Macronutrient Analysis of Target-Pooled Donor Breast Milk and Corresponding Growth in Very Low Birth Weight Infants
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Sample
2.2. Milk Collection and Analysis
2.3. Standardized VLBW Feeding Protocol
2.4. Enteral Intake Data
2.5. Anthropometric Data
2.6. Statistical Analysis
3. Results
3.1. Study Infants
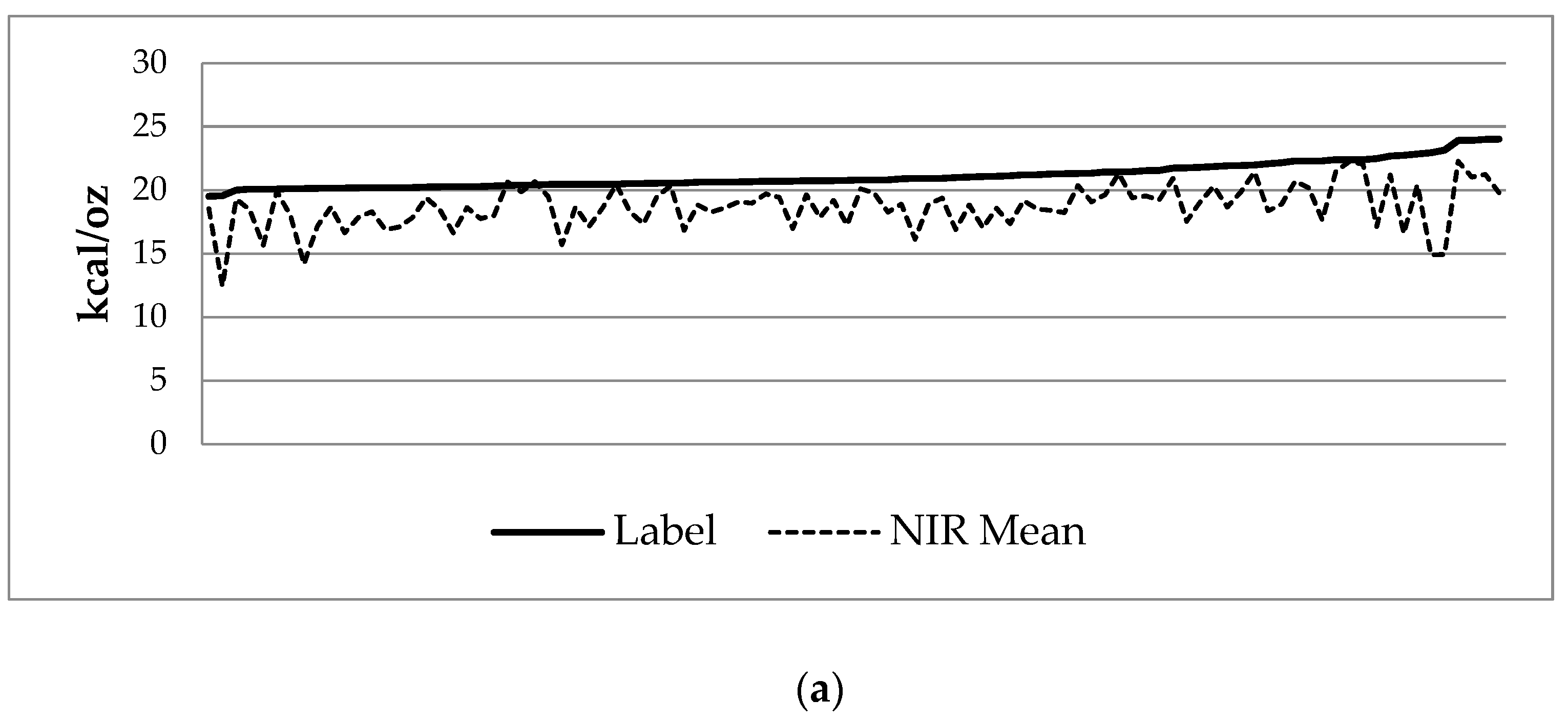
3.2. Donor Milk Analysis
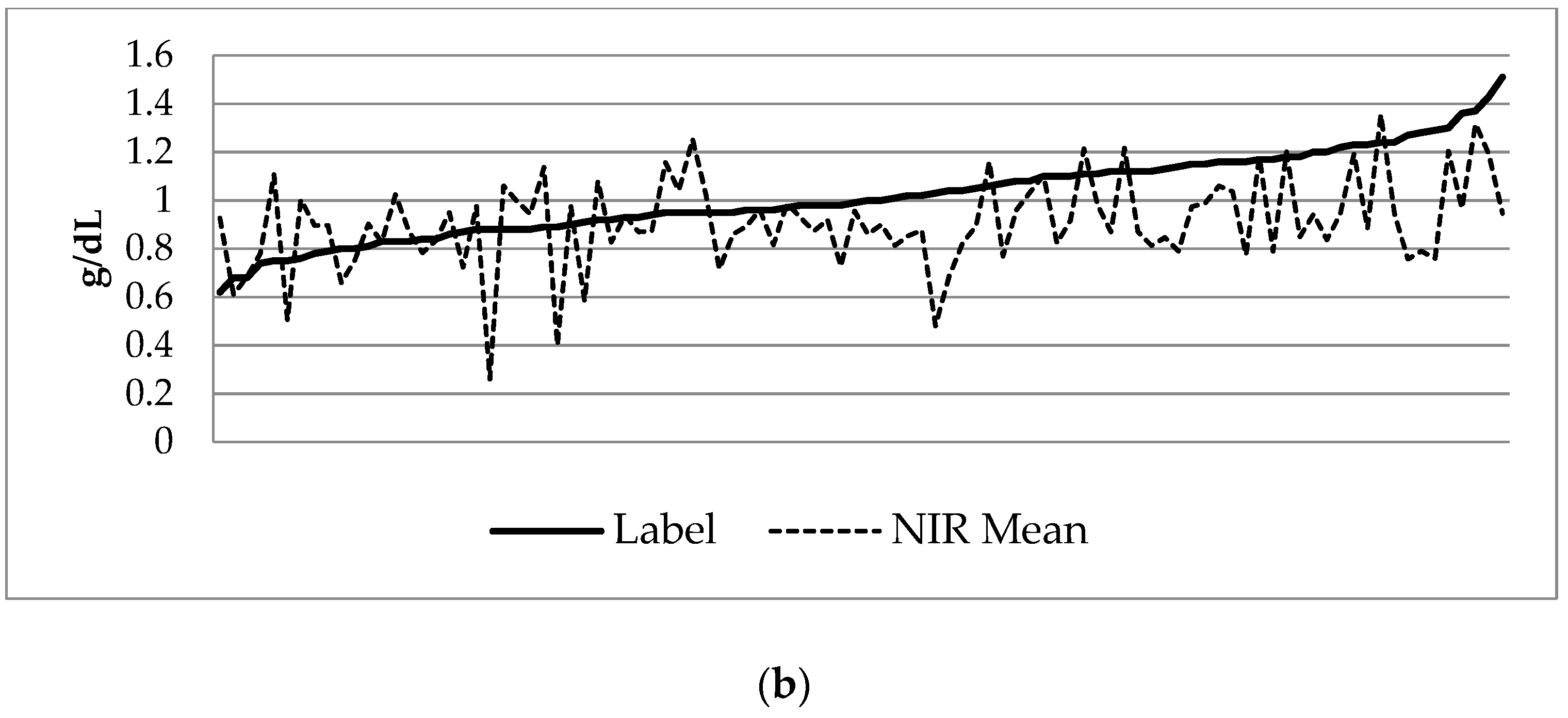
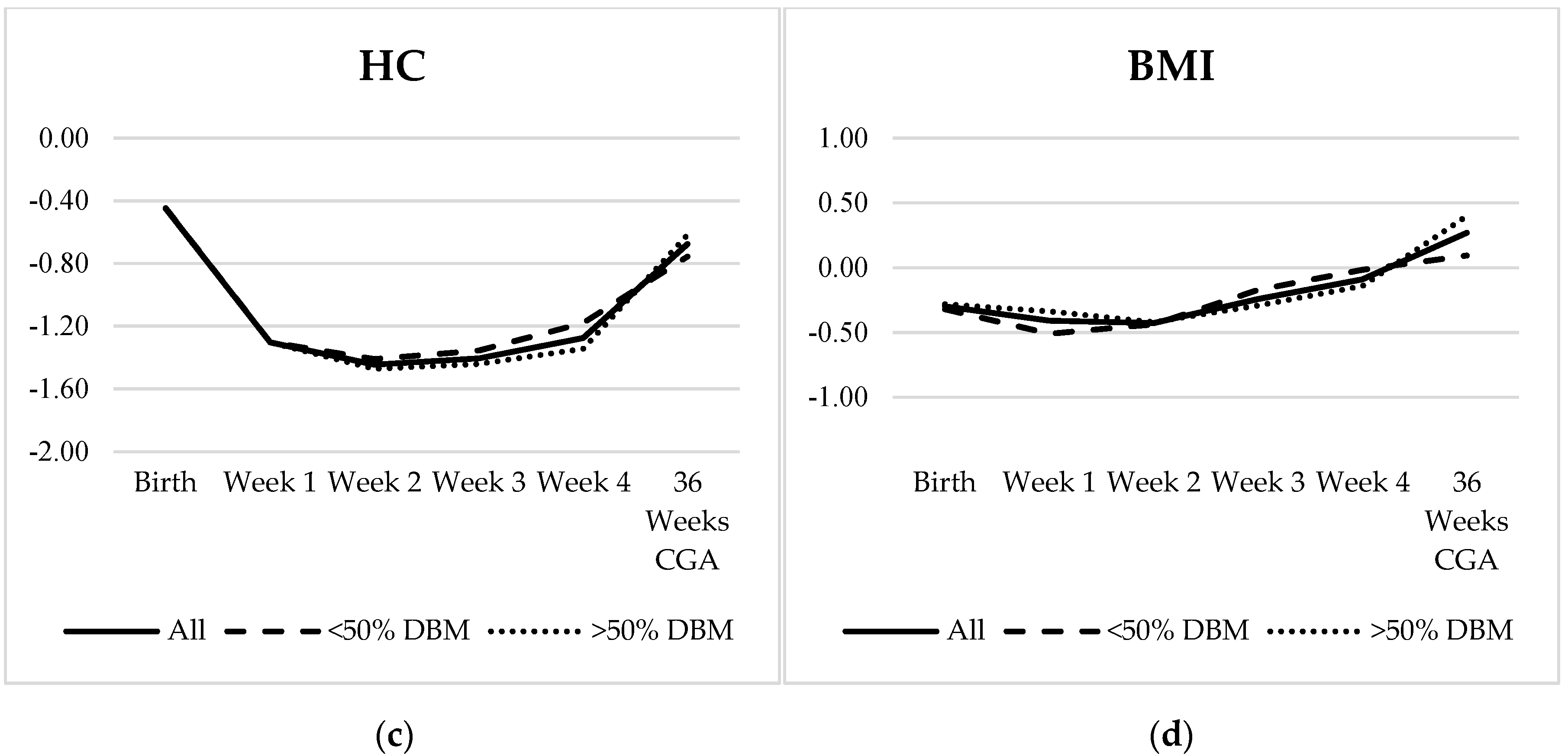
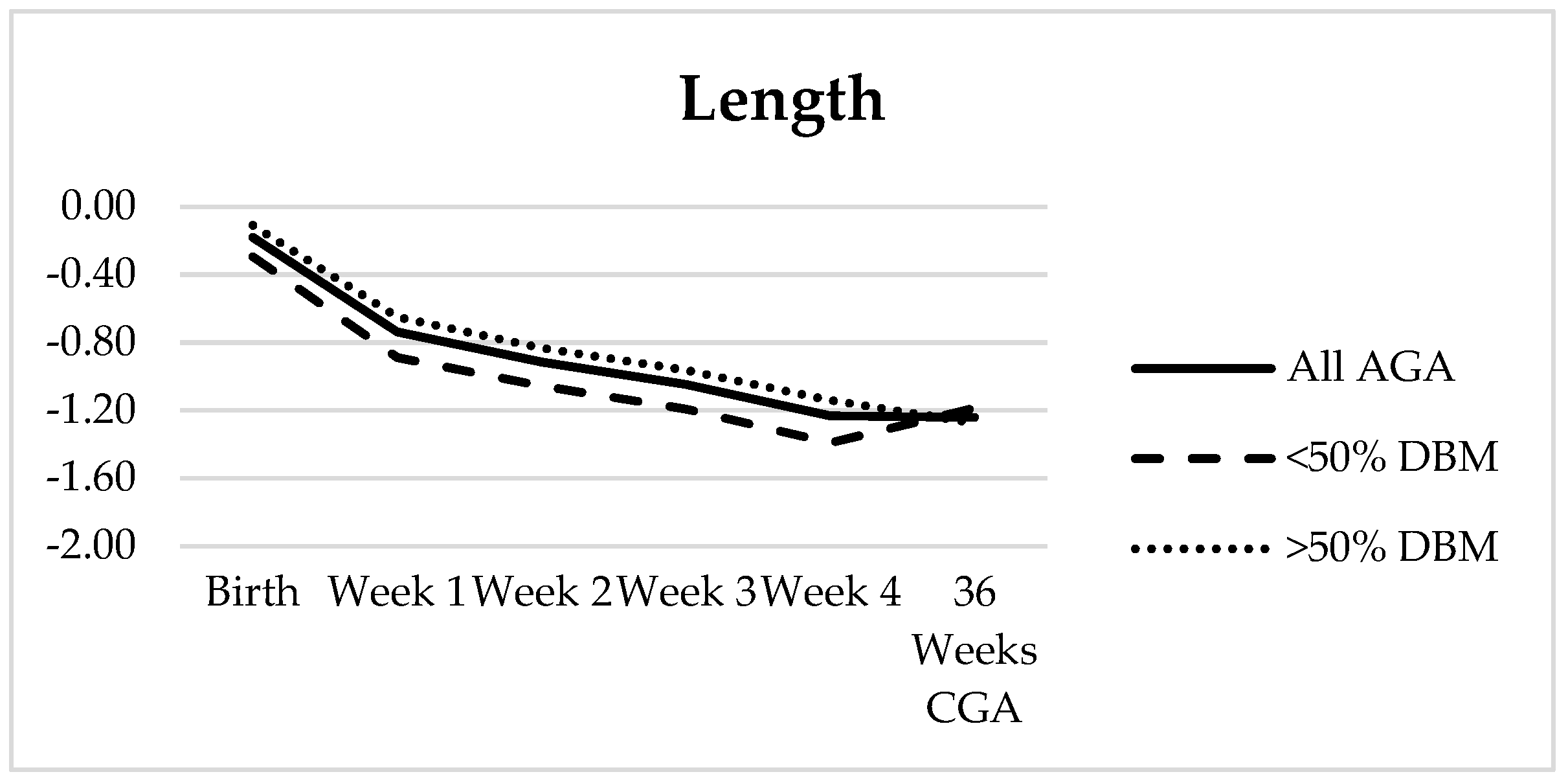
3.3. Growth Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tudehope, D.I. Human Milk and the Nutritional Needs of Preterm Infants. J. Pediatr. 2013, 162, S17–S25. [Google Scholar] [CrossRef] [PubMed]
- Ballard, O.; Morrow, A.L. Human Milk Composition. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Ramel, S.E.; Demerath, E.W.; Gray, H.L.; Younge, N.; Boys, C.; Georgieff, M.K. The relationship of poor linear growth velocity with neonatal illness and two-year neurodevelopment in preterm infants. Neonatology 2012, 102, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Stephens, B.E.; Vohr, B.R. Protein Intake and Neurodevelopmental Outcomes. Clin. Perinatol. 2014, 41, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Stephens, B.E.; Walden, R.V.; Gargus, R.A.; Tucker, R.; McKinley, L.; Mance, M.; Nye, J.; Vohr, B.R. First-Week protein and energy intakes are associated with 18-month developmental outcomes in extremely low birth weight infants. Pediatrics 2009, 123, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Radmacher, P.G.; Lewis, S.L.; Adamkin, D.H. Individualizing fortification of human milk using real time human milk analysis. J. Neonatal-Perinat. Med. 2013, 6, 319–323. [Google Scholar] [CrossRef]
- Ziegler, E.E. Human milk and human milk fortifiers. World Rev. Nutr. Diet. 2014, 110, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Agostoni, C.; Buonocore, G.; Carnielli, V.P.; De Curtis, M.; Darmaun, D.; Decsi, T.; Domellof, M.; Embleton, N.D.; Fusch, C.; Genzel-Boroviczeny, O.; et al. Enteral nutrient supply for preterm infants: Commentary from the European Society of Paediatric Gastroenterology, Hepatology and Nutrition Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2010, 50, 85–91. [Google Scholar] [CrossRef]
- Abbott Nutrition. Similac Human Milk Fortifier Hydrolyzed Protein Concentrated Liquid; Abbott: Columbus, OH, USA, 2016. [Google Scholar]
- Mead Johnson Nutrition. Enfamil Human Milk Fortifier Acidified Liquid. Available online: https://www.meadjohnson.com/pediatrics/us-en/product-information/products/newborns/enfamil-human-milk-fortifier-acidified-liquid (accessed on 19 October 2015).
- Schanler, R.J. Randomized trial of donor human milk versus preterm formula as substitutes for mothers’ own milk in the feeding of extremely premature infants. Pediatrics 2005, 116, 400–406. [Google Scholar] [CrossRef]
- Cristofalo, E.A.; Schanler, R.J.; Blanco, C.L.; Sullivan, S.; Trawoeger, R.; Kiechl-Kohlendorfer, U.; Dudell, G.; Rechtman, D.J.; Lee, M.L.; Lucas, A.; et al. Randomized Trial of Exclusive Human Milk versus Preterm Formula Diets in Extremely Premature Infants. J. Pediatr. 2013, 163, 1592–1595. [Google Scholar] [CrossRef]
- O’Connor, D.L.; Gibbins, S.; Kiss, A.; Bando, N.; Brennan-Donnan, J.; Ng, E.; Campbell, D.M.; Vaz, S.; Fusch, C.; Asztalos, E.; et al. Effect of supplemental donor human milk compared with preterm formula on neurodevelopment of very low-birth-weight infants at 18 months: A Randomized Clinical Trial. JAMA 2016, 316, 1897–1905. [Google Scholar] [CrossRef]
- Hair, A.B.; Hawthorne, K.M.; Chetta, K.E.; Abrams, S.A. Human milk feeding supports adequate growth in infants ≤ 1250 grams birth weight. BMC Res. Notes 2013, 6, 459. [Google Scholar] [CrossRef] [PubMed]
- Sisk, P.M.; Lambeth, T.M.; Rojas, M.A.; Lightbourne, T.; Barahona, M.; Anthony, E.; Auringer, S.T. Necrotizing Enterocolitis and Growth in Preterm Infants Fed Predominantly Maternal Milk, Pasteurized Donor Milk, or Preterm Formula: A Retrospective Study. Am. J. Perinatol. 2017, 34, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.E.; Lawson, M.L.; Ferguson, A.N.; Cantrell, R.; Grabich, S.C.; Zemel, B.S.; Clark, R.H. BMI Curves for Preterm Infants. Pediatrics 2015, 135, e572–e581. [Google Scholar] [CrossRef]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef]
- John, A.; Sun, R.; Maillart, L.; Schaefer, A.; Hamilton Spence, E.; Perrin, M.T. Macronutrient variability in human milk from donors to a milk bank: Implications for feeding preterm infants. PLoS ONE 2019, 14, e0210610. [Google Scholar] [CrossRef] [PubMed]
- Piemontese, P.; Mallardi, D.; Liotto, N.; Tabasso, C.; Menis, C.; Perrone, M.; Roggero, P.; Mosca, F. Macronutrient content of pooled donor human milk before and after Holder pasteurization. BMC Pediatr. 2019, 19, 58. [Google Scholar] [CrossRef] [PubMed]
- Vieira, A.A.; Soares, F.V.M.; Pimenta, H.P.; Abranches, A.D.; Moreira, M.E.L. Analysis of the influence of pasteurization, freezing/thawing, and offer processes on human milk’s macronutrient concentrations. Early Hum. Dev. 2011, 87, 577–580. [Google Scholar] [CrossRef]
- Colaizy, T.T.; Carlson, S.; Saftlas, A.F.; Morriss, F.H., Jr. Growth in VLBW infants fed predominantly fortified maternal and donor human milk diets: A retrospective cohort study. BMC Pediatr. 2012, 12, 124. [Google Scholar] [CrossRef]
- Fusch, G.; Rochow, N.; Choi, A.; Fusch, S.; Poeschl, S.; Ubah, A.O.; Lee, S.-Y.; Raja, P.; Fusch, C. Rapid measurement of macronutrients in breast milk: How reliable are infrared milk analyzers? Clin. Nutr. 2015, 34, 465–476. [Google Scholar] [CrossRef]
- Sauer, C.W.; Kim, J.H. Human milk macronutrient analysis using point-of-care near-infrared spectrophotometry. J. Perinatol. 2010, 31, 339–343. [Google Scholar] [CrossRef]
- Fusch, G.; Kwan, C.; Rochow, N.; Fusch, C. Milk analysis using milk analyzers in a standardized setting (MAMAS) study: Preliminary results. Monatsschrift Kinderheilkd. 2016, 164, 233–390. [Google Scholar]
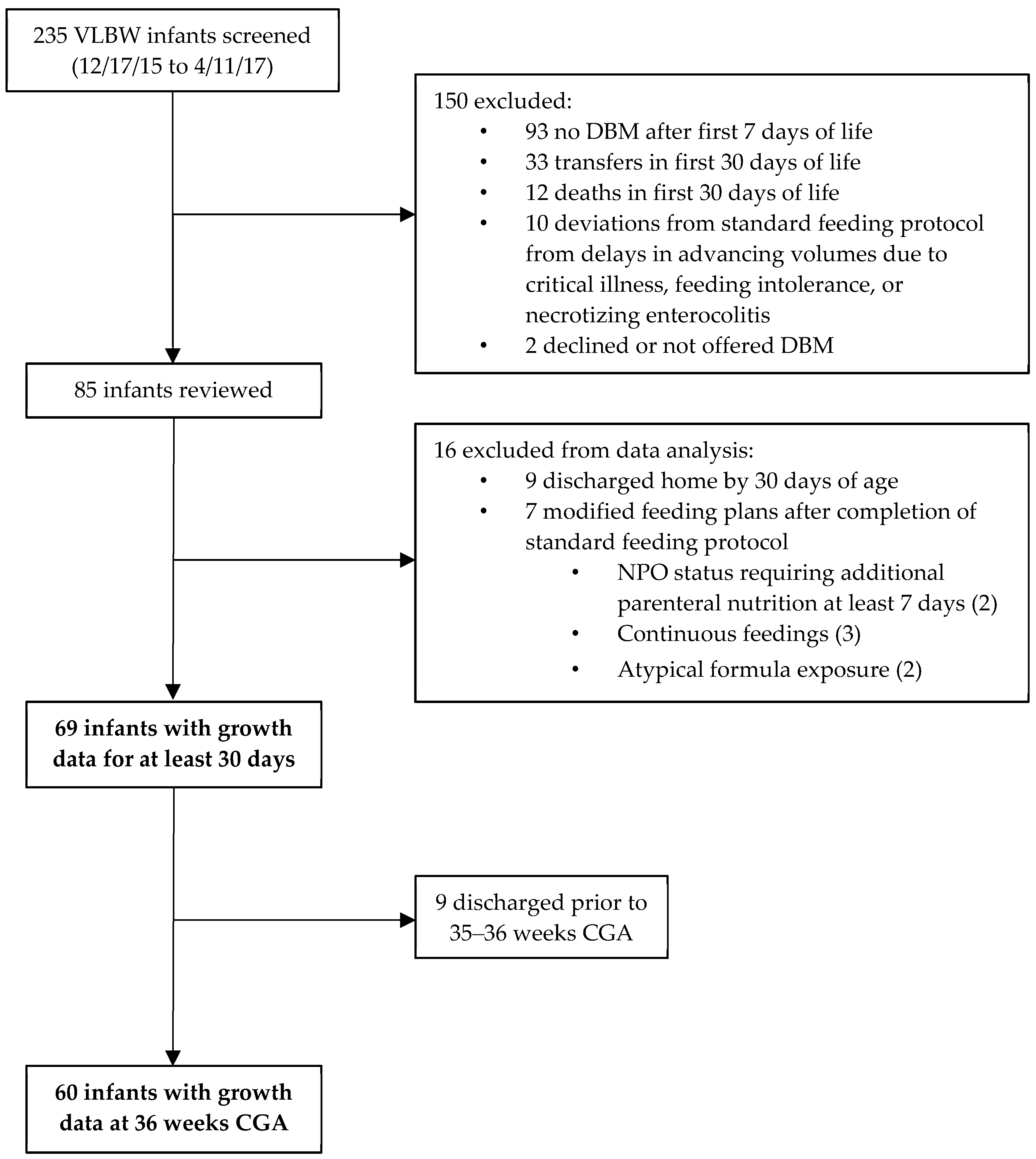
| All Infants (n = 85) | Infants with 30 Days Growth Data (n = 69) | Infants Receiving <50% DBM (n = 27) | Infants Receiving >50% DBM (n = 42) | p-Value 1 | |
|---|---|---|---|---|---|
| Male (%) | 47 (55.3%) | 38 (55.1%) | 17 (63.0%) | 21 (50%) | 0.291 |
| Gestational Age (weeks) | 29.4 ± 2.4 | 28.9 ± 2.0 | 29.6 ± 2.1 | 28.4 ± 1.8 | 0.011 |
| Birth Weight (g) | 1101.2 ± 266.5 | 1064.4 ± 260.0 | 1112.1 ± 264.5 | 1033.8 ± 255.5 | 0.225 |
| SGA (%) | 24 (28.2%) | 11 (15.9%) | 7 (25.9%) | 4 (9.5%) | 0.069 |
| Days on DBM | 26.5 ± 6.7 | 27.8 ± 5.4 | 24.9 ± 7.6 | 29.6 ± 1.7 | <0.001 |
| Infants on DBM at 30 days (%) | 56 (65.9%) 2 | 50 (72.5%) | 15 (55.6%) | 35 (83.3%) | 0.012 |
| Infants Needing >24 kcal/oz (%) | 59 (69.4%) | 49 (71.0%) | 14 (51.9%) | 35 (83.3%) | 0.005 |
| Calories (kcal/oz) | Protein (g/dL) | Fat (g/dL) | Carbohydrate (g/dL) | |
|---|---|---|---|---|
| Minimum | 12.43 | 0.26 | 1.48 | 6.29 |
| Maximum | 22.27 | 1.36 | 4.51 | 8.48 |
| Mean | 18.70 | 0.91 | 3.11 | 7.71 |
| SD | 1.75 | 0.19 | 0.57 | 0.38 |
| Mean Coefficient of Variation | 1.61% | 6.81% | 2.68% | 1.81% |
| Calories (kcal/oz) | Protein (g/dL) | |||||
|---|---|---|---|---|---|---|
| Label | NIR | Difference | Label | NIR | Difference | |
| Minimum | 19.51 | 12.43 | −8.22 | 0.62 | 0.26 | −0.62 |
| Maximum | 24.01 | 22.27 | 0.29 | 1.51 | 1.36 | 0.36 |
| Mean | 21.13 | 18.70 | −2.43 1 | 1.02 | 0.91 | −0.11 1 |
| SD | 1.01 | 1.75 | 1.66 | 0.18 | 0.19 | 0.21 |
| Overall (n = 69) | SGA (n = 11) | AGA (n = 58) | p-Value | <50% DBM (n = 27) | >50% DBM (n = 42) | p-Value | |
|---|---|---|---|---|---|---|---|
| Weight (g/kg/day) | |||||||
| Week 1 | 8.84 ± 6.81 | 15.07 ± 9.42 | 7.66 ± 5.56 | <0.001 | 8.50 ± 7.12 | 9.06 ± 6.68 | 0.742 |
| Week 2 | 12.95 ± 5.75 | 17.00 ± 6.96 | 12.18 ± 5.21 | <0.010 | 14.88 ± 6.08 | 11.71 ± 5.23 | 0.024 |
| Week 3 | 16.58 ± 5.13 | 18.45 ± 5.91 | 16.23 ± 4.95 | 0.189 | 17.21 ± 3.38 | 16.17 ± 6.01 | 0.415 |
| Week 4 | 16.11 ± 4.71 | 16.98 ± 3.71 | 15.94 ± 4.89 | 0.507 | 16.00 ± 3.50 | 16.18 ± 5.39 | 0.882 |
| Length (cm/week) | |||||||
| Week 1 | 0.59 ± 0.85 | 0.60 ± 0.87 | 0.59 ± 0.86 | 0.982 | 0.45 ± 0.81 | 0.68 ± 0.88 | 0.299 |
| Week 2 | 0.97 ± 0.72 | 1.32 ± 0.72 | 0.91 ± 0.70 | 0.080 | 1.05 ± 0.65 | 0.92 ± 0.76 | 0.478 |
| Week 3 | 0.95 ± 0.78 | 0.70 ± 0.96 | 1.00 ± 0.74 | 0.244 | 0.94 ± 0.85 | 0.96 ± 0.75 | 0.914 |
| Week 4 | 1.03 ± 0.65 | 1.31 ± 0.45 | 0.97 ± 0.66 | 0.114 | 0.95 ± 0.67 | 1.08 ± 0.63 | 0.455 |
| HC (cm/week) | |||||||
| Week 1 | 0.14 ± 0.77 | 0.39 ± 0.91 | 0.09 ± 0.74 | 0.235 | 0.06 ± 0.73 | 0.19 ± 0.80 | 0.490 |
| Week 2 | 0.67 ± 0.55 | 0.91 ± 0.58 | 0.63 ± 0.54 | 0.124 | 0.77 ± 0.58 | 0.61 ± 0.53 | 0.245 |
| Week 3 | 1.01 ± 0.54 | 1.20 ± 0.42 | 0.98 ± 0.56 | 0.216 | 0.96 ± 0.55 | 1.05 ± 0.53 | 0.525 |
| Week 4 | 1.01 ± 0.60 | 1.12 ± 0.59 | 0.99 ± 0.61 | 0.514 | 1.07 ± 0.59 | 0.97 ± 0.61 | 0.498 |
| Weight | HC | Length | BMI | |
|---|---|---|---|---|
| Birth | −0.52 ± 0.89 | −0.45 ± 1.10 | −0.51 ± 1.06 | −0.30 ± 1.09 |
| Week 1 | −0.88 ± 0.81 | −1.30 ± 0.96 | −1.14 ± 1.06 | −0.41 ± 0.95 |
| Week 2 | −1.04 ± 0.77 | −1.45 ± 0.98 | −1.28 ± 1.02 | −0.43 ± 0.88 |
| Week 3 | −1.09 ± 0.79 | −1.41 ± 0.95 | −1.44 ± 1.05 | −0.24 ± 0.82 |
| Week 4 | −1.13 ± 0.83 | −1.28 ± 0.96 | −1.60 ± 1.00 | −0.09 ± 0.81 |
| 36 Weeks CGA | −1.03 ± 1.03 | −0.68 ± 0.90 | −1.64 ± 1.13 | 0.27 ± 0.85 |
| Net Change | −0.51 ± 0.47 | −0.23 ± 0.69 | −1.01 ± 0.57 | 0.60 ± 0.93 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, T.T.; Schroder, P.E.; Poindexter, B.B. Macronutrient Analysis of Target-Pooled Donor Breast Milk and Corresponding Growth in Very Low Birth Weight Infants. Nutrients 2019, 11, 1884. https://doi.org/10.3390/nu11081884
Fu TT, Schroder PE, Poindexter BB. Macronutrient Analysis of Target-Pooled Donor Breast Milk and Corresponding Growth in Very Low Birth Weight Infants. Nutrients. 2019; 11(8):1884. https://doi.org/10.3390/nu11081884
Chicago/Turabian StyleFu, Ting Ting, Paige E. Schroder, and Brenda B. Poindexter. 2019. "Macronutrient Analysis of Target-Pooled Donor Breast Milk and Corresponding Growth in Very Low Birth Weight Infants" Nutrients 11, no. 8: 1884. https://doi.org/10.3390/nu11081884
APA StyleFu, T. T., Schroder, P. E., & Poindexter, B. B. (2019). Macronutrient Analysis of Target-Pooled Donor Breast Milk and Corresponding Growth in Very Low Birth Weight Infants. Nutrients, 11(8), 1884. https://doi.org/10.3390/nu11081884






