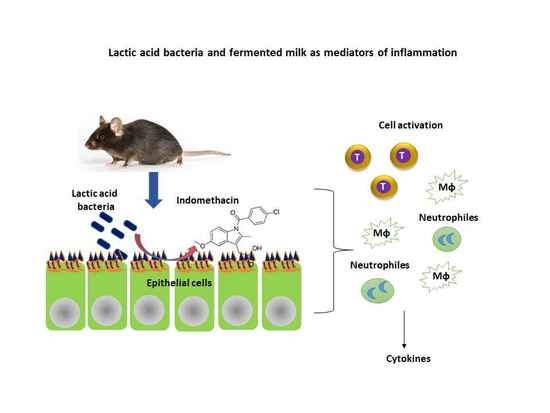
Milk Fermented with Lactobacillus fermentum Ameliorates Indomethacin-Induced Intestinal Inflammation: An Exploratory Study
Abstract
1. Introduction
2. Methods
2.1. Preparation of Fermented Milk
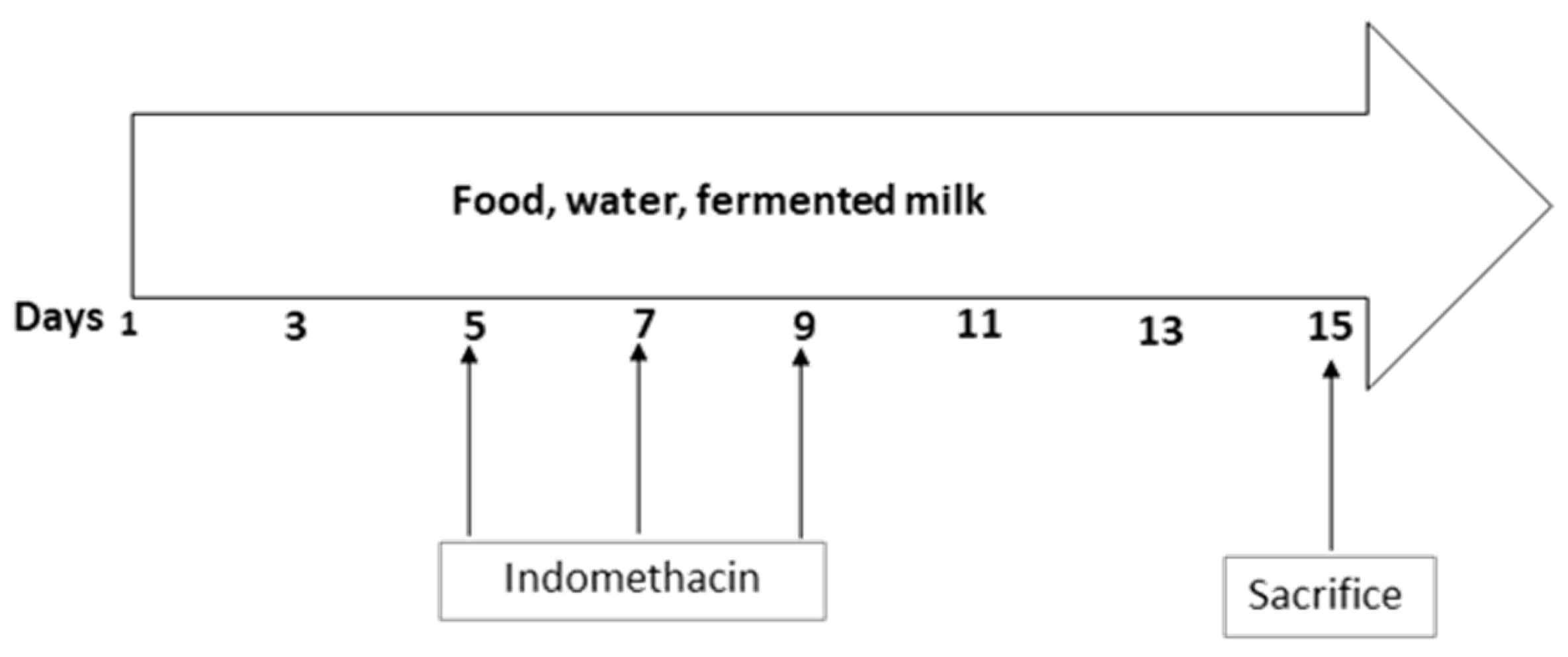
2.2. Animal Study and Induction of Inflammation
2.3. Biological Samples
2.4. Cytokines
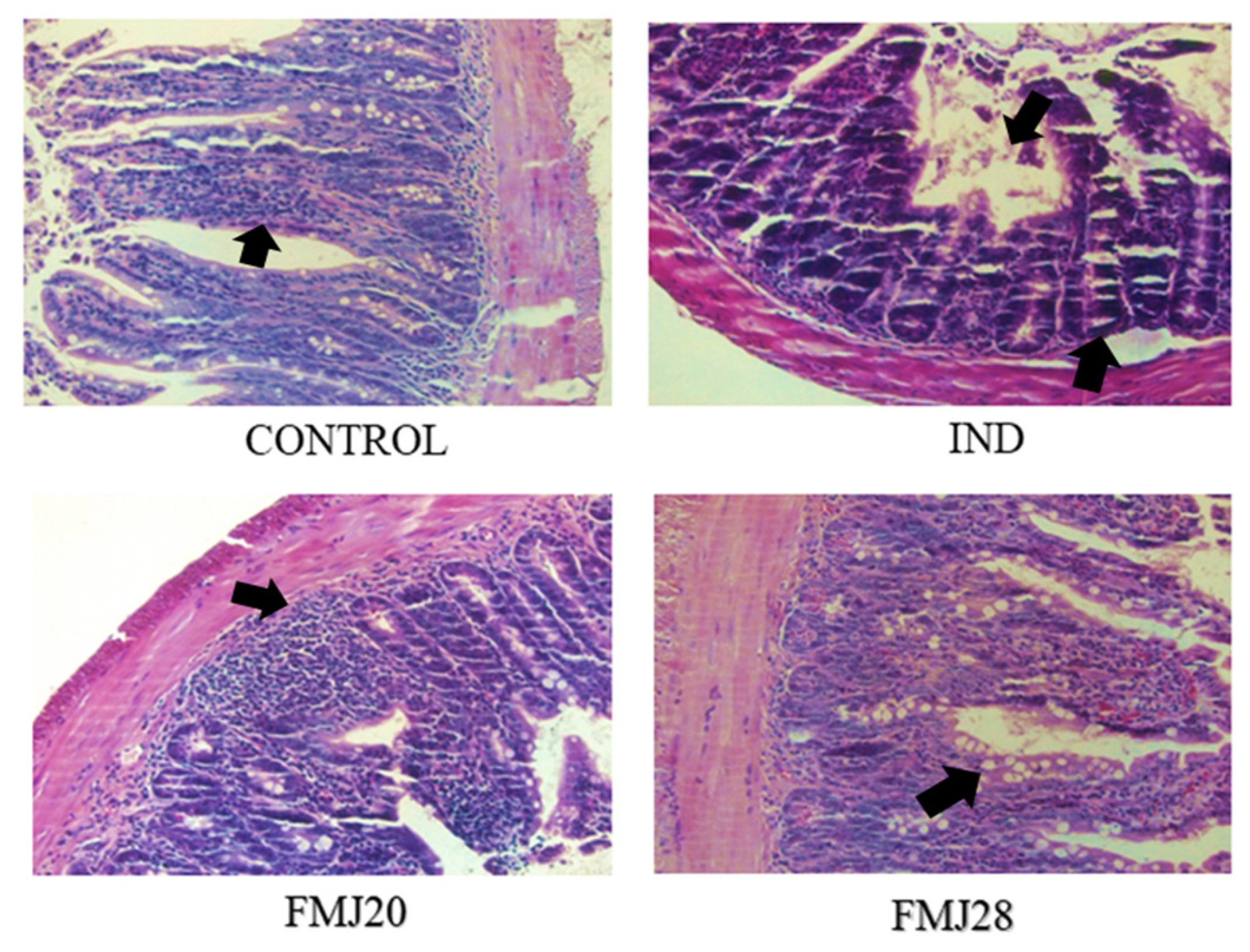
2.5. Histological Analysis
2.6. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matsui, H.; Shimokawa, O.; Kaneko, T.; Nagano, Y.; Rai, K.; Hyodo, I. The pathophysiology of non-steroidal anti-inflammatory drug (NSAID)-induced mucosal injuries in stomach and small intestine. J. Clin. Biochem. Nutr. 2011, 48, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Fujimori, S.; Seo, T.; Gudis, K.; Tanaka, S.; Mitsu, K.; Kobayashi, T.; Ehara, A.; Yonezawa, M.; Tatsuguchi, A.; Sakamoto, C. Diagnosis and treatment of obscure gastrointestinal bleeding using combined capsule endoscopy and double balloon endoscopy: 1-year follow-up study. Endoscopy 2007, 39, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Sekine, Y.; Sato, Y.; Higashizawa, T.; Miyata, T.; Lino, S.; Ido, K.; Sugano, K. Total enteroscopy with a nonsurgical steerable double-balloon method. Gastrointest. Endosc. 2001, 53, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Maiden, L.; Thjodleifsson, B.; Seigal, A.; Bjarnason, I.I.; Scott, D.; Birgisson, S.; Bjarnason, I. Long-term effects of nonsteroidal anti-inflammatory drugs and cyclooxygenase-2 selective agents on the small bowel: A cross-sectional capsule enteroscopy study. Clin. Gastroenterol. Hepatol. 2007, 5, 1040–1045. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Nishio, H.; Tanigawa, T.; Yamagami, H.; Okazaki, H.; Watanabe, K.; Tominaga, K.; Fujiwara, Y.; Oshitani, N.; Asahara, T.; et al. Probiotic Lactobacillus casei strain Shirota prevents indomethacin-induced small intestinal injury: Involvement of lactic acid. Am. J. Gastrointest. Liver Physiol. 2009, 297, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Naito, Y.; Takagi, T.; Yamada, S.; Horie, R.; Inue, K.; Harusato, A.; Hirata, I.; Omatsu, T.; Mizushima, K.; et al. Role of tumor necrosis factor-α in the pathogenesis of indomethacin-induced small intestinal injury in mice. Int. J. Mol. Med. 2011, 27, 353–359. [Google Scholar]
- Komiyama, Y.; Nakae, S.; Matsuki, T.; Nambu, A.; Ishigame, H.; Kakuta, S.; Sudo, K.; Iwakura, Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2006, 177, 566–573. [Google Scholar] [CrossRef]
- Naito, Y.; Takagi, T.; Handa, O.; Ishikaea, T.; Nakagawa, S.; Yamaguchi, T.; Yoshida, N.; Minami, M.; Kita, M. Enhanced intestinal inflammation induced by dextran sulfate sodium in tumor necrosis factor-alpha deficient mice. J. Gastroenterol. Hepatol. 2003, 18, 560–569. [Google Scholar] [CrossRef]
- Nandi, J.; Saud, B.; Zinkievich, J.M.; Yang, Z.J.; Levine, R.A. TNF-alpha modulates iNOS expression in an experimental rat model of indomethacin-induced jejunoileitis. Mol. Cell Biochem. 2010, 336, 17–24. [Google Scholar] [CrossRef]
- Ito, R.; Kita, M.; Shin-Ya, M.; Kishida, T.; Urano, A.; Takada, R.; Sakagami, J.; Imanishi, J.; Iwakura, Y.; Okanoue, T.; et al. Involvement of IL-17A in the pathogenesis of DSS-induced colitis in mice. Biochem. Biophys. Res. Commun. 2008, 377, 12–16. [Google Scholar] [CrossRef]
- Yamada, S.; Yuji, N.; Tomohisa, T.; Katsura, M.; Yasuko, H.; Ryusuke, H. Reduced small-intestinal injury induced by indomethacin in interleukin-17A-deficient mice. J. Gastroenterol. Hepatol. 2011, 26, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.W.; Kim, H.M.; Yang, K.M.; Kim, S.A.; Kim, S.K.; An, M.J.; Park, J.J.; Lee, S.K.; Kim, T.I.; Kim, W.H.; et al. Bifidobacterium lactis inhibits NF-kappaB in intestinal epithelial cells and prevents acute colitis and colitis-associated colon cancer in mice. Inflamm. Bowel Dis. 2010, 16, 1514–1525. [Google Scholar] [CrossRef] [PubMed]
- Li, S.-H.; Hsu, W.-F.; Chang, J.-S.; Shih, C.-K. Combination of Lactobacillus acidophilus and Bifidobacterium animals subsp lactis shows a stronger anti-inflammatory effect than individual strains in HT-29 cells. Nutrients 2019, 11, 969. [Google Scholar] [CrossRef] [PubMed]
- Kamil, R.; Geier, M.S.; Butler, R.N.; Howarth, G.S. Lactobacillus rhamnosus GG exacerbates intestinal ulceration in a model of indomethacin-induced enteropathy. Dig. Dis. Sci. 2007, 52, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Gotteland, M.; Cruchet, S.; Verbeke, S. Effect of Lactobacillus ingestion on the gastrointestinal mucosal barrier alterations induced by indomethacin in humans. Aliment. Pharmacol. Ther. 2001, 15, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Montalto, M.; Gallo, A.; Curigliano, V.; D’Onofrio, F.; Santoro, L.; Covino, M.; Dalvai, S.; Gasbarrini, A.; Gasbarrini, G. Clinical trial: The effects of a probiotic mixture on non-steroidal anti-inflammatory drug enteropathy: A randomized, double-blind, cross-over, placebo-controlled study. Aliment. Pharmacol. Ther. 2010, 32, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Kume, H.; Okazaki, K.; Takahashi, T.; Yamaji, T. Protective effect of an immune-modulating diet comprising whey peptides and fermented milk products on indomethacin-induced small-bowel disorders in rats. Clin. Nutr. 2014, 33, 1140–1146. [Google Scholar] [CrossRef] [PubMed]
- Santiago-López, L.; Hernández-Mendoza, A.; Mata-Haro, V.; Vallejo-Cordoba, B.; González-Córdova, A.F. Immune response induced by fermented milk with potential probiotic strains isolated from artisanal Cocido cheese. Food Agric. Immunol. 2018, 1, 911–929. [Google Scholar] [CrossRef]
- Sosa-Castañeda, J.; Hernández-Mendoza, A.; Astizarán-Garcia, H.; Garcia, H.S.; Estrada-Montoya, M.C.; González-Córdova, A.F.; Vallejo-Cordoba, B. Screening of Lactobacillus strains for their ability to produce conjugated linoleic acid in milk and to adhere to the intestinal tract. J. Dairy Sci. 2015, 98, 6651–6659. [Google Scholar]
- Reyes-Díaz, A.; Mata-Haro, V.; Hernández, J.; González-Córdova, A.F.; Hernández-Mendoza, A.; Reyes-Díaz, R.; Torres-Llanez, M.J.; Beltrán-Barrientos, L.M.; Vallejo-Cordoba, B. Milk fermented by specific Lactobacillus strains regulates the serum levels of IL-6, TNFα, and IL-10 cytokines in a LPS-stimulated murine model. Nutrients 2018, 10, 691. [Google Scholar] [CrossRef]
- Santiago-López, L.; Hernández-Mendoza, A.; Mata-Haro, V.; Vallejo-Córdoba, B.; Wall-Medrano, A.; Astizarán-Garcia, H.; Estrada-Montoya, MC.; González-Córdova, A.F. Effect of milk fermented with Lactobacillus fermentum on the inflammatory response in mice. Nutrients 2018, 10, 1039. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Gaithersburg, MA, USA, 2006. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 21st ed.; Latimer, G.W., Jr., Ed.; Association of Official Analytical Chemists: Gaithersburg, MA, USA, 2019. [Google Scholar]
- Nanda-Kumar, N.S.; Balamurugan, R.; Jayakanthan, K.; Pulimood, A.; Pugazhendhi, S.; Ramakrishna, B.S. Probiotic administration alters the gut flora and attenuates colitis in mice administered dextran sodium sulfate. J. Gastroenterol. Hepatol. 2008, 23, 1834–1839. [Google Scholar] [CrossRef] [PubMed]
- Neurath, M.F. IL-23 in inflammatory bowel diseases and colon cancer. Cytokine Growth Factor Rev. 2019, 45, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Dennehy, K.M.; Willment, J.A.; Williams, D.L.; Brown, G.D. Reciprocal regulation of IL-23 and IL-12 following co-activation of dectin-1 and TLR signaling pathways. Eur. J. Immunol. 2009, 39, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Cao, A.; Yao, S.; Evans-Marin, H.L.; Liu, H.; Wu, W.; Carlsen, E.D.; Dann, S.M.; Soong, L.; Sun, J.; et al. mTOR mediates IL-23 induction of neutrophil IL-17 and IL-22 production. J. Immunol. 2016, 196, 4390–4399. [Google Scholar] [CrossRef]
- Bamias, G.; Arseneau, K.A.; Cominelli, F. Cytokines and mucosal immunity. Curr. Opin. Gastroenterol. 2015, 30, 547–552. [Google Scholar] [CrossRef] [PubMed]
- Müzes, G.; Molnár, B.; Tulassay, Z.; Sipos, F. Changes of the cytokine profile in inflammatory bowel diseases. World J. Gastroenterol. 2012, 18, 5848–5861. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. Inducible Foxp3+ regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl. Acad. Sci. USA 2010, 107, 12204–12209. [Google Scholar] [CrossRef]
- Jeon, S.G.; Kayama, H.; Ueda, Y.; Takahashi, T.; Asahara, T.; Tsuji, H.; Tsuji, N.M.; Kiyono, H.; Ma, J.S.; Kusu, T.; et al. Probiotic Bifidobacterium breve induces IL-10-producing Tr1 cells in the colon. PLoS Phatog. 2012, 8, e1002714. [Google Scholar] [CrossRef]
- Harusato, A.; Naito, Y.; Takagi, T.; Yamada, S.; Mizushima, K.; Hirai, Y.; Horie, R.; Inoue, K.; Fukumoto, K.; Hirata, I.; et al. Inhibition of bach1 ameliorates indomethacin-induced intestinal injury in mice. J. Physiol. Pharmacol. 2009, 60, 149–154. [Google Scholar]
- Ricciotti, E.; FitzGerald, G. Prostaglandins and Inflammation. Arterioscler. Thromb. Vasc. Biol. 2012, 31, 986–1000. [Google Scholar] [CrossRef] [PubMed]
- Robert, A.; Asano, T. Resistance of germ-free rats to indomethacin-induced intestinal inflammation. Prostaglandins. 1977, 14, 333–341. [Google Scholar] [CrossRef]
- Watanabe, T.; Higuchi, K.; Kobata, A.; Nishio, H.; Tanigawa, T.; Shiba, M.; Tominaga, K.; Fujiwara, Y.; Oshitani, N.; Asahara, T.; et al. Non-steroidal anti-inflammatory drug-induced small intestinal damage is toll-like receptor 4 dependent. Gut 2008, 57, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Omatsu, T.; Naito, Y.; Handa, O.; Hayashi, N.; Mizushima, K.; Qin, Y.; Hirata, I.; Adachi, S.; Okayama, T.; Kishimoto, E.; et al. Involvement of reactive oxygen species in indomethacin-induced apoptosis of small intestinal epithelial cells. J. Gastroenterol. 2009, 19, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Corridoni, D.; Pastorelli, L.; Mattioli, B.; Lcovei, S.; Ishikawa, D.; Arseneau, K.O.; Chieppa, M.; Cominelli, F.; Pizarro, T.T. Probiotic bacteria regulate intestinal epithelial permeability in experimental ileitis by a TNF-dependent mechanism. PLoS ONE 2012, 7, e42067. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Hara, T.; Hori, T.; Mitsuyama, K.; Nagaoka, M.; Tomiyasu, N.; Suzuki, A.; Sata, M. Probiotic Lactobacillus-induced improvement in murine chronic inflammatory bowel disease is associated with the down-regulation of pro-inflammatory cytokines in lamina propria mononuclear cells. J. Clin. Exp. Immunol. 2005, 140, 417–426. [Google Scholar] [CrossRef] [PubMed]
- Bjarnason, I. The use of fecal calprotectin in inflammatory bowel disease. Gastroenterol. Hepatol. 2017, 13, 53–56. [Google Scholar]


| Group | Weight | Weight (mg) | Length (cm) | ||
|---|---|---|---|---|---|
| Loss (g) | Spleen | Kidney | Liver | Intestine | |
| Control | −3.7 ± 3.4 a | 140 ± 97.6 a | 453 ± 92.2 a | 1580 ± 313.8 a | 50.5 ± 4.5 a |
| IND | −5.8 ± 3.7 b | 288 ± 74.1 a | 500 ± 352.4 a | 1764 ± 1045.1 a | 45.9 ± 8.0 a |
| FMJ20 | −6.3 ± 2.8 b,c | 294 ± 74.0 a | 652 ± 99.9 a | 2318 ± 363.0 a | 45.0 ± 4.6 a |
| FMJ28 | −6.9 ± 3.6 b,c | 116± 131.8 a | 545 ± 366.7 a | 1274 ± 1146.0 a | 43.6 ± 2.0 a |
| Group | Food Consumption (g) | ||||||
|---|---|---|---|---|---|---|---|
| Days | |||||||
| 1 | 3 | 5 | 7 | 9 | 12 | 14 | |
| Control | 7.24 ± 2.23 a | 8.08 ±1.25 a | 6.28 ± 2.15 a | 7.03 ±1.75 a | 6.15 ± 2.11 a | 6.17 ± 1.45 a | 6.23 ± 1.89 a |
| IND | 8.24 ± 1.34 a | 7.08 ± 1.25 a | 7.28 ± 2.45 a | 6.03 ± 2.34 b | 5.15 ± 1.67 b | 5.17 ± 1.56 b | 5.23 ± 1.23 b |
| FMJ20 | 6.95 ± 1.45 a | 7.15 ± 1.78 a | 7.38 ± 1.23 a | 5.92 ± 1.67 c | 6.02 ± 1.98 c | 7.18 ± 1.27 a | 6.01 ± 1.38 a |
| FMJ28 | 8.13 ± 1.98 a | 8.71 ± 1.56 a | 6.47 ± 1.49 a | 6.97 ± 1.78 b | 6.08 ± 1.23 c | 6.86 ± 1.45 a | 7.23 ± 2.22 a |
| Cytokines | Concentration (pg/mL) | |||
|---|---|---|---|---|
| Control | IND | FMJ20 | FMJ28 | |
| IL-2 | 6.78 a | 4.33 a | 4.76 a | 5.18 a |
| IL-4 | 3.60 a | 5.83 a | 5.67 a | 5.49 a |
| IL-10 | ND | ND | ND | ND |
| TNFα | 6.33 a | 3.47 a | 3.38 a | 4.37 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santiago-López, L.; Hernández-Mendoza, A.; Vallejo-Cordoba, B.; Mata-Haro, V.; Wall-Medrano, A.; González-Córdova, A.F. Milk Fermented with Lactobacillus fermentum Ameliorates Indomethacin-Induced Intestinal Inflammation: An Exploratory Study. Nutrients 2019, 11, 1610. https://doi.org/10.3390/nu11071610
Santiago-López L, Hernández-Mendoza A, Vallejo-Cordoba B, Mata-Haro V, Wall-Medrano A, González-Córdova AF. Milk Fermented with Lactobacillus fermentum Ameliorates Indomethacin-Induced Intestinal Inflammation: An Exploratory Study. Nutrients. 2019; 11(7):1610. https://doi.org/10.3390/nu11071610
Chicago/Turabian StyleSantiago-López, Lourdes, Adrián Hernández-Mendoza, Belinda Vallejo-Cordoba, Verónica Mata-Haro, Abraham Wall-Medrano, and Aarón F. González-Córdova. 2019. "Milk Fermented with Lactobacillus fermentum Ameliorates Indomethacin-Induced Intestinal Inflammation: An Exploratory Study" Nutrients 11, no. 7: 1610. https://doi.org/10.3390/nu11071610
APA StyleSantiago-López, L., Hernández-Mendoza, A., Vallejo-Cordoba, B., Mata-Haro, V., Wall-Medrano, A., & González-Córdova, A. F. (2019). Milk Fermented with Lactobacillus fermentum Ameliorates Indomethacin-Induced Intestinal Inflammation: An Exploratory Study. Nutrients, 11(7), 1610. https://doi.org/10.3390/nu11071610