Abstract
Research has investigated 25-hydroxyvitamin D (25(OH)D) levels in the Atopic Dermatitis (AD) population, as well as changes in AD severity after vitamin D (VitD) supplementation. We performed an up-to-date systematic review and meta-analysis of these findings. Electronic searches of MEDLINE, EMBASE and COCHRANE up to February 2018 were performed. Observational studies comparing 25(OH)D between AD patients and controls, as well as trials documenting baseline serum 25(OH)D levels and clinical severity by either SCORAD/EASI scores, were included. Of the 1085 articles retrieved, sixteen were included. A meta-analysis of eleven studies of AD patients vs. healthy controls (HC) found a mean difference of −14 nmol/L (95% CI −25 to −2) for all studies and −16 nmol/L (95% CI −31 to −1) for the paediatric studies alone. A meta-analysis of three VitD supplementation trials found lower SCORAD by −11 points (95% CI −13 to −9, p < 0.00001). This surpasses the Minimal Clinical Important Difference for AD of 9.0 points (by 22%). There were greater improvements in trials lasting three months and the mean weighted dose of all trials was 1500–1600 IU/daily. Overall, the AD population, especially the paediatric subset, may be at high-risk for lower serum 25(OH)D. Supplementation with around 1600 IU/daily results in a clinically meaningful AD severity reduction.
1. Introduction
Atopic Dermatitis (AD) is a chronic recurrent inflammatory disease of the skin characterised by pruritus and inflamed lesions, involving specific areas of the body and causing generalised xerotic skin. As the disease progresses from acute to subacute, to chronic stages, excoriation from scratching and a propensity to secondary infections leads to oozing lesions and further pruritus. Severity of pruritis is associated with quality of life. Scores on the PO-SCORAD (patient oriented SCORAD) questionnaire, which includes a visual analogue scale for pruritis severity, are associated with measures of quality of life [1,2].
Eighty-five percent of AD is seen in children, of which 30% continue to suffer in their adult years [3]. There are an estimated 15 million suffers in the United Kingdom (UK) [4] and in the United States (US); 25% of children and 7% of adults have AD [3]. The incidence of AD is growing world-wide, especially in urbanised countries, with a higher rate in northern latitudes during the winter months [5]. The financial strain of AD at the level of individual, family and the public healthcare system cannot be underestimated. A 4.2 billion USD per year cost was estimated for the US alone with individual healthcare costs for AD patients higher by between 28.3% and 67.9% compared to non-AD patients [6,7].
AD pathology involves a complex interplay of barrier issues of skin and various dysfunctions in host innate and adaptive immune systems. These include high IgE, eosinophil and distinct T-helper cell populations as well as cytokine dysmodulation [8,9,10]. Bacterial and viral infestation from Staphylococcus Aureus and Herpes Simplex infections exacerbate pre-existing AD. However, factors contributing to long-term remission of AD are currently unknown [11,12]. Presently, due to no identified clinical biomarkers, quantitative and qualitative clinical tools are used to gauge severity of clinical presentation, with the Scoring Atopic Dermatitis index (SCORAD) being the most validated and commonly used in clinical research. The severity scale for SCORAD is pegged at Mild AD <25 points, Moderate AD >25 points and Severe AD >50 points [13,14].
There is much interest in the potential role of vitamin D deficiency in the development of AD, from multiple lines of research evidence. First, research has documented the aggravation of AD in winter, especially in higher latitude countries where serum 25(OH)D tends to be particularly low in this season [5]. Second, improvement in AD symptoms in patients has been observed in research studies on VitD supplementation [15,16]. Third, genetic polymorphisms including those of the Vitamin D Receptor (VDR) and a filaggrin gene mutation (up to 50% of the AD population, depending on specific mutation) have been identified as contributors to the development of AD [17,18].
Of note, Vitamin D3 (VitD3) is known to play a role in the skin barrier function, as it modulates structural proteins of the cornified dermis layer, regulating the glycoseramides essential for the hydrating protective lipid barrier which keeps the skin moisturized [19]. It modulates innate immunity via the production of the anti-microbial peptides (AMPs) cathelicidin and defensin which can help reduce skin infection risk [20]. In addition, Amon et al. (2018) discussed how vitamin D has inhibitory effects on monocyte production (via Toll-like receptors) and well as inhibiting dendritic cell activity and increasing mast cell release of IL10 [21]. They also discussed how vitamin D reduces the release of proinflammatory cytokines from Th1 cells and inhibits the release of IgE by reducing B cell function [21]. These mechanisms would theoretically aid the reduction of chronic inflammation in the skin.
The optimum 25(OH)D level for the prevention of, or rehabilitation of, inflammatory skin diseases is yet unknown. At present, for bone health, the US Endocrine Society recommends that serum 25(OH)D levels <50 nmol/L (20 ng/mL) are classified as deficient and 53–73 nmol/L (21–29 ng/mL) as insufficient. The UK Scientific Advisory Committee on Nutrition recommendations are more conservative, suggesting 25 nmol/L or higher as a population protective level for bone health [22]. These are population estimates and the physiological need for vitamin D may be higher in some clinical conditions. More research is now required to assess the optimum serum 25(OH)D concentration specifically required for those with AD, as well as assessing the current 25(OH)D status in AD patients.
Two previous systematic reviews and meta-analysis in 2016 on vitamin D and AD [12,23] have found a lower serum 25(OH)D in AD patients compared with non-AD patients [12] as well as a reduced severity of disease in AD patients after vitamin D supplementation [12,23]. However, new research has been conducted since the publication of these reviews and so there is a clear need for this systematic review and meta-analysis to be updated. A recent systematic review reviewed the area [24] but did not include a meta-analysis so there was no updated effect size using data from recent trials. The aim of this work therefore was to provide an updated review of observational and intervention trial data on the role of VitD in AD, including all published studies up to February 2018. First, we assessed the mean difference, in observational studies, between 25(OH)D concentration in AD patients and HC, with a sub-analysis of adult and paediatric populations separately. This was to gauge differences in 25(OH)D from normal which may be of clinical importance. Second, we assessed the impact of VitD supplementation on AD severity (change in SCORAD index), quantifying the role of VitD dosage and trial duration, to both support further research and advise clinical guidelines.
2. Methods
2.1. Search Procedures
A systematic search was conducted on the MEDLINE database via Pubmed (https://www.ncbi.nlm.nih.gov/pubmed/). The EMBASE database (https://www.embase.com/login) and the COCHRANE DATABASE for registered trials (http://cochranelibrary-wiley.com/cochranelibrary/search?searchRow.searchOptions.searchProducts=clinicalTrialsDoi) were also searched. For search terms, MESH i.e., Medical Subject Headings (MESH) were used along with pre-text terms. Search terms used for all three databases included: “Vitamin D AND Atopic Dermatitis”; “Vitamin D AND Eczema”; “25 Hydroxy Vit D AND Atopic Dermatitis”; “Vitamin D AND Atopic Dermatitis AND children”.
Following the recommendations by the Cochrane Database of Systematic Reviews (http://www.cochranelibrary.com/), reference lists searches were made to double-check for appropriate papers not previously located in the electronic searches. We also searched for grey literature such as abstracts from conference presentations. Researchers with abstract publications in the field were contacted via email and phone calls to request complete study results and data sets. Researchers of fully published papers were also contacted for any missing data. The searches covered papers from January 1963 to February 2018. In studies which included both adult and child participants, published data and raw unpublished data of both adult and child participants were obtained to enable sub-analysis for the adult and child populations. This review did not require ethical approval as it only involved analysis of already collected data.
2.2. Eligibility Criteria for Inclusion and Data Extraction
Observational studies (case-control design) as well as interventional studies, including randomized double-blind placebo control trials, non-randomized placebo control trials, clinical intervention and audit trials were assessed. We included all human studies published in the English language. Inclusion criteria were as follows: Age group >1 year, including both males and females. Exclusion criteria were as follows: Pregnant women, infants <1 year. Intervention studies were only included if they had an assessment of serum 25(OH)D levels at baseline. For both observational and intervention studies, the SCORAD and/or the EASI score needed to be included in the study for inclusion in the systematic review. This helped support clear mathematically calculable data in terms of proof of AD and severity of presence of AD in observational studies and improvement or exacerbation of AD during the interventional trials. For interventional studies, this enabled calculation of an effect size for the effect of VitD supplementation on AD severity, giving a quantifiable estimate to assess the clinical relevance of the results. Search procedures were documented using the PRISMA protocol.
2.3. Primary Outcomes
- From observational studies, serum 25(OH)D levels in AD patients versus HC.
- From interventional trials, changes in SCORAD or EASI score in the VitD supplemented AD group compared to the placebo supplemented AD group.
2.4. Secondary Outcomes
- The relationship between serum 25(OH)D levels in AD with SCORAD or EASI score.
- Effects of VitD supplementation on secondary infections of skin in AD.
- The relationship between serum 25(OH)D levels, serum IgE levels and total eosinophil count (TEC) in the AD population and HC, including changes in these post-supplementation.
- In interventional trials only: Effects of the co-usage of topical steroids.
- The relationship, in AD patients, between serum 25(OH)D levels and cathelicidin LL-37 or Cathelicidin Antimicrobial Peptide (CAMP) and changes post-supplementation.
- The relationship, in AD patients, between serum 25(OH)D levels and serum cytokines, with changes post-supplementation.
- The relationship between serum 25(OH)D levels and atopic sensitisation.
2.5. Statistical Analysis
The p-value, confidence interval, and effect estimate of all primary outcomes were extracted. For observational studies, included in the meta-analysis of comparison of 25(OH)D levels in AD and HC, the mean ± standard deviation (SD) of 25(OH)D levels of both groups were extracted, as well as n (number of participants) in each group. Serum 25(OH)D concentrations were inputted into the meta-analyses in nmol/L. The serum 25(OH)D concentrations in two studies were presented in nmol/L [25,26]. The other nine studies [27,28,29,30,31,32,33,34,35] presented 25(OH)D concentrations in ng/mL but were converted to nmol/L using the standard formula: nmol/L = 2.5 ng/mL.
For interventional studies, as well as the number of participants (n) in the intervention group and the control group, the SCORAD score was extracted at baseline and after intervention (mean ± SD). In one study [36], the post intervention score was represented as a percentage of improvement so the mean ± SD were calculated accordingly for this study. For supplementation trials, VitD dose and trial duration were also extracted.
Review Manager (Rev Man 5.3; Cochrane Collaboration, London, UK) was used to perform the meta-analyses. The Newcastle–Ottawa Scale and the Cochrane Risk of Bias Scale were used to assess the quality of observational studies and interventional studies, respectively. The I2 statistic was used to assess heterogeneity between study outcomes and, due to significant between-study heterogeneity, the meta-analyses were conducted using the random effects model.
A planned a priori sub-analysis, for adult and paediatric specific data, was undertaken for the meta-analysis of serum 25(OH)D levels in AD and HC. Data from the two studies with data for separate age groups [31,32] were extracted from their published papers and additional raw data were also supplied by their research teams. The meta-analysis of interventional studies was undertaken using changes in SCORAD score between baseline and post-VitD supplementation, a weighted mean dose across this meta-analysis was calculated. This was calculated manually by multiplying each trial dose by the weighting given for that study in the meta-analysis and summing up the dosage to represent the 100% weighted mean dose. A planned a priori sub-analysis of interventional trials was done to assess the difference in SCORAD score changes in relation to VitD dosage and time period of trials. Inspection of funnel plots were undertaken to assess potential publication bias (subject to restrictions incurred by the number of studies available for analysis). Sensitivity analyses were also conducted when the analysis contained more than two studies.
3. Results
3.1. Systematic Review
Results of the systematic literature searches are shown in Figure 1. The textual Systematic Review assessing results for each outcome and its relation with 25(OH)D status can be viewed in the Supplemental File. Table 1, Table 2, Table 3 and Table 4 illustrate the characteristics and outcomes of the included studies. Results of the Newcastle–Ottawa Scale and the Cochrane Risk of Bias Scale scoring can be seen in Supplementary Tables S1 and S2.
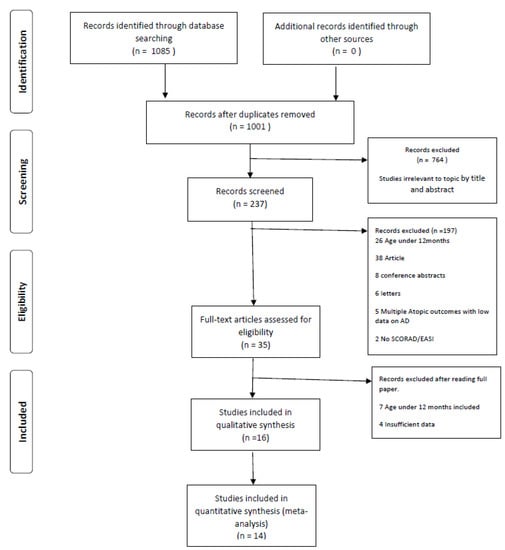
Figure 1.
PRISMA 2009 Flow Diagram to show results of the search process and inclusions/exclusions.

Table 1.
Observational case-control studies of serum 25(OH)D levels in atopic dermatitis individuals compared to healthy controls.

Table 2.
Showing data of serum 25(0H)D levels in the atopic dermatitis participants compared to healthy controls in included studies.

Table 3.
Interventional studies of vitamin D supplementation in atopic dermatitis.

Table 4.
Data of interventional studies included in the meta-analysis.
3.2. Meta-Analysis
3.2.1. Serum VitD in AD Compared to HC, with Sub-Analysis of the Paediatric Population
Eleven studies were suitable for inclusion in the meta-analysis to compare serum 25(OH)D in AD compared to HC (Figure 2). Three studied a mixed population of adult and paediatric populations [31,32,35]. One study [33] studied an adult only population whilst seven studies documented serum 25(OH)D levels in paediatric populations only [25,26,27,28,29,30,34]. Figure 2 summarizes the meta-analysis for serum 25(OH)D in AD and HC, including eleven studies. The results showed a statistically significantly lower 25(OH)D concentration in AD patients than HC by −14 nmol/L (95% CI −25 to −2, p = 0.02; I2 = 99%). Very high heterogeneity was noted so a random effects model was used.
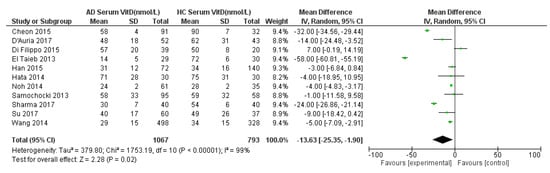
Figure 2.
Forest Plot for meta-analysis of serum 25(OH)D levels in atopic dermatitis population compared with healthy controls (nmol/L). References: Cheon 2015 [27]. D’Auria 2017 [28]. Di Filippo 2015 [29]. El Taeib 2013 [30]. Han 2015 [31]. Hata 2014 [35]. Noh 2014 [32]. Samochocki 2013 [33]. Sharma 2017 [25]. Su 2017 [34]. Wang 2014 [26].
A sensitivity analysis was performed (Table S3). The effect size stayed very stable when studies were removed in turn, except for being lower (around a 9 nmol/L group difference) when El Taieb et al. (2013) [30] was removed. Also, the removal of Cheon et al. (2015) [27], Wang et al. (2014) [26], and Sharma et al. (2017) [25] rendered the effect size not statistically significant. A further sensitivity analysis was done specifically for studies from common regions and ethnicities, including 3 studies from South Korea and two studies from Italy (Table S4). The effect size was not statistically significant when including only the Korean studies, although the magnitude of the effect was similar to when all studies were included (25(OH)D status was still around 14 nmol/L lower in the AD than in the non-AD group). The inclusion of only the Italian studies still gave a statistically significant result, with a similar effect size to that for all studies (16 nmol/L group difference).
A sub-analysis by age was also conducted, separating the purely adult AD and paediatric AD populations with their same age HC (Figure 3). The sub-analysis for the paediatric population showed a statistically significantly lower serum 25(OH)D in AD children by 16 nmol/L compared to HC: −16 (95% CI −31 to −1, p = 0.05, I2 = 99%). Considerable heterogeneity was noted (I2 = 99%) so a random effects model was used. The sub-analysis for the adult AD population did not show a statistically significant result: −2 nmol/L (95% CI −5 to 1, p = 0.15, I2 = 0%). The effect size for the adult population studies may need to be interpreted with caution due to presence of few included studies and a small population (n = 168 AD vs. n = 151 HC) due to limited data available.
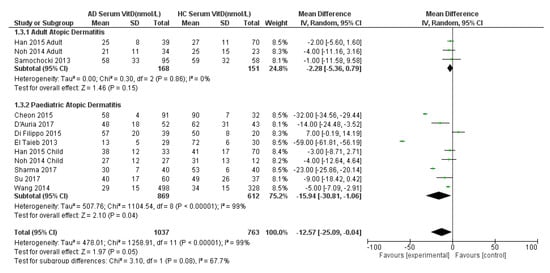
Figure 3.
Forest Plot of comparison of serum 25(OH)D levels (nmol/L) in adult and paediatric atopic dermatitis populations versus their age-matched healthy controls, with sub-analysis by age group. References: Cheon 2015 [27]. D’Auria 2017 [28]. Di Filippo 2015 [29]. El Taeib 2013 [30]. Han 2015 [31]. Hata 2014 [35]. Noh 2014 [32]. Samochocki 2013 [33]. Sharma 2017 [25]. Su 2017 [34]. Wang 2014 [26].
Finally, the overall adult and child sub-analysis showed a statistically significantly lower 25(OH)D mean difference in AD patients by −13 nmol/L (95% CI −25 to −0.04, p = 0.08; test for subgroup difference I2 = 68%, p = 0.08) (Figure 3). Of note, this result differs slightly from Figure 2 due to the differential weighting of each study when split by age group and then the adult and child estimates are pooled compared with when there is no split by age group.
Funnel plots for the analyses represented in Figure 1 and Figure 2 were conducted (Supplementary Figures S1 and S2). Both plots showed asymmetry with relatively few studies with positive effect sizes (i.e., case serum 25(OH)D higher than control) suggesting possible publication bias.
3.2.2. VitD Interventional Trials and Change in Clinical AD Severity (SCORAD)
A meta-analysis was conducted for five interventional trials in AD cases with the primary outcome of change in SCORAD index after intervention compared to baseline (Figure 4). Two trials were from a combined adult and paediatric population [39,40]. One trial was from a purely adult population [33] while two trials were from paediatric AD populations [29,36]. The analysis was performed as two subgroups as the results from repeated measures interventions (i.e., patients are their own control) could not be statistically combined with studies that involved two groups of individuals (intervention vs. placebo).
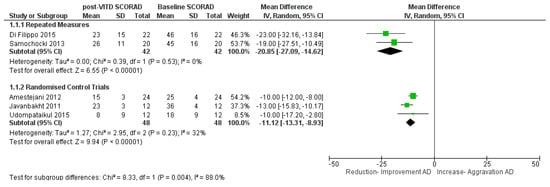
Figure 4.
Meta-analysis of vitamin D intervention trials in atopic dermatitis: Comparison of clinical SCORAD index at baseline and post-vitamin D supplementation. References: Amestejani 2012 [39]. Di Filippo 2015 [29]. Javanbakht 2011 [40]. Samochocki 2013 [33]. Udompatailkul 2015 [36].
For the repeated measures interventions, there was a highly statistically significant reduction in SCORAD by 21 points on intervention with VitD, (mean difference = −21 points (95% CI −27 to −15, p < 0.0001; I2 = 0%) with a weighted mean dose of 1500 IU/daily. For the randomised control trials, there was a highly statistically significant reduction in SCORAD by 11 points on intervention with VitD, (mean difference = −11 points (95% CI −13 to −9, p < 0.0001; I = 32%) with a weighted mean dose of 1600 IU/daily (Figure 4). The random effects model was used for the analyses as substantial heterogeneity was seen between studies for the randomised control trials. A sensitivity analysis was performed (Table S5), removal of each study in turn only had a small effect on effect size and no effect on statistical significance.
Though the number of studies were few, all studies showed improvement in SCORAD index on supplementation with VitD. It should be noted that in these five studies the AD population consisted mostly of mild and moderate AD with few severe cases in two of the studies.
A sub-analysis by dosage and duration for the five trials (Supplementary Figure S3) showed a greater change in Di Filippo et al. (2015) [29] and Samochocki et al. (2013) [33], which were both of three month duration and repeated measures, compared with the other trials which were randomised control trials and only one to two months duration. In the paediatric Di Filippo et al. (2015) [29] study, VitD dosage was 1000 IU/daily compared with 2000 IU/daily in the adult Samochocki et al. (2013) [33] trial. This sub-analysis must be interpreted with caution due to the limited number of studies, with substantial heterogeneity. Overall, it is noted that the three-month trials contained mostly mild–moderate AD patients, were repeated measures studies rather than randomised control trials and showed higher mean reduction in SCORAD than the one to two month studies.
4. Discussion
Our findings show a lower serum 25(OH)D concentration by 14 nmol/L in the overall adult and paediatric AD population than in HC, with lower serum 25(OH)D also in the AD paediatric population by 16 nmol/L. There was no difference in the adult population alone, the effect size for which did not reach statistical significance. Therefore, our study shows that the AD population have lower 25(OH)D concentration than their healthy peers, particularly for children. Our results suggest that the AD paediatric population may be an “at-risk group” for VitD insufficiency. As per US Endocrine Society guidelines, all individuals at risk of vitD insufficiency must be assessed routinely for 25(OH)D status [37]. This should be considered as best practice during the diagnosis and treatment of the AD paediatric population. VitD supplementation may be considered by the clinician taking into account baseline 25(OH)D status and possible contraindications (e.g., endocrine dysfunction).
Pooling results from repeated measures clinical trials in AD patients, post-supplementation we found a highly statistically significant difference between supplement and placebo groups in SCORAD of −21 points, using dosages of 1000–2000 IU daily for three months. Similarly, pooling results from VitD randomised control trials in AD patients, post-supplementation we found a highly statistically significant difference between supplement and placebo groups in SCORAD of −11 points, using dosages of 1000–2000 IU daily for one to two months.
In intervention trials, the minimal difference or improvement set as a measure of effectivity of the intervention is called the Minimal Clinical Important Difference or MCID. For the treatment of atopic dermatitis, the MCID of the SCORAD score which translates to clinical relevance is a reduction of 9 points [42]. Our effect size of −11 to 21 points exceeds this threshold, suggesting clinical relevance. Therefore, we have found clear evidence for a clinically meaningful reduction in AD disease severity after VitD supplementation. Of note, the baseline average (mean or median) 25(OH)D for all intervention trials in the meta-analysis was <50 nmol/L, which would be classified as deficient [33,37]. This shows that the trials included individuals who were truly deficient in 25(OH)D at baseline, so were not (on average) supplementing individuals who already had sufficient 25(OH)D status. After supplementation, this average 25(OH)D was >50 nmol/L in all trials except one [33].
Bearing in mind the clinical background of the each patient, including individual 25(OH)D concentration and any existing endocrine issues, this research supports the empirical supplementation of daily VitD doses of approximately 1500–1600 IU/daily to AD patients, taking into account the baseline vitamin D levels and eventual endocrine issues or other concomitant diseases that contraindicate vitamin D supplementation.
This weighted mean of 1500–1600 IU (38–40 micrograms) daily dosage falls well below the European Food Standards Agency (EFSA) Tolerable Upper Intake Levels (UL) of 100 micrograms per day for all adults as well as children aged 11–17 years [43]. It also falls below the UL for 1–10 year old children (50 micrograms per day) [43] but is higher than the UL for infants (25 micrograms per day) [43]. Clinical biochemical monitoring should be undertaken of children receiving 1500–1600 IU (38–40 micrograms per day) due to it being closer to their UL (50 micrograms per day) and infants should definitely not be given 1500–1600 IU/daily. Indeed, the UL is not a target, and is based on population (not individual) safety. Clinicians should make their own judgements about safe intakes for their individual patients, bearing in mind the effective dose suggested by this study (1500–1600 IU/daily).
In our analysis, larger results were seen in trials of three month duration but from the limited data available we were not able to assess whether this was due to the 3 month duration itself, or if it was simply that all the three month studies were repeated measures studies, rather than full randomised placebo control trials. Nevertheless, it stands to logical reason that supplementing for three months, rather than one to two months, is likely to lead to a better clinical response.
In terms of previous systematic reviews and meta-analyses in the field, our work supports the findings of systematic reviews by Kim et al. (2016) [12], Kim and Bae (2016) [23], Huang et al. (2018) [24] and Vaughn et al. (2019) [44] in terms of finding a lower 25(OH)D status in AD patients than controls, and finding an effect of vitamin D supplementation on symptom severity.
Our textual systematic review indicated that most interventional trials have documented a reduction in skin infection after VitD supplementation. Some observational evidence also suggested an association between lower 25(OH)D concentration and increased cutaneous secondary-colonisation of S. aureus and herpes, suggesting that increasing 25(OH)D levels in the AD population may support the reduction of and prevention of secondary cutaneous infections, albeit this was based on a small number of studies and there was not enough data to perform a meta-analysis.
In terms of biological mechanisms, it is feasible that VitD could affect the severity of AD, including number of infections. VitD is known to modulate innate and adaptive immune responses [45]. The physiological role of VitD in supporting healthy skin [20], as well as the fact that lower 25(OH)D concentrations are known to correlate with increased allergic sensitisation [46], higher IgE level [47], and lower serum cathelicidin levels [48], suggest a role of VitD in modulating AD severity. Moreover, studies involving the disruption of the VDR have showed lower levels of involucrin, profilaggrin and loricin barrier proteins [49]. Improvement in 25(OH)D leads to upregulation of functional human cathelicidin (hCAP18) in keratinocytes from AD patients, as well as from those from patients with psoriasis and normal skin [50]. In support of the above mechanisms, Albenali et al. (2016) [38] showed that higher IgE levels, higher virulence and colonisation of S. aureus were recorded when serum 25(OH)D levels were low. A significantly increased risk of having skin lesions with methicillin-resistant S. aureus (MRSA) has been found in persons with VitD deficiency [51,52,53]. Udompataikul et al. (2015) [36] found a reduction in S. aureus colonization in a paediatric population on VitD supplementation while Samochocki et al. (2013) [33] found no incidence of infection in their adult supplemented population. Albenali et al. (2016) [38] observed a 4-fold upregulation of LL-37 in the stratum corneum on VitD supplementation and a reduction in AD complicated by eczema herpeticum.
Secondary infections and re-infections in AD are notoriously challenging to treat, with excess use of topical and oral antibiotics increasing the risk of microbial antibiotic resistance. A recently published study [54] analysed eleven-year nationally representative data and calculated the morbidity, mortality and cost of secondary infections in AD to be in excess of 11 to 228 million USD annually. Improving 25(OH)D levels in AD may support the war on antibiotic resistance by reducing the risk and severity of cutaneous infections. However, a lot more research is needed on this subject due to the small amount of currently published literature.
In terms of strengths, our systematic review and meta-analysis is the most up to date available on the role of VitD in AD in both adults and children. It calculated pooled effect sizes in terms of mean difference in serum 25(OH)D levels between the AD population and HC. Our effect size is also larger than the mean difference found in the other meta-analysis by Kim and Bae (2016) [23]. Our review is the first to document clinically relevant changes in disease severity (as assessed by SCORAD) after VitD supplementation.
In terms of limitations, it is important to note that the data from trials in our analysis included mainly mild and moderate AD with only a few severe cases. Also, no data from infants (<1 year of age) or pregnant women were included. The specific reduction in SCORAD seen, and difference in 25(OH)D between AD patients and HC may differ in these groups from that found in this review. Finally, our results are based on a mean weighted dose of around 1500–1600 IU per day and SCORAD reductions observed seen are likely to differ with higher or lower doses.
Three trials [35,38,41] could not be included in the meta-analysis due to no reporting of the standard deviation for SCORAD. These happened to be the higher dose trials and so this limited our analysis to trials with dosage ranges of 1000 IU–2000 IU/daily. Six trials confirmed the form of VitD given as VitD3 or VitD2, but two trials did not report the form of VitD used. The longest trials were only of three months duration hence the effect of longer-term supplementation could not be analysed. Our meta-analysis of 25(OH)D concentration in AD compared to HC was limited by the small sample size, especially for the adult population. Similarly, the meta-analysis of the interventional studies was limited by the small number of trials suitable for inclusion.
Quality analysis of the interventional studies showed four higher scoring randomised double blind clinical trials with mention of adequate randomisation and blinding [35,36,39,40]. In terms of the other studies, one study was designed as a clinical evaluation study and so was not a randomised clinical trial, [38] and Samochocki et al. (2013) [33] did not mention randomisation but confirmed blinding of both participants and researchers. Di Filippo et al. (2015) [29], Albenali et al. (2016) [38] and Tsotra et al. (2017) [41] did not mention randomisation or blinding of participants.
There was some potential evidence of publication bias in that there was asymmetry in the funnel plots, with very few studies having positive effect sizes (i.e., cases having higher serum 25(OH)D than controls). However, the funnel plots only contained a small number of studies (n = 11 or n = 12) so they must be interpreted with caution. The number of interventional studies were too few to judge publication bias so there may still be a possibility of unpublished studies with null findings, despite best efforts being made to locate unpublished data. In some trials, the limited information on form of VitD supplemented (D2 vs. D3) and the absence of information on ingredient type and source of the D2 or D3 prevented further analysis in this regard.
In terms of further research, there is an urgent need for longer term, well conducted trials in distinct age categories, at different severity levels and also for different histopathological disease stages. Intervention trials with VitD dosage titration based on the severity of the disease, concomitant cytokines, the cell landscape and dermal cathelicidin levels are also needed. Trials designed to understand the link between VitD supplementation, skin barrier function and innate immunity to reduce secondary cutaneous infections in AD would help provide evidence that may justify the need for VitD supplementation to modulate the prevalence of microbe colonisation and reduce the need for antibiotics in these patients. Studies assessing effects of VitD supplementation on topical steroid usage would also be useful as reduced usage of steroids would have cost benefits. If optimal serum 25(OH)D levels could indirectly support the reduction of antibiotic usage and curtail antibiotic resistance, further research in this area is clearly urgently required. Particularly, research investigating and quantifying the effect of VitD on gut and skin microbiota may support supplementation as a possible preventative and adjuvant treatment strategy.
Further trials with specifically vitamin D3 vs. D2 may provide data to support form dosage and time period of the supplementation for fastest recovery with least risk. Assessing the source and ingredients in vitamin D supplements used in trials may shed new light on a population known for sensitisation especially in the younger years. VitD3 forms of supplements are likely to support a more efficient increase in serum 25(OH) levels [55] but are usually derived from lanolin (from sheep’s wool).
Finally, the textual systematic review suggested that studies of VitD supplementation in mild to moderate AD did not show changes in pruritus (based on SCORAD), skin xerosis, lichenification, skin conductance and moisture levels of skin [36]. This suggests the need for investigating other possible treatment strategies including, possibly targeting specific cytokines, such as monoclonal antibodies against IL-31 to reduce pruritus, as well as the use of nutritional factors, to support AD therapy.
5. Conclusions
Our study shows significantly lower 25(OH)D levels in the AD population, especially the paediatric subset. Monitoring of 25(OH)D levels in AD patients is warranted, especially in children. VitD supplementation trials showed clinically relevant improvements at a weighted average dose of 1500–1600 IU for up to 3 months. Clinicians should consider appropriate supplementation after evaluating patients’ 25(OH)D concentration and medical history. Further research is required to establish the efficacy of vitamin D2 vs. D3 in reducing AD severity, as well as the effects of VitD supplementation on infection rates, including superinfections and topical steroid usage.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6643/11/8/1854/s1, Figure S1: Funnel Plot for meta-analysis of serum 25(OH)D levels in Atopic Dermatitis population compared with healthy controls (nmol/L), Figure S2: Funnel Plot of comparison of serum 25(OH)D levels in adult and paediatric Atopic Dermatitis population (nmol/L) versus their age-matched healthy controls, with sub-analysis by age group, Figure S3: Forest Plot for sub-analysis of vitamin D intervention trials in Atopic Dermatitis based on vitamin D dosage and time period, Table S1: Quality analysis of included observational studies using the Newcastle-Ottawa Scale, Table S2: Quality Analysis of included Interventional Studies using Cochrane Risk of Bias Scale, Table S3: Sensitivity Analysis of Meta-analysis of comparison of Serum 25(OH)D levels of AD vs. NonAD with each study exclusion, Table S4: Sensitivity analysis of Meta-analysis of comparison of Serum 25(OH)D levels of AD vs. Non-AD excluding studies from common regions and ethnicities, Table S5: Sensitivity Analysis of RCT Intervention trials of VitD supplementation in Atopic Dermatitis.
Author Contributions
Conceptualization, S.R.H.-H., S.A.L.-N. and A.L.D.; Data curation, S.R.H.-H.; Formal analysis, S.R.H.-H., W.H.S.W., M.H.K.H. and A.L.D.; Methodology, S.R.H.-H. and A.L.D.; Project administration, S.R.H.-H.; Supervision, S.A.L.-N. and A.L.D.; Writing—original draft, S.R.H.-H.; Writing—review & editing, S.R.H.-H., S.A.L.-N., W.H.S.W., M.H.K.H. and A.L.D.
Funding
No funding was provided for this research.
Conflicts of Interest
S.L.-N. discloses that she is Research Director of D3-TEX limited which holds the UK and Gulf Corporation Council (GCC) patents for the use of UVB transparent clothing to prevent vitamin D deficiency. SLN has received consultancy fees from the following companies/organisations: National Dairy Council UK, Yoplait, Kelloggs, Danone, Nestle. All other authors (ALD, SRHH, WHSW, MHKH) have nothing to disclose and there are no other relationships or activities that could appear to have influenced the submitted work.
References
- Coutanceau, C.; Stalder, J.F. Analysis of correlations between patient-oriented SCORAD (PO-SCORAD) and other assessment scores of atopic dermatitis severity and quality of life. Dermatology 2014, 229, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Boccardi, D.; D’Auria, E.; Turati, F.; DI, M.V.; Sortino, S.; Riva, E.; Cerri, A. Disease severity and quality of life in children with atopic dermatitis: PO-SCORAD in clinical practice. Minerva Pediatr. 2017, 69, 373–380. [Google Scholar] [PubMed]
- Boguniewicz, M.; Fonacier, L.; Guttman-Yassky, E.; Ong, P.Y.; Silverberg, J.; Farrar, J.R. Atopic dermatitis yardstick: Practical recommendations for an evolving therapeutic landscape. Ann. Allergy Asthma Immunol. 2018, 120, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, S. Guide to treatments used for atopic dermatitis in adults. Prescriber 2016, 27, 30–39. [Google Scholar] [CrossRef]
- Weiland, S.K.; Husing, A.; Strachan, D.P.; Rzehak, P.; Pearce, N. Climate and the prevalence of symptoms of asthma, allergic rhinitis, and atopic eczema in children. Occup. Environ. Med. 2004, 61, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Drucker, A.M.; Wang, A.R.; Li, W.-Q.; Sevetson, E.; Block, J.K.; Qureshi, A.A. The Burden of Atopic Dermatitis: Summary of a Report for the National Eczema Association. J. Investig. Dermatol. 2017, 137, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Miao, R.; Wang, L.; Chao, J.; Yuce, H.; Wei, W. Burden of Atopic Dermatitis in the United States: Analysis of Healthcare Claims Data in the Commercial, Medicare, and Medi-Cal Databases. Adv. Ther. 2017, 34, 1989–2006. [Google Scholar] [CrossRef] [PubMed]
- Tokura, Y. Extrinsic and intrinsic types of atopic dermatitis. J. Dermatol. Sci. 2010, 58, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Yang, T.; Wu, Z.; Zhong, J.; Huang, Y.; Huang, T.; Zheng, E. Differentiation of T-helper cells in distinct phases of atopic dermatitis involves Th1/Th2 and Th17/Treg. Eur. J. Inflamm. 2017, 15, 46–52. [Google Scholar] [CrossRef]
- D’Auria, E.; Banderali, G.; Barberi, S.; Gualandri, L.; Pietra, B.; Riva, E.; Cerri, A. Atopic dermatitis: Recent insight on pathogenesis and novel therapeutic target. Asian Pac. J. Allergy Immunol. 2016, 34, 98–108. [Google Scholar]
- Von Kobyletzki, L.; Svensson, A.; Apfelbacher, C.; Schmitt, J. Factors that predict remission of infant atopic dermatitis: A systematic review. Acta Derm. Venereol. 2015, 95, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Kim, S.N.; Lee, Y.W.; Choe, Y.B.; Ahn, K.J. Vitamin D status and efficacy of vitamin D supplementation in atopic dermatitis: A systematic review and meta-analysis. Nutrients 2016, 8, 789. [Google Scholar] [CrossRef] [PubMed]
- Kunz, B.; Oranje, A.P.; Labrèze, L.; Stalder, J.F.; Ring, J.; Taïeb, A. Clinical Validation and Guidelines for the SCORAD Index: Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology 1997, 195, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Wollenberg, A.; Oranje, A.; Deleuran, M.; Simon, D.; Szalai, Z.; Kunz, B.; Svensson, A.; Barbarot, S.; Kobyletzki, L.; Taieb, A.; et al. ETFAD/EADV Eczema task force 2015 position paper on diagnosis and treatment of atopic dermatitis in adult and paediatric patients. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 729–747. [Google Scholar] [CrossRef] [PubMed]
- Sidbury, R.; Sullivan, A.; Thadhani, R.; Camargo, C. Randomized controlled trial of vitamin D supplementation for winter-related atopic dermatitis in Boston: A pilot study. Br. J. Dermatol. 2008, 159, 245–247. [Google Scholar] [CrossRef] [PubMed]
- Camargo, C.A.; Ganmaa, D.; Sidbury, R.; Erdenedelger, K.; Radnaakhand, N.; Khandsuren, B. Randomized trial of vitamin D supplementation for winter-related atopic dermatitis in children. J. Allergy Clin. Immunol. 2014, 134, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chang, C.; Lu, Q. The Genetics and Epigenetics of Atopic Dermatitis-Filaggrin and Other Polymorphisms. Clin. Rev. Allergy Immunol. 2016, 51, 315–328. [Google Scholar] [CrossRef] [PubMed]
- Bieber, T. Atopic dermatitis. N. Engl. J. Med. 2008, 358, 1483–1494. [Google Scholar] [CrossRef]
- Oda, Y.; Uchida, Y.; Moradian, S.; Crumrine, D.; Elias, P.M.; Bikle, D.D. Vitamin D receptor and coactivators SRC2 and 3 regulate epidermis-specific sphingolipid production and permeability barrier formation. J. Investig. Dermatol. 2009, 129, 1367–1378. [Google Scholar] [CrossRef]
- Umar, M.; Sastry, K.S.; Al Ali, F.; Al-Khulaifi, M.; Wang, E.; Chouchane, A.I. Vitamin D and the Pathophysiology of Inflammatory Skin Diseases. Skin Pharmacol. Physiol. 2018, 31, 74–86. [Google Scholar] [CrossRef]
- Amon, U.; Baier, L.; Yaguboglu, R.; Ennis, M.; Holick, M.F.; Amon, J. Serum 25-hydroxyvitamin D levels in patients with skin diseases including psoriasis, infections, and atopic dermatitis. Dermatoendocrinology 2018, 10, e1442159. [Google Scholar] [CrossRef] [PubMed]
- SACN. Vitamin D and Health. Available online: https://www.gov.uk/government/groups/scientific-advisory-committee-on-nutrition (accessed on 21 October 2018).
- Kim, G.; Bae, J.H. Vitamin D and atopic dermatitis: A systematic review and meta-analysis. Nutrition 2016, 32, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.M.; Lara-Corrales, I.; Pope, E. Effects of Vitamin D levels and supplementation on atopic dermatitis: A systematic review. Pediatr. Dermatol. 2018, 35, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kaur, T.; Malhotra, S.K.; Rai, J.; Chaudhari, S. Correlation of Vitamin D3 Levels and SCORAD Index in Atopic Dermatits: A Case Control Study. J. Clin. Diagn. Res. 2017, 11, WC01–WC03. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Hon, K.L.; Kong, A.P.; Pong, H.N.; Wong, G.W.; Leung, T.F. Vitamin D deficiency is associated with diagnosis and severity of childhood atopic dermatitis. Pediatr. Allergy Immunol. 2014, 25, 30–35. [Google Scholar] [CrossRef]
- Cheon, B.R.; Shin, J.E.; Kim, Y.J.; Shim, J.W.; Kim, D.S.; Jung, H.L.; Park, M.S.; Shim, J.Y. Relationship between serum 25-hydroxyvitamin D and interleukin-31 levels, and the severity of atopic dermatitis in children. Korean J. Pediatr. 2015, 58, 96–101. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, E.; Barberi, S.; Cerri, A.; Boccardi, D.; Turati, F.; Sortino, S.; Banderali, G.; Ciprandi, G. Vitamin D status and body mass index in children with atopic dermatitis: A pilot study in Italian children. Immunol. Lett. 2017, 181, 31–35. [Google Scholar] [CrossRef]
- Di Filippo, P.; Scaparrotta, A.; Rapino, D.; Cingolani, A.; Attanasi, M.; Petrosino, M.I.; Chuang, K.; Di Pillo, S.; Chiarelli, F. Vitamin D supplementation modulates the immune system and improves atopic dermatitis in children. Int. Arch. Allergy Immunol. 2015, 166, 91–96. [Google Scholar] [CrossRef]
- El Taieb, M.A.; Fayed, H.M.; Aly, S.S.; Ibrahim, A.K. Assessment of serum 25-hydroxyvitamin d levels in children with atopic dermatitis: Correlation with SCORAD index. Dermatitis 2013, 24, 296–301. [Google Scholar] [CrossRef]
- Han, T.Y.; Kong, T.S.; Kim, M.H.; Chae, J.D.; Lee, J.H.; Son, S.J. Vitamin D Status and Its Association with the SCORAD Score and Serum LL-37 Level in Korean Adults and Children with Atopic Dermatitis. Ann. Dermatol. 2015, 27, 10–14. [Google Scholar] [CrossRef]
- Noh, S.; Park, C.O.; Bae, J.M.; Lee, J.; Shin, J.U.; Hong, C.S.; Lee, K.H. Lower vitamin D status is closely correlated with eczema of the head and neck. J. Allergy Clin. Immunol. 2014, 133, 1767–1770. [Google Scholar] [CrossRef] [PubMed]
- Samochocki, Z.; Bogaczewicz, J.; Jeziorkowska, R.; Sysa-Jedrzejowska, A.; Glinska, O.; Karczmarewicz, E.; McCauliffe, D.P.; Wozniacka, A. Vitamin D effects in atopic dermatitis. J. Am. Acad. Dermatol. 2013, 69, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Su, O.; Bahali, A.G.; Demir, A.D.; Ozkaya, D.B.; Uzuner, S.; Dizman, D.; Onsun, N. The relationship between severity of disease and vitamin D levels in children with atopic dermatitis. Adv. Dermatol. Allergol. 2017, 34, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.; Audish, D.; Kotol, P.; Coda, A.; Kabigting, F.; Miller, J.; Alexandrescu, D.; Boguniewicz, M.; Taylor, P.; Aertker, L.; et al. A randomized controlled double-blind investigation of the effects of vitamin D dietary supplementation in subjects with atopic dermatitis. J. Eur. Acad. Dermatol. Venereol. 2014, 28, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Udompataikul, M.; Huajai, S.; Chalermchai, T.; Taweechotipatr, M.; Kamanamool, N. The effects of oral vitamin D supplement on atopic dermatitis: A clinical trial with staphylococcus aureus colonization determination. J. Med. Assoc. Thail. 2015, 98, S23–S30. [Google Scholar]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Albenali, L.H.; Danby, S.; Moustafa, M.; Brown, K.; Chittock, J.; Shackley, F.; Cork, M.J. Vitamin D and antimicrobial peptide levels in patients with atopic dermatitis and atopic dermatitis complicated by eczema herpeticum: A pilot study. J. Allergy Clin. Immunol. 2016, 138, 1715–1719. [Google Scholar] [CrossRef]
- Amestejani, M.; Salehi, B.; Vasigh, M.; Sobhkhiz, A.; Karami, M.; Alinia, H.; Kamrava, S.; Shamspour, N.; Ghalehbaghi, B.; Behzadi, A. Vitamin D supplementation in the treatment of atopic dermatitis: A clinical trial study. J. Drugs Dermatol. 2012, 11, 327–330. [Google Scholar]
- Javanbakht, M.; Keshavarz, S.; Djalali, M.; Siassi, F.; Eshraghian, M.; Firooz, A.; Seirafi, H.; Ehsani, A.; Chamari, M.; Mirshafiey, A. Randomized controlled trial using vitamins E and D supplementation in atopic dermatitis. J. Dermatol. Treat. 2011, 22, 144–150. [Google Scholar] [CrossRef]
- Tsotra, K.; Garoufi, A.; Kossiva, L.; Gourgiotis, D.; Tsoukatou, T.; Katsantoni, E.; Stavropoulos, P. The impact of vitamin D supplementation on serum cathelicidin levels and the clinical course of atopic dermatitis in children. Minerva Pediatr. 2017. [Google Scholar] [CrossRef]
- Schram, M.E.; Spuls, P.I.; Leeflang, M.M.; Lindeboom, R.; Bos, J.D.; Schmitt, J. EASI, (objective) SCORAD and POEM for atopic eczema: Responsiveness and minimal clinically important difference. Allergy 2012, 67, 99–106. [Google Scholar] [CrossRef] [PubMed]
- EFSA. Scientific Opinion on the Tolerable Upper Intake of Vitamin D. EFSA J. 2012, 10, 2813. [Google Scholar]
- Vaughn, A.R.; Foolad, N.; Maarouf, M.; Tran, K.A.; Shi, V.Y. Micronutrients in Atopic Dermatitis: A Systematic Review. J. Altern. Complement. Med. 2019, 25, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Gois, P.H.F.; Ferreira, D.; Olenski, S.; Seguro, A.C. Vitamin D and Infectious Diseases: Simple Bystander or Contributing Factor? Nutrients 2017, 9, 651. [Google Scholar] [CrossRef] [PubMed]
- Sharief, S.; Jariwala, S.; Kumar, J.; Muntner, P.; Melamed, M.L. Vitamin D levels and food and environmental allergies in the United States: Results from the National Health and Nutrition Examination Survey 2005–2006. J. Allergy Clin. Immunol. 2011, 127, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Yip, K.H.; Kolesnikoff, N.; Yu, C.; Hauschild, N.; Taing, H.; Biggs, L.; Goltzman, D.; Gregory, P.A.; Anderson, P.H.; Samuel, M.S.; et al. Mechanisms of vitamin D(3) metabolite repression of IgE-dependent mast cell activation. J. Allergy Clin. Immunol. 2014, 133, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Leaf, D.E.; Croy, H.E.; Abrahams, S.J.; Raed, A.; Waikar, S.S. Cathelicidin antimicrobial protein, vitamin D, and risk of death in critically ill patients. Crit. Care 2015, 19, 80. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Komuves, L.; Yu, Q.C.; Elalieh, H.; Ng, D.C.; Leary, C.; Chang, S.; Crumrine, D.; Yoshizawa, T.; Kato, S.; et al. Lack of the vitamin D receptor is associated with reduced epidermal differentiation and hair follicle growth. J. Investig. Dermatol. 2002, 118, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Mallbris, L.; Carlen, L.; Wei, T.; Heilborn, J.; Nilsson, M.F.; Granath, F.; Stahle, M. Injury downregulates the expression of the human cathelicidin protein hCAP18/LL-37 in atopic dermatitis. Exp. Dermatol. 2010, 19, 442–449. [Google Scholar] [CrossRef]
- Matheson, E.M.; Mainous, A.G., 3rd; Hueston, W.J.; Diaz, V.A.; Everett, C.J. Vitamin D and methicillin-resistant Staphylococcus aureus nasal carriage. Scand. J. Infect. Dis. 2010, 42, 455–460. [Google Scholar] [CrossRef]
- Gilaberte, Y.; Sanmartin, R.; Aspiroz, C.; Hernandez-Martin, A.; Benito, D.; Sanz-Puertolas, P.; Alonso, M.; Torrelo, A.; Torres, C. Correlation Between Serum 25-Hydroxyvitamin D and Virulence Genes of Staphylococcus aureus Isolates Colonizing Children with Atopic Dermatitis. Pediatr. Dermatol. 2015, 32, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.W.; Hogan, P.G.; Hunstad, D.A.; Fritz, S.A. Vitamin D sufficiency and Staphylococcus aureus infection in children. Pediatr. Infect. Dis. J. 2015, 34, 544–545. [Google Scholar] [CrossRef] [PubMed]
- Narla, S.; Silverberg, J.I. Association between atopic dermatitis and serious cutaneous, multiorgan and systemic infections in US adults. Ann. Allergy Asthma Immunol. 2018, 120, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Tripkovic, L.; Wilson, L.R.; Hart, K.; Johnsen, S.; De Lusignan, S.; Smith, C.P.; Bucca, G.; Penson, S.; Chope, G.; Elliott, R.; et al. Daily supplementation with 15 μg vitamin D2 compared with vitamin D3 to increase wintertime 25-hydroxyvitamin D status in healthy South Asian and white European women: A 12-wk randomized, placebo-controlled food-fortification trial. Am. J. Clin. Nutr. 2017, 106, 481–490. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).