Assessing and Managing the Metabolic Syndrome in Children and Adolescents
Abstract
1. Introduction
2. What is MetS?
3. Clinical Measures of MetS
3.1. Evaluation among Adults
3.2. Evaluation among Children
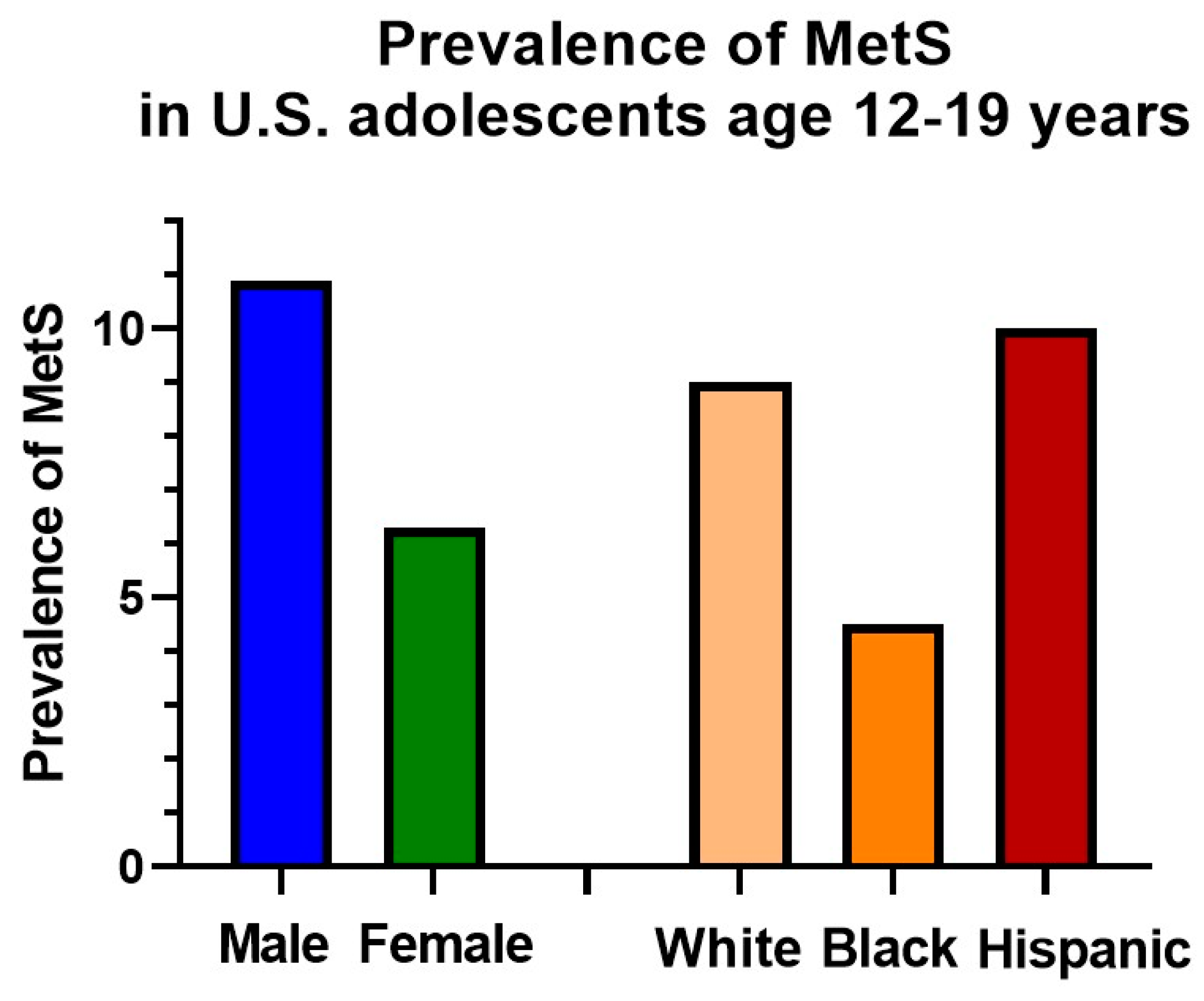
4. Epidemiology
5. Long-Term Risks
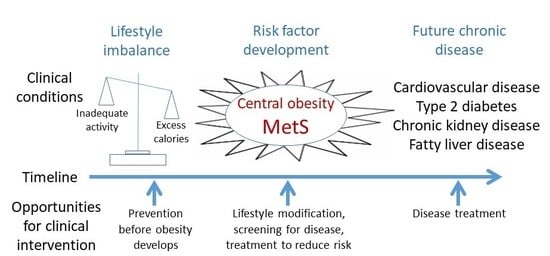
6. An Emphasis on Prevention
7. Use of Criteria in Clinical Settings
Identify Individuals at Highest Need for Assessment
8. Intervention
8.1. Dietary Changes
8.2. Physical Activity Changes
8.3. Combined Intervention Approaches
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Berenson, G.S.; Srinivasan, S.R.; Bao, W.; Newman, W.P.; Wattigney, W.A. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. The Bogalusa Heart Study. N. Engl. J. Med. 1998, 338, 1650–1656. [Google Scholar] [CrossRef] [PubMed]
- DeBoer, M.D. Obesity, systemic inflammation, and increased risk for cardiovascular disease and diabetes among adolescents: A need for screening tools to target interventions. Nutrition 2013, 29, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Afshin, A.; Forouzanfar, M.H.; Reitsma, M.B.; Sur, P.; Estep, K.; Lee, A.; Marczak, L.; Mokdad, A.H.; Moradi-Lakeh, M.; Naghavi, M.; et al. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N. Engl. J. Med. 2017, 377, 13–27. [Google Scholar]
- Kelishadi, R. Childhood overweight, obesity, and the metabolic syndrome in developing countries. Epidemiol. Rev. 2007, 29, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Inflammatory mechanisms in the regulation of insulin resistance. Mol. Med. 2008, 14, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Shulman, G.I. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N. Engl. J. Med. 2014, 371, 2237–2238. [Google Scholar] [CrossRef]
- Morrison, J.A.; Friedman, L.A.; Gray-McGuire, C. Metabolic syndrome in childhood predicts adult cardiovascular disease 25 years later: The Princeton Lipid Research Clinics follow-up study. Pediatrics 2007, 120, 340–345. [Google Scholar] [CrossRef]
- Morrison, J.A.; Friedman, L.A.; Wang, P.; Glueck, C.J. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J. Pediatr. 2008, 152, 201–206. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef]
- de Ferranti, S.; Mozaffarian, D. The perfect storm: Obesity, adipocyte dysfunction, and metabolic consequences. Clin. Chem. 2008, 54, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Defronzo, R.A. Banting Lecture. From the triumvirate to the ominous octet: A new paradigm for the treatment of type 2 diabetes mellitus. Diabetes 2009, 58, 773–795. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Cleeman, J.I.; Daniels, S.R.; Donato, K.A.; Eckel, R.H.; Franklin, B.A.; Gordon, D.J.; Krauss, R.M.; Savage, P.J.; Smith, S.C.J.; et al. Diagnosis and management of the metabolic syndrome—An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005, 112, 2735–2752. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome-a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z.; Cleeman, J.I.; Donato, K.A.; Fruchart, J.C.; James, W.P.T.; Loria, C.M.; Smith, S.C.J. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 2009, 120, 1640–1645. [Google Scholar] [PubMed]
- Gurka, M.J.; Lilly, C.L.; Norman, O.M.; DeBoer, M.D. An Examination of Sex and Racial/Ethnic Differences in the Metabolic Syndrome among Adults: A Confirmatory Factor Analysis and a Resulting Continuous Severity Score. Metabolism 2014, 63, 218–225. [Google Scholar] [CrossRef]
- Eisenmann, J.C. On the use of a continuous metabolic syndrome score in pediatric research. Cardiovasc. Diabetol. 2008, 7, 17. [Google Scholar] [CrossRef]
- Vishnu, A.; Gurka, M.J.; DeBoer, M.D. The severity of the metabolic syndrome increases over time within individuals, independent of baseline metabolic syndrome status and medication use: The Atherosclerosis Risk in Communities Study. Atherosclerosis 2015, 243, 278–285. [Google Scholar] [CrossRef]
- DeBoer, M.D.; Gurka, M.J.; Golden, S.H.; Musani, S.K.; Sims, M.; Vishnu, A.; Guo, Y.; Cardel, M.; Pearson, T.A. Independent Associations between Metabolic Syndrome Severity & Future Coronary Heart Disease by Sex and Race. J. Am. Coll. Card. 2017, 69, 1204–1205. [Google Scholar]
- Gurka, M.J.; Golden, S.H.; Musani, S.K.; Sims, M.; Vishnu, A.; Guo, Y.; Cardel, M.; Pearson, T.A.; DeBoer, M.D. Independent associations between a metabolic syndrome severity score and future diabetes by sex and race: The Atherosclerosis Risk in Communities Study and Jackson Heart Study. Diabetologia 2017, 60, 1261–1270. [Google Scholar] [CrossRef] [PubMed]
- DeBoer, M.D.; Filipp, S.L.; Gurka, M.J. Use of a Metabolic Syndrome Severity Z Score to Track Risk During Treatment of Prediabetes: An Analysis of the Diabetes Prevention Program. Diabetes Care 2018, 41, 2421–2430. [Google Scholar] [CrossRef] [PubMed]
- Reinehr, T.; de Sousa, G.; Toschke, A.M.; Andler, W. Comparison of metabolic syndrome prevalence using eight different definitions: A critical approach. Arch. Dis. Child. 2007, 92, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Li, C.; Cook, S.; Choi, H.K. Serum concentrations of uric acid and the metabolic syndrome among US children and adolescents. Circulation 2007, 115, 2526–2532. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.R.; Redden, D.T.; Pietrobelli, A.; Allison, D.B. Waist circumference percentiles in nationally representative samples of African-American, European-American, and Mexican-American children and adolescents. J. Pediatr. 2004, 145, 439–444. [Google Scholar] [CrossRef] [PubMed]
- National High Blood Pressure Education Program Working Group. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 2004, 114, 555–576. [Google Scholar] [CrossRef]
- Zimmet, P.; Alberti, K.G.M.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S.; et al. The metabolic syndrome in children and adolescents—An IDF consensus report. Pediatr. Diabetes 2007, 8, 299–306. [Google Scholar] [CrossRef]
- Jolliffe, C.J.; Janssen, I. Development of age-specific adolescent metabolic syndrome criteria that are linked to the Adult Treatment Panel III and International Diabetes Federation criteria. J. Am. Coll. Cardiol. 2007, 49, 891–898. [Google Scholar] [CrossRef]
- Gurka, M.J.; Ice, C.L.; Sun, S.S.; DeBoer, M.D. A confirmatory factor analysis of the metabolic syndrome in adolescents: An examination of sex and racial/ethnic differences. Cardiovasc. Diabetol. 2012, 11, 128. [Google Scholar] [CrossRef]
- DeBoer, M.D.; Gurka, M.J.; Morrison, J.A.; Woo, J.G. Inter-relationships between the severity of metabolic syndrome, insulin and adiponectin and their relationship to future type 2 diabetes and cardiovascular disease. Int. J. Obes. 2016, 40, 1353–1359. [Google Scholar] [CrossRef]
- Lee, A.M.; Gurka, M.J.; DeBoer, M.D. Trends in Metabolic Syndrome Severity and Lifestyle Factors Among Adolescents. Pediatrics 2016, 137, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.M.; Fermin, C.R.; Filipp, S.L.; Gurka, M.J.; DeBoer, M.D. Examining trends in prediabetes and its relationship with the metabolic syndrome in US adolescents, 1999–2014. Acta Diabetol. 2017, 54, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, J.K.; Yanoff, L.B.; Easter, B.D.; Brady, S.M.; Keil, M.F.; Roberts, M.D.; Sebring, N.G.; Han, J.C.; Yanovski, S.Z.; Hubbard, V.S.; et al. The Stability of Metabolic Syndrome in Children and Adolescents. J. Clin. Endocrinol. Metab. 2009, 94, 4828–4834. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ford, E.S.; Huang, T.T.-K.; Sun, S.S.; Goodman, E. Patterns of change in cardiometabolic risk factors associated with the metabolic syndrome among children and adolescents: The Fels Longitudinal Study. J. Pediatr. 2009, 155, S5.e9–S5.e16. [Google Scholar] [CrossRef] [PubMed]
- Scheitel, M.R.; Kessler, M.E.; Shellum, J.L.; Peters, S.G.; Milliner, D.S.; Liu, H.; Elayavilli, R.K.; Poterack, K.A.; Miksch, T.A.; Boysen, J.J.; et al. Effect of a Novel Clinical Decision Support Tool on the Efficiency and Accuracy of Treatment Recommendations for Cholesterol Management. Appl. Clin. Inform. 2017, 8, 124–136. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Magge, S.N.; Goodman, E.; Armstrong, S.C. The Metabolic Syndrome in Children and Adolescents: Shifting the Focus to Cardiometabolic Risk Factor Clustering. Pediatrics 2017, 140, e20171603. [Google Scholar] [CrossRef] [PubMed]
- Messiah, S.E.; Arheart, K.L.; Luke, B.; Lipshultz, S.E.; Miller, T.L. Relationship between body mass index and metabolic syndrome risk factors among US 8-to 14-year-olds, 1999 to 2002. J. Pediatr. 2008, 153, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Walker, S.E.; Gurka, M.J.; Oliver, M.N.; Johns, D.W.; DeBoer, M.D. Racial/ethnic discrepancies in the metabolic syndrome begin in childhood and persist after adjustment for environmental factors. Nutr. Metab. Cardiovasc. Dis. 2012, 22, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Li, C.; Zhao, G.; Pearson, W.S.; Mokdad, A.H. Prevalence of the metabolic syndrome among U.S. adolescents using the definition from the International Diabetes Federation. Diabetes Care 2008, 31, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.A.; Friedman, L.A.; Harlan, W.R.; Harlan, L.C.; Barton, B.A.; Schreiber, G.B.; Klein, D.J. Development of the metabolic syndrome in black and white adolescent girls: A longitudinal assessment. Pediatrics 2005, 116, 1178–1182. [Google Scholar] [CrossRef] [PubMed]
- DeBoer, M.D.; Gurka, M.J. Ability among adolescents for the metabolic syndrome to predict elevations in factors associated with type 2 diabetes and cardiovascular disease: Data from the national health and nutrition examination survey 1999-2006. Metab. Syndr. Relat. Disord. 2010, 8, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Aradillas-García, C.; Rodríguez-Morán, M.; Garay-Sevilla, M.E.; Malacara, J.M.; Rascon-Pacheco, R.A.; Guerrero-Romero, F. Distribution of the homeostasis model assessment of insulin resistance in Mexican children and adolescents. Eur. J. Endocrinol. 2012, 166, 301–306. [Google Scholar] [CrossRef] [PubMed]
- DeBoer, M.D. Underdiagnosis of Metabolic Syndrome in Non-Hispanic Black Adolescents: A Call for Ethnic-Specific Criteria. Curr. Cardiovasc. Risk Rep. 2010, 4, 302–310. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Friend, A.; Craig, L.; Turner, S. The prevalence of metabolic syndrome in children: A systematic review of the literature. Metab. Syndr. Relat. Disord. 2013, 11, 71–80. [Google Scholar] [CrossRef] [PubMed]
- DeBoer, M.D.; Filipp, S.L.; Gurka, M.J. Geographical variation in the prevalence of obesity and metabolic syndrome among US adolescents. Pediatr. Obes. 2019, 14, e12483. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Goel, K.; Shah, P.; Misra, A. Childhood obesity in developing countries: Epidemiology, determinants, and prevention. Endocr. Rev. 2012, 33, 48–70. [Google Scholar] [CrossRef] [PubMed]
- Cossrow, N.; Falkner, B. Race/ethnic issues in obesity and obesity-related comorbidities. J. Clin. Endocrinol. Metab. 2004, 89, 2590–2594. [Google Scholar] [CrossRef]
- Magnussen, C.G.; Koskinen, J.; Chen, W.; Thomson, R.; Schmidt, M.D.; Srinivasan, S.R.; Kivimaki, M.; Mattsson, N.; Kähönen, M.; Laitinen, T.; et al. Pediatric metabolic syndrome predicts adulthood metabolic syndrome, subclinical atherosclerosis, and type 2 diabetes mellitus but is no better than body mass index alone: The Bogalusa Heart Study and the Cardiovascular Risk in Young Finns Study. Circulation 2010, 122, 1604–1611. [Google Scholar] [CrossRef]
- DeBoer, M.D.; Gurka, M.J.; Woo, J.G.; Morrison, J.A. Severity of the metabolic syndrome as a predictor of type 2 diabetes between childhood and adulthood: The Princeton Lipid Research Cohort Study. Diabetologia 2015, 58, 2745–2752. [Google Scholar] [CrossRef]
- DeBoer, M.D.; Gurka, M.J.; Woo, J.G.; Morrison, J.A. Severity of Metabolic Syndrome as a Predictor of Cardiovascular Disease Between Childhood and Adulthood: The Princeton Lipid Research Cohort Study. J. Amer. Coll. Card. 2015, 66, 755–757. [Google Scholar] [CrossRef]
- Deboer, M.D.; Wiener, R.C.; Barnes, B.H.; Gurka, M.J. Ethnic differences in the link between insulin resistance and elevated ALT. Pediatrics 2013, 132, e718–e726. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.M.; Charlton, J.R.; Carmody, J.B.; Gurka, M.J.; DeBoer, M.D. Metabolic risk factors in nondiabetic adolescents with glomerular hyperfiltration. Nephrol. Dial. Transpl. 2016, 32, 1517–1524. [Google Scholar] [CrossRef] [PubMed]
- DeBoer, M.D.; Filipp, S.L.; Musani, S.K.; Sims, M.; Okusa, M.D.; Gurka, M.J. Metabolic Syndrome Severity and Risk of CKD and Worsened GFR: The Jackson Heart Study. Kidney Blood Press. Res. 2018, 43, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Nader, P.R.; Bradley, R.H.; Houts, R.M.; McRitchie, S.L.; O’Brien, M. Moderate-to-vigorous physical activity from ages 9 to 15 years. JAMA 2008, 300, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Scharf, R.J.; DeBoer, M.D. Sugar-Sweetened Beverages and Children’s Health. Annu. Rev. Public Health 2016, 37, 273–293. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Interim Report of the Commission on Ending Childhood Obesity; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- O′Connor, E.A.; Evans, C.V.; Burda, B.U.; Walsh, E.S.; Eder, M.; Lozano, P. Screening for Obesity and Intervention for Weight Management in Children and Adolescents: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2017, 317, 2427–2444. [Google Scholar] [CrossRef] [PubMed]
- Morrison, J.A.; Glueck, C.J.; Horn, P.S.; Wang, P. Childhood predictors of adult type 2 diabetes at 9 and 26-year follow-ups. Arch. Pediatr. Adolesc. Med. 2010, 164, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Daniels, S.R.; Greer, F.R. Lipid screening and cardiovascular health in childhood. Pediatrics 2008, 122, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Brickman, W.J.; Huang, J.; Silverman, B.L.; Metzger, B.E. Acanthosis nigricans identifies youth at high risk for metabolic abnormalities. J. Pediatr. 2010, 156, 87–92. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care 2018, 41, S13–S27. [Google Scholar] [CrossRef]
- Bean, M.K.; Powell, P.; Quinoy, A.; Ingersoll, K.; Wickham, E.P., III; Mazzeo, S.E. Motivational interviewing targeting diet and physical activity improves adherence to paediatric obesity treatment: Results from the MI Values randomized controlled trial. Pediatr. Obes. 2015, 10, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.F.; Fonseca, S.C.; Garcia Rosa, M.L.; Yokoo, E.M. Relationship between ultra-processed foods and metabolic syndrome in adolescents from a Brazilian Family Doctor Program. Public Health Nutr. 2012, 15, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.-F.; Lin, W.-T.; Huang, H.-L.; Lee, C.-Y.; Wu, P.-W.; Chiu, Y.-W.; Huang, C.-C.; Tsai, S.; Lin, C.-L.; Lee, C.-H. Consumption of sugar-sweetened beverages is associated with components of the metabolic syndrome in adolescents. Nutrients 2014, 6, 2088–2103. [Google Scholar] [CrossRef] [PubMed]
- Ebbeling, C.B.; Ludwig, D.S. Sugar-sweetened beverages, genetic risk, and obesity. N. Engl. J. Med. 2013, 368, 287. [Google Scholar] [PubMed]
- CDC. 2008 Physical Activity Guidelines Americans. Available online: https://health.gov/paguidelines/pdf/paguide.pdf (accessed on 1 August 2019).
- Ekelund, U.; Anderssen, S.A.; Froberg, K.; Sardinha, L.B.; Andersen, L.B.; Brage, S. Independent associations of physical activity and cardiorespiratory fitness with metabolic risk factors in children: The European youth heart study. Diabetologia 2007, 50, 1832–1840. [Google Scholar] [CrossRef] [PubMed]
- Stabelini Neto, A.; de Campos, W.; Dos Santos, G.C.; Junior, O.M. Metabolic syndrome risk score and time expended in moderate to vigorous physical activity in adolescents. BMC Pediatr. 2014, 14, 42. [Google Scholar] [CrossRef] [PubMed]
- Guinhouya, B.C.; Samouda, H.; Zitouni, D.; Vilhelm, C.; Hubert, H. Evidence of the influence of physical activity on the metabolic syndrome and/or on insulin resistance in pediatric populations: A systematic review. Int. J. Pediatr. Obes. 2011, 6, 361–388. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Vélez, R.; García-Hermoso, A.; Agostinis-Sobrinho, C.A.; Mota, J.; Santos, R.; Correa-Bautista, J.E.; Amaya-Tambo, D.C.; Villa-González, E. Cycling to School and Body Composition, Physical Fitness, and Metabolic Syndrome in Children and Adolescents. J. Pediatr. 2017, 188, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Blundell, J.E.; Stubbs, R.J.; Hughes, D.A.; Whybrow, S.; King, N.A. Cross talk between physical activity and appetite control: Does physical activity stimulate appetite? Proc. Nutr. Soc. 2003, 62, 651–661. [Google Scholar] [CrossRef]
- Martin, C.K.; Heilbronn, L.K.; De Jonge, L.; Delany, J.P.; Volaufova, J.; Anton, S.D.; Redman, L.M.; Smith, S.R.; Ravussin, E.; Jonge, L. Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity 2007, 15, 2964–2973. [Google Scholar] [CrossRef]
- Caranti, D.A.; De Mello, M.T.; Prado, W.L.; Tock, L.; Siqueira, K.O.; De Piano, A.; Lofrano, M.C.; Cristofalo, D.M.; Lederman, H.; Tufik, S.; et al. Short and long-term beneficial effects of a multidisciplinary therapy for the control of metabolic syndrome in obese adolescents. Metabolism 2007, 56, 1293–1300. [Google Scholar] [CrossRef]
- Leite, N.; Milano, G.; Cieslak, F.; Lopes, W.; Rodacki, A.; Radominski, R. Effects of physical exercise and nutritional guidance on metabolic syndrome in obese adolescents. Braz. J. Phys. Ther. 2009, 12, 73–81. [Google Scholar] [CrossRef]
- WHO. Global Strategy on Diet, Physical Activity and Health. Available online: http://www.who.int/dietphysicalactivity/factsheet_young_people/en/ (accessed on 1 August 2019).
- Walker, S.E.; Smolkin, M.E.; O′Leary, M.L.; Cluett, S.B.; Norwood, V.F.; DeBoer, M.D.; Gurka, M.J. Predictors of Retention and BMI Loss or Stabilization in Obese Youth Enrolled in a Weight Loss Intervention. Obes. Res. Clin. Pract. 2012, 6, e330–e339. [Google Scholar] [CrossRef]
- Wu, T.; Gao, X.; Chen, M.; Van Dam, R.M. Long-term effectiveness of diet-plus-exercise interventions vs. diet-only interventions for weight loss: A meta-analysis. Obes. Rev. 2009, 10, 313–323. [Google Scholar] [CrossRef]

| Central Obesity (WC) | High BP (mmHg) | High Triglycerides (mg/dL) | Low HDL (mg/dL) | High Fasting Glucose |
|---|---|---|---|---|
| WC ≥ 90th percentile [25] | Systolic or diastolic DBP ≥ 90% for age, sex, height [26] | TG ≥ 110 mg/dL (≥1.24 mmol/L) | HDL ≤ 40 mg/dL (<1.03 mmol/L) | ≥100 mg/dL (5.6 mmol/L) or known T2DM |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
DeBoer, M.D. Assessing and Managing the Metabolic Syndrome in Children and Adolescents. Nutrients 2019, 11, 1788. https://doi.org/10.3390/nu11081788
DeBoer MD. Assessing and Managing the Metabolic Syndrome in Children and Adolescents. Nutrients. 2019; 11(8):1788. https://doi.org/10.3390/nu11081788
Chicago/Turabian StyleDeBoer, Mark D. 2019. "Assessing and Managing the Metabolic Syndrome in Children and Adolescents" Nutrients 11, no. 8: 1788. https://doi.org/10.3390/nu11081788
APA StyleDeBoer, M. D. (2019). Assessing and Managing the Metabolic Syndrome in Children and Adolescents. Nutrients, 11(8), 1788. https://doi.org/10.3390/nu11081788