Functional Ingredients based on Nutritional Phenolics. A Case Study against Inflammation: Lippia Genus
Abstract
1. Introduction
2. Biosynthesis and Chemical Structure of Phenolic Compounds
3. Inflammation and Phenolic Compounds
4. Functional Ingredients based on Phenolic Compounds with Anti-Inflammatory Properties: Genus Lippia, A Case Study
4.1. Phytochemicals Present in Lippia Plants: Phenolic Compounds and Anti-Inflammatory Properties
4.2. New Trends in Dietary Phenolics: Functional Ingredients and Nutraceuticals from the Lippia genus
4.2.1. Advanced Extraction Technique for Retrieval of Bioactive Compounds from the Lippia Genus
4.2.2. Encapsulation Techniques
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Galland, L. Diet and Inflammation. Nutr. Clin. Pract. 2010, 25, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Shivappa, N.; Hebert, J.R.; Marcos, A.; Diaz, L.-E.; Gomez, S.; Nova, E.; Michels, N.; Arouca, A.; González-Gil, E.; Frederic, G.; et al. Association between dietary inflammatory index and inflammatory markers in the HELENA study. Mol. Nutr. Food Res. 2017, 61, 1600707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tsao, R. Dietary polyphenols, oxidative stress and antioxidant and anti-inflammatory effects. Curr. Opin. Food Sci. 2016, 8, 33–42. [Google Scholar] [CrossRef]
- De la Luz Cádiz Gurrea, M.; Alañón Pardo, E.; Arráez Román, D.; Fernández Arroyo, S.; Micol Molina, V.; Roche, E.; Segura Carretero, A. Bioactive Compounds from Lippia citriodora: Application in Diseases Prevention. In Occurrences, Structure, Biosynthesis, and Health Benefits Based on Their Evidences of Medicinal Phytochemicals in Vegetables and Fruits; Motohashi, N., Ed.; Nova Science Publisher: New York, NY, USA, 2017; Volume 7, pp. 183–220. ISBN 978-1-53611-982-4. [Google Scholar]
- Al-Salami, H.; Mooranian, A.; Negrulj, R.; Chen-Tan, N.; Al-Sallami, H.; Fang, Z.; Mukkur, T.; Mikov, M.; Golocorbin-Kon, S.; Watts, G.; et al. Microencapsulation as a novel delivery method for the potential antidiabetic drug, Probucol. Drug Des. Dev. Ther. 2014, 8, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Boonchu, T.; Utama-ang, N. Optimization of extraction and microencapsulation of bioactive compounds from red grape (Vitis vinifera L.) pomace. J. Food Sci. Technol. 2015, 52, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Grigor, J.M.; Zhang, D.; Quantick, P.C.; Shahidi, F. Comparison of Volatiles, Phenolics, Sugars, Antioxidant Vitamins, and Sensory Quality of Different Colored Carrot Varieties. J. Agric. Food Chem. 2001, 49, 1410–1416. [Google Scholar] [CrossRef] [PubMed]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Bravo, L.; Sources, D.; Significance, N. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 2009, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Randhir, R.; Lin, Y.-T.; Shetty, K. Phenolics, their antioxidant and antimicrobial activity in dark germinated fenugreek sprouts in response to peptide and phytochemical elicitors. Asia Pac. J. Clin. Nutr. 2004, 13, 295–307. [Google Scholar] [PubMed]
- Harborne, J.B.; Jeffrey, B. Plant Phenolics; Academic Press: Cambridge, MA, USA, 1989; ISBN 9780124610118. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Sekher Pannala, A.; Chan, T.S.; O’Brien, P.J.; Rice-Evans, C.A. Flavonoid B-ring chemistry and antioxidant activity: Fast reaction kinetics. Biochem. Biophys. Res. Commun. 2001, 282, 1161–1168. [Google Scholar] [CrossRef]
- Lattanzio, V. Phenolic Compounds: Introduction. In Natural Products; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1543–1580. [Google Scholar]
- Farah, A.; Donangelo, C.M. Phenolic compounds in coffee—Minireview. Braz. J. Plant Physiol. 2006, 18, 23–36. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Clifford, M.N. Dietary hydroxybenzoic acid derivatives—Nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1024–1032. [Google Scholar] [CrossRef]
- Nile, S.H.; Ko, E.Y.; Kim, D.H.; Keum, Y.-S. Screening of ferulic acid related compounds as inhibitors of xanthine oxidase and cyclooxygenase-2 with anti-inflammatory activity. Rev. Bras. Farmacogn. 2016, 26, 50–55. [Google Scholar] [CrossRef]
- Lu, Z.; Nie, G.; Belton, P.S.; Tang, H.; Zhao, B. Structure–activity relationship analysis of antioxidant ability and neuroprotective effect of gallic acid derivatives. Neurochem. Int. 2006, 48, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Taofiq, O.; Calhelha, R.C.; Heleno, S.; Barros, L.; Martins, A.; Santos-Buelga, C.; Queiroz, M.J.R.P.; Ferreira, I.C.F.R. The contribution of phenolic acids to the anti-inflammatory activity of mushrooms: Screening in phenolic extracts, individual parent molecules and synthesized glucuronated and methylated derivatives. Food Res. Int. 2015, 76, 821–827. [Google Scholar] [CrossRef] [PubMed]
- Wojdylo, A.; Oszmianski, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Chen, L.; Teng, H.; Xie, Z.; Cao, H.; Cheang, W.S.; Skalicka-Woniak, K.; Georgiev, M.I.; Xiao, J. Modifications of dietary flavonoids towards improved bioactivity: An update on structure–activity relationship. Crit. Rev. Food Sci. Nutr. 2018, 58, 513–527. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Ricci, A.; Olejar, K.J.; Parpinello, G.P.; Mattioli, A.U.; Teslić, N.; Kilmartin, P.A.; Versari, A. Antioxidant activity of commercial food grade tannins exemplified in a wine model. Food Addit. Contam. Part A 2016, 33, 1761–1774. [Google Scholar] [CrossRef]
- Rubió, L.; Motilva, M.J.; Romero, M.P. Recent Advances in Biologically Active Compounds in Herbs and Spices: A Review of the Most Effective Antioxidant and Anti-Inflammatory Active Principles. Crit. Rev. Food Sci. Nutr. 2013, 53, 943–953. [Google Scholar] [CrossRef]
- Taofiq, O.; Martins, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Anti-inflammatory potential of mushroom extracts and isolated metabolites. Trends Food Sci. Technol. 2016, 50, 193–210. [Google Scholar] [CrossRef]
- Ambriz-Pérez, D.L.; Leyva-López, N.; Gutierrez-Grijalva, E.P.; Heredia, J.B. Phenolic compounds: Natural alternative in inflammation treatment. A Review. Cogent Food Agric. 2016, 2, 1131412. [Google Scholar]
- Koeberle, A.; Werz, O. Multi-target approach for natural products in inflammation. Drug Discov. Today 2015, 19, 1871–1882. [Google Scholar] [CrossRef]
- Nagula, R.L.; Wairkar, S. Recent advances in topical delivery of flavonoids: A review. J. Control Release 2019, 296, 190–201. [Google Scholar] [CrossRef]
- Pragasam, S.J.; Venkatesan, V.; Rasool, M. Immunomodulatory and Anti-inflammatory Effect of p-Coumaric Acid, a Common Dietary Polyphenol on Experimental Inflammation in Rats. Inflammation 2013, 36, 169–176. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, H.; Yang, T.; Ye, Y.; Shan, J.; Yin, Z.; Luo, L. Chlorogenic acid protects mice against lipopolysaccharide-induced acute lung injury. Injury 2010, 41, 746–752. [Google Scholar] [CrossRef]
- Rocha, J.; Eduardo-Figueira, M.; Barateiro, A.; Fernandes, A.; Brites, D.; Bronze, R.; Duarte, C.M.; Serra, A.T.; Pinto, R.; Freitas, M.; et al. Anti-inflammatory Effect of Rosmarinic Acid and an Extract of Rosmarinus officinalis in Rat Models of Local and Systemic Inflammation. Basic Clin. Pharmacol. Toxicol. 2015, 116, 398–413. [Google Scholar] [CrossRef]
- Rehman, S.U.; Ali, T.; Alam, S.I.; Ullah, R.; Zeb, A.; Lee, K.W.; Rutten, B.P.F.; Kim, M.O. Ferulic Acid Rescues LPS-Induced Neurotoxicity via Modulation of the TLR4 Receptor in the Mouse Hippocampus. Mol. Neurobiol. 2019, 56, 2774–2790. [Google Scholar] [CrossRef]
- Jing, W.; Chunhua, M.; Shumin, W. Effects of acteoside on lipopolysaccharide-induced inflammation in acute lung injury via regulation of NF-κB pathway in vivo and in vitro. Toxicol. Appl. Pharmacol. 2015, 285, 128–135. [Google Scholar] [CrossRef]
- Al-Harbi, N.O.; Imam, F.; Al-Harbi, M.M.; Al-Shabanah, O.A.; Alotaibi, M.R.; Sobeai, H.M.; Afzal, M.; Kazmi, I.; Al Rikabi, A.C. Rutin inhibits carfilzomib-induced oxidative stress and inflammation via the NOS-mediated NF-κB signaling pathway. Inflammopharmacology 2019, 1–11. [Google Scholar] [CrossRef]
- Yelumalai, S.; Giribabu, N.; Karim, K.; Omar, S.Z.; Salleh, N. Bin In vivo administration of quercetin ameliorates sperm oxidative stress, inflammation, preserves sperm morphology and functions in streptozotocin-nicotinamide induced adult male diabetic rats. Arch. Med. Sci. 2019, 15, 240–249. [Google Scholar] [CrossRef]
- Jung, J.-H.; Kang, J.-I.; Kim, H.-S. Effect of quercetin on impaired immune function in mice exposed to irradiation. Nutr. Res. Pract. 2012, 6, 301–307. [Google Scholar] [CrossRef]
- Yang, Y.-L.; Cheng, X.; Li, W.-H.; Liu, M.; Wang, Y.-H.; Du, G.-H. Kaempferol Attenuates LPS-Induced Striatum Injury in Mice Involving Anti-Neuroinflammation, Maintaining BBB Integrity, and Down-Regulating the HMGB1/TLR4 Pathway. Int. J. Mol. Sci. 2019, 20, 491. [Google Scholar] [CrossRef]
- Sun, Z.; Li, Q.; Hou, R.; Sun, H.; Tang, Q.; Wang, H.; Hao, Z.; Kang, S.; Xu, T.; Wu, S. Kaempferol-3-O-glucorhamnoside inhibits inflammatory responses via MAPK and NF-κB pathways in vitro and in vivo. Toxicol. Appl. Pharmacol. 2019, 364, 22–28. [Google Scholar] [CrossRef]
- Fei, J.; Liang, B.; Jiang, C.; Ni, H.; Wang, L. Luteolin inhibits IL-1β-induced inflammation in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomed. Pharmacother. 2019, 109, 1586–1592. [Google Scholar] [CrossRef]
- Li, Q.; Tian, Z.; Wang, M.; Kou, J.; Wang, C.; Rong, X.; Li, J.; Xie, X.; Pang, X. Luteoloside attenuates neuroinflammation in focal cerebral ischemia in rats via regulation of the PPARγ/Nrf2/NF-κB signaling pathway. Int. Immunopharmacol. 2019, 66, 309–316. [Google Scholar] [CrossRef]
- Wu, D.; Wang, Y.; Zhang, H.; Du, M.; Li, T. Acacetin attenuates mice endotoxin-induced acute lung injury via augmentation of heme oxygenase-1 activity. Inflammopharmacology 2018, 26, 635–643. [Google Scholar] [CrossRef]
- Prince, P.D.; Fischerman, L.; Toblli, J.E.; Fraga, C.G.; Galleano, M. LPS-induced renal inflammation is prevented by (−)-epicatechin in rats. Redox Biol. 2017, 11, 342–349. [Google Scholar] [CrossRef]
- Shanmugam, T.; Selvaraj, M.; Poomalai, S. Epigallocatechin gallate potentially abrogates fluoride induced lung oxidative stress, inflammation via Nrf2/Keap1 signaling pathway in rats: An in-vivo and in-silico study. Int. Immunopharmacol. 2016, 39, 128–139. [Google Scholar] [CrossRef]
- Fikry, E.M.; Gad, A.M.; Eid, A.H.; Arab, H.H. Caffeic acid and ellagic acid ameliorate adjuvant-induced arthritis in rats via targeting inflammatory signals, chitinase-3-like protein-1 and angiogenesis. Biomed. Pharmacother. 2019, 110, 878–886. [Google Scholar] [CrossRef]
- Calixto-Campos, C.; Carvalho, T.T.; Hohmann, M.S.N.; Pinho-Ribeiro, F.A.; Fattori, V.; Manchope, M.F.; Zarpelon, A.C.; Baracat, M.M.; Georgetti, S.R.; Casagrande, R.; et al. Vanillic Acid Inhibits Inflammatory Pain by Inhibiting Neutrophil Recruitment, Oxidative Stress, Cytokine Production, and NFκB Activation in Mice. J. Nat. Prod. 2015, 78, 1799–1808. [Google Scholar] [CrossRef]
- Domitrović, R.; Rashed, K.; Cvijanović, O.; Vladimir-Knežević, S.; Škoda, M.; Višnić, A. Myricitrin exhibits antioxidant, anti-inflammatory and antifibrotic activity in carbon tetrachloride-intoxicated mice. Chem. Biol. Interact. 2015, 230, 21–29. [Google Scholar] [CrossRef]
- Silva, S.; Sepodes, B.; Rocha, J.; Direito, R.; Fernandes, A.; Brites, D.; Freitas, M.; Fernandes, E.; Bronze, M.R.; Figueira, M.E. Protective effects of hydroxytyrosol-supplemented refined olive oil in animal models of acute inflammation and rheumatoid arthritis. J. Nutr. Biochem. 2015, 26, 360–368. [Google Scholar] [CrossRef]
- De la Luz Cádiz-Gurrea, M.; Fernández-Arroyo, S.; Joven, J.; Segura-Carretero, A. Comprehensive characterization by UHPLC-ESI-Q-TOF-MS from an Eryngium bourgatii extract and their antioxidant and anti-inflammatory activities. Food Res. Int. 2013, 50, 197–204. [Google Scholar]
- De la Luz Cádiz-Gurrea, M.; Borrás-Linares, I.; Lozano-Sánchez, J.; Joven, J.; Fernández-Arroyo, S.; Segura-Carretero, A. Cocoa and grape seed byproducts as a source of antioxidant and anti-inflammatory proanthocyanidins. Int. J. Mol. Sci. 2017, 18, 376. [Google Scholar]
- Monk, J.M.; Zhang, C.P.; Wu, W.; Zarepoor, L.; Lu, J.T.; Liu, R.; Peter Pauls, K.; Wood, G.A.; Tsao, R.; Robinson, L.E.; et al. White and dark kidney beans reduce colonic mucosal damage and inflammation in response to dextran sodium sulfate. J. Nutr. Biochem. 2015, 26, 752–760. [Google Scholar] [CrossRef]
- Chandran, R.; Abrahamse, H.; Parimelazhagan, T. Cytotoxic, analgesic and anti-inflammatory properties of Syzygium calophyllifolium bark. Biomed. Pharmacother. 2018, 103, 1079–1085. [Google Scholar] [CrossRef]
- Appel, K.; Meiser, P.; Millán, E.; Collado, J.A.; Rose, T.; Gras, C.C.; Carle, R.; Muñoz, E. Chokeberry (Aronia melanocarpa (Michx.) Elliot) concentrate inhibits NF-κB and synergizes with selenium to inhibit the release of pro-inflammatory mediators in macrophages. Fitoterapia 2015, 105, 73–82. [Google Scholar] [CrossRef]
- Joseph, S.V.; Edirisinghe, I.; Burton-Freeman, B.M. Fruit Polyphenols: A Review of Anti-inflammatory Effects in Humans. Crit. Rev. Food Sci. Nutr. 2016, 56, 419–444. [Google Scholar] [CrossRef]
- Shi, M.; Loftus, H.; McAinch, A.J.; Su, X.Q. Blueberry as a source of bioactive compounds for the treatment of obesity, type 2 diabetes and chronic inflammation. J. Funct. Foods 2017, 30, 16–29. [Google Scholar] [CrossRef]
- Medina-Remón, A.; Casas, R.; Tressserra-Rimbau, A.; Ros, E.; Martínez-González, M.A.; Fitó, M.; Corella, D.; Salas-Salvadó, J.; Lamuela-Raventos, R.M.; Estruch, R. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: A substudy of the PREDIMED trial. Br. J. Clin. Pharmacol. 2017, 83, 114–128. [Google Scholar] [CrossRef]
- Oliviero, F.; Scanu, A.; Zamudio-Cuevas, Y.; Punzi, L.; Spinella, P. Anti-inflammatory effects of polyphenols in arthritis. J. Sci. Food Agric. 2018, 98, 1653–1659. [Google Scholar] [CrossRef]
- Tangney, C.C.; Rasmussen, H.E. Polyphenols, inflammation, and cardiovascular disease. Curr. Atheroscler. Rep. 2013, 15, 324. [Google Scholar] [CrossRef]
- Buchwald-Werner, S.; Naka, I.; Wilhelm, M.; Schütz, E.; Schoen, C.; Reule, C. Effects of lemon verbena extract (Recoverben®) supplementation on muscle strength and recovery after exhaustive exercise: A randomized, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2018, 15, 5. [Google Scholar] [CrossRef]
- Domenech, M.; Casas, R.; Ruiz-León, A.M.; Sobrino, J.; Ros, E.; Estruch, R. Effects of a New Nutraceutical Combination (Aquilea Colesterol®) on the Lipid Profile and Inflammatory Biomarkers: A Randomized Control Trial. Nutrients 2019, 11, 949. [Google Scholar] [CrossRef]
- Yimam, M.; Horm, T.; Wright, L.; Jiao, P.; Hong, M.; Brownell, L.; Jia, Q. UP1306: A Composition Containing Standardized Extracts of Acacia catechu and Morus alba for Arthritis Management. Nutrients 2019, 11, 272. [Google Scholar] [CrossRef]
- Meiss, M.S.; Sanchez-Hidalgo, M.; Alarcón-de-la-Lastra, C.; Fernández-Bolaños, J.M.; Oreffo, R.O.; de Andres, M.C. FRI0508 Olive oil polyphenols as new nutraceutical in treatment of osteoarthritis. In Proceedings of the Cartilage, Synovium and Bone; BMJ Publishing Group Ltd.: London, UK; European League Against Rheumatism: Zürich, Switzerland, 2019. [Google Scholar]
- Menghini, L.; Ferrante, C.; Leporini, L.; Recinella, L.; Chiavaroli, A.; Leone, S.; Pintore, G.; Vacca, M.; Orlando, G.; Brunetti, L. An Hydroalcoholic Chamomile Extract Modulates Inflammatory and Immune Response in HT29 Cells and Isolated Rat Colon. Phyther. Res. 2016, 30, 1513–1518. [Google Scholar] [CrossRef]
- Hosseini, B.; Saedisomeolia, A.; Wood, L.G.; Yaseri, M.; Tavasoli, S. Effects of pomegranate extract supplementation on inflammation in overweight and obese individuals: A randomized controlled clinical trial. Complement. Ther. Clin. Pract. 2016, 22, 44–50. [Google Scholar] [CrossRef]
- Zielińska, M.; Lewandowska, U.; Podsędek, A.; Cygankiewicz, A.I.; Jacenik, D.; Sałaga, M.; Kordek, R.; Krajewska, W.M.; Fichna, J. Orally available extract from Brassica oleracea var. capitata rubra attenuates experimental colitis in mouse models of inflammatory bowel diseases. J. Funct. Foods 2015, 17, 587–599. [Google Scholar] [CrossRef]
- Kenjerić, D.; Mandić, M.L.; Primorac, L.; Čačić, F.; Kenjeric, D.; Mandic, M.; Primorac, L.; Cacic, F.; Cooper, R.; Molan, P.; et al. Flavonoid pattern of sage (Salvia officinalis L.) unifloral honey. Food Chem. 2008, 110, 187–192. [Google Scholar] [CrossRef]
- Shahhoseini, R.; Beyraghdar, A.; Karimi, S.-R.; Ebadi, M.-T. Essential Oil Content and Composition of Lemon Verbena (Lippia citriodora Kunth.) during Different Phenological Stages. J. Med. Plants By-Prod. 2013, 2, 205–208. [Google Scholar]
- Argyropoulou, C.; Daferera, D.; Tarantilis, P.A.; Fasseas, C.; Polissiou, M. Chemical composition of the essential oil from leaves of Lippia citriodora H.B.K. (Verbenaceae) at two developmental stages. Biochem. Syst. Ecol. 2007, 35, 831–837. [Google Scholar] [CrossRef]
- Oliveira, D.R.; Leitão, G.G.; Bizzo, H.R.; Lopes, D.; Alviano, D.S.; Alviano, C.S.; Leitão, S.G. Chemical and antimicrobial analyses of essential oil of Lippia origanoides H.B.K. Food Chem. 2007, 101, 236–240. [Google Scholar] [CrossRef]
- Pérez, S.; Meckes, M.; Pérez, C.; Susunaga, A.; Zavala, M.A. Anti-inflammatory activity of Lippia dulcis. J. Ethnopharmacol. 2005, 102, 1–4. [Google Scholar] [CrossRef]
- Gutiérrez, S.L.G.; Chilpa, R.R.; Jaime, H.B. Medicinal plants for the treatment of “nervios”, anxiety, and depression in Mexican Traditional Medicine. Rev. Bras. Farmacogn. 2014, 24, 591–608. [Google Scholar] [CrossRef]
- Pascual, M.E.; Slowing, K.; Carretero, E.; Mata, D.S.; Villar, A. Lippia: Traditional uses, chemistry and pharmacology: A review. J. Ethnopharmacol. 2001, 76, 201–214. [Google Scholar] [CrossRef]
- Mirzaie, A.; Shandiz, S.A.S.; Noorbazargan, H.; Asgary, E.A. Evaluation of chemical composition, antioxidant, antibacterial, cytotoxic and apoptotic effects of Aloysia citrodora extract on colon cancer cell line. Tehran Univ. Med. J. 2016, 74, 168–176. [Google Scholar]
- Mauriz, E.; Vallejo, D.; Tuñón, M.J.; Rodriguez-López, J.M.; Rodríguez-Pérez, R.; Sanz-Gómez, J.; García-Fernández, M. del C. Effects of dietary supplementation with lemon verbena extracts on serum inflammatory markers of multiple sclerosis patients. Nutr. Hosp. 2014, 31, 764–771. [Google Scholar]
- Stashenko, E.E.; Jaramillo, B.E.; Martínez, J.R. Comparison of different extraction methods for the analysis of volatile secondary metabolites of Lippia alba (Mill.) N.E. Brown, grown in Colombia, and evaluation of its in vitro antioxidant activity. J. Chromatogr. A 2004, 1025, 93–103. [Google Scholar] [CrossRef]
- Gomes, A.F.; Almeida, M.P.; Leite, M.F.; Schwaiger, S.; Stuppner, H.; Halabalaki, M.; Amaral, J.G.; David, J.M. Seasonal variation in the chemical composition of two chemotypes of Lippia alba. Food Chem. 2017, 273, 186–193. [Google Scholar] [CrossRef]
- Sena Filho, J.G.; Duringer, J.M.; Uchoa, D.E.A.; Xavier, H.S.; Barbosa Filho, J.M.; Braz Filho, R. Distribution of iridoid glucosides in plants from the genus Lippia (Verbenaceae): An investigation of Lippia alba (Mill.) N.E. Brown. Nat. Prod. Commun. 2007, 2, 715–716. [Google Scholar]
- Leyva-Jiménez, F.J.; Lozano-Sánchez, J.; Borrás-Linares, I.; Arráez-Román, D.; Segura-Carretero, A. Comparative study of conventional and pressurized liquid extraction for recovering bioactive compounds from Lippia citriodora leaves. Food Res. Int. 2018, 109, 213–222. [Google Scholar] [CrossRef]
- Kim, N.-S.; Lee, D.-S. Headspace solid-phase microextraction for characterization of fragrances of lemon verbena (Aloysia triphylla) by gas chromatography-mass spectrometry. J. Sep. Sci. 2004, 27, 96–100. [Google Scholar] [CrossRef]
- Maroyi, A. Lippia javanica (Burm.f.) Spreng.: Traditional and Commercial Uses and Phytochemical and Pharmacological Significance in the African and Indian Subcontinent. Evid. Based Complement. Altern. Med. 2017, 2017, 6746071. [Google Scholar] [CrossRef]
- Parodi, T.V.; de Castagna Vargas, A.P.; Krewer, C.; de Moraes Flores, É.M.; Baldisserotto, B.; Heinzmann, B.M.; de Oliveira, J.V.; Popiolski, A.S.; Minozzo, M. Chemical Composition and Antibacterial Activity of Aloysia triphylla (L’Hérit) Britton Extracts Obtained by Pressurized CO2 Extraction. Braz. Arch. Biol. Technol. 2013, 56, 283–292. [Google Scholar] [CrossRef]
- Pérez Zamora, C.; Torres, C.; Nuñez, M. Antimicrobial Activity and Chemical Composition of Essential Oils from Verbenaceae Species Growing in South America. Molecules 2018, 23, 544. [Google Scholar]
- Parodi, T.V.; Cunha, M.A.; Heldwein, C.G.; de Souza, D.M.; Martins, Á.C.; Garcia, L.D.; Junior, W.W.; Monserrat, J.M.; Schmidt, D.; Caron, B.O.; et al. The anesthetic efficacy of eugenol and the essential oils of Lippia alba and Aloysia triphylla in post-larvae and sub-adults of Litopenaeus vannamei (Crustacea, Penaeidae). Comp. Biochem. Physiol. Part. C Toxicol. Pharmacol. 2012, 155, 462–468. [Google Scholar] [CrossRef]
- Sarrazin, S.; da Silva, L.; de Assunção, A.; Oliveira, R.; Calao, V.; da Silva, R.; Stashenko, E.; Maia, J.; Mourão, R. Antimicrobial and Seasonal Evaluation of the Carvacrol-Chemotype Oil from Lippia origanoides Kunth. Molecules 2015, 20, 1860–1871. [Google Scholar] [CrossRef]
- Viljoen, A.M.; Subramoney, S.; van Vuuren, S.F.; Başer, K.H.C.; Demirci, B. The composition, geographical variation and antimicrobial activity of Lippia javanica (Verbenaceae) leaf essential oils. J. Ethnopharmacol. 2005, 96, 271–277. [Google Scholar] [CrossRef]
- Lira, P.D.L.; van Baren, C.M.; Retta, D.; Bandoni, A.L.; Gil, A.; Gattuso, M.; Gattuso, S. Characterization of Lemon Verbena (Aloysia citriodora Palau) from Argentina by the Essential Oil. J. Essent. Oil Res. 2008, 20, 350–353. [Google Scholar] [CrossRef]
- Quirantes-Piné, R.; Arráez-Román, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Characterization of phenolic and other polar compounds in a lemon verbena extract by capillary electrophoresis-electrospray ionization-mass spectrometry. J. Sep. Sci. 2010, 33, 2818–2827. [Google Scholar] [CrossRef]
- Hennebelle, T.; Sahpaz, S.; Joseph, H.; Bailleul, F. Ethnopharmacology of Lippia alba. J. Ethnopharmacol. 2008, 116, 211–222. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Mukhopadhyay, S.; Robbins, R.J.; Harnly, J.M. Identification and quantification of flavonoids of Mexican oregano (Lippia graveolens) by LC-DAD-ESI/MS analysis. J. Food Compos. Anal. 2007, 20, 361–369. [Google Scholar] [CrossRef]
- Quirantes-Piné, R.; Herranz-López, M.; Funes, L.; Borrás-Linares, I.; Micol, V.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenylpropanoids and their metabolites are the major compounds responsible for blood-cell protection against oxidative stress after administration of Lippia citriodora in rats. Phytomedicine 2013, 20, 1112–1118. [Google Scholar] [CrossRef]
- De la Luz Cádiz-Gurrea, M.; Olivares-Vicente, M.; Herranz-López, M.; Román-Arráez, D.; Fernández-Arroyo, S.; Micol, V.; Segura-Carretero, A. Bioassay-guided purification of Lippia citriodora polyphenols with AMPK modulatory activity. J. Funct. Foods 2018, 46, 514–520. [Google Scholar]
- Wang, T.; Zhang, Y.; Chen, Y.; Wang, S.; Dong, Y.; Wang, T.; Qu, L.; Li, N. Bioactive constituents from the aerial parts of Lippia triphylla. Molecules 2015, 20, 21946–21959. [Google Scholar]
- Merfort, I. Review of the analytical techniques for sesquiterpenes and sesquiterpene lactones. J. Chromatogr. A 2002, 967, 115–130. [Google Scholar] [CrossRef]
- Koumaglo, K.H.; Akpagana, K.; Glitho, A.I.; Garneau, F.-X.; Gagnon, H.; Jean, F.-I.; Moudachirou, M.; Addae-Mensah, I. Geranial and Neral, Major Constituents of Lippia multiflora Moldenke Leaf Oil. J. Essent. Oil Res. 1996, 8, 237–240. [Google Scholar] [CrossRef]
- Koumaglo, K.; Ayedoun, A.; Akpagana, K.; Moudachirou, M.; Bouchet, P. Antifungal Activity of Essential Oils Extracted in the African States of Togo and Benin; Cryptogamie Mycologie: Montpellier, France, 1997. [Google Scholar]
- Valentin, A.; Pélissier, Y.; Benoit, F.; Marion, C.; Kone, D.; Mallie, M.; Bastide, J.-M.; Bessière, J.-M. Composition and antimalarial activity in vitro of volatile components of lippia multiflora. Phytochemistry 1995, 40, 1439–1442. [Google Scholar] [CrossRef]
- Vogt, T. Phenylpropanoid biosynthesis. Mol. Plant. 2010, 3, 2–20. [Google Scholar] [CrossRef]
- Kaneda, N.; Lee, I.-S.; Gupta, M.P.; Soejarto, D.D.; Kinghorn, A.D. (+)-4β-Hydroxyhernandulcin, A New Sweet Sesquiterpene from the Leaves and Flowers of Lippia dulcis. J. Nat. Prod. 1992, 55, 1136–1141. [Google Scholar] [CrossRef]
- Taoubi, K.; Fauvel, M.; Gleye, J.; Moulis, C.; Fourasté, I. Phenylpropanoid Glycosides from Lantana camara and Lippia multiflora. Planta Med. 1997, 63, 192–193. [Google Scholar] [CrossRef]
- Nakamura, T.; Okuyama, E.; Tsukada, A.; Yamazaki, M.; Satake, M.; Nishibe, S.; Deyama, T.; Moriya, A.; Maruno, M.; Nishimura, H. Acteoside as the Analgesic Principle of Cedron (Lippia hriphylla), a Peruvian Medicinal Plant. Chem. Pharm. Bull. 1997, 45, 499–504. [Google Scholar] [CrossRef]
- Hennebelle, T.; Sahpaz, S.; Gressier, B.; Joseph, H.; Bailleul, F. Antioxidant and neurosedative properties of polyphenols and iridoids fromLippia alba. Phyther. Res. 2008, 22, 256–258. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A.; Harborne, J.B.; Self, R. Twelve 6-oxygenated flavone sulphates from Lippia nodiflora andL. canescens. Phytochemistry 1987, 26, 2281–2284. [Google Scholar] [CrossRef]
- Dominguez, S.X.; Sánchez, H.V.; Suárez, M.; Baldas, J.H.; del Rosario González, M. Chemical Constituents of Lippia graveolens. Planta Med. 1989, 55, 208–209. [Google Scholar] [CrossRef]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Herranz-López, M.; Barrajón-Catalán, E.; Segura-Carretero, A.; Menéndez, J.A.; Joven, J.; Micol, V. Lemon verbena (Lippia citriodora) polyphenols alleviate obesity-related disturbances in hypertrophic adipocytes through AMPK-dependent mechanisms. Phytomedicine 2015, 22, 605–614. [Google Scholar] [CrossRef]
- De la Luz Cádiz-Gurrea, M.; Micol, V.; Joven, J.; Segura-Carretero, A.; Fernández-Arroyo, S. Different behavior of polyphenols in energy metabolism of lipopolysaccharide-stimulated cells. Food Res. Int. 2018, 118, 96–100. [Google Scholar] [CrossRef]
- Palazzo, M.; Schiavitto, M.; Cinone, M.; Vizzarri, F. Rabbit metabolic response and selected meat quality traits: Evaluation of dietary PLX ® 23 and LycoBeads ® feed supplement. J. Anim. Physiol. Anim. Nutr. 2019, 103, 383–394. [Google Scholar] [CrossRef]
- Transparency Market Research. Polyphenols Market by Product (Grape Seed, Green Tea, Apple and Others), by Application (Functional Beverages, Functional Food, Dietary Supplements and Others)—Global Industry Analysis, Size, Share, Growth, Trends and Forecast (2012–2018); Transparency Market Research: Albany, NY, USA, 2013. [Google Scholar]
- Bilia, A.R.; Giomi, M.; Innocenti, M.; Gallori, S.; Vincieri, F.F. HPLC-DAD-ESI-MS analysis of the constituents of aqueous preparations of verbena and lemon verbena and evaluation of the antioxidant activity. J. Pharm. Biomed. Anal. 2008, 46, 463–470. [Google Scholar] [CrossRef]
- Choupani, M.; Arabshahi Delouee, S.; Alami, M. Antioxidant Properties of Various Solvent Extracts of Lemon Verbena (Lippia Citriodora) Leaves. Int. J. Adv. Biol. Biomed. Res. 2014, 2, 1340–1346. [Google Scholar]
- Nuñez, M.B.; Torres, C.A.; Aguado, M.I.; Bela, A.J.; Dudik, H.N.; Bregni, C. Polyphenols and antimicrobial activity in extracts of Lippia alba (Mill.). Int. J. Med. Arom. Plantas 2012, 2, 361–368. [Google Scholar]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.H. Green Extraction Methods for Polyphenols from Plant Matrices and Their Byproducts: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- Smith, R.M. Before the injection—Modern methods of sample preparation for separation techniques. J. Chromatogr. A 2003, 1000, 3–27. [Google Scholar] [CrossRef]
- Braga, M.E.M.; Ehlert, P.A.D.; Ming, L.C.; Meireles, M.A.A. Supercritical fluid extraction from Lippia alba: Global yields, kinetic data, and extract chemical composition. J. Supercrit. Fluids 2005, 34, 149–156. [Google Scholar] [CrossRef]
- Garmus, T.T.; Paviani, L.C.; Queiroga, C.L.; Cabral, F.A. Extraction of phenolic compounds from pepper-rosmarin (Lippia sidoides Cham.) leaves by sequential extraction in fixed bed extractor using supercritical CO2, ethanol and water as solvents. J. Supercrit. Fluids 2015, 99, 68–75. [Google Scholar] [CrossRef]
- Rocchetti, G.; Blasi, F.; Montesano, D.; Ghisoni, S.; Marcotullio, M.C.; Sabatini, S.; Cossignani, L.; Lucini, L. Impact of conventional/non-conventional extraction methods on the untargeted phenolic profile of Moringa oleifera leaves. Food Res. Int. 2019, 115, 319–327. [Google Scholar] [CrossRef]
- Pimentel-Moral, S.; Borrás-Linares, I.; Lozano-Sánchez, J.; Arráez-Román, D.; Martínez-Férez, A.; Segura-Carretero, A. Microwave-assisted extraction for Hibiscus sabdariffa bioactive compounds. J. Pharm. Biomed. Anal. 2018, 156, 313–322. [Google Scholar] [CrossRef]
- Ivanović, M.; Alañón, M.E.; Arráez-Román, D.; Segura-Carretero, A. Enhanced and green extraction of bioactive compounds from Lippia citriodora by tailor-made natural deep eutectic solvents. Food Res. Int. 2018, 111, 67–76. [Google Scholar] [CrossRef]
- Etxeberria, U.; Arias, N.; Boqué, N.; Macarulla, M.T.; Portillo, M.P.; Martínez, J.A.; Milagro, F.I. Reshaping faecal gut microbiota composition by the intake of trans-resveratrol and quercetin in high-fat sucrose diet-fed rats. J. Nutr. Biochem. 2015, 26, 651–660. [Google Scholar] [CrossRef]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef]
- Kasote, D.M.; Jayaprakasha, G. Encapsulation of Polyphenols: An Effective Way to Enhance Their Bioavailability for Gut Health; American Chemical Society: Washington, DC, USA, 2018. [Google Scholar]
- Gibbs, F.; Selim, K.; Inteaz, A.; Catherine, N.; Mulligan, B. Encapsulation in the food industry: A review. Int. J. Food Sci. Nutr. 1999, 50, 213–224. [Google Scholar]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, preparation, and applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef]
- Colletier, J.-P.; Chaize, B.; Winterhalter, M.; Fournier, D. Protein encapsulation in liposomes: Efficiency depends on interactions between protein and phospholipid bilayer. BMC Biotechnol. 2002, 2, 9. [Google Scholar] [CrossRef]
- Bonechi, C.; Donati, A.; Tamasi, G.; Pardini, A.; Rostom, H.; Leone, G.; Lamponi, S.; Consumi, M.; Magnani, A.; Rossi, C. Chemical characterization of liposomes containing nutraceutical compounds: Tyrosol, hydroxytyrosol and oleuropein. Biophys. Chem. 2019, 246, 25–34. [Google Scholar] [CrossRef]
- Mazzacuva, F.; Sinico, C.; Bilia, A. Enhanced skin permeation of verbascoside-cyclodextrin complex loaded into liposomes. Planta Med. 2010, 76, P228. [Google Scholar] [CrossRef]
- Desai, K.G.H.; Jin Park, H. Recent Developments in Microencapsulation of Food Ingredients. Dry. Technol. 2005, 23, 1361–1394. [Google Scholar] [CrossRef]
- Fang, Z.; Bhandari, B. Encapsulation of polyphenols—A review. Trends Food Sci. Technol. 2010, 21, 510–523. [Google Scholar] [CrossRef]
- González, E.; Gómez-Caravaca, A.M.; Giménez, B.; Cebrián, R.; Maqueda, M.; Martínez-Férez, A.; Segura-Carretero, A.; Robert, P. Evolution of the phenolic compounds profile of olive leaf extract encapsulated by spray-drying during in vitro gastrointestinal digestion. Food Chem. 2019, 279, 40–48. [Google Scholar] [CrossRef]
- Tsali, A.; Goula, A.M. Valorization of grape pomace: Encapsulation and storage stability of its phenolic extract. Powder Technol. 2018, 340, 194–207. [Google Scholar] [CrossRef]
- Pacheco, C.; González, E.; Robert, P.; Parada, J. Retention and pre-colon bioaccessibility of oleuropein in starchy food matrices, and the effect of microencapsulation by using inulin. J. Funct. Foods 2018, 41, 112–117. [Google Scholar] [CrossRef]
- Arana-Sánchez, A.; Estarrón-Espinosa, M.; Obledo-Vázquez, E.N.; Padilla-Camberos, E.; Silva-Vázquez, R.; Lugo-Cervantes, E. Antimicrobial and antioxidant activities of Mexican oregano essential oils (Lippia graveolens H. B. K.) with different composition when microencapsulated inβ-cyclodextrin. Lett. Appl. Microbiol. 2010, 50, 585–590. [Google Scholar] [CrossRef]
- Minemoto, Y.; Adachi, S.; Matsuno, R. Effect of Relative Humidity during Storage on the Autoxidation of Linoleic Acid Encapsulated with a Polysaccharide by Hot-Air-Drying and Freeze-Drying. Food Sci. Technol. Res. 2001, 7, 91–93. [Google Scholar] [CrossRef][Green Version]
- Minemoto, Y.; Adachi, S.; Matsuno, R. Comparison of Oxidation of Methyl Linoleate Encapsulated with Gum Arabic by Hot-Air-Drying and Freeze-Drying. J. Agric. Food Chem. 1997, 45, 4530–4534. [Google Scholar] [CrossRef]
- Papoutsis, K.; Golding, J.; Vuong, Q.; Pristijono, P.; Stathopoulos, C.; Scarlett, C.; Bowyer, M. Encapsulation of Citrus By-Product Extracts by Spray-Drying and Freeze-Drying Using Combinations of Maltodextrin with Soybean Protein and ι-Carrageenan. Foods 2018, 7, 115. [Google Scholar] [CrossRef]
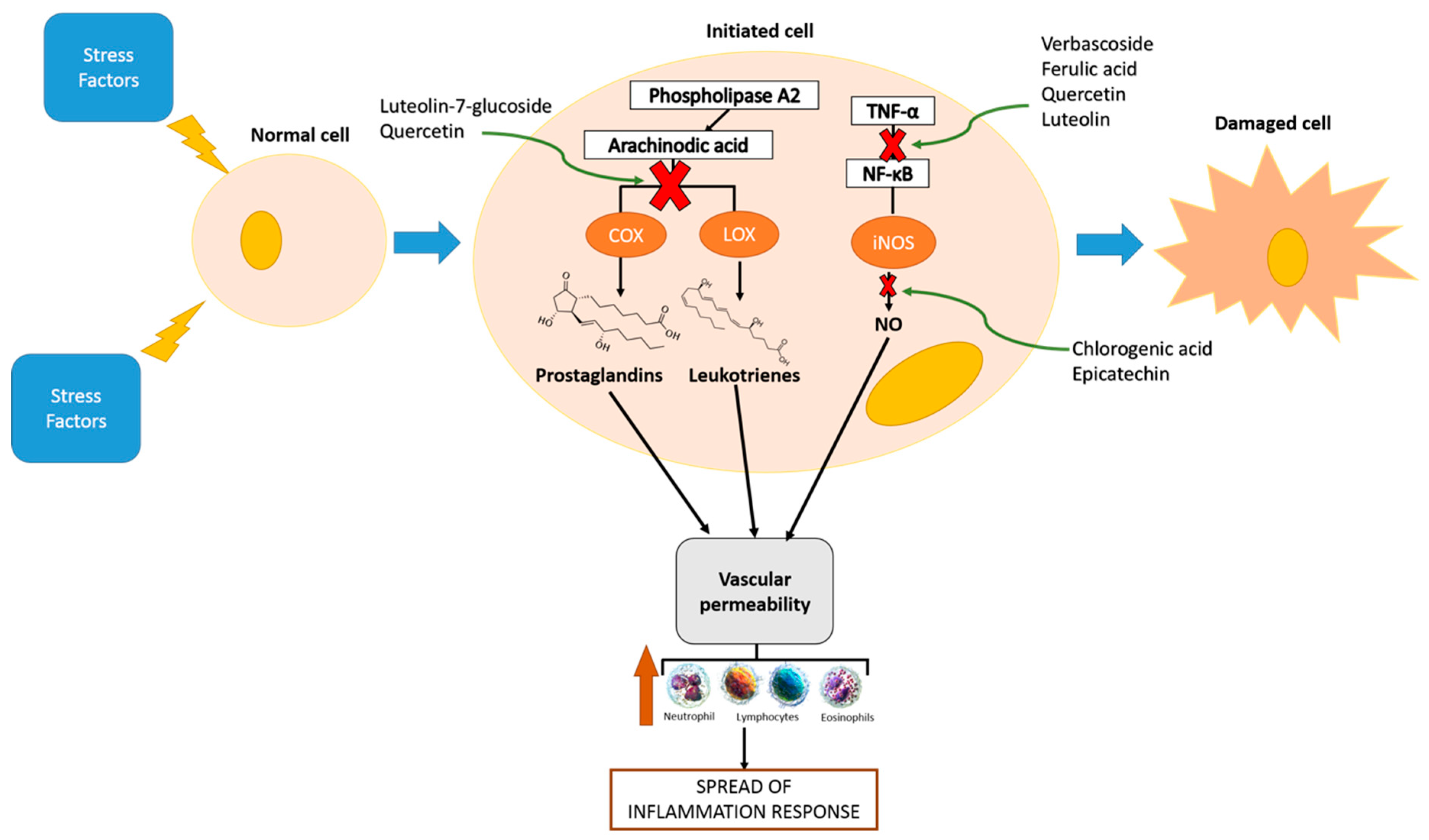
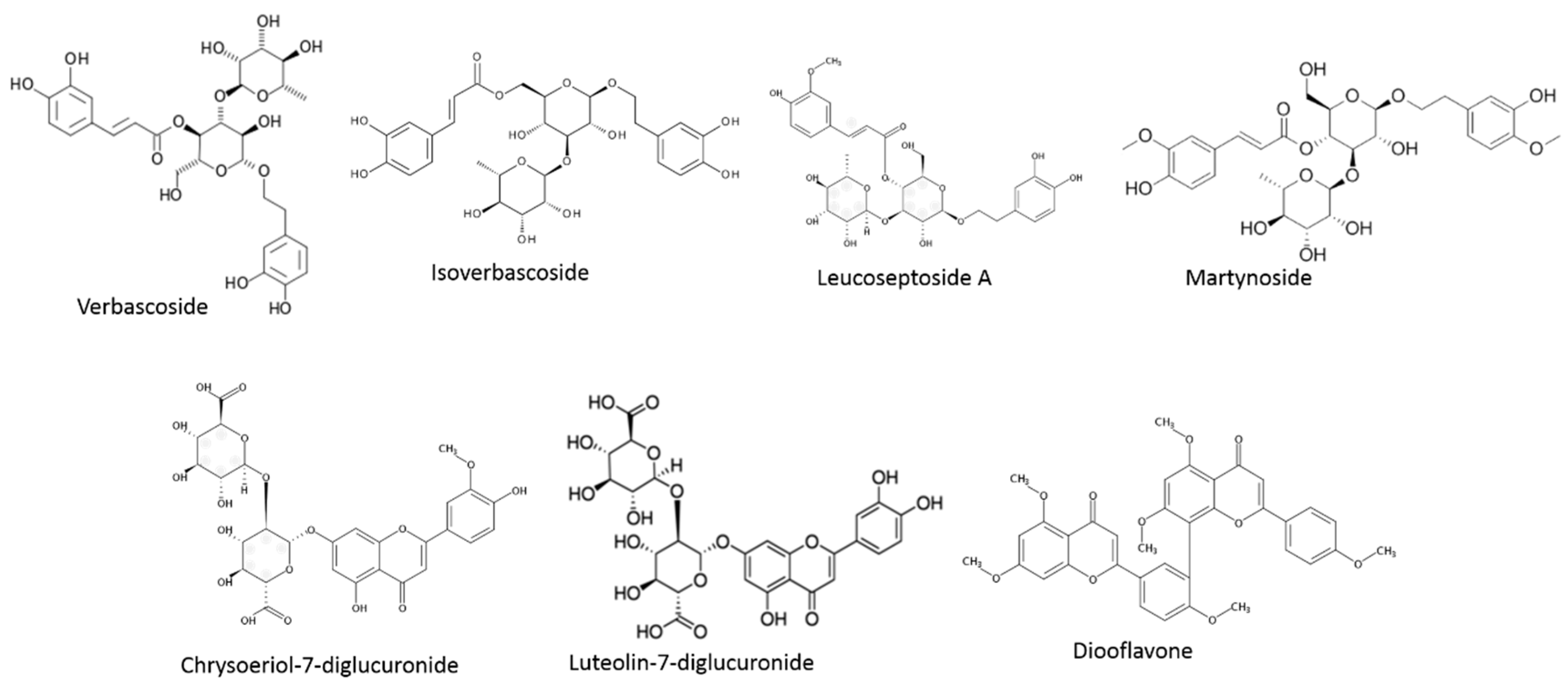
| Class | Phenolic Compounds | Biomarkers Studied | Mechanisms | Doses | Model | Reference |
|---|---|---|---|---|---|---|
| Phenolic acids | p-coumaric acid | IL-12, TNF-α, and IL-1β | Decreased TNF-α expression Decreased circulating immune complexes | 100 mg/kg body weight | Adjuvant-induced arthritic rats | [29] |
| Chlorogenic acid | NO | Decreased the activity of iNOS | 5, 20, and 50 mg/kg body weight | LPS-induced acute lung injury rats | [30] | |
| Rosmarinic acid | TNF-α, Il-1β, and IL-6 | Decreased neutrophil activity Decreased MMP-9 activity Modulation of NF-κB | 10, 25, and 50 mg/kg body weight | Carrageenin-induced paw oedema rat | [31] | |
| Ferulic acid | iNOS, COX-2, TNF-α, and IL-1β | Decreased JNK/NF-κB | 20 mg/kg body weight | LPS-induced neuroinflammation rats | [32] | |
| Verbascoside | TNF-α, IL-1β, and IL-6 | Decreased IκBα Decreased NF-κB Decreased IKK-α Decreased IKKβ | 30 and 60 mg/kg body weight | LPS-induced acute lung injury rats | [33] | |
| Flavonol | Rutin | NOS-2, NF-κB, IkBa, and IL-17 | Increased NF-κB Increased IκBα phosphorylation | 10, 20, and 40 mg/kg body weight | CFZ-induced nephrotoxicity rats | [34] |
| Flavonol | Quercetin | IL-1β, IL-6, and TNF-α | Decreased LPO, NF-κβ, and TNF-α. | 10, 20, 25, 40, and 50 mg/kg body weight | Streptozotocin-nicotinamide induced diabetic rats Induced inflammation by radiation mice | [35,36] |
| Flavonol | Kaempferol | IL-1β, IL-6, TNF-α, MCP-1, and ICAM-1 | Decreased HMGB1/TLR4 inflammatory pathway | 20 and 50 mg/kg body weight | LPS-induced striatum injury mice | [37] |
| Flavanol | Kaempferol-3-O-glucorhamnoside | TNF-α, IL-6, IL-1β, and PGE2 | Decreased NF-κB and MAP kinase phosphorylation | 50, 100 or 200 μg/kg | Klebsiella pneumoniae infected mice | [38] |
| Flavone | Luteolin | IL-1β, NO, PGE2, TNF-α, NOS, COX-2,MMP-1, MMP-2, MMP-3, MMP-8, and MMP-9 | Decreased NF-κB | 10 mg/kg body weight | MIA-induced osteoarthritis mice | [39] |
| Flavone | Luteolin-7-glucoside | IL-1β, TNF-α, iNOS, and COX-2 | Decreased NF-κB Increased PPARγ Increased Nrf2 | 20, 40, 80 mg/kg body weight | Cerebral ischemia-reperfusion induced neuroinflammation rats | [40] |
| Flavone | Acacetin | TNF-α, IL-1β | Decreased TNF-α Increased HO-1 | 50 mg/kg body weight | LPS-induced acute lung injury rats | [41] |
| Flavan-3-ol | Epicatechin | TNF-α, iNOS, and IL-6 | Decreased TLR4 upregulation Decreased NOX activation Decreased NF-kB activation. | 80 mg/kg body weight | LPS-induced renal inflammation rats | [42] |
| Flavanol | Epigallocatechin gallate | TNF-α, IL-1β, IL-6, CINC-3 and iNOS | Increased Nrf2 Increased HO-1 | 40 mg/kg body weight | Fluoride induced lung oxidative stress rats | [43] |
| Ellagitannin | Ellagic acid | NO, caspase-3, MMP-9, IL-1β | Decreased NF-κB | 50 mg/kg body weight | FCA-induced arthritis rats | [44] |
| Source | Composition | Effect | Reference |
|---|---|---|---|
| L. citriodora | Mainly verbascoside and other phenylpropanoids | Anti-inflammatory and reducing muscle damage after sport | [58] |
| Not revealed | Phytosterols, RYR, hydroxytyrosol, and vitamin E | Anti-inflammatory in patients with hypercholesterolemia | [59] |
| Acacia catechu and Morus alba | Catechin, stilbenes, and flavonoids | Anti-arthritis effects | [60] |
| Olive oil | Oleocanthal, ligstroside aglycone, docosahexaenoic, and eicosapentaenoic acids | Anti-osteoarthritis | [61] |
| Chamomile | Apigenin, apigenin-7-glucoside, and apigenin-7-(6-acetil)glucoside. | Inflammatory in bowel diseases | [62] |
| Pomegranate fruit | Mainly ellagic acid | Anti-inflammatory in obese patients | [63] |
| Brassica oleracea | Anthocyanins | Inflammatory in bowel diseases | [64] |
| Chemical Group | Compound | Species | Reference |
|---|---|---|---|
| Iridoids | Carioptoside | Lippia alba | [76] |
| Gardoside | L. citriodora | [77] | |
| Durandoside I | L. citriodora | [77] | |
| Hydroxyl campsiside | L. citriodora | [77] | |
| Ixoside | L. citriodora | [77] | |
| Lipedoside A I | L. citriodora | [77] | |
| Lippioside I | L. citriodora | [77] | |
| Lippioside II | L. citriodora | [77] | |
| Loganic acid | L. alba | [76] | |
| Manuleoside H | L. citriodora | [77] | |
| Myxospyroside | L. citriodora | [77] | |
| Shanzhiside | L. alba | [76] | |
| Secologanin | L. alba | [76] | |
| Secoxyloganin | L. alba | [76] | |
| teucardoside | L. citriodora | [77] | |
| Theviridoside | Lippia javanica | [76] | |
| Theveside | L. citriodora | [77] | |
| Terpenes | Camfor | L. citriodora/javanica | [78,79] |
| Caryophyllene | L. citriodora/javanica | [67,79] | |
| Carvacrol | L. citriodora/origanoides | [67] | |
| Cimonene | L. citriodora | [78] | |
| Citral | L. citriodora/dulcis | [80,81] | |
| Citronelal | L. citriodora | [5] | |
| Curcumene | L. citriodora | [82] | |
| Eucalyptol | L. javanica | [79] | |
| Escualen | L. citriodora | [5] | |
| Geranial | L. citriodora/javanica | [67,79] | |
| Geraniol | L. citriodora/javanica | [67] | |
| Heptacosanol | L. citriodora | [82] | |
| Ipsdienone | L. javanica | [79] | |
| Limonene | L. citriodora/javanica | [79,83] | |
| Linalool | L. citriodora/javanica | [79,84] | |
| Myrcenone | L. citriodora/javanica | [79,84] | |
| Neral | L. citriodora | [5] | |
| Nerol | L. citriodora | [85] | |
| Nonenal | L. citriodora/javanica | [67,79] | |
| Sabinene | L. citriodora/javanica | [78,79] | |
| Spathulenol | L. javanica | [79] | |
| Thymol | L. citriodora/origanoides | [83] | |
| α-Terpineol | L. citriodora | [5] | |
| β-Caryophyllene | L. citriodora/dulcis | [81,83] | |
| β-Cymene | L. citriodora | [83] | |
| p-Cymene | L. origanoides | [81] | |
| γ-Terpinene | L. citriodora/javanica | [67,79] | |
| Flavonoids | Acacetin-7-diglucuronide | L. citriodora | [86] |
| Apigenin | L. citriodora/graveolens/javanica | [79] | |
| Apigenin-7-diglucuronide | L. alba/citriodora | [86,87] | |
| Carssifolioside | L. javanica | [79] | |
| Chrysoeriol | L. citriodora/javanica | [67,79] | |
| Chrysoeriol-7-diglucuronide | L. citriodora | [86] | |
| Cirsiliol | L. citriodora | [67] | |
| Cirsimaritin | L. citriodora | [67] | |
| Dimethylscutellarein | Lippia graveolens | [88] | |
| Diosmetin | L. citriodora | [67] | |
| Eriodictyol | L. graveolens | [88] | |
| Eriodictyol -7-glucoside | L. graveolens | [88] | |
| Eupafolin | L. citriodora | [67] | |
| Eupaforin | L. citriodora | [67] | |
| Eupatorin | L. javanica | [79] | |
| Galangin | L. graveolens | [88] | |
| Genkawin | L. javanica | [79] | |
| Hipidulin | L. citriodora | [67] | |
| Hydroxyluteolin | L. citriodora/graveolens | [88] | |
| Hydroxyluteolin 7-O-hexoside | L. graveolens | [88] | |
| Hydroxyluteolin 7-O-rhamnoside | L. graveolens | [88] | |
| Isothymusin | L. javanica | [79] | |
| Jaceosidin | L. citriodora | [86] | |
| Luteolin | L. citriodora/javanica | [67,79] | |
| Luteolin-7-glucoside | L. alba/citriodora/graveolens | [86,87,88] | |
| Methylscutellarein | L. graveolens | [88] | |
| Methoxylutrolin | L. javanica | [79] | |
| Naringenin | L. graveolens | [88] | |
| Nepetin | L. citriodora | [67] | |
| Nepitrin | L. citriodora | [67] | |
| Quercetin | L. citriodora/graveolens | [67,88] | |
| Pectolinarigenin | L. citriodora | [67] | |
| Phloretin | L. graveolens | [88] | |
| Phloretin-6-glucoside | L. graveolens | [88] | |
| Pinocembrin | L. graveolens | [88] | |
| Sakuranetin | L. graveolens | [88] | |
| Salvigenin | L. citriodora/javanica | [67,79] | |
| Scutellarein | L. graveolens | [88] | |
| Scutellarein-7-hexoside | L. graveolens | [88] | |
| Taxifolin | L. graveolens | [88] | |
| Tricin | L. javanica | [79] | |
| Phenylpropanoids | Calceolarioside E | L. alba | [87] |
| Cistanoside F | L. alba/citriodora | [86,87] | |
| Decaffeoylverbascoside | L. alba | [87] | |
| Eukovoside | L. citriodora | [89] | |
| Hydroxy-verbascoside | L. citriodora | [89] | |
| Hydroxy-isoverbascoside | L. citriodora | [89] | |
| Forsythoside A | L. citriodora | [89] | |
| Forsythoside B | L. alba | [87] | |
| Isonuomioside | L. alba | [87] | |
| Isoverbascoside | L. citriodora/graveolens/javanica | [79] | |
| Lariciresino glucopyranoside | L. citriodora | [77] | |
| Leucoseptoside | L. citriodora | [90] | |
| Lippianoside B | Lippia tryiphylla | [91] | |
| Martynoside | L. citriodora | [90] | |
| Osmanthisude B | L. citriodora | [77] | |
| Verbascoside | L. citriodora/graveolens/javanica | [79,88] | |
| Verbascoside A | L. citriodora | [77] | |
| Verbascoside | L. citriodora | [89] | |
| Oxipilins | Tuberonic acid glucoside | L. citriodora | [89] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leyva-Jiménez, F.J.; Lozano-Sánchez, J.; Cádiz-Gurrea, M.d.l.L.; Arráez-Román, D.; Segura-Carretero, A. Functional Ingredients based on Nutritional Phenolics. A Case Study against Inflammation: Lippia Genus. Nutrients 2019, 11, 1646. https://doi.org/10.3390/nu11071646
Leyva-Jiménez FJ, Lozano-Sánchez J, Cádiz-Gurrea MdlL, Arráez-Román D, Segura-Carretero A. Functional Ingredients based on Nutritional Phenolics. A Case Study against Inflammation: Lippia Genus. Nutrients. 2019; 11(7):1646. https://doi.org/10.3390/nu11071646
Chicago/Turabian StyleLeyva-Jiménez, Francisco Javier, Jesús Lozano-Sánchez, María de la Luz Cádiz-Gurrea, David Arráez-Román, and Antonio Segura-Carretero. 2019. "Functional Ingredients based on Nutritional Phenolics. A Case Study against Inflammation: Lippia Genus" Nutrients 11, no. 7: 1646. https://doi.org/10.3390/nu11071646
APA StyleLeyva-Jiménez, F. J., Lozano-Sánchez, J., Cádiz-Gurrea, M. d. l. L., Arráez-Román, D., & Segura-Carretero, A. (2019). Functional Ingredients based on Nutritional Phenolics. A Case Study against Inflammation: Lippia Genus. Nutrients, 11(7), 1646. https://doi.org/10.3390/nu11071646









