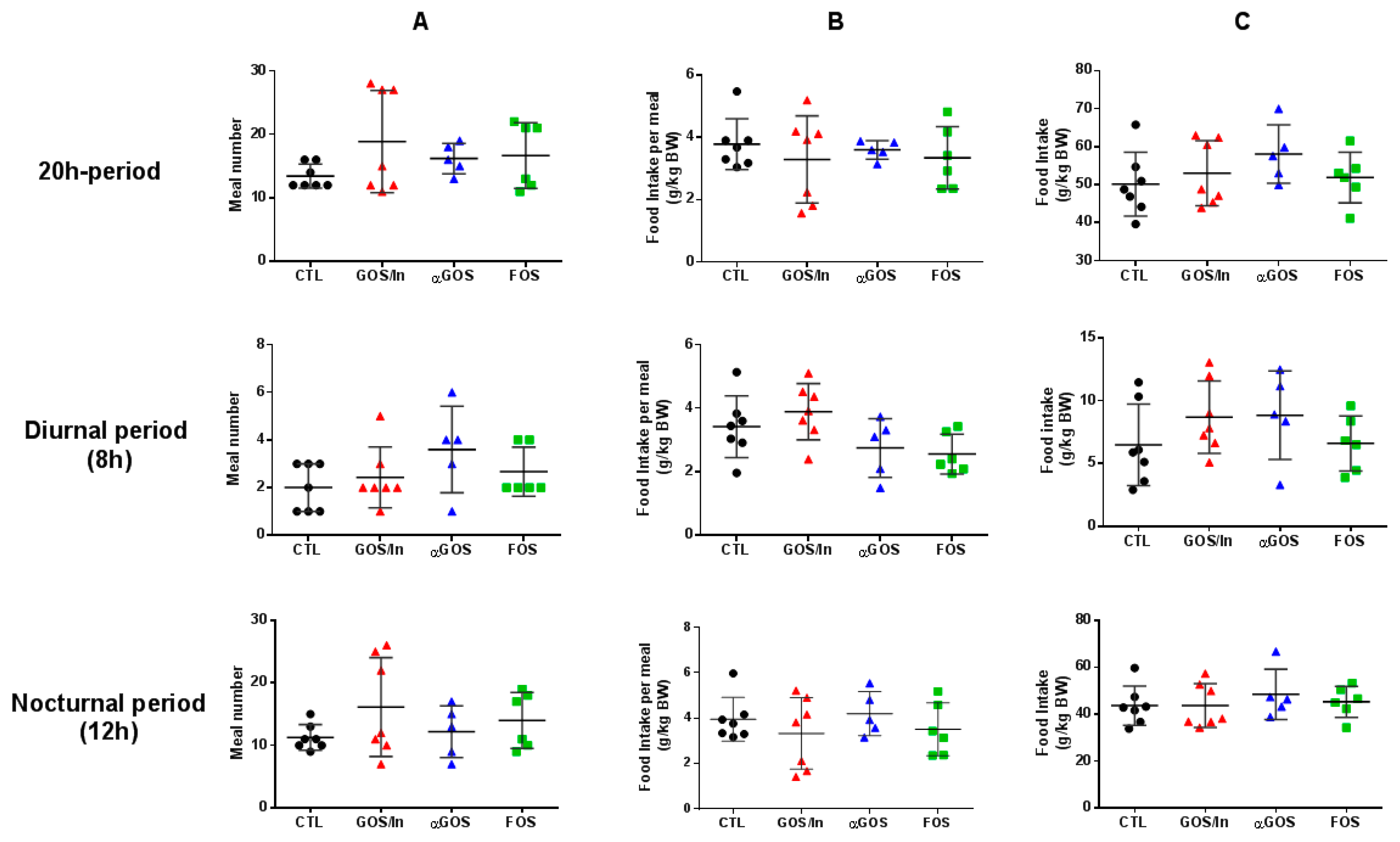
2.4.1. Meal Pattern
2.4.2. Taste Preference
2.5. Fasting-Refeeding Test, Kinetics of GLP-1 and PPY Release and Response to Glucose
2.6. Plasma Gastrointestinal Peptides
2.7. Fermentation End-Products
2.8. Immunochemistry
2.9. Quantitative Real-Time PCR
2.10. Bacterial 16S rDNA Sequencing of Cecal Contents
2.11. Statistical Analysis
3. Results
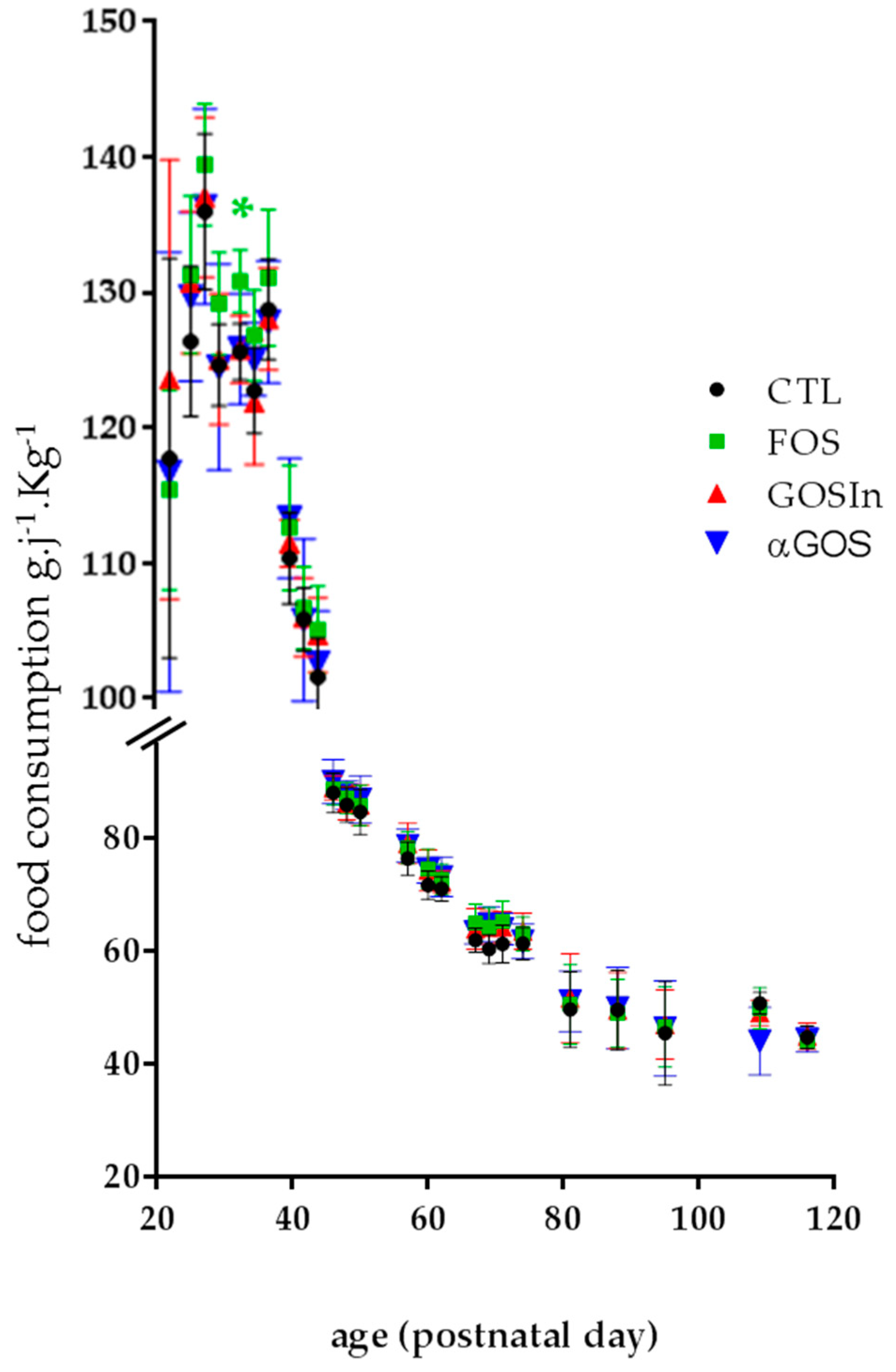
3.1. Neonatal OS Supplementation Did Not Substantially Affect Rat Growth
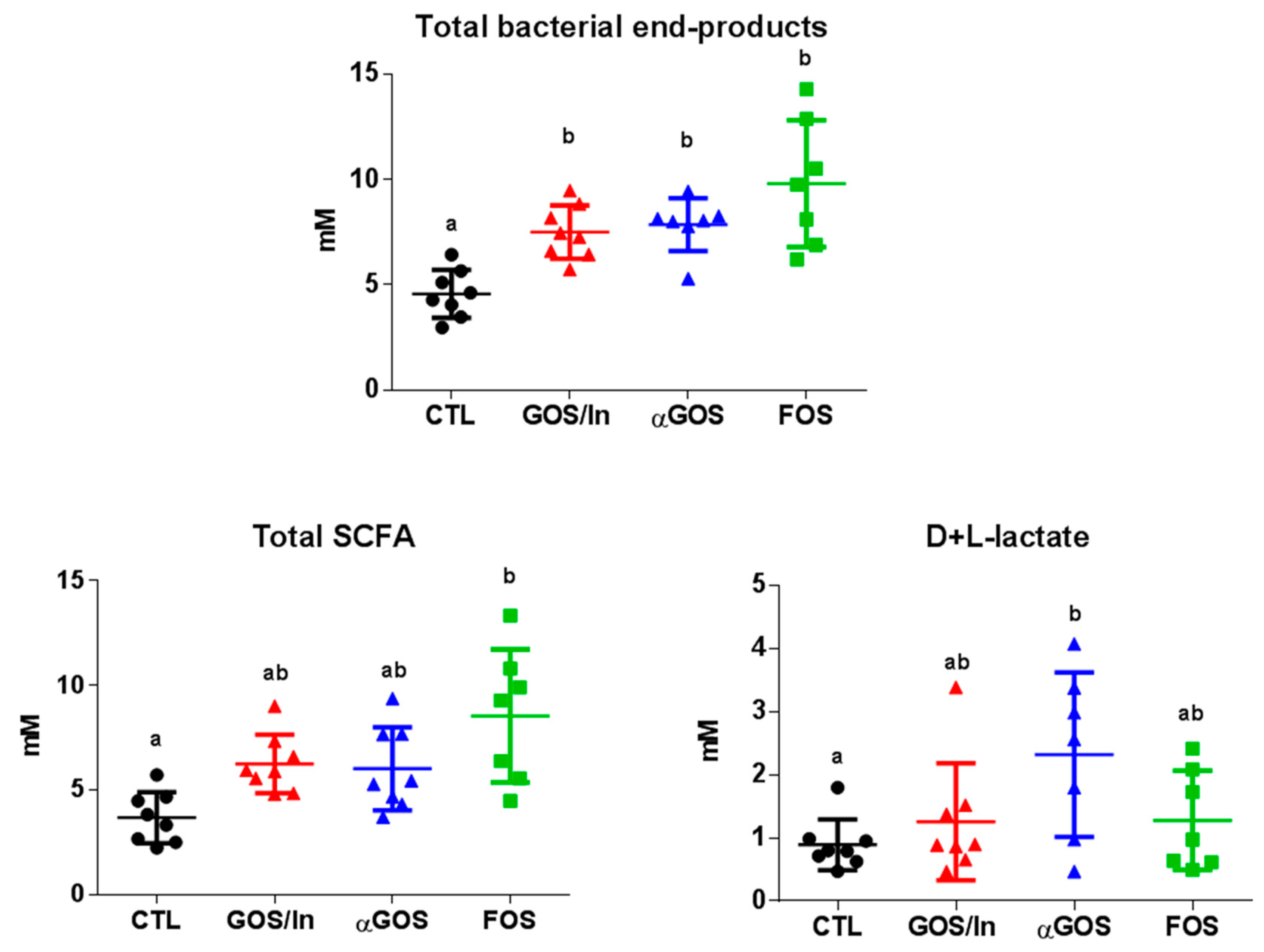
3.2. Neonatal OS Supplementation Exerted a Marked Immediate Impact on Intestinal Environment
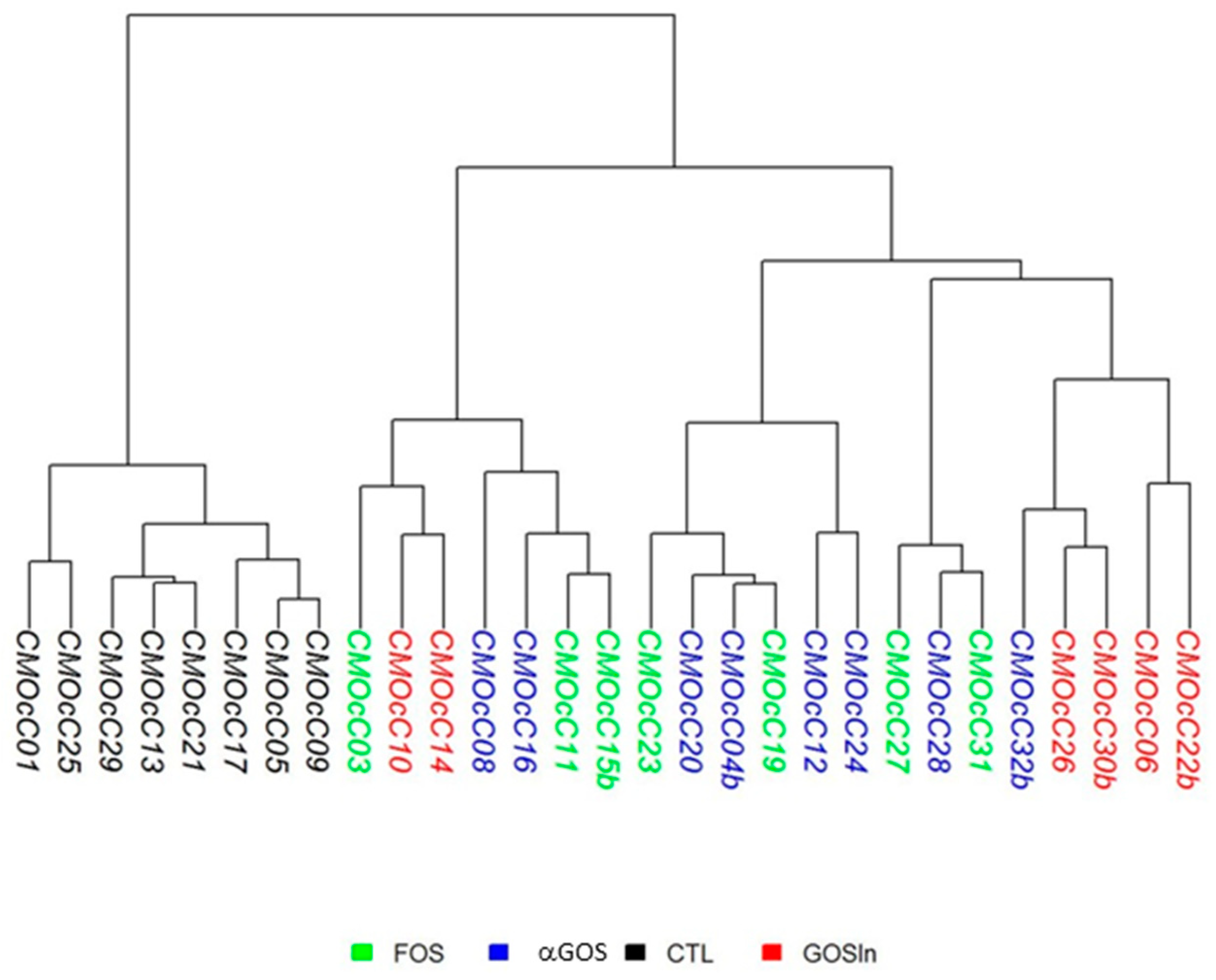
3.2.1. OS Supplementation Modified Both Composition and Activity of Neonatal Intestinal Microbiota
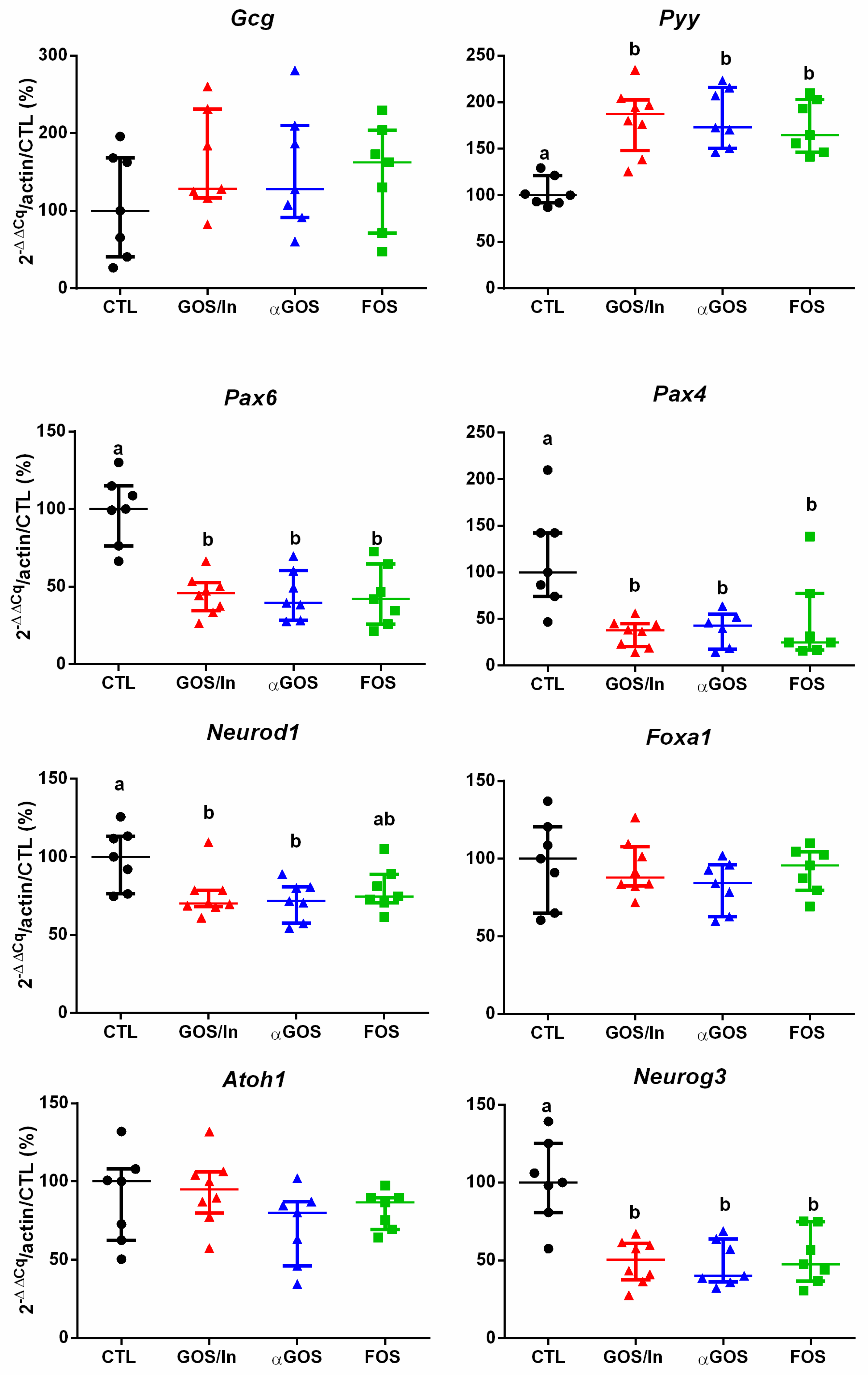
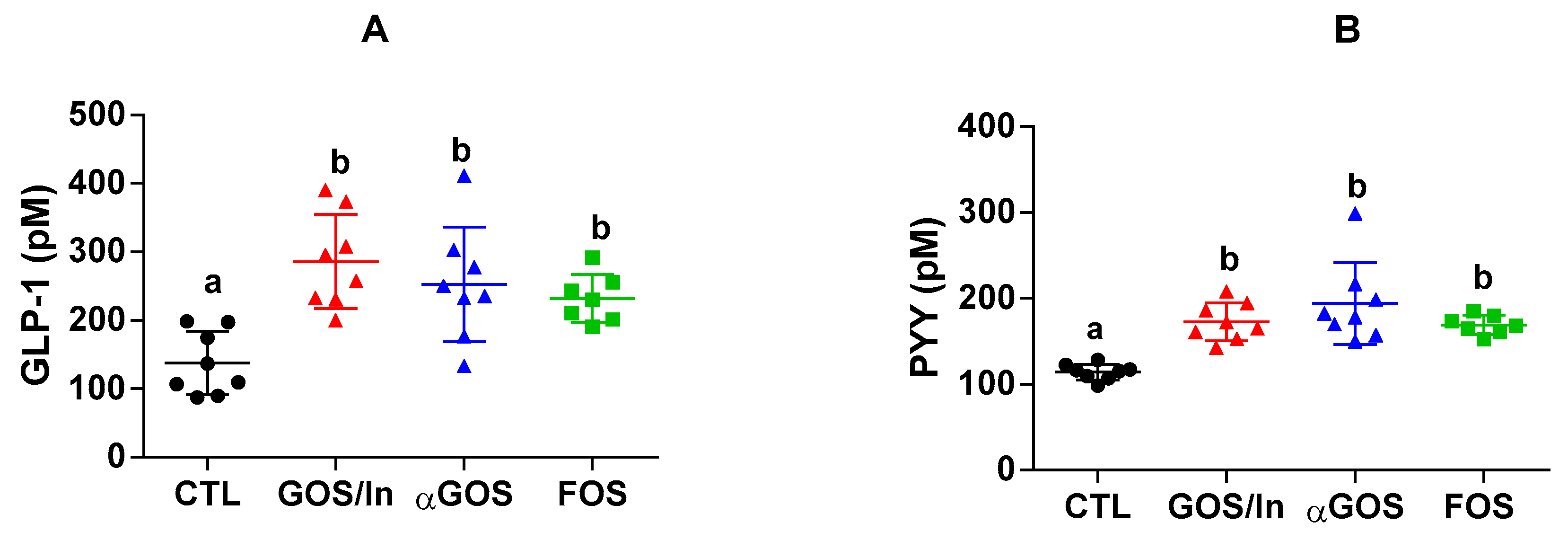
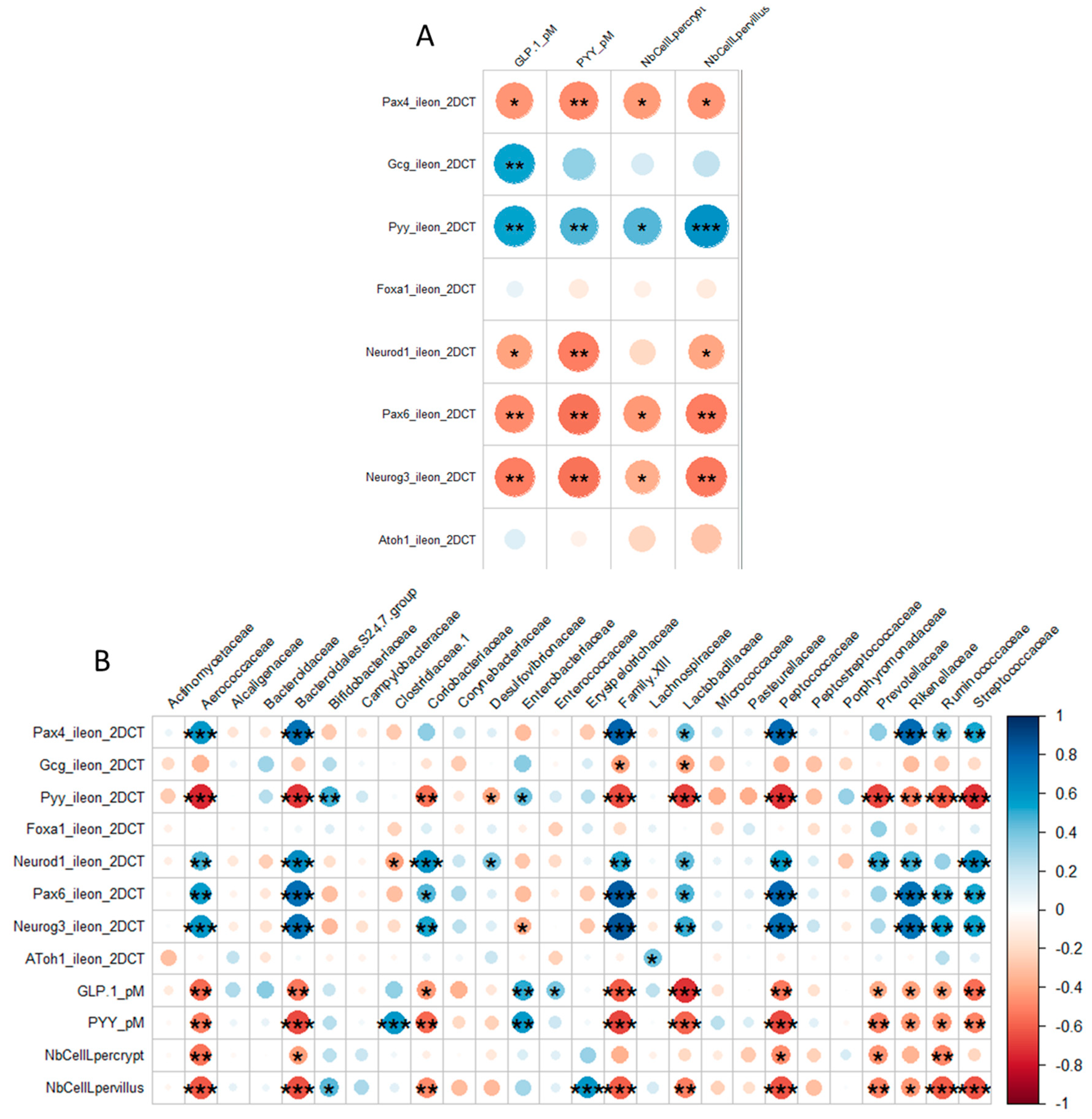
3.2.2. OS Supplementation Modified both Differentiation and Activity of the Neonatal EEC
3.3. Neonatal OS Supplementation Had No Significant Long-Term Consequences
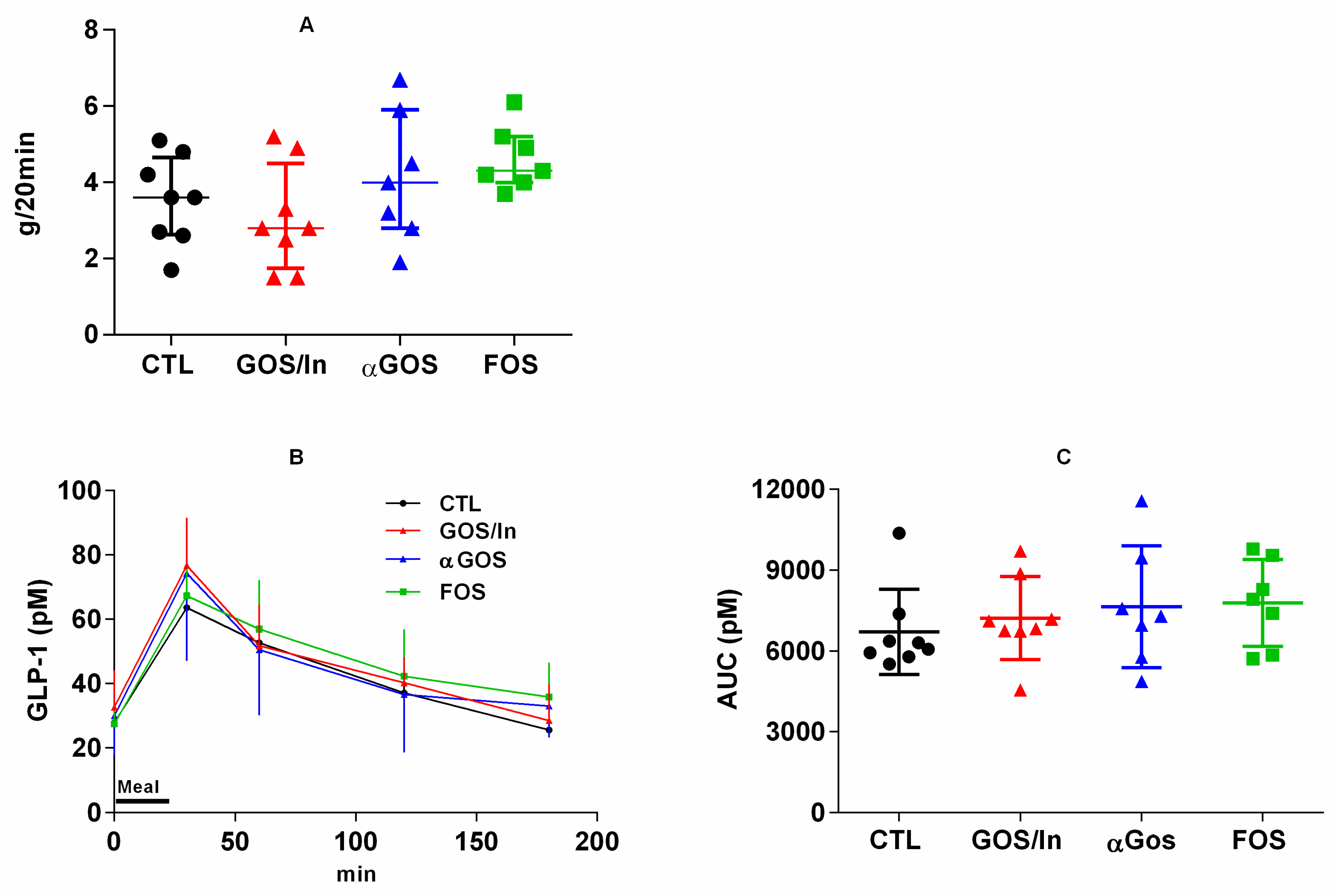
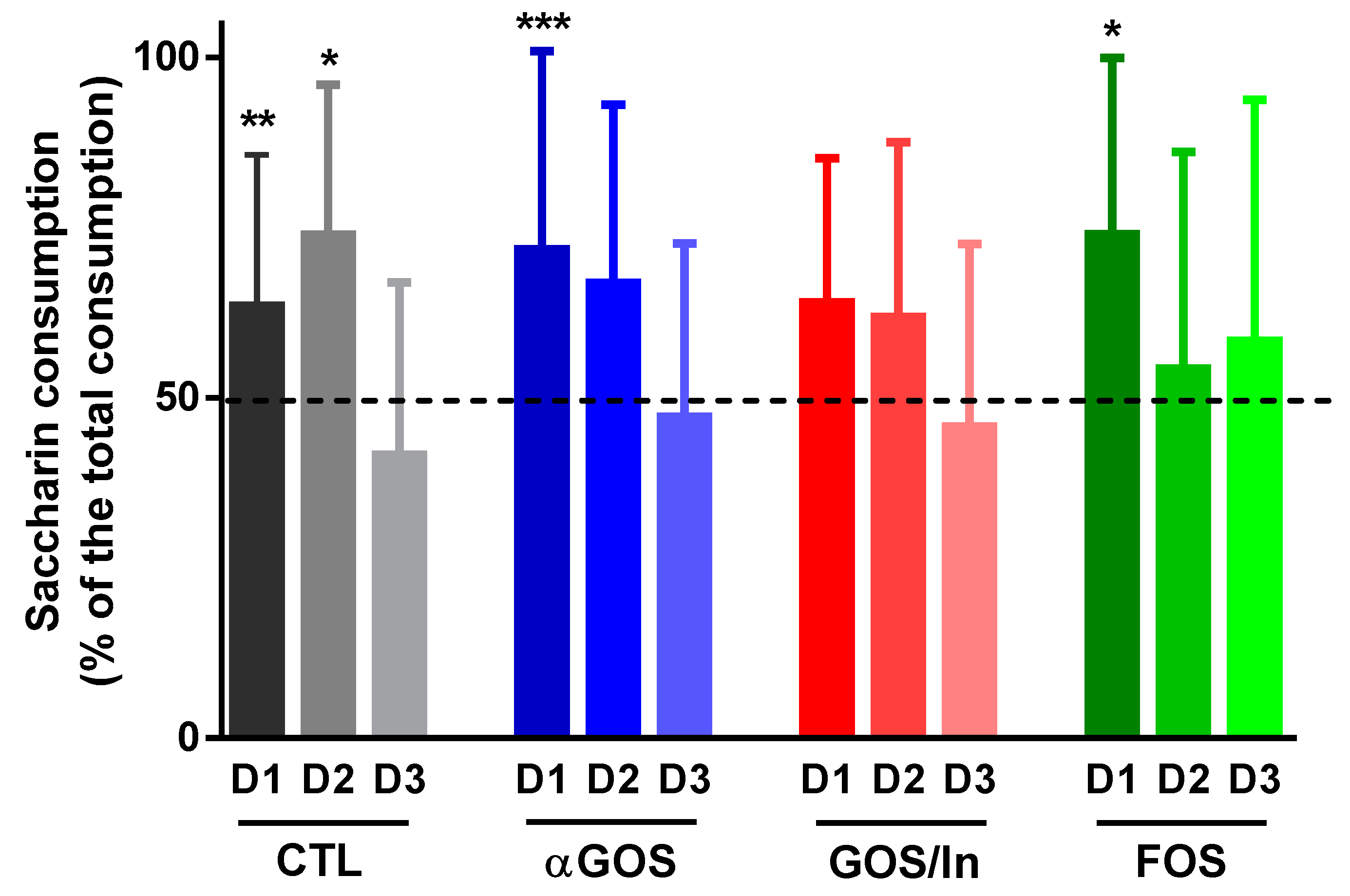
3.3.1. Neonatal OS Supplementation Did Not Significantly Program Enteropeptide Production or Eating Behavior in Adulthood
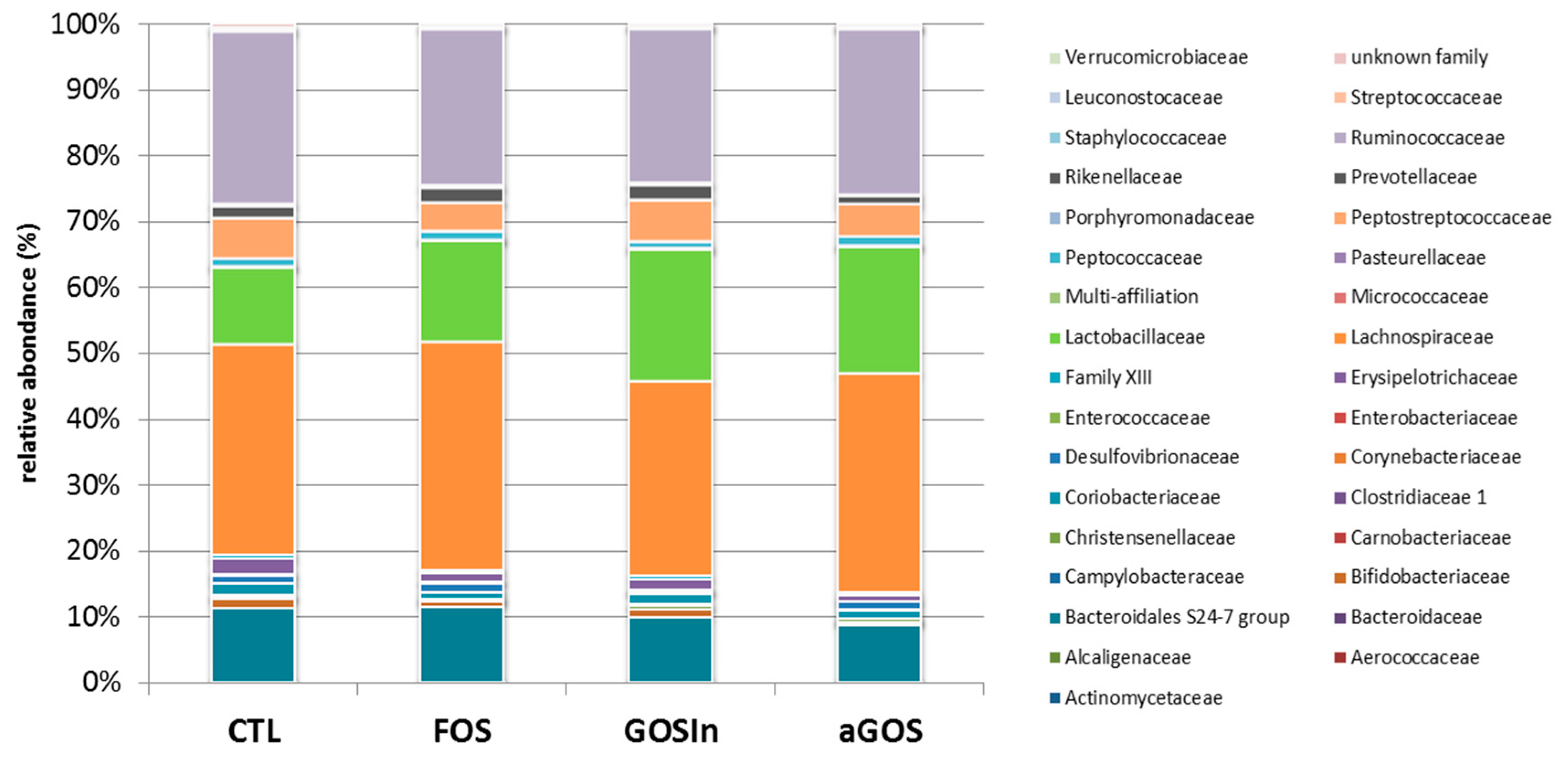
3.3.2. Neonatal OS Supplementation Did Not Significantly Program Adult Intestinal Microbiota
4. Discussion
4.1. Neonatal OS Supplementation Affected Intestinal Microbiota Despite Its Immaturity
4.2. OS Supplementation May Stimulate Ileal EECs to Produce GLP-1/PYY While Acting in Feedback on Endocrine Precursors
4.3. What are the Putative Mediators of the Massive Effect of Neonatal OS Supplementation on Ileal L-Cells?
4.4. OS impact on Eating Behavior, Usually Observed Simultaneously with Their Consumption, Does Not Seem to Be Programmable
4.5. Is Programming of the Microbiota Subject to Particular Timing?
4.6. All OS Studied Performed Similarly Despite Differences in Their Chemical Characteristics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lindsay, A.C.; Sitthisongkram, S.; Greaney, M.L.; Wallington, S.F.; Ruengdej, P. Non-Responsive Feeding Practices, Unhealthy Eating Behaviors, and Risk of Child Overweight and Obesity in Southeast Asia: A Systematic Review. Int. J. Env. Res. Public Health 2017, 14, 436. [Google Scholar] [CrossRef] [PubMed]
- MacLean, P.S.; Blundell, J.E.; Mennella, J.A.; Batterham, R.L. Biological control of appetite: A daunting complexity. Obesity 2017, 25, S8–S16. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, H.-R.; Sutton, G.M.; Townsend, R.L.; Patterson, L.M.; Zheng, H. Brainstem mechanisms integrating gut-derived satiety signals and descending forebrain information in the control of meal size. Physiol. Behav. 2006, 89, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Gaetani, S.; Romano, A.; Provensi, G.; Ricca, V.; Lutz, T.; Passani, M.B. Eating disorders: From bench to bedside and back. J. Neurochem. 2016, 139, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Cripps, R.L.; Martin-Gronert, M.S.; Ozanne, S.E. Fetal and perinatal programming of appetite. Clin. Sci. 2005, 109, 1–11. [Google Scholar] [CrossRef]
- Ross, M.G.; Desai, M. Developmental programming of appetite/satiety. Ann. Nutr. Metab. 2014, 64, 36–44. [Google Scholar] [CrossRef]
- Desai, M.; Gayle, D.; Han, G.; Ross, M.G. Programmed hyperphagia due to reduced anorexigenic mechanisms in intrauterine growth-restricted offspring. Reprod. Sci. 2007, 14, 329–337. [Google Scholar] [CrossRef]
- Coupé, B.; Delamaire, E.; Hoebler, C.; Grit, I.; Even, P.; Fromentin, G.; Darmaun, D.; Parnet, P. Hypothalamus integrity and appetite regulation in low birth weight rats reared artificially on a high-protein milk formula. J. Nutr. Biochem. 2011, 22, 956–963. [Google Scholar] [CrossRef]
- Ndjim, M.; Poinsignon, C.; Parnet, P.; Le Dréan, G. Loss of Vagal Sensitivity to Cholecystokinin in Rats Born with Intrauterine Growth Retardation and Consequence on Food Intake. Front. Endocrinol. 2017, 8, 65. [Google Scholar] [CrossRef]
- Martin Agnoux, A.; Alexandre-Gouabau, M.-C.; Le Dréan, G.; Antignac, J.-P.; Parnet, P. Relative contribution of foetal and post-natal nutritional periods on feeding regulation in adult rats. Acta Physiol. 2014, 210, 188–201. [Google Scholar] [CrossRef]
- Van Deutekom, A.W.; Chinapaw, M.J.M.; Jansma, E.P.; Vrijkotte, T.G.M.; Gemke, R.J.B.J. The Association of Birth Weight and Infant Growth with Energy Balance-Related Behavior—A Systematic Review and Best-Evidence Synthesis of Human Studies. PLoS ONE 2017, 12, e0168186. [Google Scholar] [CrossRef] [PubMed]
- Lussana, F.; Painter, R.C.; Ocke, M.C.; Buller, H.R.; Bossuyt, P.M.; Roseboom, T.J. Prenatal exposure to the Dutch famine is associated with a preference for fatty foods and a more atherogenic lipid profile. Am. J. Clin. Nutr. 2008, 88, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- Perälä, M.-M.; Männistö, S.; Kaartinen, N.E.; Kajantie, E.; Osmond, C.; Barker, D.J.P.; Valsta, L.M.; Eriksson, J.G. Body size at birth is associated with food and nutrient intake in adulthood. PLoS ONE 2012, 7, e46139. [Google Scholar] [CrossRef] [PubMed]
- van Deutekom, A.W.; Chinapaw, M.J.M.; Vrijkotte, T.G.M.; Gemke, R.J.B.J. The association of birth weight and postnatal growth with energy intake and eating behavior at 5 years of age—A birth cohort study. Int. J. Behav. Nutr. Phys. Act. 2016, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- De Cosmi, V.; Scaglioni, S.; Agostoni, C. Early Taste Experiences and Later Food Choices. Nutrients 2017, 9, 107. [Google Scholar] [CrossRef] [PubMed]
- Reimer, R.A.; McBurney, M.I. Dietary fiber modulates intestinal proglucagon messenger ribonucleic acid and postprandial secretion of glucagon-like peptide-1 and insulin in rats. Endocrinology 1996, 137, 3948–3956. [Google Scholar] [CrossRef] [PubMed]
- Kok, N.N.; Morgan, L.M.; Williams, C.M.; Roberfroid, M.B.; Thissen, J.P.; Delzenne, N.M. Insulin, glucagon-like peptide 1, glucose-dependent insulinotropic polypeptide and insulin-like growth factor I as putative mediators of the hypolipidemic effect of oligofructose in rats. J. Nutr. 1998, 128, 1099–1103. [Google Scholar] [CrossRef] [PubMed]
- Van de Wouw, M.; Schellekens, H.; Dinan, T.G.; Cryan, J.F. Microbiota-Gut-Brain Axis: Modulator of Host Metabolism and Appetite. J. Nutr. 2017, 147, 727–745. [Google Scholar] [CrossRef] [PubMed]
- Glenny, E.M.; Bulik-Sullivan, E.C.; Tang, Q.; Bulik, C.M.; Carroll, I.M. Eating Disorders and the Intestinal Microbiota: Mechanisms of Energy Homeostasis and Behavioral Influence. Curr. Psych. Rep. 2017, 19, 51. [Google Scholar] [CrossRef]
- Morita, C.; Tsuji, H.; Hata, T.; Gondo, M.; Takakura, S.; Kawai, K.; Yoshihara, K.; Ogata, K.; Nomoto, K.; Miyazaki, K.; et al. Gut Dysbiosis in Patients with Anorexia Nervosa. PLoS ONE 2015, 10, e0145274. [Google Scholar] [CrossRef]
- Rabot, S.; Membrez, M.; Bruneau, A.; Gérard, P.; Harach, T.; Moser, M.; Raymond, F.; Mansourian, R.; Chou, C.J. Germ-free C57BL/6J mice are resistant to high-fat diet-induced insulin resistance and have altered cholesterol metabolism. FASEB. J. 2010, 24, 4948–4959. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; Neyrinck, A.M.; Maton, N.; Delzenne, N.M. Oligofructose promotes satiety in rats fed a high-fat diet: Involvement of glucagon-like Peptide-1. Obes. Res. 2005, 13, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Maurer, A.D.; Chen, Q.; McPherson, C.; Reimer, R.A. Changes in satiety hormones and expression of genes involved in glucose and lipid metabolism in rats weaned onto diets high in fibre or protein reflect susceptibility to increased fat mass in adulthood. J. Physiol. 2009, 587, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Morel, F.B.; Oozeer, R.; Piloquet, H.; Moyon, T.; Pagniez, A.; Knol, J.; Darmaun, D.; Michel, C. Preweaning modulation of intestinal microbiota by oligosaccharides or amoxicillin can contribute to programming of adult microbiota in rats. Nutrition 2015, 31, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.; Chen, L.; Savignac, H.M.; Tzortzis, G.; Anthony, D.C.; Burnet, P.W.J. Neonatal prebiotic (BGOS) supplementation increases the levels of synaptophysin, GluN2A-subunits and BDNF proteins in the adult rat hippocampus. Synapse 2016, 70, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Estienne, M.; Claustre, J.; Clain-Gardechaux, G.; Paquet, A.; Taché, Y.; Fioramonti, J.; Plaisancié, P. Maternal deprivation alters epithelial secretory cell lineages in rat duodenum: Role of CRF-related peptides. Gut 2010, 59, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Mischke, M.; Plösch, T. More than just a gut instinct-the potential interplay between a baby’s nutrition, its gut microbiome, and the epigenome. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R1065–R1069. [Google Scholar] [CrossRef]
- Forssberg, H. Microbiome programming of brain development: Implications for neurodevelopmental disorders. Dev. Med. Child. Neurol. 2019, 61, 744–749. [Google Scholar] [CrossRef]
- Savignac, H.M.; Couch, Y.; Stratford, M.; Bannerman, D.M.; Tzortzis, G.; Anthony, D.C.; Burnet, P.W.J. Prebiotic administration normalizes lipopolysaccharide (LPS)-induced anxiety and cortical 5-HT2A receptor and IL1-β levels in male mice. Brain Behav. Immun. 2016, 52, 120–131. [Google Scholar] [CrossRef]
- Burokas, A.; Arboleya, S.; Moloney, R.D.; Peterson, V.L.; Murphy, K.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol. Psych. 2017, 82, 472–487. [Google Scholar] [CrossRef]
- Macfarlane, G.T.; Steed, H.; Macfarlane, S. Bacterial metabolism and health-related effects of galacto-oligosaccharides and other prebiotics. J. Appl. Microbiol. 2008, 104, 305–344. [Google Scholar] [CrossRef]
- Skórka, A.; Pieścik-Lech, M.; Kołodziej, M.; Szajewska, H. Infant formulae supplemented with prebiotics: Are they better than unsupplemented formulae? An updated systematic review. Br. J. Nutr. 2018, 119, 810–825. [Google Scholar] [CrossRef]
- Delzenne, N.M.; Cani, P.D.; Neyrinck, A.M. Modulation of glucagon-like peptide 1 and energy metabolism by inulin and oligofructose: Experimental data. J. Nutr. 2007, 137, 2547S–2551S. [Google Scholar] [CrossRef]
- Overduin, J.; Schoterman, M.H.C.; Calame, W.; Schonewille, A.J.; Ten Bruggencate, S.J.M. Dietary galacto-oligosaccharides and calcium: Effects on energy intake, fat-pad weight and satiety-related, gastrointestinal hormones in rats. Br. J. Nutr. 2013, 109, 1338–1348. [Google Scholar] [CrossRef]
- Sabater, C.; Prodanov, M.; Olano, A.; Corzo, N.; Montilla, A. Quantification of prebiotics in commercial infant formulas. Food Chem. 2016, 194, 6–11. [Google Scholar] [CrossRef]
- Silva, M.T. Saccharin aversion in the rat following adrenalectomy. Physiol. Behav. 1977, 19, 239–244. [Google Scholar] [CrossRef]
- Fança-Berthon, P.; Hoebler, C.; Mouzet, E.; David, A.; Michel, C. Intrauterine growth restriction not only modifies the cecocolonic microbiota in neonatal rats but also affects its activity in young adult rats. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 402–413. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Lozupone, C.A.; Turnbaugh, P.J.; Fierer, N.; Knight, R. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc. Natl. Acad. Sci. USA 2011, 108, 4516–4522. [Google Scholar] [CrossRef]
- Escudié, F.; Auer, L.; Bernard, M.; Mariadassou, M.; Cauquil, L.; Vidal, K.; Maman, S.; Hernandez-Raquet, G.; Combes, S.; Pascal, G. FROGS: Find, Rapidly, OTUs with Galaxy Solution. Bioinformatics 2018, 34, 1287–1294. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: https://www.R-project.org/ (accessed on 10 May 2019).
- Sample, C.H.; Davidson, T.L. Considering sex differences in the cognitive controls of feeding. Physiol. Behav. 2018, 187, 97–107. [Google Scholar] [CrossRef]
- Pueta, M.; Abate, P.; Haymal, O.B.; Spear, N.E.; Molina, J.C. Ethanol exposure during late gestation and nursing in the rat: Effects upon maternal care, ethanol metabolism and infantile milk intake. Pharmacol. Biochem. Behav. 2008, 91, 21–31. [Google Scholar] [CrossRef]
- Sevrin, T.; Alexandre-Gouabau, M.C.; Darmaun, D.; Palvadeau, A.; André, A.; Nguyen, P.; Ouguerram, K.; Boquien, C.Y. Use of water turnover method to measure mother’s milk flow in a rat model: Application to dams receiving a low protein diet during gestation and lactation. PLoS ONE 2017, 12, e0180550. [Google Scholar] [CrossRef]
- Fehlbaum, S.; Prudence, K.; Kieboom, J.; Heerikhuisen, M.; van den Broek, T.; Schuren, F.H.J.; Steinert, R.E.; Raederstorff, D. In Vitro Fermentation of Selected Prebiotics and Their Effects on the Composition and Activity of the Adult Gut Microbiota. Int. J. Mol. Sci. 2018, 19, 3097. [Google Scholar] [CrossRef]
- Liu, F.; Li, P.; Chen, M.; Luo, Y.; Prabhakar, M.; Zheng, H.; He, Y.; Qi, Q.; Long, H.; Zhang, Y.; et al. Fructooligosaccharide (FOS) and Galactooligosaccharide (GOS) Increase Bifidobacterium but Reduce Butyrate Producing Bacteria with Adverse Glycemic Metabolism in healthy young population. Sci. Rep. 2017, 7, 11789. [Google Scholar] [CrossRef]
- Wang, L.; Hu, L.; Yan, S.; Jiang, T.; Fang, S.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Effects of different oligosaccharides at various dosages on the composition of gut microbiota and short-chain fatty acids in mice with constipation. Food Funct. 2017, 8, 1966–1978. [Google Scholar] [CrossRef]
- Le Blay, G.; Michel, C.; Blottière, H.M.; Cherbut, C. Prolonged intake of fructo-oligosaccharides induces a short-term elevation of lactic acid-producing bacteria and a persistent increase in cecal butyrate in rats. J. Nutr. 1999, 129, 2231–2235. [Google Scholar] [CrossRef]
- Midtvedt, A.C.; Midtvedt, T. Production of short chain fatty acids by the intestinal microflora during the first 2 years of human life. J. Pediatr. Gastroenterol. Nutr. 1992, 15, 395–403. [Google Scholar] [CrossRef]
- Cani, P.D.; Dewever, C.; Delzenne, N.M. Inulin-type fructans modulate gastrointestinal peptides involved in appetite regulation (glucagon-like peptide-1 and ghrelin) in rats. Br. J. Nutr. 2004, 92, 521–526. [Google Scholar] [CrossRef]
- Cani, P.D.; Hoste, S.; Guiot, Y.; Delzenne, N.M. Dietary non-digestible carbohydrates promote L-cell differentiation in the proximal colon of rats. Br. J. Nutr. 2007, 98, 32–37. [Google Scholar] [CrossRef]
- Parnell, J.A.; Reimer, R.A. Prebiotic fibres dose-dependently increase satiety hormones and alter Bacteroidetes and Firmicutes in lean and obese JCR:LA-cp rats. Br. J. Nutr. 2012, 107, 601–613. [Google Scholar] [CrossRef]
- Li, H.J.; Ray, S.K.; Singh, N.K.; Johnston, B.; Leiter, A.B. Basic helix-loop-helix transcription factors and enteroendocrine cell differentiation. Diabetes Obes. Metab. 2011, 13, 5–12. [Google Scholar] [CrossRef]
- Engelstoft, M.S.; Egerod, K.L.; Lund, M.L.; Schwartz, T.W. Enteroendocrine cell types revisited. Curr. Opin. Pharm. 2013, 13, 912–921. [Google Scholar] [CrossRef]
- Grigoryan, M.; Kedees, M.H.; Charron, M.J.; Guz, Y.; Teitelman, G. Regulation of mouse intestinal L cell progenitors proliferation by the glucagon family of peptides. Endocrinology 2012, 153, 3076–3088. [Google Scholar] [CrossRef]
- Habib, A.M.; Richards, P.; Cairns, L.S.; Rogers, G.J.; Bannon, C.A.M.; Parker, H.E.; Morley, T.C.E.; Yeo, G.S.H.; Reimann, F.; Gribble, F.M. Overlap of endocrine hormone expression in the mouse intestine revealed by transcriptional profiling and flow cytometry. Endocrinology 2012, 153, 3054–3065. [Google Scholar] [CrossRef]
- Beumer, J.; Artegiani, B.; Post, Y.; Reimann, F.; Gribble, F.; Nguyen, T.N.; Zeng, H.; Van den Born, M.; Van Es, J.H.; Clevers, H. Enteroendocrine cells switch hormone expression along the crypt-to-villus BMP signalling gradient. Nat. Cell Biol. 2018, 20, 909–916. [Google Scholar] [CrossRef]
- Gehart, H.; van Es, J.H.; Hamer, K.; Beumer, J.; Kretzschmar, K.; Dekkers, J.F.; Rios, A.; Clevers, H. Identification of Enteroendocrine Regulators by Real-Time Single-Cell Differentiation Mapping. Cell 2019, 176, 1158–1173.e16. [Google Scholar] [CrossRef]
- Everard, A.; Lazarevic, V.; Derrien, M.; Girard, M.; Muccioli, G.G.; Muccioli, G.M.; Neyrinck, A.M.; Possemiers, S.; Van Holle, A.; François, P.; et al. Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 2011, 60, 2775–2786. [Google Scholar] [CrossRef]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef]
- Arora, T.; Akrami, R.; Pais, R.; Bergqvist, L.; Johansson, B.R.; Schwartz, T.W.; Reimann, F.; Gribble, F.M.; Bäckhed, F. Microbial regulation of the L cell transcriptome. Sci. Rep. 2018, 8, 1207. [Google Scholar] [CrossRef]
- Zhou, J.; Martin, R.J.; Tulley, R.T.; Raggio, A.M.; McCutcheon, K.L.; Shen, L.; Danna, S.C.; Tripathy, S.; Hegsted, M.; Keenan, M.J. Dietary resistant starch upregulates total GLP-1 and PYY in a sustained day-long manner through fermentation in rodents. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E1160–E1166. [Google Scholar] [CrossRef]
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Larraufie, P.; Martin-Gallausiaux, C.; Lapaque, N.; Dore, J.; Gribble, F.M.; Reimann, F.; Blottiere, H.M. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci. Rep. 2018, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Panwar, H.; Calderwood, D.; Gillespie, A.L.; Wylie, A.R.; Graham, S.F.; Grant, I.R.; Grover, S.; Green, B.D. Identification of lactic acid bacteria strains modulating incretin hormone secretion and gene expression in enteroendocrine cells. J. Funct. Foods 2016, 23, 348–358. [Google Scholar] [CrossRef]
- Cani, P.D.; Lecourt, E.; Dewulf, E.M.; Sohet, F.M.; Pachikian, B.D.; Naslain, D.; De Backer, F.; Neyrinck, A.M.; Delzenne, N.M. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am. J. Clin. Nutr. 2009, 90, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, C.; Lefevre, S.; Peters, V.; Patterson, M.; Ghatei, M.A.; Morgan, L.M.; Frost, G.S. Gut hormone release and appetite regulation in healthy non-obese participants following oligofructose intake. A dose-escalation study. Appetite 2013, 66, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Morel, F.B.; Dai, Q.; Ni, J.; Thomas, D.; Parnet, P.; Fança-Berthon, P. α-Galacto-oligosaccharides Dose-Dependently Reduce Appetite and Decrease Inflammation in Overweight Adults. J. Nutr. 2015, 145, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Reimer, R.A.; Willis, H.J.; Tunnicliffe, J.M.; Park, H.; Madsen, K.L.; Soto-Vaca, A. Inulin-type fructans and whey protein both modulate appetite but only fructans alter gut microbiota in adults with overweight/obesity: A randomized controlled trial. Mol. Nutr Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J., Jr.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G.; et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef]
- Frost, G.; Sleeth, M.L.; Sahuri-Arisoylu, M.; Lizarbe, B.; Cerdan, S.; Brody, L.; Anastasovska, J.; Ghourab, S.; Hankir, M.; Zhang, S.; et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat. Commun. 2014, 5, 3611. [Google Scholar] [CrossRef]
- Li, Z.; Yi, C.X.; Katiraei, S.; Kooijman, S.; Zhou, E.; Chung, C.K.; Gao, Y.; van den Heuvel, J.K.; Meijer, O.C.; Berbée, J.F.P.; et al. Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut 2018, 67, 1269–1279. [Google Scholar] [CrossRef]
- Perry, R.J.; Peng, L.; Barry, N.A.; Cline, G.W.; Zhang, D.; Cardone, R.L.; Petersen, K.F.; Kibbey, R.G.; Goodman, A.L.; Shulman, G.I. Acetate mediates a microbiome-brain-β-cell axis to promote metabolic syndrome. Nature 2016, 534, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Darzi, J.; Frost, G.S.; Robertson, M.D. Do SCFA have a role in appetite regulation? Proc. Nutr. Soc. 2011, 70, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.S.; Chambers, E.S.; Alhabeeb, H.; Chhina, N.; Morrison, D.J.; Preston, T.; Tedford, C.; Fitzpatrick, J.; Irani, C.; Busza, A.; et al. Increased colonic propionate reduces anticipatory reward responses in the human striatum to high-energy foods. Am. J. Clin. Nutr. 2016, 104, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Viardot, A.; Psichas, A.; Morrison, D.J.; Murphy, K.G.; Zac-Varghese, S.E.K.; MacDougall, K.; Preston, T.; Tedford, C.; Finlayson, G.S.; et al. Effects of targeted delivery of propionate to the human colon on appetite regulation, body weight maintenance and adiposity in overweight adults. Gut 2015, 64, 1744–1754. [Google Scholar] [CrossRef] [PubMed]
- Tomas, J.; Langella, P.; Cherbuy, C. The intestinal microbiota in the rat model: Major breakthroughs from new technologies. Anim. Health Res. Rev. 2012, 13, 54–63. [Google Scholar] [CrossRef]
- Fujiwara, R.; Takemura, N.; Watanabe, J.; Sonoyama, K. Maternal consumption of fructo-oligosaccharide diminishes the severity of skin inflammation in offspring of NC/Nga mice. Br. J. Nutr. 2010, 103, 530–538. [Google Scholar] [CrossRef]
- Le Bourgot, C.; Ferret-Bernard, S.; Apper, E.; Taminiau, B.; Cahu, A.; Le Normand, L.; Respondek, F.; Le Huërou-Luron, I.; Blat, S. Perinatal short-chain fructooligosaccharides program intestinal microbiota and improve enteroinsular axis function and inflammatory status in high-fat diet-fed adult pigs. FASEB J. 2019, 33, 301–313. [Google Scholar] [CrossRef]
- Koenig, J.E.; Spor, A.; Scalfone, N.; Fricker, A.D.; Stombaugh, J.; Knight, R.; Angenent, L.T.; Ley, R.E. Succession of microbial consortia in the developing infant gut microbiome. Proc. Natl. Acad. Sci. USA 2011, 108, 4578–4585. [Google Scholar] [CrossRef]
- Falony, G.; Joossens, M.; Vieira-Silva, S.; Wang, J.; Darzi, Y.; Faust, K.; Kurilshikov, A.; Bonder, M.J.; Valles-Colomer, M.; Vandeputte, D.; et al. Population-level analysis of gut microbiome variation. Science 2016, 352, 560–564. [Google Scholar] [CrossRef]
- Mackie, R.I.; Sghir, A.; Gaskins, H.R. Developmental microbial ecology of the neonatal gastrointestinal tract. Am. J. Clin. Nutr. 1999, 69, 1035S–1045S. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J.; Michel, C. How to Manipulate the Microbiota: Prebiotics. Adv. Exp. Med. Biol. 2016, 902, 119–142. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.C.; Edwards, C.A.; Morrison, D.J. Impact of Glycosidic Bond Configuration on Short Chain Fatty Acid Production from Model Fermentable Carbohydrates by the Human Gut Microbiota. Nutrients 2017, 9, 26. [Google Scholar] [CrossRef]

| CTL | FOS | GOS/In | αGOS | |
|---|---|---|---|---|
| GOS syrup (VivinalGOS, FrieslandCampina Domo, LE Amersfoort, The Netherlands) | 0.65 | |||
| Inulin powder (Raftiline HP, BENEO-Orafti S.A., Tienen, Belgium) | 0.03 | |||
| FOS powder (Beneo P95, BENEO-Orafti S.A., Tienen, Belgium) | 0.34 | |||
| αGOS powder (Olygose, Venette, France) | 0.32 | |||
| α-Lactose monohydrate (L3625, Sigma-Aldrich, St. Quentin Fallavier, France) | 0.096 | 0.096 | 0.096 | |
| D(+)-glucose monohydrate (108342, Merck Santé SAS, Fontenay sous Bois, France) | 0.087 | 0.082 | 0.087 | |
| D(+)-galactose monohydrate (104058, Merck Santé SAS, Fontenay sous Bois, France) | 0.005 | 0.005 | 0.004 | |
| D(−)-fructose (F0127, Sigma-Aldrich, St. Quentin Fallavier, France) | 0.015 | 0.015 | 0.015 | |
| Saccharose (S9378, Sigma-Aldrich, St. Quentin Fallavier, France) | 0.002 | 0.002 | 0.002 | |
| Total oligosaccharides § | 0.30 | 0.30 | 0.30 | |
| Total digestible sugars § ‼ | 0.20 | 0.20 | 0.20 | 0.20 |
| Treatment | BW Gain PND0-14 | BW Gain PND0-20 |
|---|---|---|
| CTL | 30.4 ± 4.2 1 | 50.5 ± 6.0 |
| FOS | 28.0 ± 3.4 | 45.7 ± 4.6 |
| GOS/In | 29.4 ± 3.3 | 49.6 ± 5.8 |
| αGOS | 27.9 ± 2.7 | 46.8 ± 5.1 |
| Family | CTL | FOS | GOSIn | αGOS |
|---|---|---|---|---|
| Actinomycetaceae | 0.095 ± 0.084 1 | 0.028 ± 0.032 | 0.076 ± 0.056 | 0.068 ± 0.058 |
| Aerococcaceae | 0.086 ± 0.029 a,2 | 0.011 ± 0.011b | 0.015 ± 0.012 b | 0.020 ± 0.019 b |
| Alcaligenaceae | 0.020 ± 0.041 | 0.030 ± 0.047 | 0.378 ± 0.576 | 0.036 ± 0.053 |
| Bacteroidaceae | 2.352 ± 0.991 | 6.510 ± 10.047 | 6.837 ± 5.729 | 3.719 ± 5.492 |
| Bacteroidales.S24.7 group | 6.812 ± 2.953 a | 0.053 ± 0.080 b | 0.098 ± 0.094 b | 0.084 ± 0.103 b |
| Bifidobacteriaceae | 0.624 ± 0.45 a | 17.188 ± 12.735 b | 7.894 ± 7.947 ab | 13.577 ± 10.631 b |
| Campylobacteraceae | 0.009 ± 0.024 | 0.093 ± 0.220 | 0.066 ± 0.151 | 0.294 ± 0.546 |
| Clostridiaceae.1 | 0.273 ± 0.146 | 2.413 ± 3.231 | 5.509 ± 8.749 | 5.044 ± 4.581 |
| Coriobacteriaceae | 0.108 ± 0.039 a | 0.039 ± 0.034 b | 0.036 ± 0.041 b | 0.023 ± 0.018 b |
| Corynebacteriaceae | 0.032 ± 0.023 | 0.007 ± 0.011 | 0.020 ± 0.030 | 0.012 ± 0.023 |
| Desulfovibrionaceae | 0.098 ± 0.182 | 0.000 ± 0.000 | 0.003 ± 0.008 | 0.006 ± 0.014 |
| Enterobacteriaceae | 13.86 ± 5.97 a | 23.48 ± 12.23 ab | 19.51 ± 6.69 b | 33.42 ± 11.99 b |
| Enterococcaceae | 0.435 ± 0.707 | 0.145 ± 0.203 | 2.892 ± 6.293 | 0.542 ± 0.771 |
| Erysipelotrichaceae | 0.682 ± 0.387 | 4.080 ± 3.988 | 3.774 ± 4.950 | 2.766 ± 3.002 |
| Family.XIII | 0.062 ± 0.030 a | 0.004 ± 0.008 b | 0.000 ± 0.000 b | 0.001 ± 0.003 b |
| Lachnospiraceae | 6.327 ± 2.300 a | 9.787 ± 6.180 ab | 15.298 ± 9.544 b | 4.962 ± 4.587 b |
| Lactobacillaceae | 57.47 ± 8.72 a | 28.74 ± 10.84 b | 24.47 ± 5.71 b | 31.13 ± 11.24 b |
| Micrococcaceae | 0.140 ± 0.064 | 0.075 ± 0.074 | 0.071 ± 0.053 | 0.110 ± 0.105 |
| Pasteurellaceae | 0.582 ± 0.581 | 0.236 ± 0.235 | 0.456 ± 0.297 | 0.394 ± 0.446 |
| Peptococcaceae | 0.396 ± 0.182 a | 0.006 ± 0.015 b | 0.015 ± 0.019 b | 0.007 ± 0.021 b |
| Peptostreptococcaceae | 0.747 ± 0.485 | 0.471 ± 0.262 | 0.543 ± 0.108 | 0.640 ± 0.379 |
| Porphyromonadaceae | 1.242 ± 1.153 | 5.924 ± 9.747 | 9.826 ± 15.228 | 2.055 ± 5.475 |
| Prevotellaceae | 2.136 ± 1.540 a | 0.014 ± 0.016 b | 0.011 ± 0.018 b | 0.028 ± 0.060 b |
| Rikenellaceae | 0.034 ± 0.039 a | 0.001 ± 0.004 b | 0.000 ± 0.000 b | 0.001 ± 0.003 b |
| Ruminococcaceae | 3.242 ± 0.743 a | 0.135 ± 0.147 b | 1.610 ± 2.622 b | 0.406 ± 0.665 b |
| Streptococcaceae | 2.118 ± 0.620 a | 0.510 ± 0.316 b | 0.586 ± 0.156 b | 0.643 ± 0.450 b |
| Treatment | Acetate | Propionate | Butyrate | pH |
|---|---|---|---|---|
| CTL | 3.17 ± 1.05 1,a,2 | 0.39 ± 0.16 | 0.07 ± 0.04 | 6.9 ±0.3 a |
| GOS/In | 5.82 ± 1.32 b | 0.33 ± 0.21 | 0.10 ± 0.09 | 6.3 ±0.2 b |
| αGOS | 5.69 ± 1.77 ab | 0.28 ± 0.27 | 0.05 ± 0.00 | 6.1 ±0.2 b |
| FOS | 8.00 ± 2.94 b | 0.47 ± 0.37 | 0.06 ± 0.04 | 6.2 ±0.2 b |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Dréan, G.; Pocheron, A.-L.; Billard, H.; Grit, I.; Pagniez, A.; Parnet, P.; Chappuis, E.; Rolli-Derkinderen, M.; Michel, C. Neonatal Consumption of Oligosaccharides Greatly Increases L-Cell Density without Significant Consequence for Adult Eating Behavior. Nutrients 2019, 11, 1967. https://doi.org/10.3390/nu11091967
Le Dréan G, Pocheron A-L, Billard H, Grit I, Pagniez A, Parnet P, Chappuis E, Rolli-Derkinderen M, Michel C. Neonatal Consumption of Oligosaccharides Greatly Increases L-Cell Density without Significant Consequence for Adult Eating Behavior. Nutrients. 2019; 11(9):1967. https://doi.org/10.3390/nu11091967
Chicago/Turabian StyleLe Dréan, Gwenola, Anne-Lise Pocheron, Hélène Billard, Isabelle Grit, Anthony Pagniez, Patricia Parnet, Eric Chappuis, Malvyne Rolli-Derkinderen, and Catherine Michel. 2019. "Neonatal Consumption of Oligosaccharides Greatly Increases L-Cell Density without Significant Consequence for Adult Eating Behavior" Nutrients 11, no. 9: 1967. https://doi.org/10.3390/nu11091967
APA StyleLe Dréan, G., Pocheron, A.-L., Billard, H., Grit, I., Pagniez, A., Parnet, P., Chappuis, E., Rolli-Derkinderen, M., & Michel, C. (2019). Neonatal Consumption of Oligosaccharides Greatly Increases L-Cell Density without Significant Consequence for Adult Eating Behavior. Nutrients, 11(9), 1967. https://doi.org/10.3390/nu11091967






