Neuroprotective Effect of Schisandra Chinensis on Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Parkinsonian Syndrome in C57BL/6 Mice
Abstract
1. Introduction
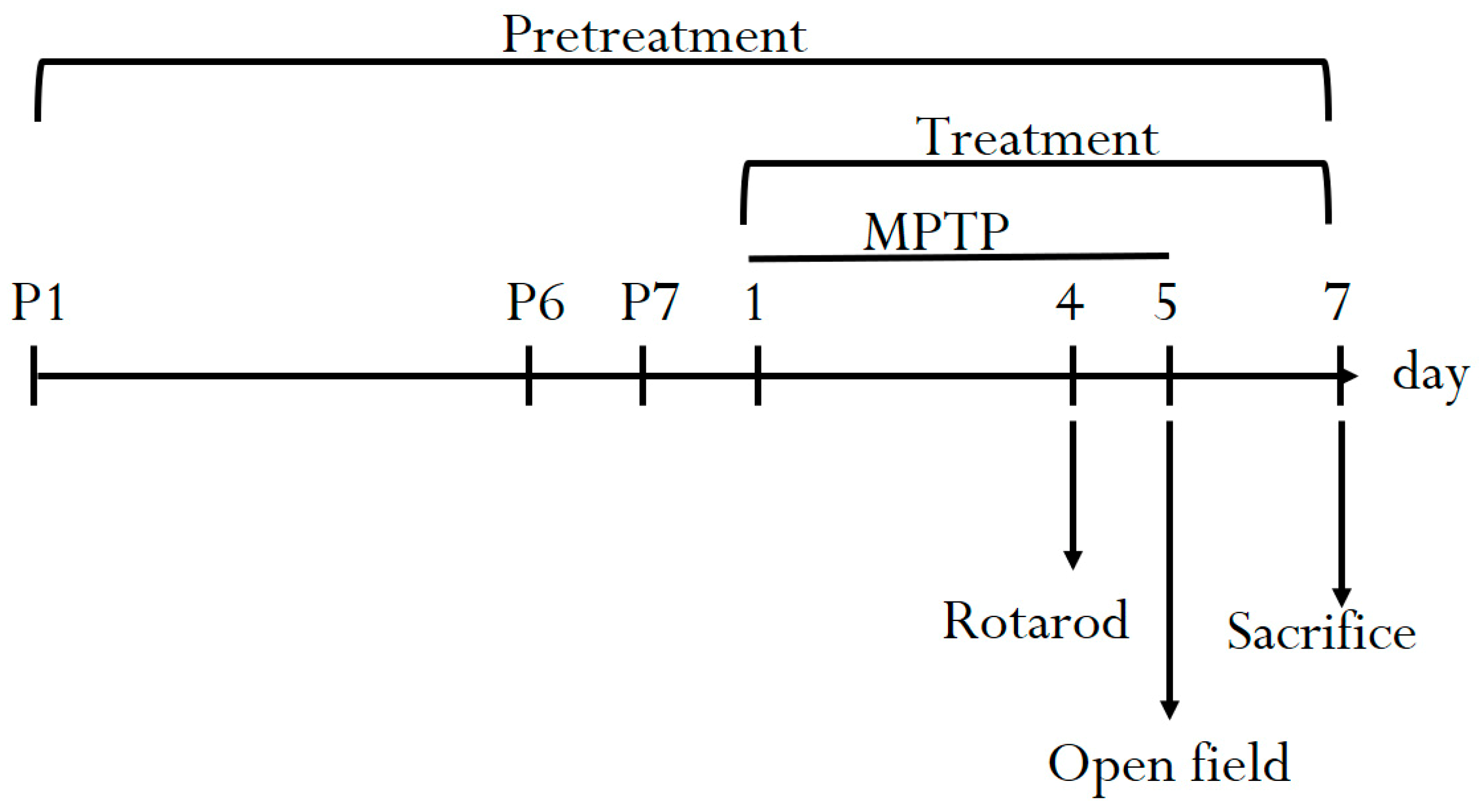
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Herbal Preparation of Aqueous Ethanol Extract from S. Chinensis
2.3. Experimental Animals
2.4. Apparatuses, Analytical Conditions and Sample Preparation for Neurochemicals
2.5. Behavioral Test
2.5.1. Open Field Test
2.5.2. Rotarod Test
2.6. Estimation of SOD, CAT and GPx Activity
2.7. Immunohistochemistry
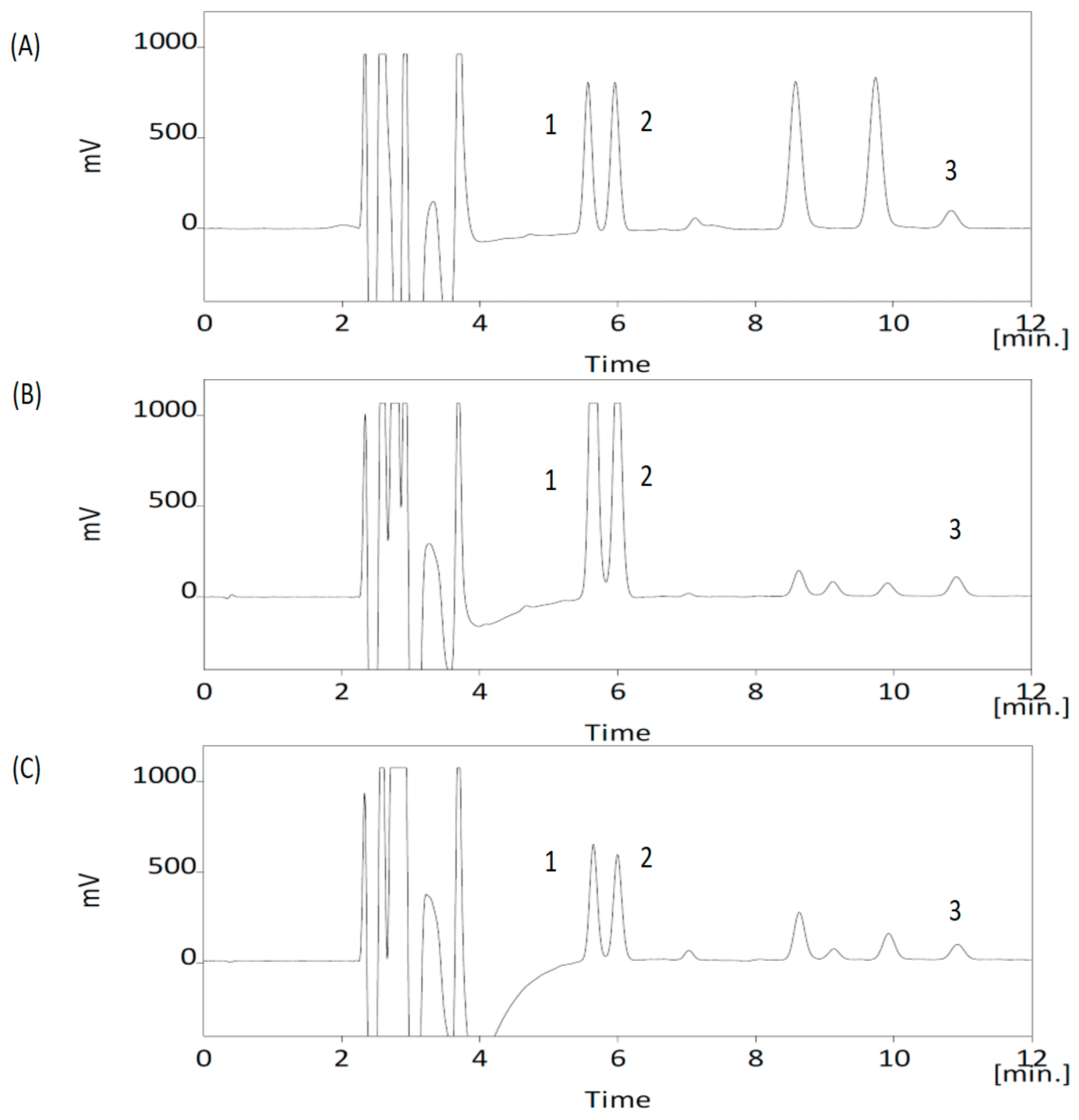
2.8. Method Validation for the Neurochemicals
2.9. Statistical Analysis
3. Results
3.1. Analytical Method Validation
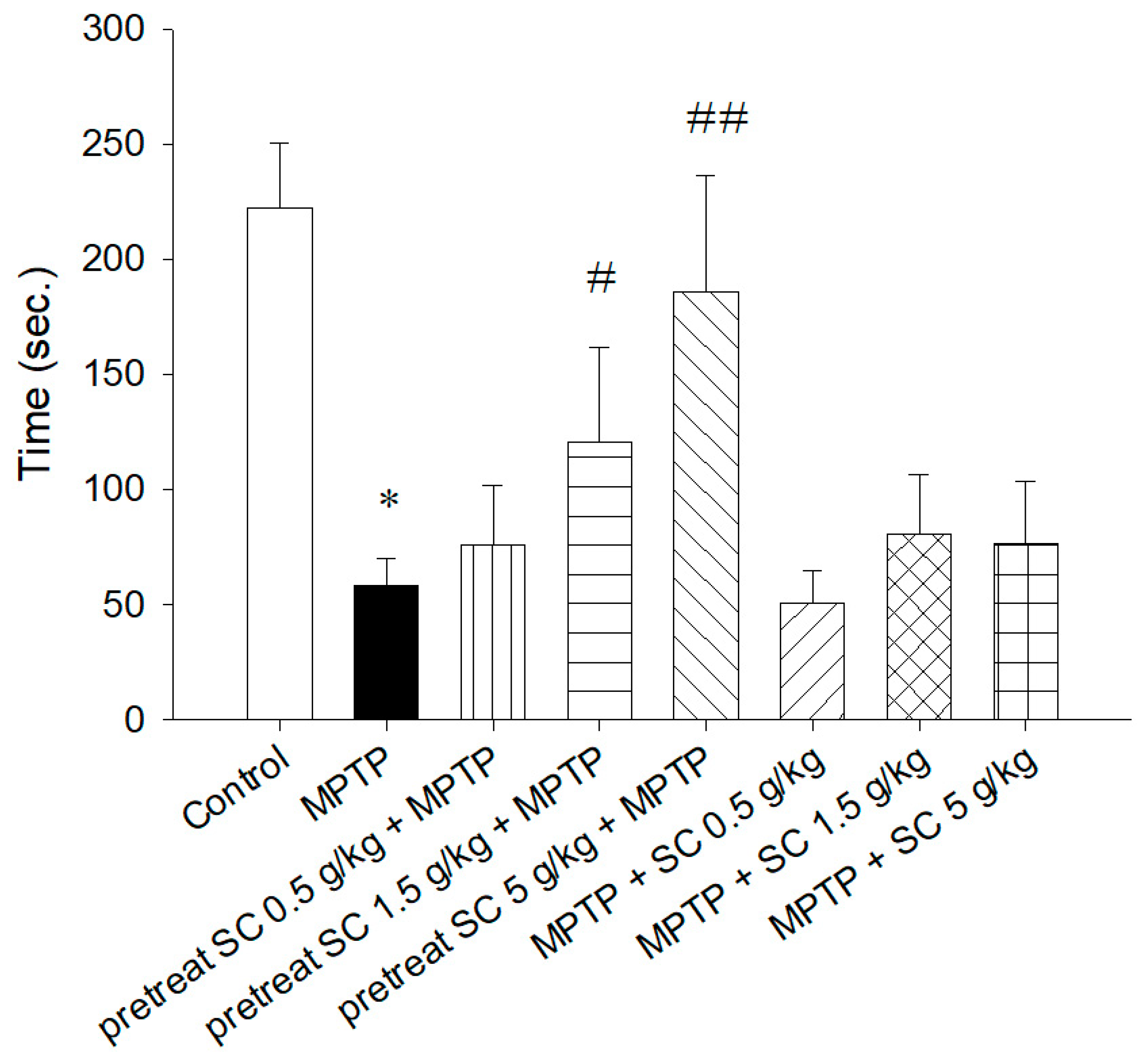
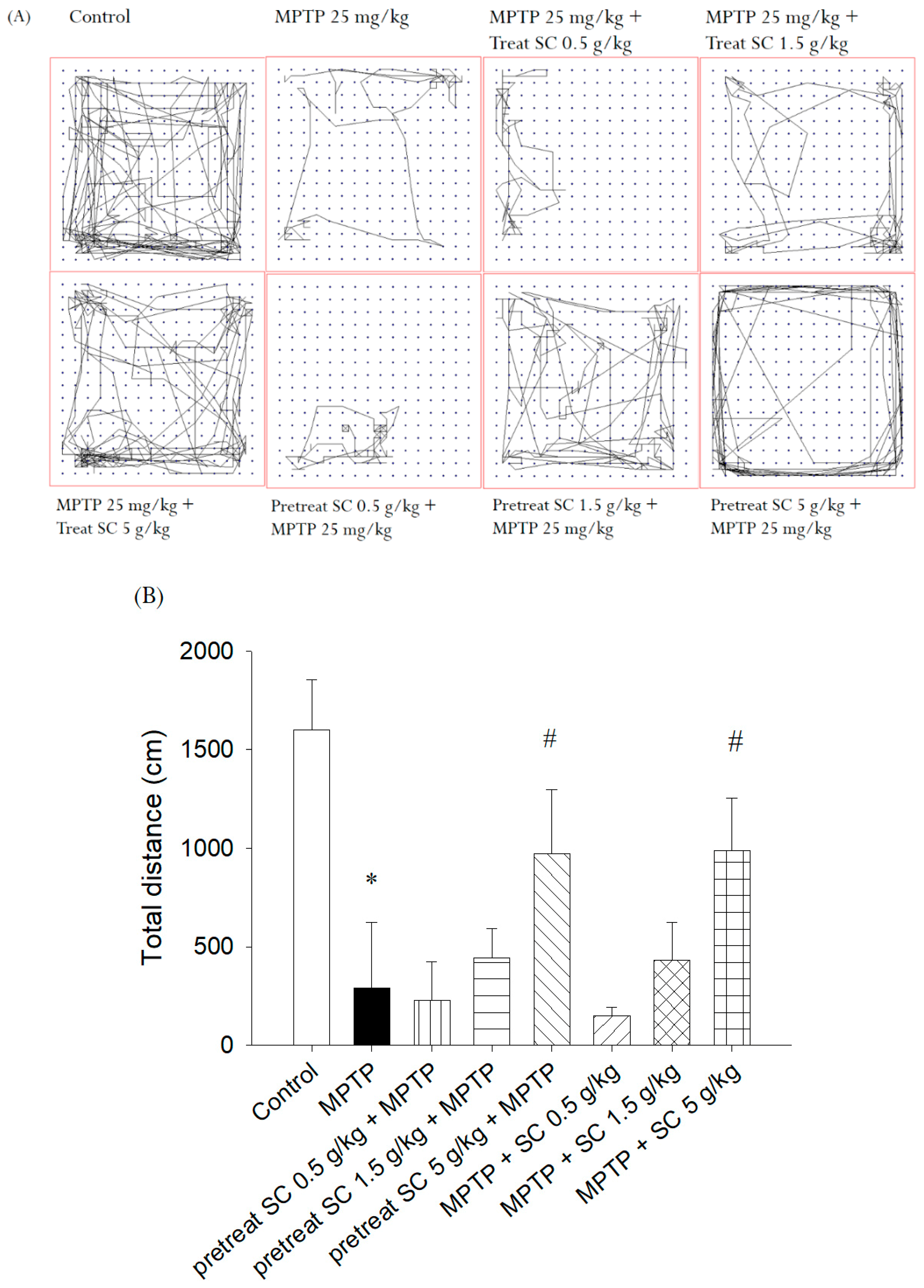
3.2. Behavioral Experiments
3.2.1. Rotarod Test
3.2.2. Open Field Test
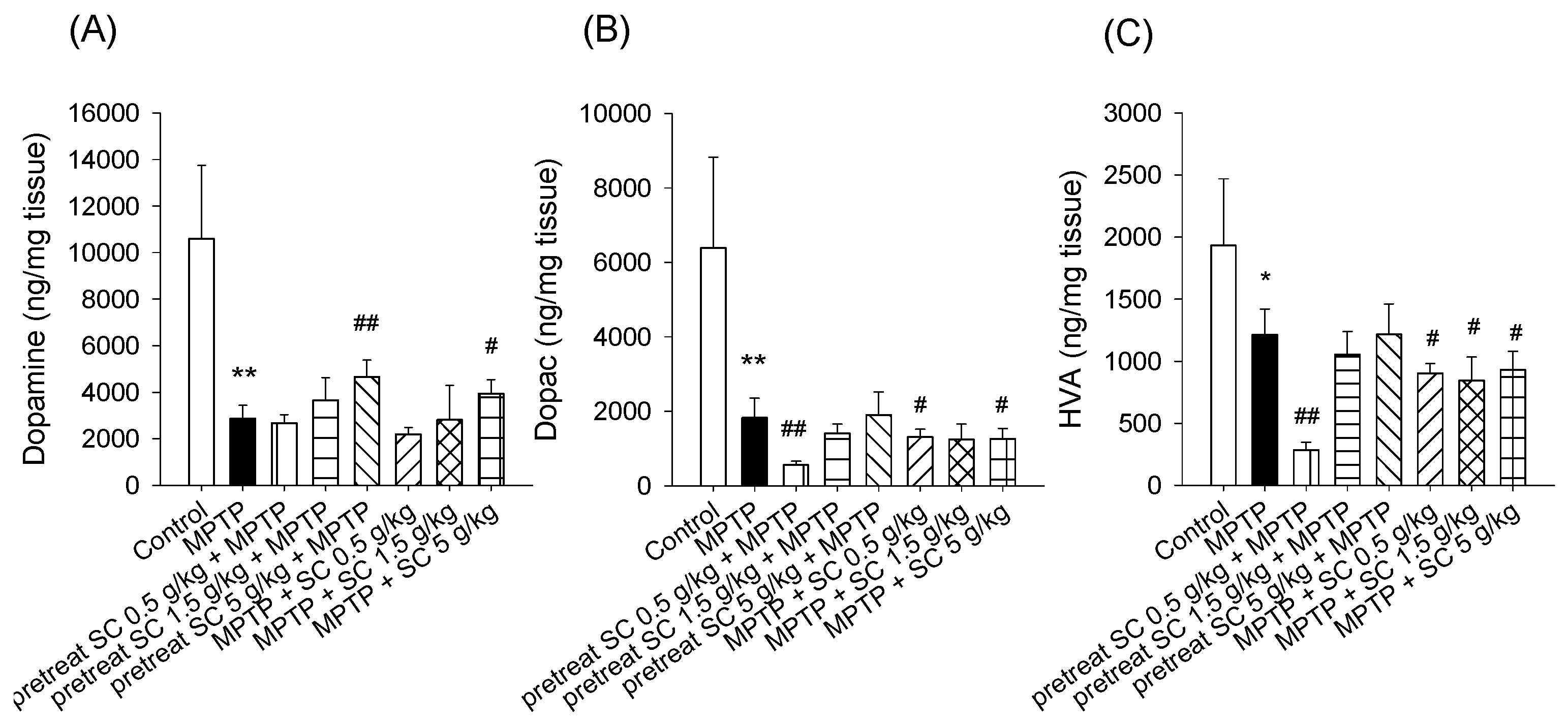
3.3. Levels of Dopamine and Dopamine Metabolites in the Striatum
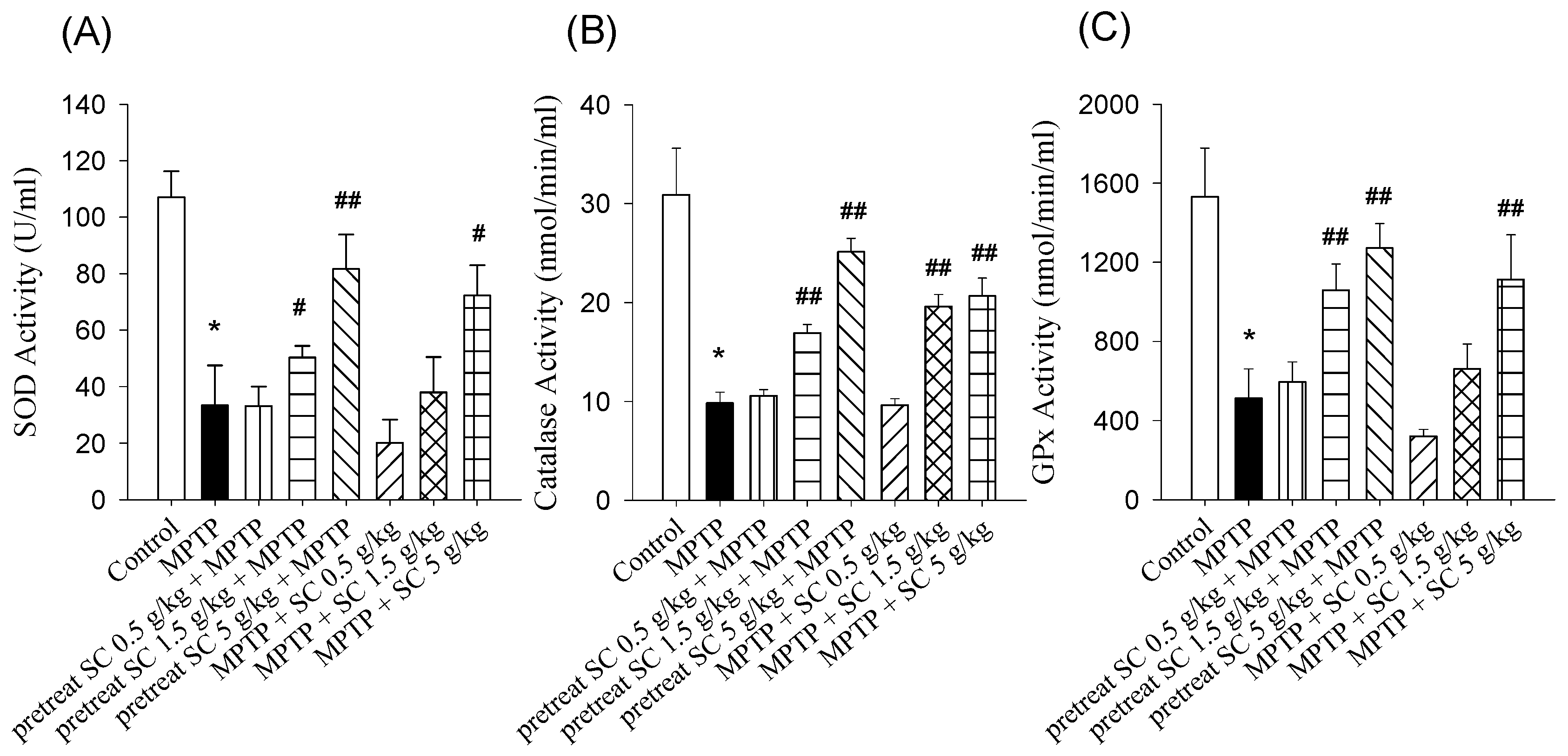
3.4. Effect on SOD, CAT and GSH
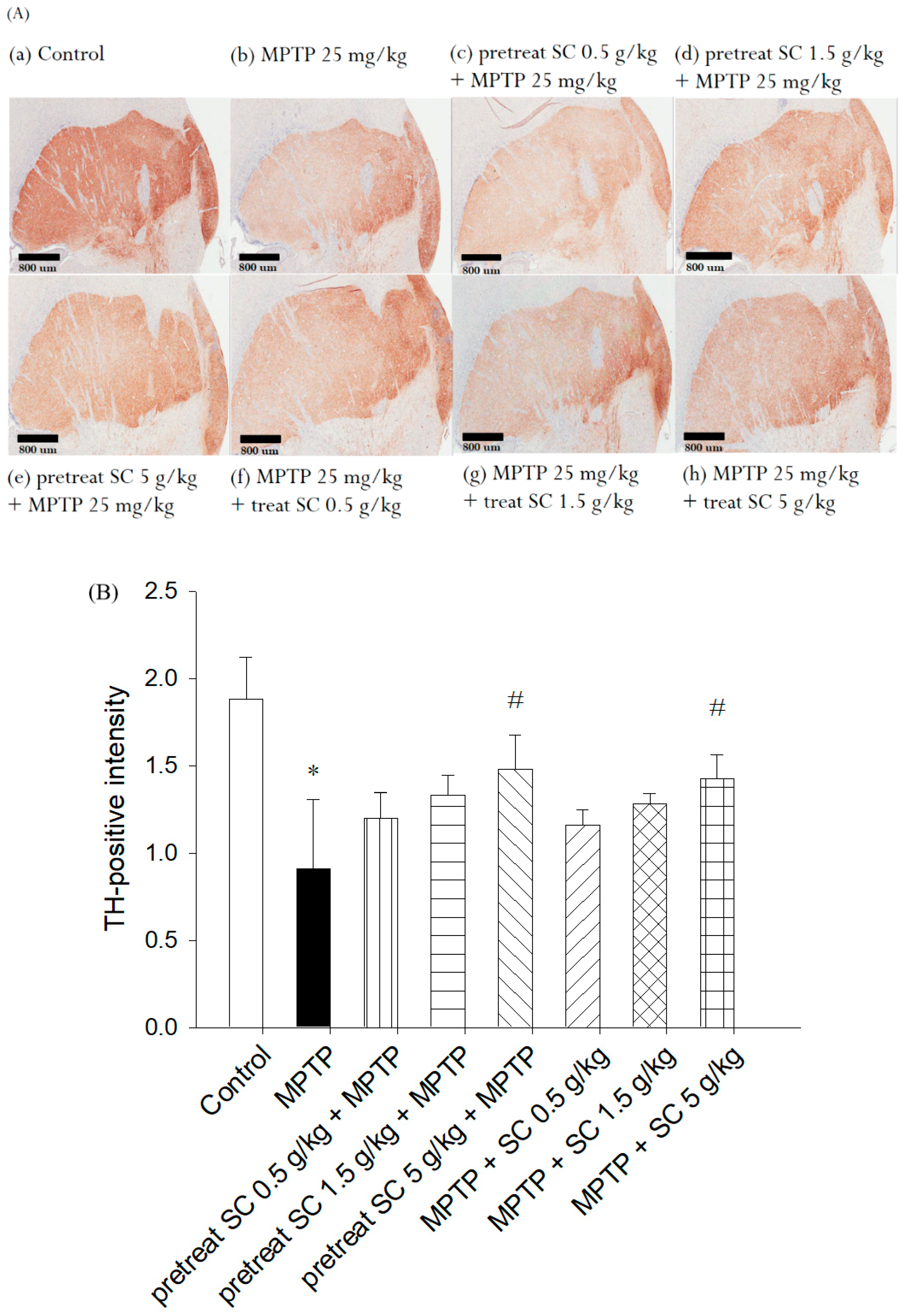
3.5. Evaluation of Immunohistochemical Staining for Tyrosine Hydroxylase
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dehay, B.; Bourdenx, M.; Gorry, P.; Przedborski, S.; Vila, M.; Hunot, S.; Singleton, A.; Olanow, C.W.; Merchant, K.M.; Bezard, E.; et al. Targeting α-synuclein for treatment of Parkinson’s disease: Mechanistic and therapeutic considerations. Lancet Neurol. 2015, 14, 855–866. [Google Scholar] [CrossRef]
- Dauer, W.; Przedborski, S. Parkinson’s disease mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Klegeris, A.; McGeer, P.L. R-(−)-Deprenyl inhibits monocytic THP-1 cell neurotoxicity independently of monoamine oxidase inhibition. Exp. Neurol. 2000, 166, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Perry, G.; Smith, M.A.; Robertson, D.; Olson, S.J.; Graham, D.G.; Montine, T.J. Parkinson’s Disease Is Associated with Oxidative Damage to Cytoplasmic DNA and RNA in Substantia Nigra Neurons. Am. J. Pathol. 1999, 154, 1423–1429. [Google Scholar] [CrossRef]
- Hartmann, A. Hartmann A Postmortem studies in Parkinson’s disease. Dialogues Clin. Neurosci. 2004, 6, 281–293. [Google Scholar] [PubMed]
- Burns, R.S.; Chiueh, C.C.; Markey, S.P.; Ebert, M.H.; Jacobowitz, D.M.; Kopin, I.J. A primate model of Parkinsonism Selective destruction of dopaminergic neurons in the pars compacta of the substantia nigra by MPTP. Proc. Natl. Acad. Sci. USA 1983, 80, 4546–4550. [Google Scholar] [CrossRef]
- Davis, G.C.; Williams, A.C.; Markey, S.P.; Ebert, M.H.; Caine, E.D.; Reichert, C.M.; Kopin, I.J. Chronic parkinsonism secondary to intravenous injection of meperidine analogues. Psychiatry Res. 1979, 1, 249–254. [Google Scholar] [CrossRef]
- Heikkila, R.E.; Hess, A.; Duvoisin, R.C. Dopaminergic neurotoxicity of 1-methyl-4-phenyl-1, 2, 5, 6-tetrahydropyridine in mice. Science 1984, 224, 1451–1453. [Google Scholar] [CrossRef]
- Speciale, S.G. MPTP: Insights into parkinsonian neurodegeneration. Neurotoxicol. Teratol. 2002, 24, 607–620. [Google Scholar] [CrossRef]
- Nagatsu, T.; Sawada, M. Molecular mechanism of the relation of monoamine oxidase B and its inhibitors to Parkinson’s disease: Possible implications of glial cells. J. Neural Transm. 2006, 71, 53–65. [Google Scholar]
- Javitch, J.A.; D’Amato, R.J.; Strittmatter, S.M.; Snyder, S.H. Parkinsonism-inducing neurotoxin, MPTP uptake of the metabolite MPP+ by dopamine neurons explains selective toxicity. Proc. Natl. Acad. Sci. USA 1985, 82, 2173–2177. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Kim, C.S.; Lee, Y.J. Astaxanthin protects against MPTP/MPP+-induced mitochondrial Dysfunction and ROS production in vivo and in vitro. Food Chem. Toxicol. 2011, 49, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Dal Ben, M.; Bongiovanni, R.; Tuniz, S.; Fioriti, E.; Tiribelli, C.; Moretti, R.; Gazzin, S. Earliest Mechanisms of Dopaminergic Neurons Sufferance in a Novel Slow Progressing Ex Vivo Model of Parkinson Disease in Rat Organotypic Cultures of Substantia Nigra. Int. J. Mol. Sci. 2019, 20, 2224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, X.; Mao, X.; Liu, A.; Liu, Z.; Li, X.; Bi, K.; Jia, Y. Pharmacological evaluation of sedative and hypnotic effects of schizandrin through the modification of pentobarbital-induced sleep behaviors in mice. Eur. J. Pharmacol. 2014, 744, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, L.; Wang, G.; He, Z.; Zhao, Y.; Xu, Y.; Gao, Y.; Zhang, L. Sedative and hypnotic effects of supercritical carbon dioxide fluid extraction from Schisandra chinensis in mice. J. Food Drug Anal. 2016, 24, 831–838. [Google Scholar] [CrossRef]
- Lee, K.; Ahn, J.H.; Lee, K.T.; Jang, D.S.; Choi, J.H. Deoxyschizandrin, Isolated from Schisandra Berries, Induces Cell Cycle Arrest in Ovarian Cancer Cells and Inhibits the Protumoural Activation of Tumour-Associated Macrophages. Nutrients 2018, 10, 91. [Google Scholar] [CrossRef]
- Jiang, Y.M.; Wang, Y.; Tan, H.S.; Yu, T.; Fan, X.M.; Chen, P.; Zeng, H.; Huang, M.; Bi, H.C. Schisandrol B protects against acetaminophen-induced acute hepatotoxicity in mice via activation of the NRF2/ARE signaling pathway. Acta Pharmacol. Sin. 2016, 37, 382–389. [Google Scholar] [CrossRef]
- Yang, G.Y.; Wang, R.R.; Mu, H.X.; Li, Y.K.; Xiao, W.L.; Yang, L.M.; Pu, J.X.; Zheng, Y.T.; Sun, H.D. Neolignans from Schisandra wilsoniana and Their Anti-human Immunodeficiency Virus-1 Activities. Chem. Pharm. Bull. (Tokyo) 2011, 59, 1344–1347. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Grandi, N.; Del Vecchio, C.; Mandas, D.; Corona, A.; Piano, D.; Esposito, F.; Parolin, C.; Tramontano, E. From the traditional Chinese medicine plant Schisandra chinensis new scaffolds effective on HIV-1 reverse transcriptase resistant to non-nucleoside inhibitors. J. Microbiol. 2015, 53, 288–293. [Google Scholar] [CrossRef]
- Huang, H.; Shen, Z.; Geng, Q.; Wu, Z.; Shi, P.; Miao, X. Protective effect of Schisandra chinensis bee pollen extract on liver and kidney injury induced by cisplatin in rats. Biomed. Pharmacother. 2017, 95, 1765–1776. [Google Scholar] [CrossRef]
- Song, Q.Y.; Gao, K.; Nan, Z.B. Highly oxygenated triterpenoids from the roots of Schisandra chinensis and their anti-inflammatory activities. J. Asian Nat. Prod. Res. 2016, 18, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Jung, C.H.; Lee, D.H. Neuroprotective effects of Schisandrin B against transient focal cerebral ischemia in Sprague-Dawley rats. Food Chem. Toxicol. 2012, 50, 4239–4245. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Bi, W.; Fan, K.; Li, T.; Yan, T.; Xiao, F.; He, B.; Bi, K.; Jia, Y. Ameliorating effect of Alpinia oxyphylla-Schisandra chinensis herb pair on cognitive impairment in a mouse model of Alzheimer’s disease. Biomed. Pharmacother. 2018, 97, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.Y.; Han, W.; Han, H.; Liu, Y.; Guan, W.; Li, X.M.; Kuang, H.X. Effects of Lignans from Schisandra chinensis Rattan Stems against Abeta1-42-Induced Memory Impairment in Rats and Neurotoxicity in Primary Neuronal Cells. Molecules 2018, 23, 870. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Rozas, G.; López-Martín, E.; Guerra, M.J.; Labandeira-García, J.L. The overall rod performance test in the MPTP-treated-mouse model of Parkinsonism. J. Neurosci. Methods 1998, 83, 165–175. [Google Scholar] [CrossRef]
- Nandi, A.; Chatterjee, I.B. Assay of superoxide dismutase activity in animal tissues. J. Biosci. 1988, 13, 305–315. [Google Scholar] [CrossRef]
- Sinha, A.K. Calorimetric assay of catalase. Anal. Biochem. 1972, 47, 389–394. [Google Scholar] [CrossRef]
- Rotruck, J.T.; Pope, A.L.; Ganther, H.E.; Swanson, A.B.; Hafeman, D.G.; Hoekstra, W.G. Selenium biochemical role as a component of glutathione peroxidase. Science 1973, 179, 588–590. [Google Scholar] [CrossRef]
- Sridharan, S.; Mohankumar, K.; Jeepipalli, S.P.; Sankaramourthy, D.; Ronsard, L.; Subramanian, K.; Thamilarasan, M.; Raja, K.; Chandra, V.K.; Sadras, S.R. Neuroprotective effect of Valeriana wallichii rhizome extract against the neurotoxin MPTP in C57BL/6 mice. Neurotoxicology 2015, 51, 172–183. [Google Scholar] [CrossRef]
- Shi, X.; Chen, Y.H.; Liu, H.; Qu, H.D. Therapeutic effects of paeonol on methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine/probenecid-induced Parkinson’s disease in mice. Mol. Med. Rep. 2016, 14, 2397–2404. [Google Scholar] [CrossRef]
- Patil, S.P.; Jain, P.D.; Sancheti, J.S.; Ghumatkar, P.J.; Tambe, R.; Sathaye, S. Neuroprotective and neurotrophic effects of Apigenin and Luteolin in MPTP induced parkinsonism in mice. Neuropharmacology 2014, 86, 192–202. [Google Scholar] [CrossRef]
- Rai, S.N.; Yadav, S.K.; Singh, D.; Singh, S.P. Ursolic acid attenuates oxidative stress in nigrostriatal tissue and improves neurobehavioral activity in MPTP-induced Parkinsonian mouse model. J. Chem. Neuroanat. 2016, 71, 41–49. [Google Scholar] [CrossRef]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef]
- Ahumada, F.; Hola, R.; Wikman, G. Effect of schisandra chinensis extract on thoroughbreds in sprine race. Equine Athl. 1991, 4, 4–5. [Google Scholar]
- Hancke, J.; Burgos, R.; Wikman, G.; Ewertz, E.; Ahumada, F. Schisandra chinensis, a potential phytodrug for recovery of sport horses. Fitoterapia 1994, 65, 113. [Google Scholar]
- Piggott, M.A.; Marshall, E.F.; Thomas, N.; Lloyd, S.; Court, J.A.; Jaros, E.; Burn, D.; Johnson, M.; Perry, R.H.; McKeith, I.G.; et al. Striatal dopaminergic markers in dementia with Lewy bodies, Alzheimer’s and PD rostrocaudal distribution. Brain 1999, 122, 1449–1468. [Google Scholar] [CrossRef]
- Gu, B.H.; Minh, N.V.; Lee, S.H.; Lim, S.W.; Lee, Y.M.; Lee, K.S.; Kim, D.K. Deoxyschisandrin inhibits H2O2-induced apoptotic cell death in intestinal epithelial cells through nuclear factor-ΚB. Int. J. Mol. Med. 2010, 26, 401–406. [Google Scholar] [CrossRef]
- Laatikainen, L.M.; Sharp, T.; Harrison, P.J.; Tunbridge, E.M. Sexually dimorphic effects of catechol-O-methyltransferase (COMT) inhibition on dopamine metabolism in multiple brain regions. PLoS ONE 2013, 8, e61839. [Google Scholar] [CrossRef]
- Di Napoli, M.; Shah, I.; Stewart, D. Molecular pathways and genetic aspects of Parkinson’s disease from bench to bedside. Expert Rev. Neurother. 2007, 7, 1693–1729. [Google Scholar] [CrossRef]
- Schapira, A.H. Mitochondria in the aetiology and pathogenesis of Parkinson’s disease. Lancet Neurol. 2008, 7, 97–109. [Google Scholar] [CrossRef]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- Sriram, K.; Pai, K.S.; Boyd, M.R.; Ravindranath, V. Evidence for generation of oxidative stress in brain by MPTP in vitro and in vivo studies in mice. Brain Res. 1997, 749, 44–52. [Google Scholar] [CrossRef]
- More, S.V.; Kumar, H.; Kang, S.M.; Song, S.Y.; Lee, K.; Choi, D.K. Advances in neuroprotective ingredients of medicinal herbs by using cellular and animal models of Parkinson’s disease. Evid. Based Complement. Alternat. Med. 2013, 2013, 957875. [Google Scholar] [CrossRef]
- Panossian, A.; Wikman, G. Pharmacology of Schisandra chinensis Bail: An overview of Russian research and uses in medicine. J. Ethnopharmacol. 2008, 118, 183–212. [Google Scholar] [CrossRef]
- Sowndhararajan, K.; Deepa, P.; Kim, M.; Park, S.J.; Kim, S. An overview of neuroprotective and cognitive enhancement properties of lignans from Schisandra chinensis. Biomed. Pharmacother. 2018, 97, 958–968. [Google Scholar] [CrossRef]
- Lüdecke, B.; Knappskog, P.M.; Clayton, P.T.; Surtees, R.A.; Clelland, J.D.; Heales, S.J.; Brand, M.P.; Bartholomé, K.; Flatmark, T. Recessively inherited L-DOPA-responsive parkinsonism in infancy caused by a point mutation in the TH gene. Hum. Mol. Genet. 1996, 5, 1023–1028. [Google Scholar] [CrossRef]
- Fukuda, T.; Takahashi, J.; Tanaka, J. TH-immunoreactive neurons are decreased in number in the cerebral cortex of PD. Neuropathology 1999, 19, 10–13. [Google Scholar] [CrossRef]
- Ghosh, A.; Roy, A.; Matras, J.; Brahmachari, S.; Gendelman, H.E.; Pahan, K. Simvastatin inhibits the activation of p21ras and prevents the loss of dopaminergic neurons in a mouse model of Parkinson’s disease. J. Neurosci. 2009, 29, 13543–13556. [Google Scholar] [CrossRef]
| Intraday | Interday | |||||
|---|---|---|---|---|---|---|
| Nominal Concentration (ng/mL) | Observed Concentration (ng/mL) | Precision (% RSD) | Accuracy (% Bias) | Observed Concentration (ng/mL) | Precision (% RSD) | Accuracy (% Bias) |
| DA | ||||||
| 5 | 4.70 ± 0.05 | 1.04 | −5.99 | 4.32 ± 0.09 | 2.01 | −13.68 |
| 10 | 10.16 ± 0.09 | 0.86 | 1.62 | 9.79 ±0.09 | 0.87 | −2.11 |
| 50 | 50.18 ± 1.14 | 2.27 | 0.37 | 49.50 ± 1.16 | 2.34 | −0.99 |
| 100 | 101.14 ± 1.87 | 1.85 | 1.14 | 103.43 ± 0.84 | 0.81 | 3.43 |
| 200 | 199.34 ± 1.15 | 0.58 | −0.33 | 198.49 ± 2.45 | 1.23 | −0.75 |
| DOPAC | ||||||
| 5 | 4.33 ± 0.08 | 1.77 | −13.43 | 4.31 ±0.01 | 2.28 | −13.90 |
| 10 | 9.98 ± 0.05 | 0.54 | −0.16 | 9.96 ± 0.08 | 0.79 | −0.36 |
| 50 | 49.99 ± 0.24 | 0.48 | −0.03 | 49.97 ± 0.29 | 0.58 | −0.07 |
| 100 | 100.45 ± 0.29 | 0.29 | 0.45 | 100.47 ±0.31 | 0.30 | 0.47 |
| 200 | 199.31 ± 0.38 | 0.19 | −0.34 | 199.10 ± 0.67 | 0.34 | −0.45 |
| HVA | ||||||
| 5 | 4.32 ± 0.08 | 1.94 | −13.55 | 4.13 ± 0.12 | 2.82 | −17.47 |
| 10 | 10.17 ± 0.20 | 1.99 | 1.74 | 10.31 ± 0.11 | 1.10 | 3.11 |
| 50 | 50.35 ± 0.22 | 0.43 | 0.70 | 50.81 ± 0.32 | 0.62 | 1.62 |
| 100 | 99.75 ± 0.06 | 0.06 | −0.25 | 99.26 ± 0.21 | 0.21 | −0.74 |
| 200 | 199.99 ± 0.06 | 0.03 | −0.01 | 200.08 ± 0.12 | 0.06 | 0.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.-L.; Tsuang, Y.-H.; Tsai, T.-H. Neuroprotective Effect of Schisandra Chinensis on Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Parkinsonian Syndrome in C57BL/6 Mice. Nutrients 2019, 11, 1671. https://doi.org/10.3390/nu11071671
Li C-L, Tsuang Y-H, Tsai T-H. Neuroprotective Effect of Schisandra Chinensis on Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Parkinsonian Syndrome in C57BL/6 Mice. Nutrients. 2019; 11(7):1671. https://doi.org/10.3390/nu11071671
Chicago/Turabian StyleLi, Chi-Lin, Yang-Hwei Tsuang, and Tung-Hu Tsai. 2019. "Neuroprotective Effect of Schisandra Chinensis on Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Parkinsonian Syndrome in C57BL/6 Mice" Nutrients 11, no. 7: 1671. https://doi.org/10.3390/nu11071671
APA StyleLi, C.-L., Tsuang, Y.-H., & Tsai, T.-H. (2019). Neuroprotective Effect of Schisandra Chinensis on Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Parkinsonian Syndrome in C57BL/6 Mice. Nutrients, 11(7), 1671. https://doi.org/10.3390/nu11071671






