Gender Differences in Phytoestrogens and the Relationship with Speed of Processing in Older Adults: A Cross-Sectional Analysis of NHANES, 1999–2002
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | body mass index |
| CDC | lefts for Disease Control and Prevention; |
| DSST | Digit Symbol Substitution Test |
| E2 | plasma estradiol |
| ERβ | estrogen receptor beta |
| GED | General Educational Development |
| GLM | general linear model |
| NHANES | National Health and Nutrition Examination Survey |
| SOP | speed of processing |
| PIR | poverty–income ratio |
| T | testosterone |
References
- Ruiz-Larrea, M.B.; Mohan, A.R.; Paganga, G.; Miller, N.J.; Bolwell, G.P.; Rice-Evans, C.A. Antioxidant activity of phytoestrogenic isoflavones. Free Radic. Res. 1997, 26, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Salzman, A.L.; Preiser, J.C.; Setchell, K.D.; Szabo, C. Isoflavone-mediated inhibition of tyrosine kinase: A novel antiinflammatory approach. J. Med. Food 1999, 2, 179–181. [Google Scholar] [CrossRef] [PubMed]
- Belcher, S.M.; Zsarnovszky, A. Estrogenic Actions in the Brain: Estrogen, Phytoestrogens, and Rapid Intracellular Signaling Mechanisms. J. Pharmacol. Exp. Ther. 2001, 299, 408–414. [Google Scholar] [PubMed]
- Lin, T.; Liu, G.A.; Perez, E.; Rainer, R.D.; Febo, M.; Cruz-Almeida, Y.; Ebner, N.C. Systemic Inflammation Mediates Age-Related Cognitive Deficits. Front. Aging Neurosci. 2018, 10, 10. [Google Scholar] [CrossRef] [PubMed]
- Warren, K.N.; Beason-Held, L.L.; Carlson, O.; Egan, J.M.; An, Y.; Doshi, J.; Davatzikos, C.; Ferrucci, L.; Resnick, S.M. Elevated Markers of Inflammation Are Associated with Longitudinal Changes in Brain Function in Older Adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2018, 73, 770–778. [Google Scholar] [CrossRef] [PubMed]
- Jantaratnotai, N.; Utaisincharoen, P.; Sanvarinda, P.; Thampithak, A.; Sanvarinda, Y. Phytoestrogens mediated anti-inflammatory effect through suppression of IRF-1 and pSTAT1 expressions in lipopolysaccharide-activated microglia. Int. Immunopharmacol. 2013, 17, 483–488. [Google Scholar] [CrossRef]
- Subedi, L.; Ji, E.; Shin, D.; Jin, J.; Yeo, J.H.; Kim, S.Y. Equol, a Dietary Daidzein Gut Metabolite Attenuates Microglial Activation and Potentiates Neuroprotection In Vitro. Nutrients 2017, 9, 207. [Google Scholar] [CrossRef]
- Ganai, A.A.; Husain, M. Genistein Alleviates Neuroinflammation and Restores Cognitive Function in Rat Model of Hepatic Encephalopathy: Underlying Mechanisms. Mol. Neurobiol. 2018, 55, 1762–1772. [Google Scholar] [CrossRef]
- McEwen, B.S.; Alves, S.E. Estrogen Actions in the Central Nervous System. Endocr. Rev. 1999, 20, 279–307. [Google Scholar] [CrossRef]
- Halbreich, U.; Kahn, L.S. Selective oestrogen receptor modulators—Current and future brain and behaviour applications. Expert Opin. Pharmacother. 2000, 1, 1385–1398. [Google Scholar] [CrossRef]
- Kuiper, G.G.; Lemmen, J.G.; Carlsson, B.; Corton, J.C.; Safe, S.H.; van der Saag, P.T.; van der Burg, B.; Gustafsson, J.A. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998, 139, 4252–4263. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Invited Review: Estrogens effects on the brain: Multiple sites and molecular mechanisms. J. Appl. Physiol. 2001, 91, 2785–2801. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.W.; Hoskin, E.; Yudkovitz, J.; Pear, L.; Wilkinson, H.A.; Hayashi, S.; Pfaff, D.W.; Ogawa, S.; Rohrer, S.P.; Schaeffer, J.M.; et al. Immunolocalization of Estrogen Receptor β in the Mouse Brain: Comparison with Estrogen Receptor α. Endocrinology 2003, 144, 2055–2067. [Google Scholar] [CrossRef]
- Agradi, E.; Vegeto, E.; Sozzi, A.; Fico, G.; Regondi, S.; Tomè, F. Traditional healthy Mediterranean diet: Estrogenic activity of plants used as food and flavoring agents. Phytother. Res. 2006, 20, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, A.; Fondell, E.; Ascherio, A.; Yuan, C.; Grodstein, F.; Willett, W. Adherence to Mediterranean diet and subjective cognitive function in men. Eur. J. Epidemiol. 2017, 33, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cox, P.A.; Metcalf, J.S. Traditional Food Items in Ogimi, Okinawa: L-Serine Content and the Potential for Neuroprotection. Curr. Nutr. Rep. 2017, 6, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Loughrey, D.G.; Lavecchia, S.; Brennan, S.; Lawlor, B.A.; Kelly, M.E. The Impact of the Mediterranean Diet on the Cognitive Functioning of Healthy Older Adults: A Systematic Review and Meta-Analysis. Adv. Nutr. 2017, 8, 571–586. [Google Scholar]
- Lampe, J.W.; Gustafson, D.R.; Hutchins, A.M.; Martini, M.C.; Li, S.; Wähälä, K.; Grandits, G.A.; Potter, J.D.; Slavin, J.L. Urinary Isoflavonoid and Lignan Excretion on a Western Diet: Relation to Soy, Vegetable, and Fruit Intake. Cancer Epidemiol. Biomark. Prev. 1999, 8, 699–707. [Google Scholar]
- Sales and Trends Soyfoods Association Health Benefits of Making Soy the Preferred Plant Based Protein. Available online: http://www.soyfoods.org/soy-products/sales-and-trends (accessed on 26 June 2019).
- Xu, X.; Harris, K.S.; Wang, H.J.; Murphy, P.A.; Hendrich, S. Bioavailability of soybean isoflavones depends upon gut microflora in women. J. Nutr. 1995, 125, 2307–2315. [Google Scholar] [CrossRef]
- Yuan, J.P.; Wang, J.H.; Liu, X. Metabolism of dietary soy isoflavones to equol by human intestinal microflora—Implications for health. Mol. Nutr. Food Res. 2007, 51, 765–781. [Google Scholar] [CrossRef]
- Song, Y.; You, N.C.Y.; Song, Y.; Kang, M.K.; Hou, L.; Wallace, R.; Eaton, C.B.; Tinker, L.F.; Liu, S. Intake of small-to-medium-chain saturated fatty acids is associated with peripheral leukocyte telomere length in postmenopausal women. J. Nutr. 2013, 143, 907–914. [Google Scholar] [CrossRef]
- Guadamuro, L.; Delgado, S.; Redruello, B.; Flórez, A.B.; Suárez, A.; Martínez-Camblor, P.; Mayo, B. Equol status and changes in fecal microbiota in menopausal women receiving long-term treatment for menopause symptoms with a soy-isoflavone concentrate. Front. Microbiol. 2015, 6, 777. [Google Scholar] [CrossRef]
- Zoetendal, E.G.; Akkermans, A.D.L.; De Vos, W.M. Temperature Gradient Gel Electrophoresis Analysis of 16S rRNA from Human Fecal Samples Reveals Stable and Host-Specific Communities of Active Bacteria. Appl. Environ. Microbiol. 1998, 64, 3854–3859. [Google Scholar]
- Kaliannan, K.; Robertson, R.C.; Murphy, K.; Stanton, C.; Kang, C.; Wang, B.; Hao, L.; Bhan, A.K.; Kang, J.X. Estrogen-mediated gut microbiome alterations influence sexual dimorphism in metabolic syndrome in mice. Microbiome 2018, 6, 205. [Google Scholar] [CrossRef]
- Horstman, A.M.; Dillon, E.L.; Urban, R.J.; Sheffield-Moore, M. The Role of Androgens and Estrogens on Healthy Aging and Longevity. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2012, 67, 1140–1152. [Google Scholar] [CrossRef]
- Leifke, E.; Gorenoi, V.; Wichers, C.; Von Zur Mühlen, A.; Von Büren, E.; Brabant, G. Age-related changes of serum sex hormones, insulin-like growth factor-1 and sex-hormone binding globulin levels in men: Cross-sectional data from a healthy male cohort. Clin. Endocrinol. 2000, 53, 689–695. [Google Scholar] [CrossRef]
- Roivainen, E. Gender differences in processing speed: A review of recent research. Learn. Individ. Differ. 2011, 21, 145–149. [Google Scholar] [CrossRef]
- Ronquillo, J.G.; Baer, M.R.; Lester, W.T. Sex-specific patterns and differences in dementia and Alzheimer’s disease using informatics approaches. J. Women Aging 2016, 28, 403–411. [Google Scholar] [CrossRef]
- Rosario, E.R.; Chang, L.; Head, E.H.; Stanczyk, F.Z.; Pike, C.J. Brain levels of sex steroid hormones in men and women during normal aging and in Alzheimer’s disease. Neurobiol. Aging 2011, 32, 604–613. [Google Scholar] [CrossRef]
- Small, B.J.; Dixon, R.A.; McArdle, J.J. Tracking cognition-health changes from 55 to 95 years of age. J. Gerontol. Ser. B Psychol. Sci. Soc. Sci. 2011, 66 (Suppl. 1), i153–i161. [Google Scholar] [CrossRef]
- Ostatníková, D.; Celec, P.; Hodosy, J.; Hampl, R.; Putz, Z.; Kúdela, M. Short-term soybean intake and its effect on steroid sex hormones and cognitive abilities. Fertil. Steril. 2007, 88, 1632–1636. [Google Scholar] [CrossRef]
- Gleason, C.E.; Carlsson, C.M.; Barnet, J.H.; Meade, S.A.; Setchell, K.D.R.; Atwood, C.S.; Johnson, S.C.; Ries, M.L.; Asthana, S. A preliminary study of the safety, feasibility and cognitive efficacy of soy isoflavone supplements in older men and women. Age Ageing 2009, 38, 86–93. [Google Scholar] [CrossRef]
- Johnson, C.L.; Paulose-Ram, R.; Ogden, C.L.; Carroll, M.D.; Kruszon-Moran, D.; Dohrmann, S.M.; Curtin, L.R. National health and nutrition examination survey: Analytic guidelines, 1999–2010. Vital. Health Stat. 2013, 2, 1–24. [Google Scholar]
- Favé, G.; Beckmann, M.; Lloyd, A.J.; Zhou, S.; Harold, G.; Lin, W.; Tailliart, K.; Xie, L.; Draper, J.; Mathers, J.C. Development and validation of a standardized protocol to monitor human dietary exposure by metabolite fingerprinting of urine samples. Metabolomics 2011, 7, 469–484. [Google Scholar] [CrossRef]
- Blair, R.M.; Appt, S.E.; Franke, A.A.; Clarkson, T.B. Treatment with antibiotics reduces plasma equol concentration in cynomolgus monkeys (Macaca fascicularis). J. Nutr. 2003, 133, 2262–2267. [Google Scholar] [CrossRef]
- Kilkkinen, A.; Stumpf, K.; Pietinen, P.; Valsta, L.M.; Tapanainen, H.; Adlercreutz, H. Determinants of serum enterolactone concentration. Am. J. Clin. Nutr. 2001, 73, 1094–1100. [Google Scholar] [CrossRef]
- Valentín-Blasini, L.; Sadowski, M.A.; Walden, D.; Caltabiano, L.; Needham, L.L.; Barr, D.B. Urinary phytoestrogen concentrations in the U.S. population (1999–2000). J. Expo. Sci. Environ. Epidemiol. 2005, 15, 509–523. [Google Scholar] [CrossRef]
- Tukey, J.W. Exploratory Data Analysis; Addison-Wesley Pub. Co.: Reading, MA, USA, 1977. [Google Scholar]
- McGill, R.; Tukey, J.W.; Larsen, W.A. Variations of Box Plots. Am. Stat. 1978, 32, 12–16. [Google Scholar]
- Wechsler, D. WAIS-R, Wechsler Adult Intelligence Scale-Revised, Manual; Psychological Corporation: New York, NY, USA, 1981. [Google Scholar]
- Hoyer, W.J.; Stawski, R.S.; Wasylyshyn, C.; Verhaeghen, P. Adult age and digit symbol substitution performance: A meta-analysis. Psychol. Aging 2004, 19, 211–214. [Google Scholar] [CrossRef]
- Matarazzo, J.D.; Herman, D.O. Base rate data for the WAIS-R: Test-retest stability and VIQ-PIQ differences. J. Clin. Neuropsychol. 1984, 6, 351–366. [Google Scholar] [CrossRef]
- Ryan, J.J.; Schnakenberg-Ott, S.D. Scoring reliability on the Wechsler Adult Intelligence Scale-Third Edition (WAIS-III). Assessment 2003, 10, 151–159. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS). Laboratory Procedure Manual. In Phytoestrogens: Genistein, Daidzein, Equol, Enterodiol, Enterolactone, O-Desmethylangolensin; Centers for Disease Control and Prevention (CDC) National Center for Health Statistics (NCHS): Hyattsville, MD, USA, 2004. [Google Scholar]
- Valentín-Blasini, L.; Blount, B.C.; Rogers, H.; Needham, L.L. HPLC-MS/MS method for the measurement of seven phytoestrogens in human serum and urine. J. Expo. Sci. Environ. Epidemiol. 2000, 10, 799. [Google Scholar] [CrossRef][Green Version]
- Valentín-Blasini, L.; Blount, B.C.; Caudill, S.P.; Needham, L.L. Urinary and serum concentrations of seven phytoestrogens in a human reference population subset. J. Expo. Sci. Environ. Epidemiol. 2003, 13, 276. [Google Scholar] [CrossRef]
- Grace, P.B.; Taylor, J.I.; Low, Y.L.; Luben, R.N.; Mulligan, A.A.; Botting, N.P.; Dowsett, M.; Welch, A.A.; Khaw, K.T.; Wareham, N.J.; et al. Phytoestrogen Concentrations in Serum and Spot Urine as Biomarkers for Dietary Phytoestrogen Intake and Their Relation to Breast Cancer Risk in European Prospective Investigation of Cancer and Nutrition-Norfolk. Cancer Epidemiol. Biomark. Prev. 2004, 13, 698–708. [Google Scholar]
- Seow, A.; Shi, C.Y.; Franke, A.A.; Hankin, J.H.; Lee, H.P.; Yu, M.C. Isoflavonoid levels in spot urine are associated with frequency of dietary soy intake in a population-based sample of middle-aged and older Chinese in Singapore. Cancer Epidemiol. Biomark. Prev. 1998, 7, 135–140. [Google Scholar]
- Chávez-Suárez, K.M.; Ortega-Vélez, M.I.; Valenzuela-Quintanar, A.I.; Galván-Portillo, M.; López-Carrillo, L.; Esparza-Romero, J.; Saucedo-Tamayo, M.S.; Robles-Burgueño, M.R.; Palma-Durán, S.A.; Gutiérrez-Coronado, M.L.; et al. Phytoestrogen Concentrations in Human Urine as Biomarkers for Dietary Phytoestrogen Intake in Mexican Women. Nutrients 2017, 9, 1078. [Google Scholar] [CrossRef]
- Arai, Y.; Uehara, M.; Sato, Y.; Kimira, M.; Eboshida, A.; Adlercreutz, H.; Watanabe, S. Comparison of Isoflavones among Dietary Intake, Plasma Concentration and Urinary Excretion for Accurate Estimation of Phytoestrogen Intake. J. Epidemiol. 2000, 10, 127–135. [Google Scholar] [CrossRef]
- Barr, D.B.; Wilder, L.C.; Caudill, S.P.; Gonzalez, A.J.; Needham, L.L.; Pirkle, J.L. Urinary Creatinine Concentrations in the U.S. Population: Implications for Urinary Biologic Monitoring Measurements. Environ. Health Perspect. 2005, 113, 192–200. [Google Scholar] [CrossRef]
- Orth, S.R. Smoking and the Kidney. JASN 2002, 13, 1663–1672. [Google Scholar] [CrossRef]
- O’Brien, K.M.; Upson, K.; Buckley, J.P. Lipid and Creatinine Adjustment to Evaluate Health Effects of Environmental Exposures. Curr. Environ. Health Rep. 2017, 4, 44–50. [Google Scholar] [CrossRef]
- O’Brien, K.M.; Upson, K.; Cook, N.R.; Weinberg, C.R. Environmental Chemicals in Urine and Blood: Improving Methods for Creatinine and Lipid Adjustment. Environ. Health Perspect. 2016, 124, 220–227. [Google Scholar] [CrossRef]
- Poverty Guidelines. Available online: https://aspe.hhs.gov/poverty-guidelines (accessed on 25 April 2017).
- SAS Institute. SAS 9.4 Macro Language: Reference, 5th ed.; SAS Institute: Cary, NC, USA, 2013. [Google Scholar]
- SAS Institute. SAS 9.4 Software; SAS Institute: Cary, NC, USA.
- Long, J.A. Interactions: Comprehensive, User-Friendly Toolkit for Probing Interactions. Available online: https://cran.r-project.org/web/packages/interactions/index.html (accessed on 12 July 2019).
- Team, R.C. The R Project for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Casini, M.L.; Marelli, G.; Papaleo, E.; Ferrari, A.; D’Ambrosio, F.; Unfer, V. Psychological assessment of the effects of treatment with phytoestrogens on postmenopausal women: A randomized, double-blind, crossover, placebo-controlled study. Fertil. Steril. 2006, 85, 972–978. [Google Scholar] [CrossRef]
- Celec, P.; Ostatníková, D.; Cagánová, M.; Zuchová, S.; Hodosy, J.; Putz, Z.; Bernadic, M.; Kúdela, M. Endocrine and cognitive effects of short-time soybean consumption in women. Gynecol. Obstet. Investig. 2005, 59, 62–66. [Google Scholar] [CrossRef]
- Duffy, R.; Wiseman, H.; File, S.E. Improved cognitive function in postmenopausal women after 12 weeks of consumption of a soya extract containing isoflavones. Pharmacol. Biochem. Behav. 2003, 75, 721–729. [Google Scholar] [CrossRef]
- Kritz-Silverstein, D.; Von Mühlen, D.; Barrett-Connor, E.; Bressel, M.A.B. Isoflavones and cognitive function in older women: The SOy and Postmenopausal Health in Aging (SOPHIA) Study. Menopause 2003, 10, 196–202. [Google Scholar] [CrossRef]
- Nakamoto, M.; Otsuka, R.; Nishita, Y.; Tange, C.; Tomida, M.; Kato, Y.; Imai, T.; Sakai, T.; Ando, F.; Shimokata, H. Soy food and isoflavone intake reduces the risk of cognitive impairment in elderly Japanese women. Eur. J. Clin. Nutr. 2018, 72, 1458–1462. [Google Scholar] [CrossRef]
- Ho, S.C.; Chan, A.S.Y.; Ho, Y.P.; So, E.K.F.; Sham, A.; Zee, B.; Woo, J.L.F. Effects of soy isoflavone supplementation on cognitive function in Chinese postmenopausal women: A double-blind, randomized, controlled trial. Menopause 2007, 14, 489–499. [Google Scholar] [CrossRef]
- Kreijkamp-Kaspers, S.; Kok, L.; Grobbee, D.E.; de Haan, E.H.F.; Aleman, A.; Lampe, J.W.; van der Schouw, Y.T. Effect of soy protein containing isoflavones on cognitive function, bone mineral density, and plasma lipids in postmenopausal women: A randomized controlled trial. JAMA 2004, 292, 65–74. [Google Scholar] [CrossRef]
- White, L.R.; Petrovitch, H.; Ross, G.W.; Masaki, K.; Hardman, J.; Nelson, J.; Davis, D.; Markesbery, W. Brain aging and midlife tofu consumption. J. Am. Coll. Nutr. 2000, 19, 242–255. [Google Scholar] [CrossRef]
- Lephart, E.D.; West, T.W.; Weber, K.S.; Rhees, R.W.; Setchell, K.D.R.; Adlercreutz, H.; Lund, T.D. Neurobehavioral effects of dietary soy phytoestrogens. Neurotoxicol. Teratol. 2002, 24, 5–16. [Google Scholar] [CrossRef]
- Lund, T.D.; West, T.W.; Tian, L.Y.; Bu, L.H.; Simmons, D.L.; Setchell, K.D.; Adlercreutz, H.; Lephart, E.D. Visual spatial memory is enhanced in female rats (but inhibited in males) by dietary soy phytoestrogens. BMC Neurosci. 2001, 2, 20. [Google Scholar]
- Lin, H.C.; Peng, C.H.; Huang, C.N.; Chiou, J.Y. Soy-Based Foods Are Negatively Associated with Cognitive Decline in Taiwan’s Elderly. J. Nutr. Sci. Vitaminol. 2018, 64, 335–339. [Google Scholar] [CrossRef]
- Thorp, A.A.; Sinn, N.; Buckley, J.D.; Coates, A.M.; Howe, P.R.C. Soya is oflavone supplementation enhances spatial working memory in men. Br. J. Nutr. 2009, 102, 1348–1354. [Google Scholar] [CrossRef]
- Celec, P.; Ostatníková, D.; Hodosy, J.; Putz, Z.; Kúdela, M. Increased one week soybean consumption affects spatial abilities but not sex hormone status in men. Int. J. Food Sci. Nutr. 2007, 58, 424–428. [Google Scholar] [CrossRef]
- File, S.E.; Jarrett, N.; Fluck, E.; Duffy, R.; Casey, K.; Wiseman, H. Eating soya improves human memory. Psychopharmacology 2001, 157, 430–436. [Google Scholar] [CrossRef]
- Kruijver, F.P.M.; Balesar, R.; Espila, A.M.; Unmehopa, U.A.; Swaab, D.F. Estrogen-receptor-beta distribution in the human hypothalamus: Similarities and differences with ER alpha distribution. J. Comp. Neurol. 2003, 466, 251–277. [Google Scholar] [CrossRef]
- Morito, K.; Hirose, T.; Kinjo, J.; Hirakawa, T.; Okawa, M.; Nohara, T.; Ogawa, S.; Inoue, S.; Muramatsu, M.; Masamune, Y. Interaction of Phytoestrogens with Estrogen Receptors α and β. Biol. Pharm. Bull. 2001, 24, 351–356. [Google Scholar] [CrossRef]
- Atkinson, C.; Skor, H.E.; Fitzgibbons, E.D.; Scholes, D.; Chen, C.; Wähälä, K.; Schwartz, S.M.; Lampe, J.W. Overnight urinary isoflavone excretion in a population of women living in the United States, and its relationship to isoflavone intake. Cancer Epidemiol. Biomark. Prev. 2002, 11, 253–260. [Google Scholar]
- Degen, G.H.; Blaszkewicz, M.; Shi, L.; Buyken, A.E.; Remer, T. Urinary isoflavone phytoestrogens in German children and adolescents—A longitudinal examination in the Donald cohort. Mol. Nutr. Food Res. 2011, 55, 359–367. [Google Scholar] [CrossRef]
- Teitelbaum, S.L.; Britton, J.A.; Calafat, A.M.; Ye, X.; Silva, M.J.; Reidy, J.A.; Galvez, M.P.; Brenner, B.L.; Wolff, M.S. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environ. Res. 2008, 106, 257–269. [Google Scholar] [CrossRef]
- Maskarinec, G.; Singh, S.; Meng, L.; Franke, A.A. Dietary soy intake and urinary isoflavone excretion among women from a multiethnic population. Cancer Epidemiol. Biomark. Prev. 1998, 7, 613–619. [Google Scholar]
- French, M.R.; Thompson, L.U.; Hawker, G.A. Validation of a Phytoestrogen Food Frequency Questionnaire with Urinary Concentrations of Isoflavones and Lignan Metabolites in Premenopausal Women. J. Am. Coll. Nutr. 2007, 26, 76–82. [Google Scholar] [CrossRef]
- Darby, D.; Walsh, K.W.; Walsh, K.W. Walsh’s Neuropsychology: A Clinical Approach; Elsevier Churchill Livingstone: Edinburgh/London, UK, 2005. [Google Scholar]
- Lezak, M.D. Neuropsychological Assessment, 3rd ed.; Oxford University Press: New York, NY, USA, 1995. [Google Scholar]
- Piccinin, A.M.; Rabbitt, P.M.A. Contribution of cognitive abilities to performance and improvement on a substitution coding task. Psychol. Aging 1999, 14, 539–551. [Google Scholar] [CrossRef]
- Landete, J.M.; Arqués, J.; Medina, M.; Gaya, P.; de Las Rivas, B.; Muñoz, R. Bioactivation of Phytoestrogens: Intestinal Bacteria and Health. Crit. Rev. Food Sci. Nutr. 2016, 56, 1826–1843. [Google Scholar] [CrossRef]
- López, P.; Sánchez, M.; Perez-Cruz, C.; Velázquez-Villegas, L.A.; Syeda, T.; Aguilar-López, M.; Rocha-Viggiano, A.K.; Del Carmen Silva-Lucero, M.; Torre-Villalvazo, I.; Noriega, L.G.; et al. Long-Term Genistein Consumption Modifies Gut Microbiota, Improving Glucose Metabolism, Metabolic Endotoxaemia and Cognitive Function in Mice Fed a High-Fat Diet. Mol. Nutr. Food Res. 2018, 62, 1800313. [Google Scholar] [CrossRef]
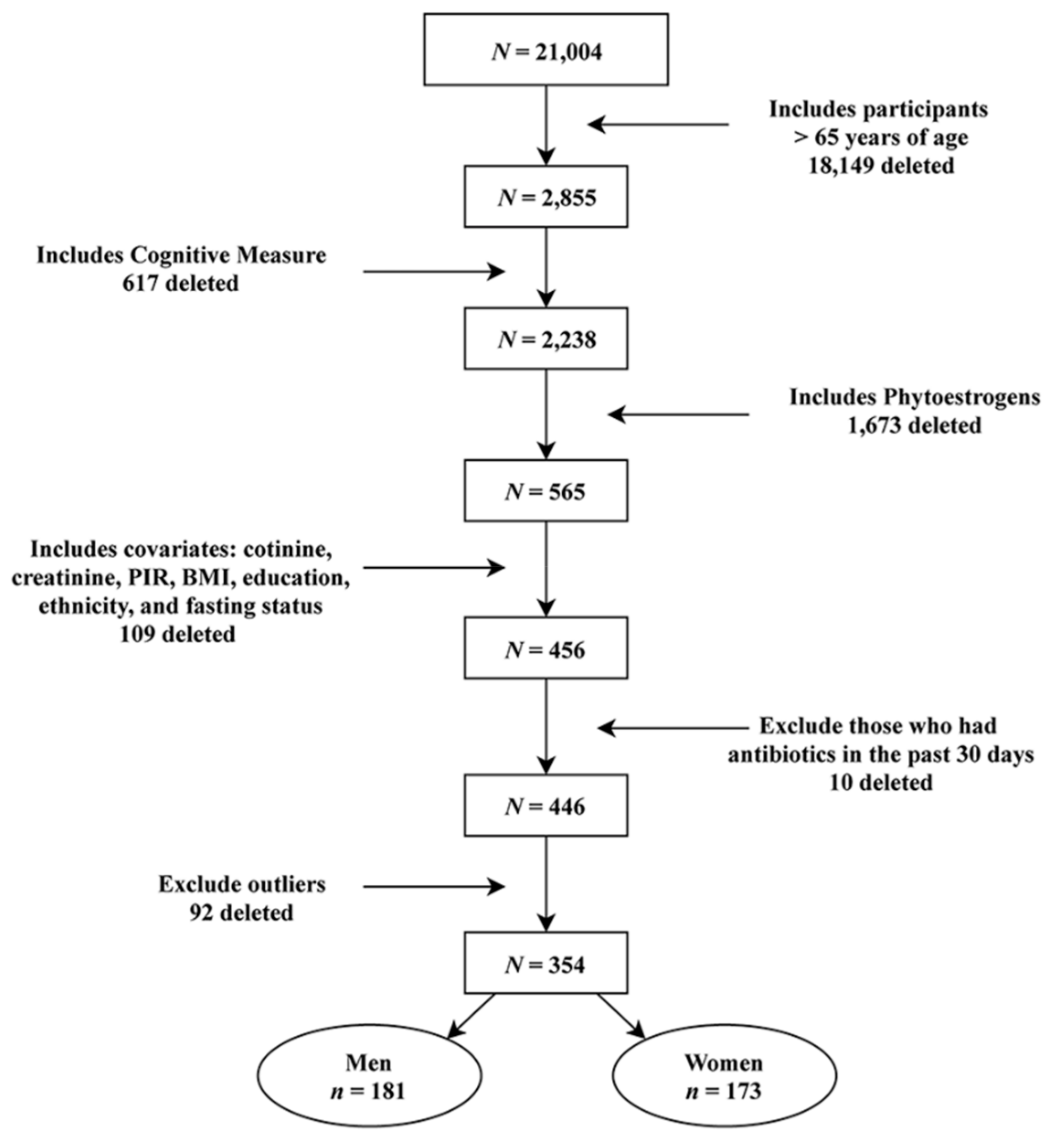
| All | Men | Women | ||||
|---|---|---|---|---|---|---|
| N = 354 | N = 181 | N = 173 | ||||
| Characteristic | M (%) | SD | M (%) | SD | M (%) | SD |
| Age (years) | 74.10 | 6.26 | 73.5 | 6.03 | 74.74 | 5.46 |
| Non-Hispanic White | (71.19) | (70.88) | (71.51) | |||
| Education | (40.06) | (39.56) | (40.12) | |||
| Body Mass Index (BMI) | 27.41 | 4.57 | 27.39 | 4.13 | 27.43 | 5.01 |
| Above Poverty Line (PIR) | (84.46) | (84.62) | (84.30) | |||
| Creatinine (mg/dL) * | 104.38 | 62.34 | 121.40 | 62.02 | 86.33 | 57.56 |
| Smoke * | (39.27) | (46.15) | (31.98) | |||
| Fasted | (63.28) | (66.48) | (59.88) | |||
| Speed of Processing * | 41.25 | 17.60 | 39.11 | 16.49 | 43.51 | 18.48 |
| Raw Scores (ng/mL) | ||||||
| O-DMA | 17.85 | 67.67 | 18.54 | 43.78 | 17.13 | 86.16 |
| Daidzein | 115.09 | 249.41 | 145.10 | 312.20 | 83.37 | 152.60 |
| Equol | 10.14 | 11.68 | 10.68 | 11.56 | 9.57 | 11.81 |
| Genistein | 64.89 | 130.26 | 70.22 | 141.00 | 59.25 | 117.90 |
| Enterolactone | 646.24 | 854.33 | 713.50 | 993.60 | 575.10 | 672.20 |
| Enterodiol | 58.15 | 71.90 | 59.60 | 72.54 | 56.61 | 71.40 |
| Adjusted | ||||||
| O-DMA | 0.68 | 2.65 | 0.75 | 2.80 | 0.62 | 2.49 |
| Daidzein | 3.83 | 2.83 | 4.10 | 2.90 | 3.54 | 2.73 |
| Equol | 2.02 | 1.43 | 2.06 | 1.43 | 1.98 | 1.43 |
| Genistein | 3.34 | 2.34 | 3.47 | 2.13 | 3.20 | 2.54 |
| Enterolactone | 6.84 | 4.61 | 6.97 | 6.25 | 6.72 | 6.07 |
| Enterodiol | 3.93 | 2.50 | 3.99 | 3.62 | 3.87 | 3.49 |
| Predictor | Estimate | Std. Error |
| Constant | 29.01 *** | 2.84 |
| Age | −5.19 *** | 0.82 |
| BMI | 1.25 | 0.84 |
| Ethnicity | −9.49 *** | 1.99 |
| Education | 9.61 *** | 1.64 |
| Smoking | −2.52 | 1.61 |
| Poverty–Income Ratio | 11.10 *** | 2.23 |
| Creatinine | −3.01 | 2.15 |
| Fasting status | 2.17 | 1.61 |
| Women | 2.96 | 2.13 |
| O-Desmethylangolensin | 0.84 | 1.24 |
| Daidzein | −0.26 | 2.04 |
| Equol | −0.22 | 1.52 |
| Genistein | −4.02 * | 2.00 |
| Enterolactone | 2.28 | 2.25 |
| Enterodiol | −1.03 | 1.82 |
| Predictor (Interactions) | ||
| O-Desmethylangolensin × Women | −0.58 | 1.82 |
| Daidzein × Women | −0.79 | 2.63 |
| Equol × Women | −0.42 | 2.02 |
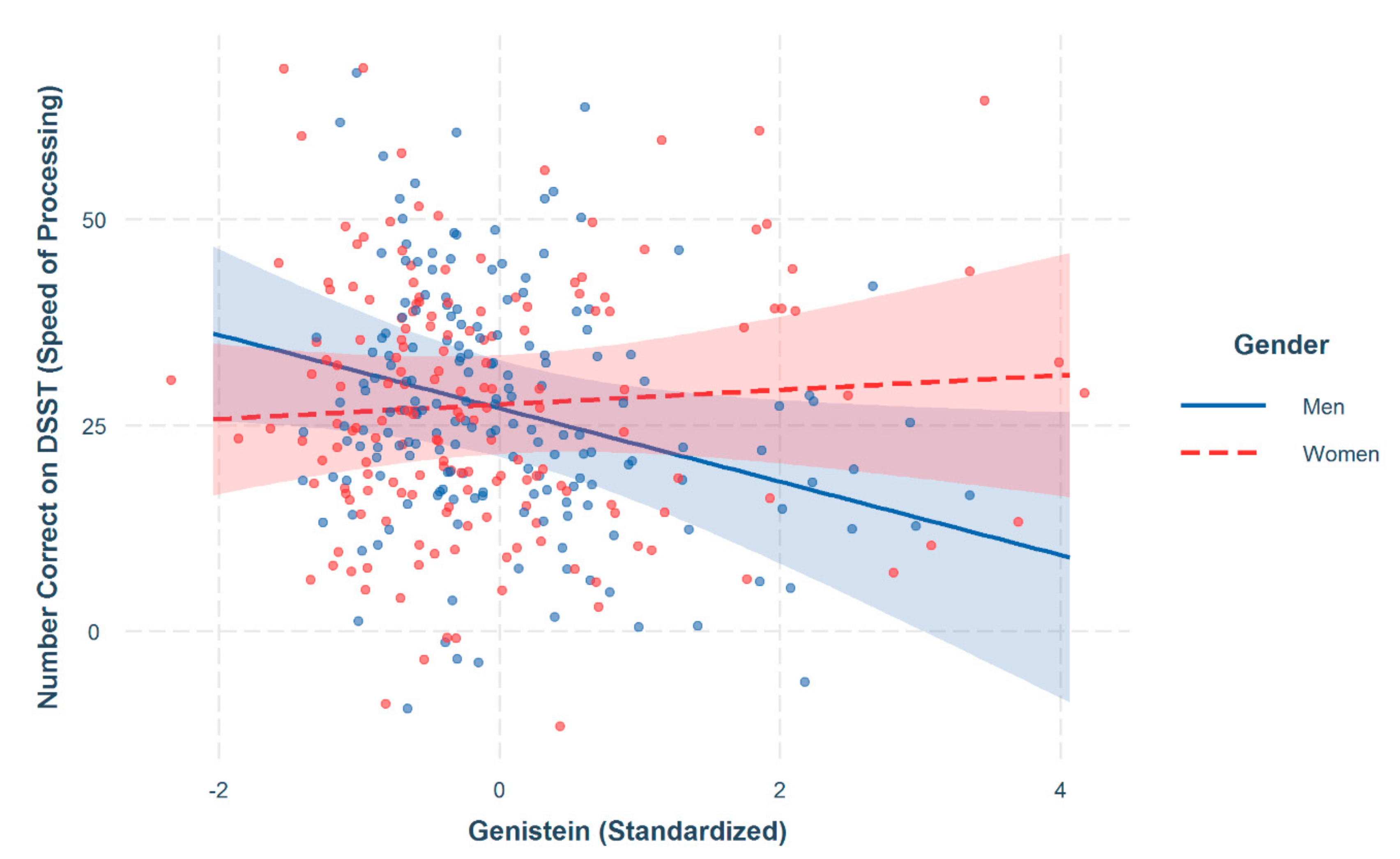
| Genistein × Women | 5.04 * | 2.51 |
| Enterolactone × Women | −0.04 | 2.88 |
| Enterodiol × Women | −3.06 | 2.45 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alwerdt, J.; Patterson, A.D.; Sliwinski, M.J. Gender Differences in Phytoestrogens and the Relationship with Speed of Processing in Older Adults: A Cross-Sectional Analysis of NHANES, 1999–2002. Nutrients 2019, 11, 1780. https://doi.org/10.3390/nu11081780
Alwerdt J, Patterson AD, Sliwinski MJ. Gender Differences in Phytoestrogens and the Relationship with Speed of Processing in Older Adults: A Cross-Sectional Analysis of NHANES, 1999–2002. Nutrients. 2019; 11(8):1780. https://doi.org/10.3390/nu11081780
Chicago/Turabian StyleAlwerdt, Jessie, Andrew D. Patterson, and Martin J. Sliwinski. 2019. "Gender Differences in Phytoestrogens and the Relationship with Speed of Processing in Older Adults: A Cross-Sectional Analysis of NHANES, 1999–2002" Nutrients 11, no. 8: 1780. https://doi.org/10.3390/nu11081780
APA StyleAlwerdt, J., Patterson, A. D., & Sliwinski, M. J. (2019). Gender Differences in Phytoestrogens and the Relationship with Speed of Processing in Older Adults: A Cross-Sectional Analysis of NHANES, 1999–2002. Nutrients, 11(8), 1780. https://doi.org/10.3390/nu11081780






