Eight Weeks of a High Dose of Curcumin Supplementation May Attenuate Performance Decrements Following Muscle-Damaging Exercise
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Experimental Protocol
2.3. Supplementation
2.4. Muscle Function Assessment
2.5. Statistical Analysis
3. Results
3.1. Isokinetic Peak Flexion Torque
3.2. Isokinetic Average Peak Flexion Torque
3.3. Isokinetic Peak Extension Torque
3.4. Isokinetic Average Peak Extension Torque
3.5. Isokinetic Peak Extension Power
3.6. Isokinetic Peak Flexion Power
3.7. Isometric Peak Torque
3.8. Isometric Average Peak Torque
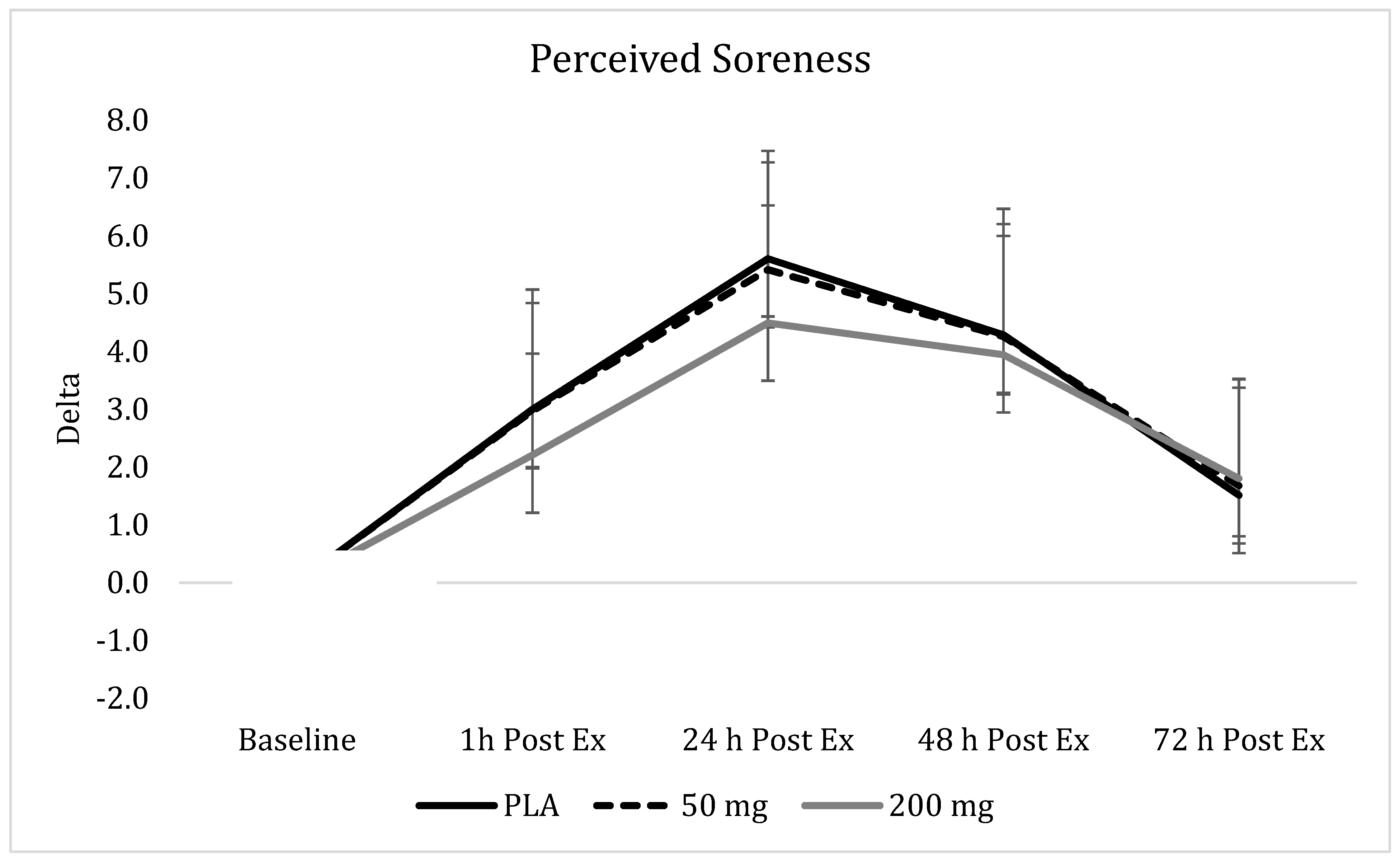
3.9. Perceived Soreness
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jobin, C.; Bradham, C.A.; Russo, M.P.; Juma, B.; Narula, A.S.; Brenner, D.A.; Sartor, R.B. Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity. J. Immunol. 1999, 163, 3474–3483. [Google Scholar]
- Shehzad, A.; Ha, T.; Subhan, F.; Lee, Y.S. New mechanisms and the anti-inflammatory role of curcumin in obesity and obesity-related metabolic diseases. Eur. J. Nutr. 2011, 50, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Howatson, G.; van Someren, K.A. The prevention and treatment of exercise-induced muscle damage. Sports Med. 2008, 38, 483–503. [Google Scholar] [CrossRef] [PubMed]
- Paulsen, G.; Mikkelsen, U.R.; Raastad, T.; Peake, J.M. Leucocytes, cytokines and satellite cells: What role do they play in muscle damage and regeneration following eccentric exercise? Exerc. Immunol. Rev. 2012, 18, 42–97. [Google Scholar] [PubMed]
- Harty, P.S.; Cottet, M.L.; Malloy, J.K.; Kerksick, C.M. Nutritional and Supplementation Strategies to Prevent and Attenuate Exercise-Induced Muscle Damage: A Brief Review. Sports Med. Open 2019, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.; Hume, P.; Maxwell, L. Delayed onset muscle soreness: Treatment strategies and performance factors. Sports Med. 2003, 33, 145–164. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. 2009, 14, 141–153. [Google Scholar]
- Davis, J.M.; Murphy, E.A.; Carmichael, M.D.; Zielinski, M.R.; Groschwitz, C.M.; Brown, A.S.; Gangemi, J.D.; Ghaffar, A.; Mayer, E.P. Curcumin effects on inflammation and performance recovery following eccentric exercise-induced muscle damage. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R2168–R2173. [Google Scholar] [CrossRef]
- Udani, J.K.; Singh, B.B.; Singh, V.J.; Sandoval, E. BounceBack capsules for reduction of DOMS after eccentric exercise: A randomized, double-blind, placebo-controlled, crossover pilot study. J. Int. Soc. Sports Nutr. 2009, 6, 14. [Google Scholar] [CrossRef]
- Drobnic, F.; Riera, J.; Appendino, G.; Togni, S.; Franceschi, F.; Valle, X.; Pons, A.; Tur, J. Reduction of delayed onset muscle soreness by a novel curcumin delivery system (Meriva(R)): A randomised, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2014, 11, 31. [Google Scholar] [CrossRef] [PubMed]
- Nicol, L.M.; Rowlands, D.S.; Fazakerly, R.; Kellett, J. Curcumin supplementation likely attenuates delayed onset muscle soreness (DOMS). Eur. J. Appl. Physiol. 2015, 115, 1769–1777. [Google Scholar] [CrossRef] [PubMed]
- Gaffey, A.; Slater, H.; Porritt, K.; Campbell, J.M. The effects of curcuminoids on musculoskeletal pain: A systematic review. JBI Database Syst. Rev. Implement. Rep. 2017, 15, 486–516. [Google Scholar]
- Nelson, K.M.; Dahlin, J.L.; Bisson, J.; Graham, J.; Pauli, G.F.; Walters, M.A. The Essential Medicinal Chemistry of Curcumin. J. Med. Chem. 2017, 60, 1620–1637. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Wahlstrom, B.; Blennow, G. A study on the fate of curcumin in the rat. Acta Pharmacol. Toxicol. (Copenh.) 1978, 43, 86–92. [Google Scholar] [CrossRef]
- Lao, C.D.; Ruffin, M.T., 4th; Normolle, D.; Heath, D.D.; Murray, S.I.; Bailey, J.M.; Boggs, M.E.; Crowell, J.; Rock, C.L.; Brenner, D.E. Dose escalation of a curcuminoid formulation. BMC Complement. Altern. Med. 2006, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Yallapu, M.M.; Jaggi, M.; Chauhan, S.C. Curcumin nanoformulations: A future nanomedicine for cancer. Drug Discov. Today 2012, 17, 71–80. [Google Scholar] [CrossRef]
- Jäger, R.; Lowery, R.P.; Calvanese, A.V.; Joy, J.M.; Purpura, M.; Wilson, J.M. Comparative absorption of curcumin formulations. Nutr. J. 2014, 13, 11. [Google Scholar] [CrossRef]
- Oliver, J.M.; Stoner, L.; Rowlands, D.S.; Caldwell, A.R.; Sanders, E.; Kreutzer, A.; Mitchell, J.B.; Purpura, M.; Jäger, R. Novel Form of Curcumin Improves Endothelial Function in Young, Healthy Individuals: A Double-Blind Placebo Controlled Study. J. Nutr. Metab. 2016, 2016, 1089653. [Google Scholar] [CrossRef]
- Thompson, W.R.; Gordon, N.F.; Pescatello, L.S. ACSM’s Guidelines for Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2010. [Google Scholar]
- Li, S.; Yuan, W.; Deng, G.; Wang, P.; Yang, P. Chemical composition and product quality control of turmeric (Curcuma longa L.). Pharm. Crops. 2011, 2, 28–54. [Google Scholar] [CrossRef]
- Gleeson, N.P.; Mercer, T.H. The utility of isokinetic dynamometry in the assessment of human muscle function. Sports Med. 1996, 21, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Wilson, G.J.; Murphy, A.J. The use of isometric tests of muscular function in athletic assessment. Sports Med. 1996, 22, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.M.; Kreider, R.B.; Willoughby, D.S. Intramuscular adaptations to eccentric exercise and antioxidant supplementation. Amino Acids 2010, 39, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Kelloff, G.J.; Crowell, J.A.; Hawk, E.T.; Steele, V.E.; Lubet, R.A.; Boone, C.W.; Covey, J.M.; Doody, L.A.; Omenn, G.S.; Greenwald, P.; et al. Strategy and planning for chemopreventive drug development: Clinical development plans II. J. Cell. Biochem. Suppl. 1996, 26, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, N.; Choi, Y.; Miyaki, A.; Tanabe, Y.; Sugawara, J.; Ajisaka, R.; Maeda, S. Curcumin ingestion and exercise training improve vascular endothelial function in postmenopausal women. Nutr. Res. 2012, 32, 795–799. [Google Scholar] [CrossRef] [PubMed]
- Sugawara, J.; Akazawa, N.; Miyaki, A.; Choi, Y.; Tanabe, Y.; Imai, T.; Maeda, S. Effect of endurance exercise training and curcumin intake on central arterial hemodynamics in postmenopausal women: Pilot study. Am. J. Hypertens. 2012, 25, 651–656. [Google Scholar] [CrossRef]
- Vitadello, M.; Germinario, E.; Ravara, B.; Libera, L.D.; Danieli-Betto, D.; Gorza, L. Curcumin counteracts loss of force and atrophy of hindlimb unloaded rat soleus by hampering neuronal nitric oxide synthase untethering from sarcolemma. J. Physiol. 2014, 592, 2637–2652. [Google Scholar] [CrossRef]
- Kerksick, C.; Taylor, L., 4th; Harvey, A.; Willoughby, D. Gender-related differences in muscle injury, oxidative stress, and apoptosis. Med. Sci. Sports Exerc. 2008, 40, 1772–1780. [Google Scholar] [CrossRef]
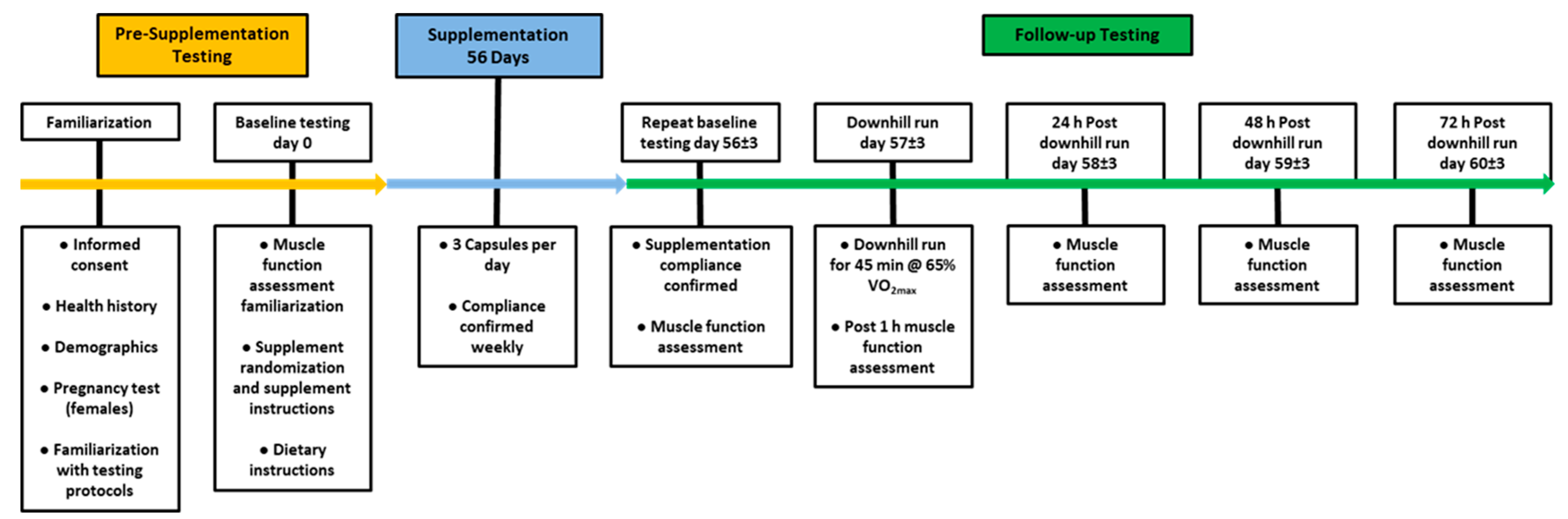
 ), 50 of mg CUR (in the form of 250 mg of CurcuWIN®) (
), 50 of mg CUR (in the form of 250 mg of CurcuWIN®) ( ), and 200 mg of CUR (in the form of 1000 mg of CurcuWIN®) (
), and 200 mg of CUR (in the form of 1000 mg of CurcuWIN®) ( ). * = Significantly different than respective baseline value.
). * = Significantly different than respective baseline value.
 ), 50 of mg CUR (in the form of 250 mg of CurcuWIN®) (
), 50 of mg CUR (in the form of 250 mg of CurcuWIN®) ( ), and 200 mg of CUR (in the form of 1000 mg of CurcuWIN®) (
), and 200 mg of CUR (in the form of 1000 mg of CurcuWIN®) ( ). * = Significantly different than respective baseline value.
). * = Significantly different than respective baseline value.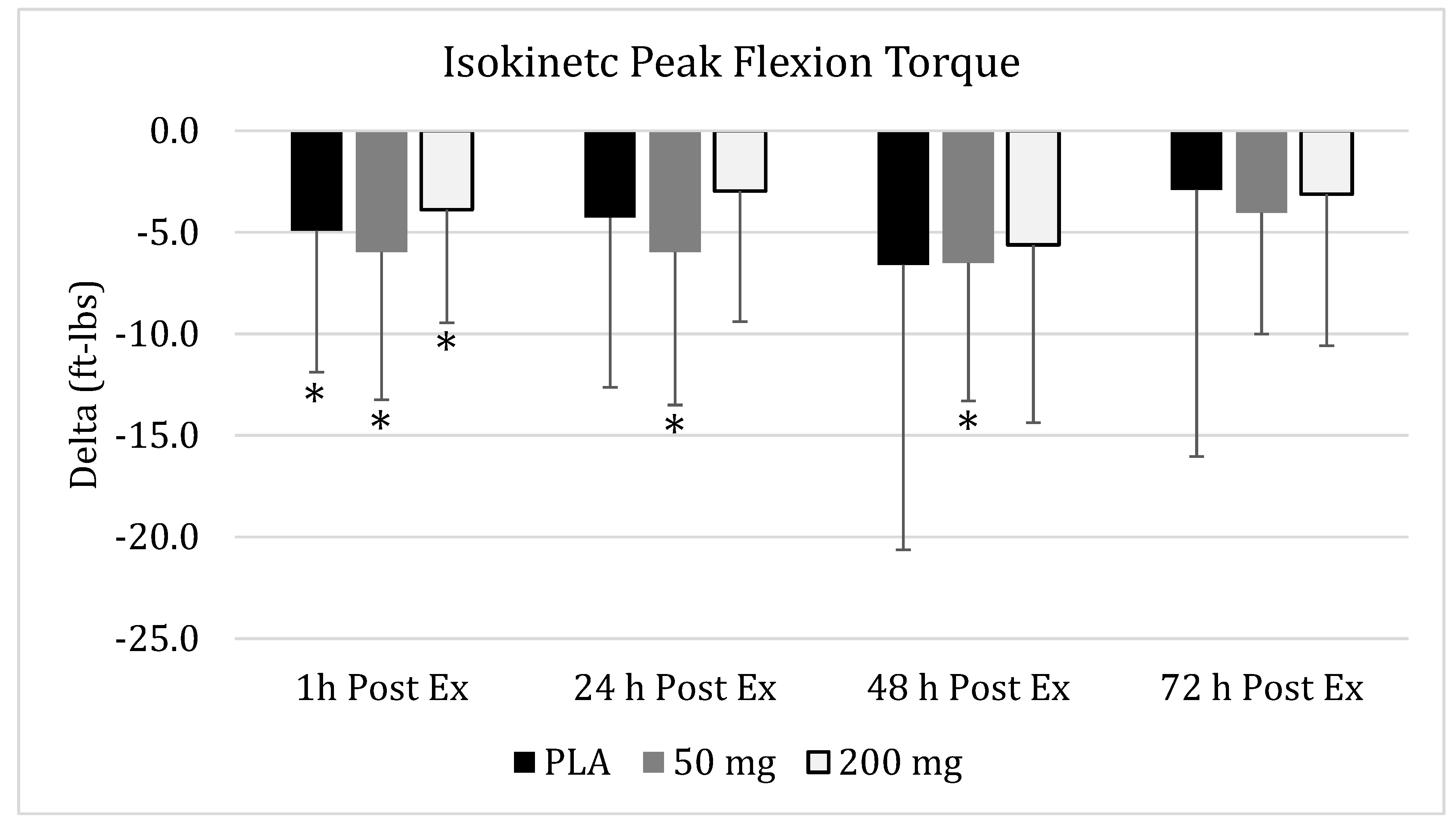
 ), 50 mg of CUR (in the form of 250 mg of CurcuWIN®) (
), 50 mg of CUR (in the form of 250 mg of CurcuWIN®) ( ), and 200 mg of CUR (in the form of 1000 mg of CurcuWIN®) (
), and 200 mg of CUR (in the form of 1000 mg of CurcuWIN®) ( ). * = Significantly different than respective baseline value.
). * = Significantly different than respective baseline value.
 ), 50 mg of CUR (in the form of 250 mg of CurcuWIN®) (
), 50 mg of CUR (in the form of 250 mg of CurcuWIN®) ( ), and 200 mg of CUR (in the form of 1000 mg of CurcuWIN®) (
), and 200 mg of CUR (in the form of 1000 mg of CurcuWIN®) ( ). * = Significantly different than respective baseline value.
). * = Significantly different than respective baseline value.
 ), 50 mg of CUR (in the form of 250 mg of CurcuWIN®) (
), 50 mg of CUR (in the form of 250 mg of CurcuWIN®) ( ), and 200 mg of CUR (in the form of 1000 mg of CurcuWIN®) (
), and 200 mg of CUR (in the form of 1000 mg of CurcuWIN®) ( ). * = Significantly different than respective baseline value.
). * = Significantly different than respective baseline value.
 ), 50 mg of CUR (in the form of 250 mg of CurcuWIN®) (
), 50 mg of CUR (in the form of 250 mg of CurcuWIN®) ( ), and 200 mg of CUR (in the form of 1000 mg of CurcuWIN®) (
), and 200 mg of CUR (in the form of 1000 mg of CurcuWIN®) ( ). * = Significantly different than respective baseline value.
). * = Significantly different than respective baseline value.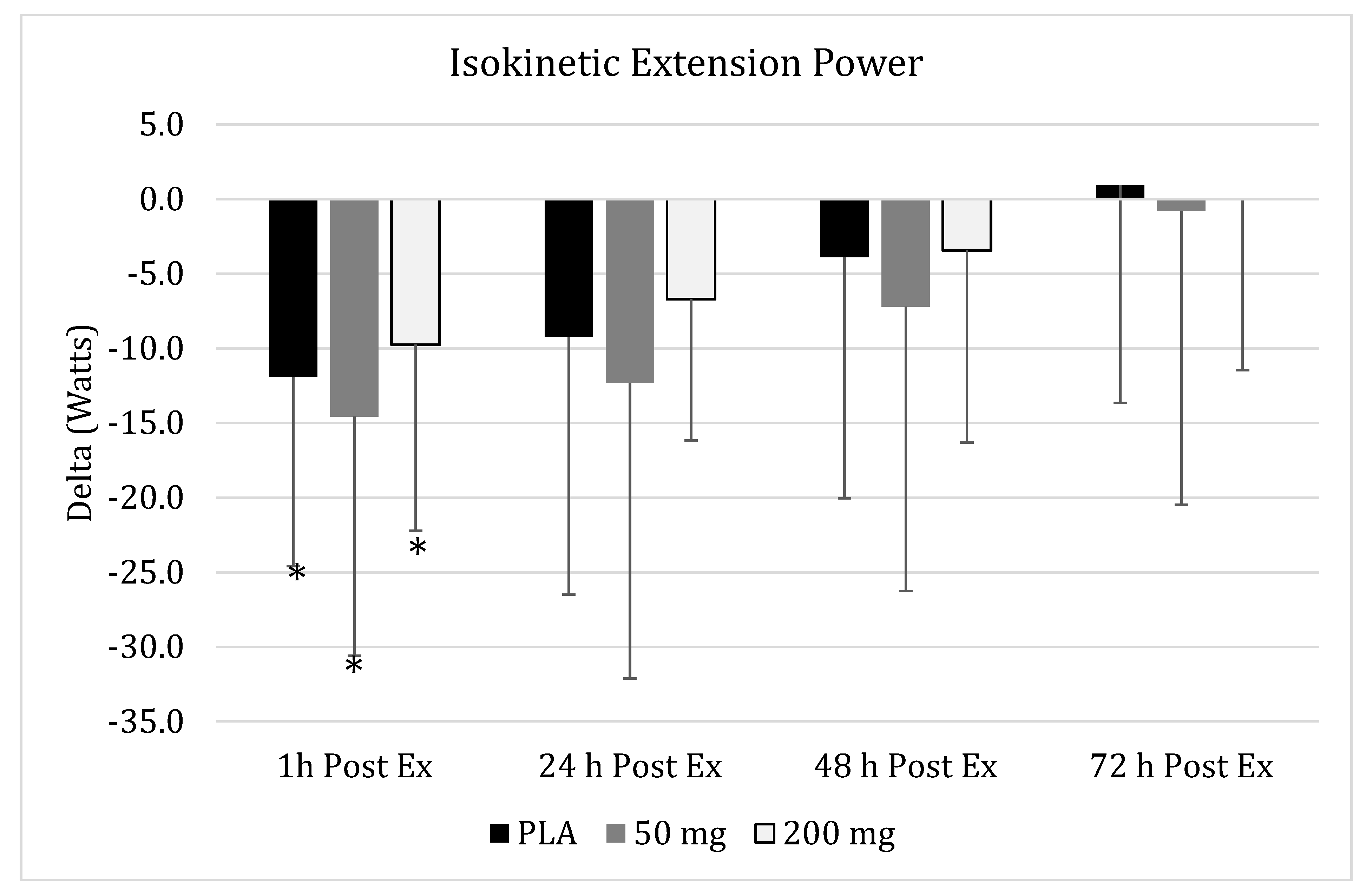
 ), 50 mg of CUR (in the form of 250 mg of CurcuWIN®) (
), 50 mg of CUR (in the form of 250 mg of CurcuWIN®) ( ), and 200 mg of CUR (in the form of 1000 mg of CurcuWIN®) (
), and 200 mg of CUR (in the form of 1000 mg of CurcuWIN®) ( ). * = Significantly different than respective baseline value.
). * = Significantly different than respective baseline value.
 ), 50 mg of CUR (in the form of 250 mg of CurcuWIN®) (
), 50 mg of CUR (in the form of 250 mg of CurcuWIN®) ( ), and 200 mg of CUR (in the form of 1000 mg of CurcuWIN®) (
), and 200 mg of CUR (in the form of 1000 mg of CurcuWIN®) ( ). * = Significantly different than respective baseline value.
). * = Significantly different than respective baseline value.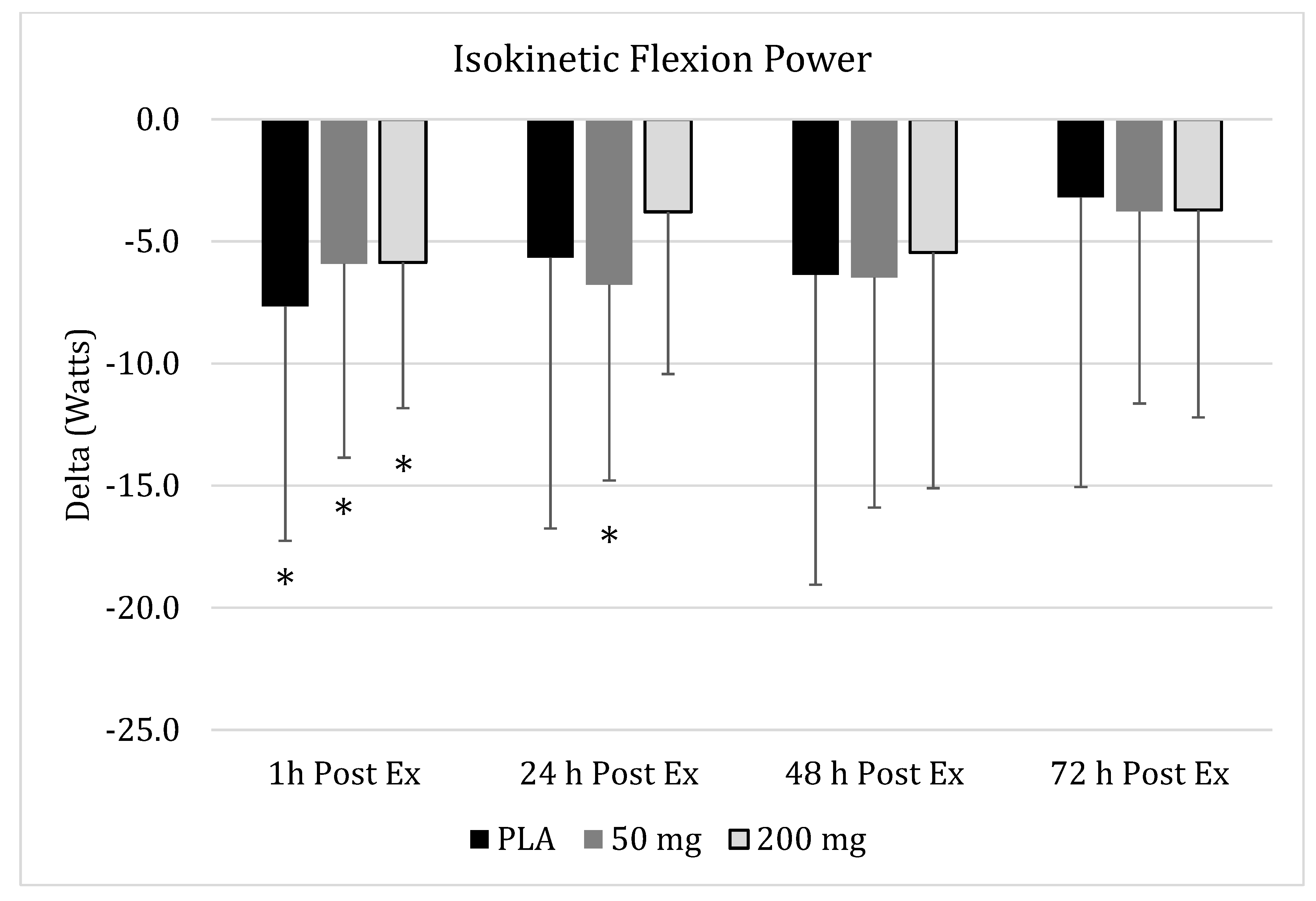
 ), 50 mg of CUR (in the form of 250 mg of CurcuWIN®) (
), 50 mg of CUR (in the form of 250 mg of CurcuWIN®) ( ), and 200 mg of CUR (in the form of 1000 mg of CurcuWIN®) (
), and 200 mg of CUR (in the form of 1000 mg of CurcuWIN®) ( ).
).
 ), 50 mg of CUR (in the form of 250 mg of CurcuWIN®) (
), 50 mg of CUR (in the form of 250 mg of CurcuWIN®) ( ), and 200 mg of CUR (in the form of 1000 mg of CurcuWIN®) (
), and 200 mg of CUR (in the form of 1000 mg of CurcuWIN®) ( ).
).
| Placebo | 50 mg | 200 mg | |
|---|---|---|---|
| Age [years] | |||
| Women (n = 11/10/11) | 20.8 ± 1.5 | 21.5 ± 3.0 | 21.5 ± 2.7 |
| Men (n = 10/10/11) | 20.8 ± 2.1 | 21.3 ± 1.8 | 21.6 ± 1.9 |
| Total (n = 21/20/22) | 20.8 ± 1.8 | 21.4 ± 2.4 | 21.6 ± 2.3 |
| Height [cm] | |||
| Women (n = 11/10/11) | 165.1 ± 5.3 | 167.6 ± 5.5 | 162.6 ± 7.8 |
| Men (n = 10/10/11) | 176.6 ± 6.4 | 178.8 ± 8.8 | 175.9 ± 5.0 |
| Total (n = 21/20/22) | 170.6 ± 8.2 | 173.2 ± 9.2 | 169.3 ± 9.4 |
| Weight [kg] | |||
| Women (n = 11/10/11) | 62.2 ± 6.0 | 61.6 ± 10.2 | 58.2 ± 8.6 |
| Men (n = 10/10/11) | 78.2 ± 9.9 | 82.8 ± 11.5 | 78.2 ± 11.6 |
| Total (n = 21/20/22) | 69.8 ± 11.3 | 72.2 ± 15.2 | 68.2 ± 14.3 |
| Body Mass Index (kg/m2) | |||
| Women (n = 11/10/11) | 22.8 ± 1.8 | 21.9 ± 3.2 | 21.9 ± 1.9 |
| Men (n = 10/10/11) | 25.1 ± 3.0 | 25.9 ± 2.6 | 25.2 ± 3.3 |
| Total (n = 21/20/22) | 23.9 ± 2.6 | 23.9 ± 3.5 | 23.6 ± 3.1 |
| Maximum Aerobic Capacity [VO2mMax in mL/kg/min] | |||
| Women (n = 11/10/11) | 39.2 ± 4.9 | 39.4 ± 4.7 | 40.9 ± 7.7 |
| Men (n = 10/10/11) | 44.4 ± 6.0 | 47.4 ± 7.4 | 46.3 ± 6.0 |
| Total (n = 21/20/22) | 41.6 ± 6.0 | 43.2 ± 7.2 | 43.6 ± 7.3 |
| Race | |||
| White | 16 (10F, 6M) | 15 (7F, 8M) | 19 (10F, 9M) |
| Asian | 3 (2 F, 1M) | 2 (1F, 1M) | 1 (1M) |
| African-American | 1 (1M) | 1 (1M) | - |
| Latino | 1 (1M) | 2 (2F) | 2 (1F, 1M) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jäger, R.; Purpura, M.; Kerksick, C.M. Eight Weeks of a High Dose of Curcumin Supplementation May Attenuate Performance Decrements Following Muscle-Damaging Exercise. Nutrients 2019, 11, 1692. https://doi.org/10.3390/nu11071692
Jäger R, Purpura M, Kerksick CM. Eight Weeks of a High Dose of Curcumin Supplementation May Attenuate Performance Decrements Following Muscle-Damaging Exercise. Nutrients. 2019; 11(7):1692. https://doi.org/10.3390/nu11071692
Chicago/Turabian StyleJäger, Ralf, Martin Purpura, and Chad M. Kerksick. 2019. "Eight Weeks of a High Dose of Curcumin Supplementation May Attenuate Performance Decrements Following Muscle-Damaging Exercise" Nutrients 11, no. 7: 1692. https://doi.org/10.3390/nu11071692
APA StyleJäger, R., Purpura, M., & Kerksick, C. M. (2019). Eight Weeks of a High Dose of Curcumin Supplementation May Attenuate Performance Decrements Following Muscle-Damaging Exercise. Nutrients, 11(7), 1692. https://doi.org/10.3390/nu11071692




