Endothelium-Dependent Vasorelaxant Effect of Prunus Persica Branch on Isolated Rat Thoracic Aorta
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Material and Extraction
2.3. Animals and Preparation of Rat Aortic Rings
2.4. Experimental Protocols
2.4.1. Effect of PPE on Endothelium-Intact and Endothelium-Denuded Aortic Rings Pre-Contracted by PE
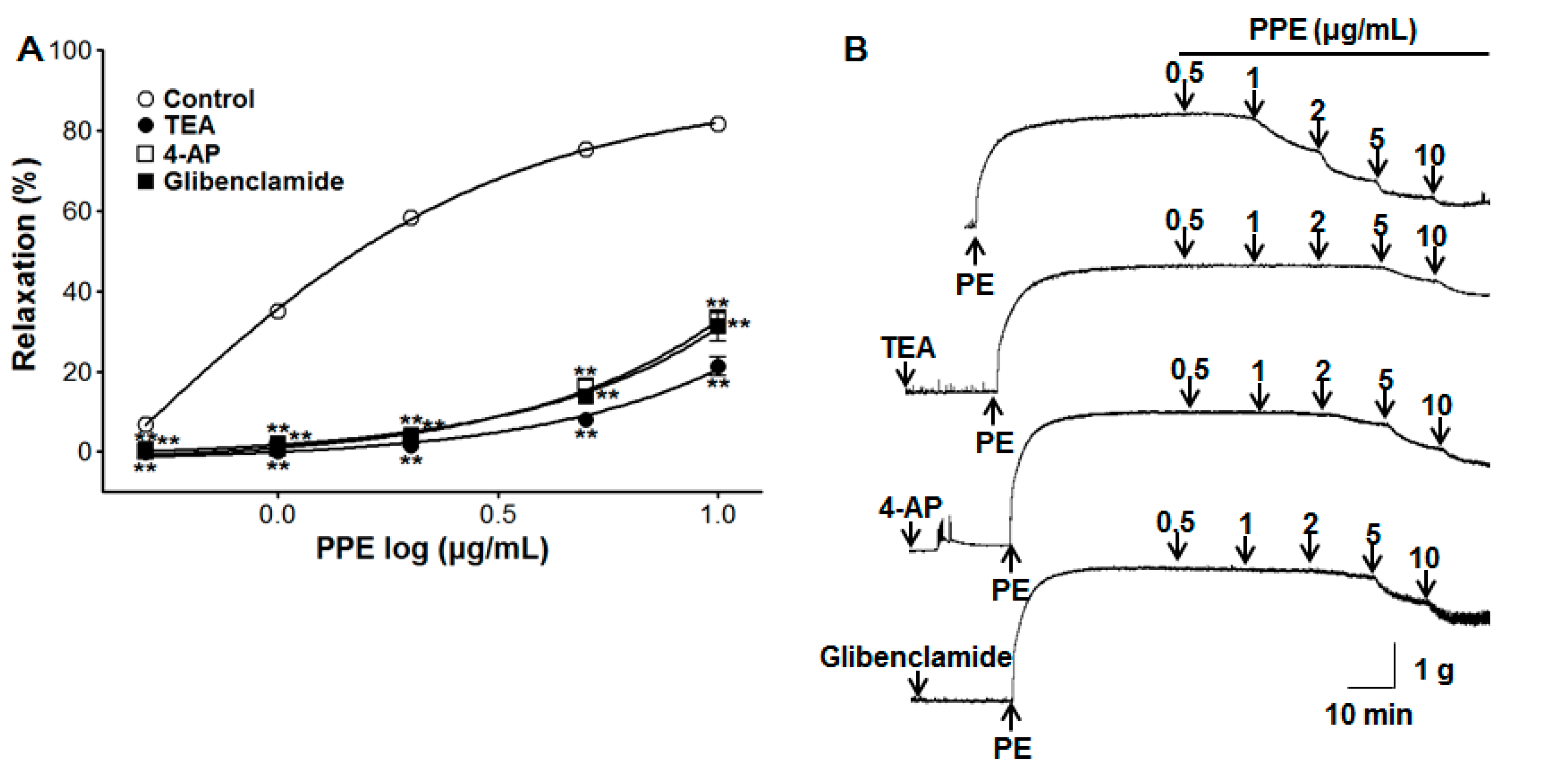
2.4.2. Effect of PPE on Endothelium-Intact Aortic Rings Pre-Incubated with L-NAME, ODQ, MB, Indomethacin, Atropine, Various Potassium Channel Blockers
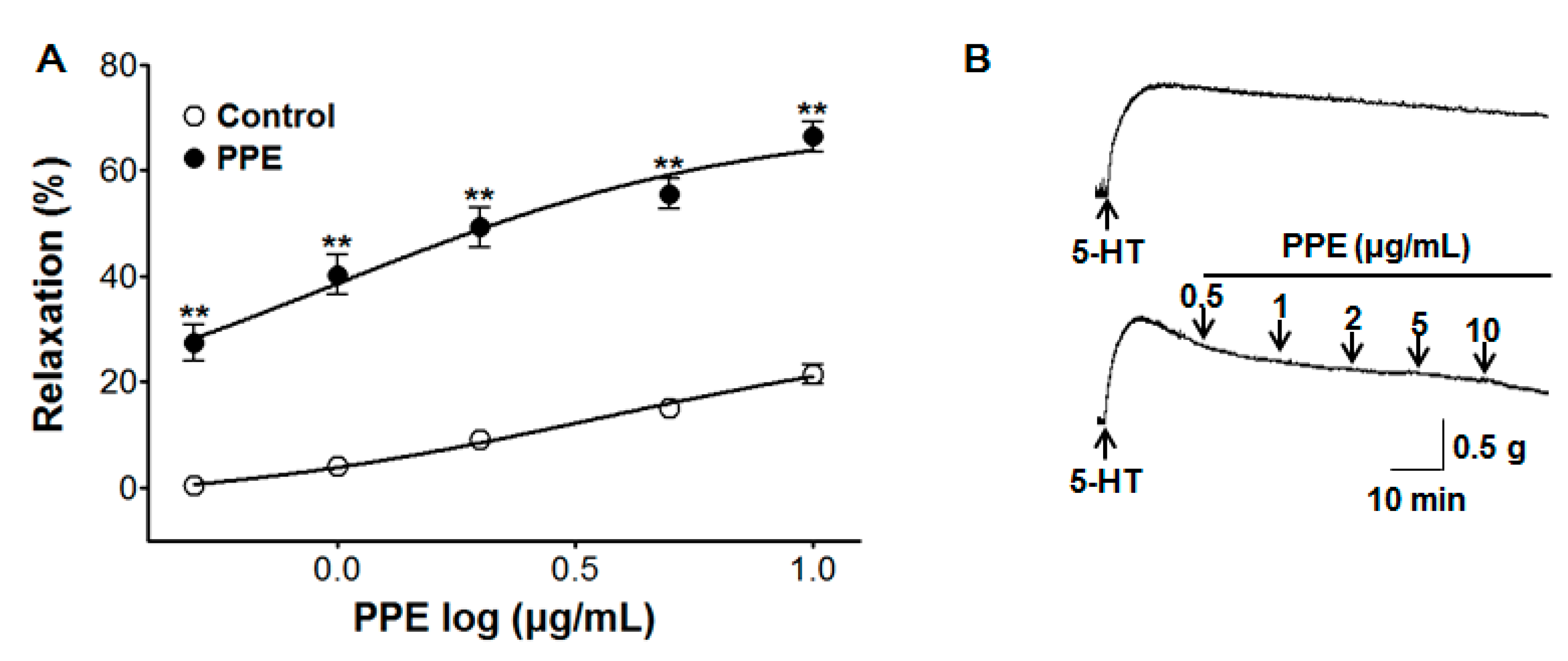
2.4.3. Effect of PPE on Endothelium-Intact Aortic Rings Pre-Contracted by 5-HT
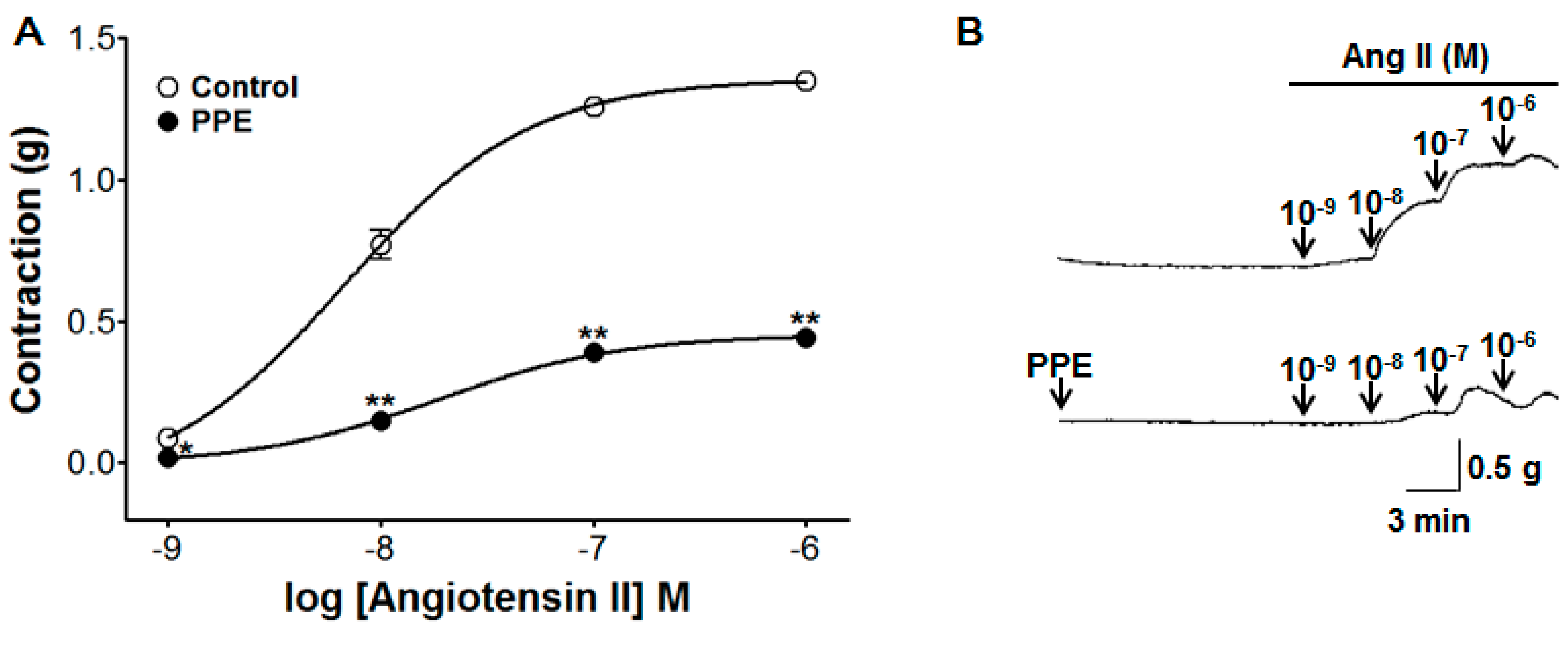
2.4.4. Effect of PPE Pre-Treatment on Ang II-Induced Contraction
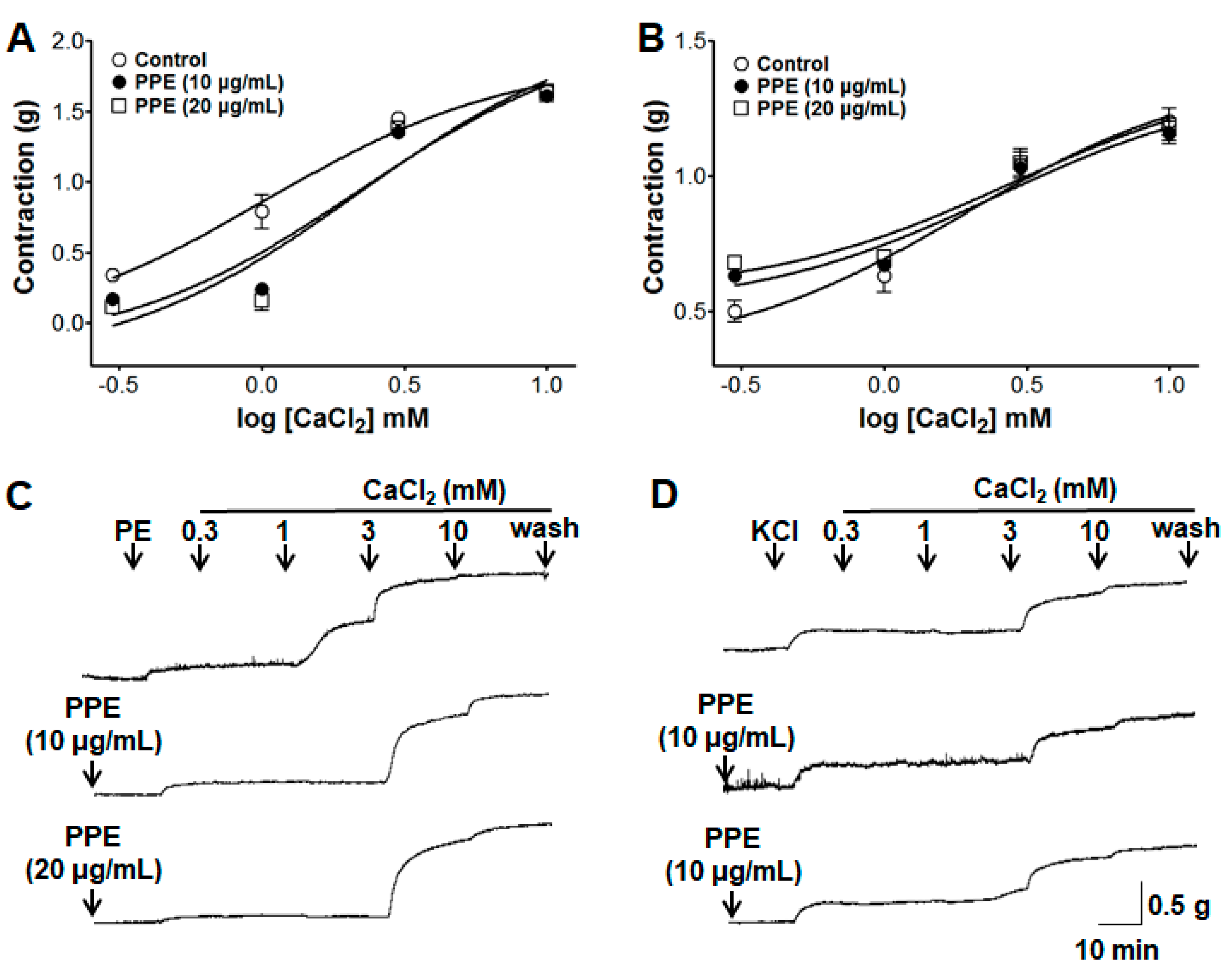
2.4.5. Effect of PPE on Extracellular Ca2+-Induced Contraction
2.4.6. Effect of PPE on Intracellular Ca2+ Release
2.5. Equation for Percentage Changes of Vasorelaxation
2.6. Data Analysis
3. Results
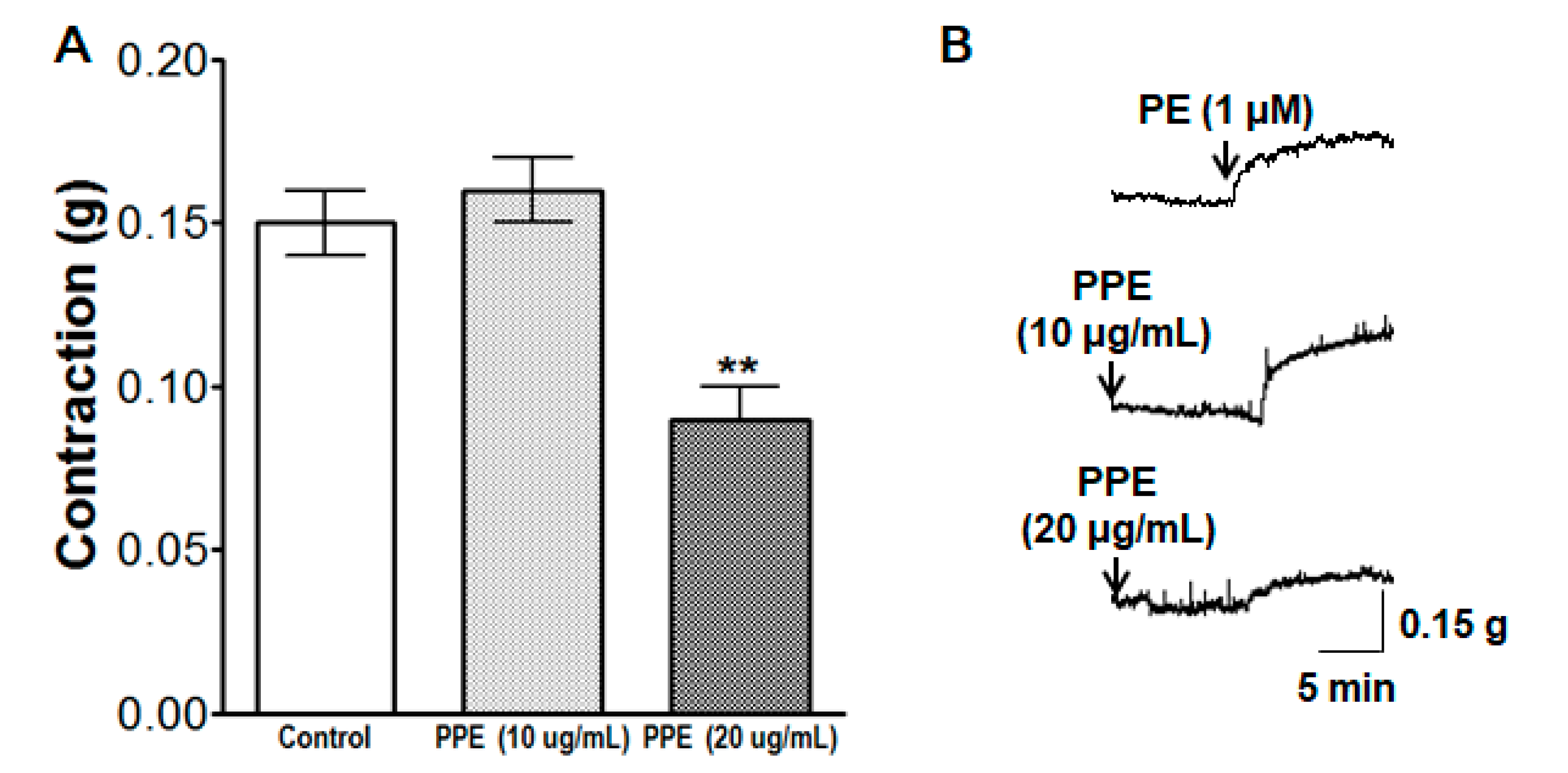
3.1. Vasorelaxant Effect of PPE on Endothelium-Intact and Endothelium-Denuded Aortic Rings Pre-Contracted by PE or KCl
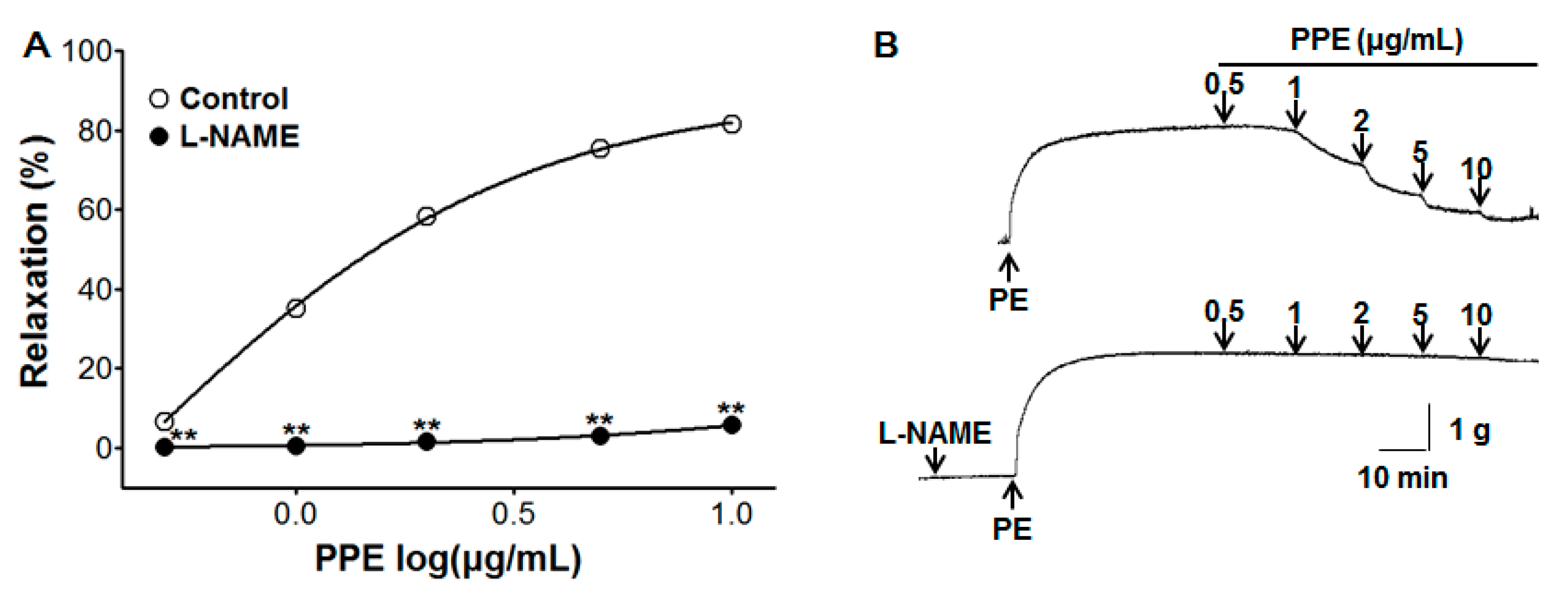
3.2. Vasorelaxant Effect of PPE on Endothelium-Intact Aortic Rings Pre-Incubated with L-NAME
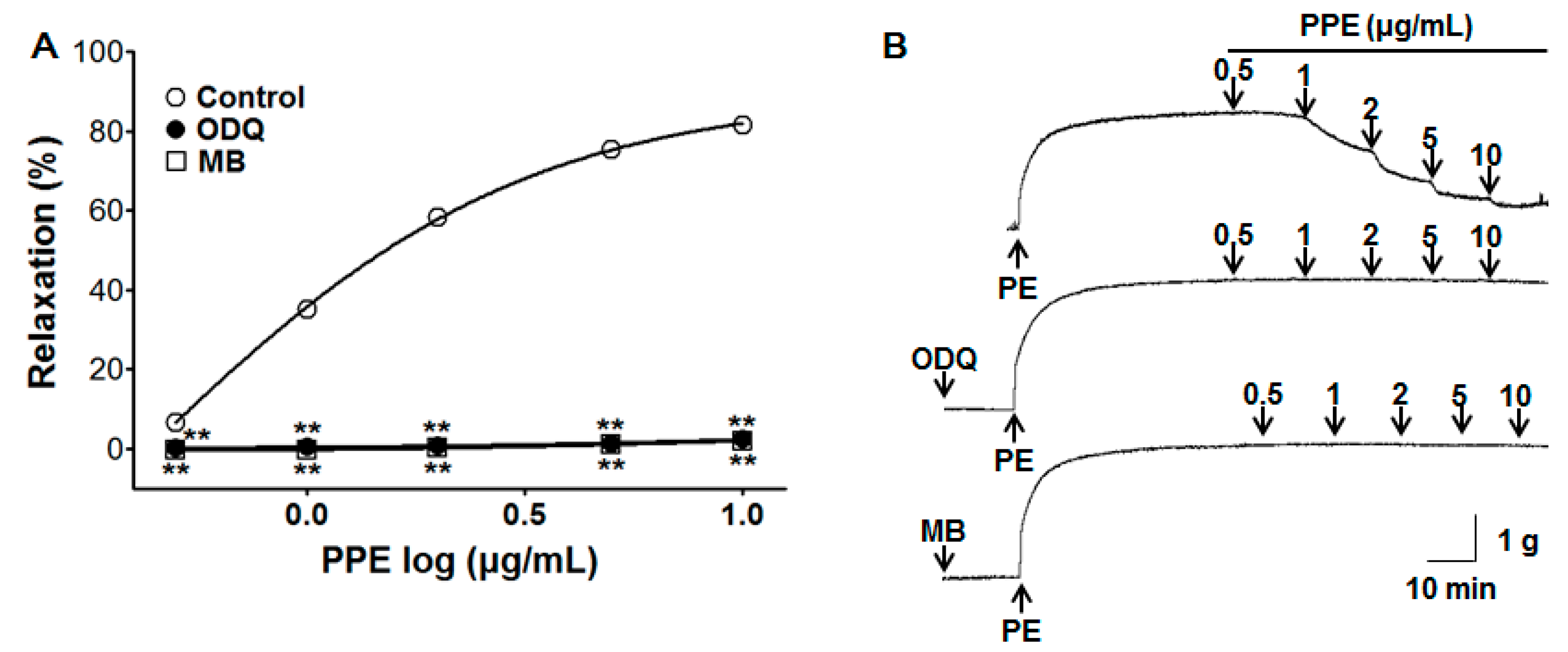
3.3. Vasorelaxant Effect of PPE on Endothelium-Intact Aortic Rings Pre-Incubated with ODQ or MB
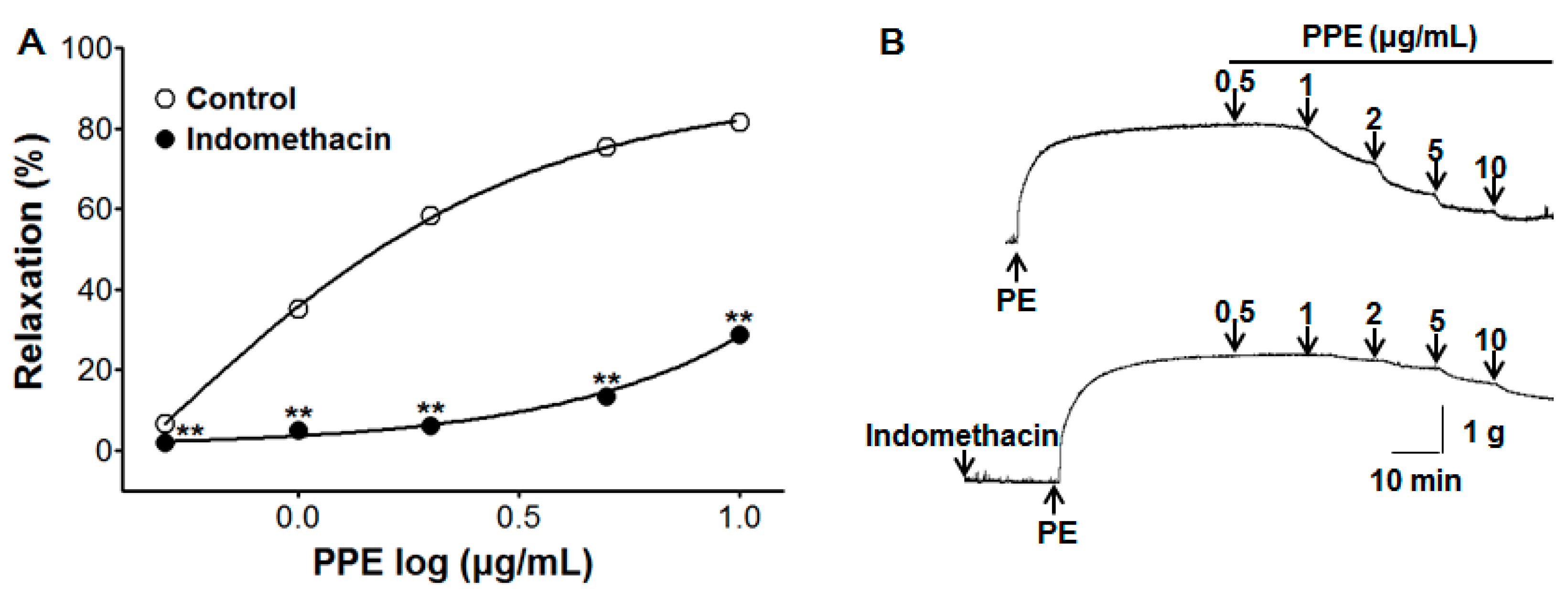
3.4. Vasorelaxant Effect of PPE on Endothelium-Intact Aortic Rings Pre-Incubated with Indomethacin
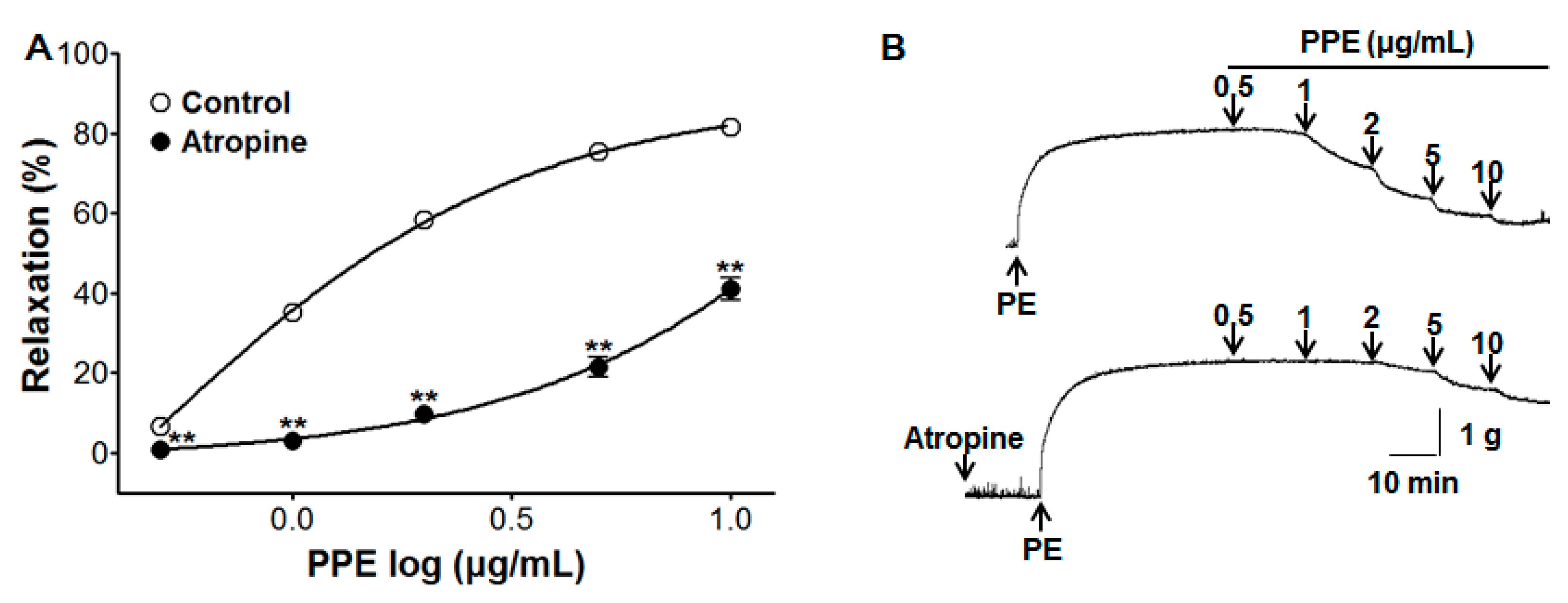
3.5. Vasorelaxant Effect of PPE on Endothelium-Intact Aortic Rings Pre-Incubated with Atropine
3.6. Vasorelaxant Effect of PPE on Endothelium-Intact Aortic Rings Pre-Incubated with Various Potassium Channel Blockers
3.7. Vasorelaxant Effect of PPE on 5-HT-Induced Contraction
3.8. Inhibitory Effect of PPE Pre-Treatment on Ang II-Induced Contraction
3.9. Inhibitory Effects of PPE on Extracellular Ca2+-Induced Contraction Through ROCCs or VDCCs
3.10. Inhibitory Effects of PPE on Intracellular Ca2+ Release
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sun, D.; Liu, J.; Xiao, L.; Liu, Y.; Wang, Z.; Li, C.; Jin, Y.; Zhao, Q.; Wen, S. Recent development of risk-prediction models for incident hypertension: An updated systematic review. PLoS ONE 2017, 12, e0187240. [Google Scholar] [CrossRef] [PubMed]
- Poulter, N.R.; Prabhakaran, D.; Caulfield, M. Hypertension. Lancet 2015, 386, 801–812. [Google Scholar] [CrossRef]
- Beeftink, M.M.A.; van der Sande, N.G.C.; Bots, M.L.; Doevendans, P.A.; Blankestijn, P.J.; Visseren, F.L.J.; Voskuil, M.; Spiering, W. Safety of temporary discontinuation of antihypertensive medication in patients with difficult-to-control hypertension. Hypertension 2017, 69, 927–932. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.P.; Hilgers, K.F.; Schmid, M.; Hübner, S.; Nadal, J.; Seitz, D.; Busch, M.; Haller, H.; Köttgen, A.; Kronenberg, F.; et al. GCKD study investigators blood pressure control in chronic kidney disease: A cross-sectional analysis from the German Chronic Kidney Disease (GCKD) study. PLoS ONE 2018, 13, e0202604. [Google Scholar] [CrossRef]
- Laurent, S. Antihypertensive drugs. Pharmacol. Res. 2017, 124, 116–125. [Google Scholar] [CrossRef]
- Nair, S.S.; Kavrekar, V.; Mishra, A. Evaluation of in vitro antidiabetic activity of selected plant extracts. Int. J. Pharm. Pharm. Sci. Inven. 2013, 2, 12–19. [Google Scholar]
- Al Disi, S.S.; Anwar, M.A.; Eid, A.H. Anti-hypertensive herbs and their mechanisms of action: Part I. Front. Pharmacol. 2016, 6, 323. [Google Scholar] [CrossRef]
- Camejo, D.; Martí, M.C.; Román, P.; Ortiz, A.; Jiménez, A. Antioxidant system and protein pattern in peach fruits at two maturation stages. J. Agric. Food Chem. 2010, 58, 11140–11147. [Google Scholar] [CrossRef]
- Common Editing Commission. Bonchohak, 3rd ed.; Younglimsa: Seoul, Korea, 2017. [Google Scholar]
- Fukuda, T.; Ito, H.; Mukainaka, T.; Tokuda, H.; Nishino, H.; Yoshida, T. Anti-tumor promoting effect of glycosides from Prunus persica seeds. Biol. Pharm. Bull. 2003, 26, 271–273. [Google Scholar] [CrossRef]
- Kazan, A.; Koyu, H.; Turu, I.C.; Yesil-Celiktas, O. Supercritical fluid extraction of Prunus persica leaves and utilization possibilities as a source of phenolic compounds. J. Supercrit. Fluids 2014, 92, 55–59. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Koo, B.-S.; Gong, D.-J.; Lee, Y.-C.; Ko, J.-H.; Kim, C.-H. Comparative effect of Prunus persica L BATSCH-water extract and tacrine (9-amino-1,2,3,4-tetrahydroacridine hydrochloride) on concentration of extracellular acetylcholine in the rat hippocampus. J. Ethnopharmacol. 2003, 87, 149–154. [Google Scholar] [CrossRef]
- Shin, T.-Y.; Park, S.-B.; Yoo, J.-S.; Kim, I.K.; Lee, H.-S.; Kwon, T.K.; Kim, M.K.; Kim, J.C.; Kim, S.-H. Anti-allergic inflammatory activity of the fruit of Prunus persica: role of calcium and NF-kappaB. Food Chem. Toxicol. 2010, 48, 2797–2802. [Google Scholar] [CrossRef] [PubMed]
- Dabbou, S.; Maatallah, S.; Castagna, A.; Guizani, M.; Sghaeir, W.; Hajlaoui, H.; Ranieri, A. Carotenoids, phenolic profile, mineral content and antioxidant properties in flesh and peel of Prunus persica fruits during two maturation stages. Plant Foods Hum. Nutr. 2017, 72, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Wang, H.; Wang, L.; Wang, L.; Zhu, M.; Ming, Y.; Zhao, S.; Fan, J.; Lai, E.Y. Ethanol extract of root of Prunus persica inhibited the growth of liver cancer cell HepG2 by inducing cell cycle arrest and migration suppression. Evid. Based. Complement. Alternat. Med. 2017, 2017, 8231936. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Ham, I.; Yang, G.; Lee, M.; Bu, Y.; Kim, H.; Choi, H.-Y. Vasorelaxant effect of Prunus yedoensis bark. BMC Complement. Altern. Med. 2013, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Keane, K.M.; George, T.W.; Constantinou, C.L.; Brown, M.A.; Clifford, T.; Howatson, G. Effects of Montmorency tart cherry (Prunus cerasus L) consumption on vascular function in men with early hypertension. Am. J. Clin. Nutr. 2016, 103, 1531–1539. [Google Scholar] [CrossRef] [PubMed]
- Luna-Vázquez, F.; Ibarra-Alvarado, C.; Rojas-Molina, A.; Romo-Mancillas, A.; López-Vallejo, F.; Solís-Gutiérrez, M.; Rojas-Molina, J.; Rivero-Cruz, F. Role of nitric oxide and hydrogen sulfide in the vasodilator effect of ursolic acid and uvaol from black cherry Prunus serotina fruits. Molecules 2016, 21, 78. [Google Scholar] [CrossRef]
- Kono, R.; Okuno, Y.; Nakamura, M.; Inada, K.; Tokuda, A.; Yamashita, M.; Hidaka, R.; Utsunomiya, H. Peach (Prunus persica) extract inhibits angiotensin II-induced signal transduction in vascular smooth muscle cells. Food Chem. 2013, 139, 371–376. [Google Scholar] [CrossRef]
- Godo, S.; Shimokawa, H. Endothelial functions. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e108–e114. [Google Scholar] [CrossRef]
- Dantas, B.; Ribeiro, T.; Assis, V.; Furtado, F.; Assis, K.; Alves, J.; Silva, T.; Camara, C.; França-Silva, M.; Veras, R. Vasorelaxation induced by a new naphthoquinone-oxime is mediated by NO-sGC-cGMP pathway. Molecules 2014, 19, 9773–9785. [Google Scholar] [CrossRef]
- Kawabe, J.; Ushikubi, F.; Hasebe, N. Prostacyclin in vascular diseases—Recent insights and future perspectives. Circ. J. 2010, 74, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Cuthbert, A.W. Some effects of atropine on smooth muscle. Br. J. Pharmacol. Chemother. 1963, 21, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Standen, N.B.; Quayle, J.M. K+ channel modulation in arterial smooth muscle. Acta Physiol. Scand. 1998, 164, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Watts, S.W. 5-HT in systemic hypertension: foe, friend or fantasy? Clin. Sci. 2005, 108, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Sung, D.J.; Noh, H.J.; Kim, J.G.; Park, S.W.; Kim, B.; Cho, H.; Bae, Y.M. Serotonin contracts the rat mesenteric artery by inhibiting 4-aminopyridine-sensitive Kv channels via the 5-HT2A receptor and Src tyrosine kinase. Exp. Mol. Med. 2013, 45, e67. [Google Scholar] [CrossRef]
- Lote, C. The renin-angiotensin system and regulation of fluid volume. Surgery 2006, 24, 154–159. [Google Scholar] [CrossRef]
- Huang, Y.; Ho, I.H. Separate activation of intracellular Ca2+ release, voltage-dependent and receptor-operated Ca2+ channels in the rat aorta. Chin. J. Physiol. 1996, 39, 1–8. [Google Scholar]
- Niazmand, S.; Fereidouni, E.; Mahmoudabady, M.; Hosseini, M. Teucrium polium-induced vasorelaxation mediated by endothelium-dependent and endothelium-independent mechanisms in isolated rat thoracic aorta. Pharmacogn. Res. 2017, 9, 372–377. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, B.; Kim, K.-W.; Lee, S.; Jo, C.; Lee, K.; Ham, I.; Choi, H.-Y. Endothelium-Dependent Vasorelaxant Effect of Prunus Persica Branch on Isolated Rat Thoracic Aorta. Nutrients 2019, 11, 1816. https://doi.org/10.3390/nu11081816
Kim B, Kim K-W, Lee S, Jo C, Lee K, Ham I, Choi H-Y. Endothelium-Dependent Vasorelaxant Effect of Prunus Persica Branch on Isolated Rat Thoracic Aorta. Nutrients. 2019; 11(8):1816. https://doi.org/10.3390/nu11081816
Chicago/Turabian StyleKim, Bumjung, Kwang-Woo Kim, Somin Lee, Cheolmin Jo, Kyungjin Lee, Inhye Ham, and Ho-Young Choi. 2019. "Endothelium-Dependent Vasorelaxant Effect of Prunus Persica Branch on Isolated Rat Thoracic Aorta" Nutrients 11, no. 8: 1816. https://doi.org/10.3390/nu11081816
APA StyleKim, B., Kim, K.-W., Lee, S., Jo, C., Lee, K., Ham, I., & Choi, H.-Y. (2019). Endothelium-Dependent Vasorelaxant Effect of Prunus Persica Branch on Isolated Rat Thoracic Aorta. Nutrients, 11(8), 1816. https://doi.org/10.3390/nu11081816




