No Effect of Tart Cherry Juice or Pomegranate Juice on Recovery from Exercise-Induced Muscle Damage in Non-Resistance Trained Men
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
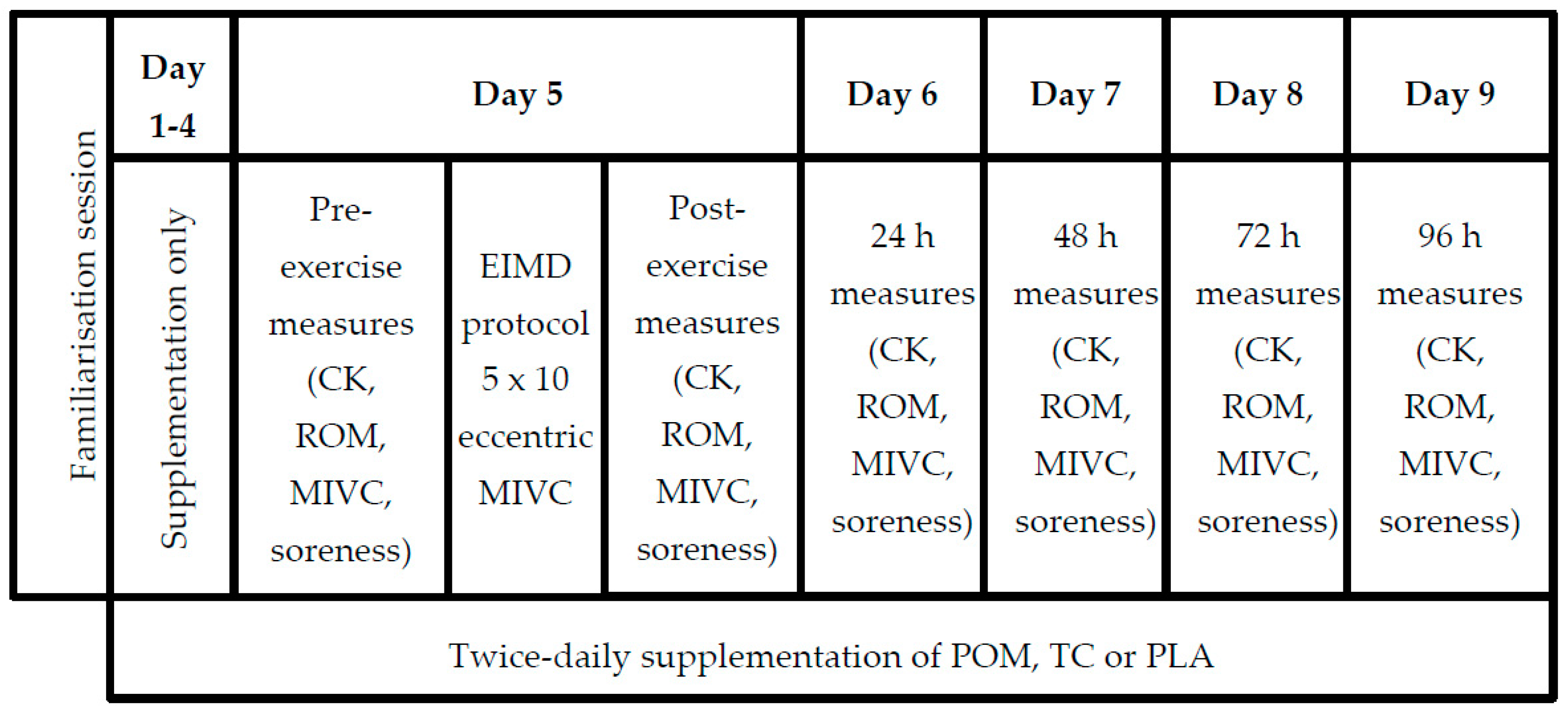
2.2. Study Design
2.3. Fruit Juices, Placebo Beverage and Post-Exercise Meal
2.4. Anthropometric Measures
2.5. Measurement of Maximal Isometric Voluntary Contractions (MIVC)
2.6. Eccentric (EIMD) Protocol
2.7. Measurement of Creatine Kinase
2.8. Measurement of Range of Motion and Elbow Flexion Soreness
2.9. Statistical Analysis
3. Results
3.1. Analysis of POM and TC
3.2. Participants
3.3. Compliance with Protocol
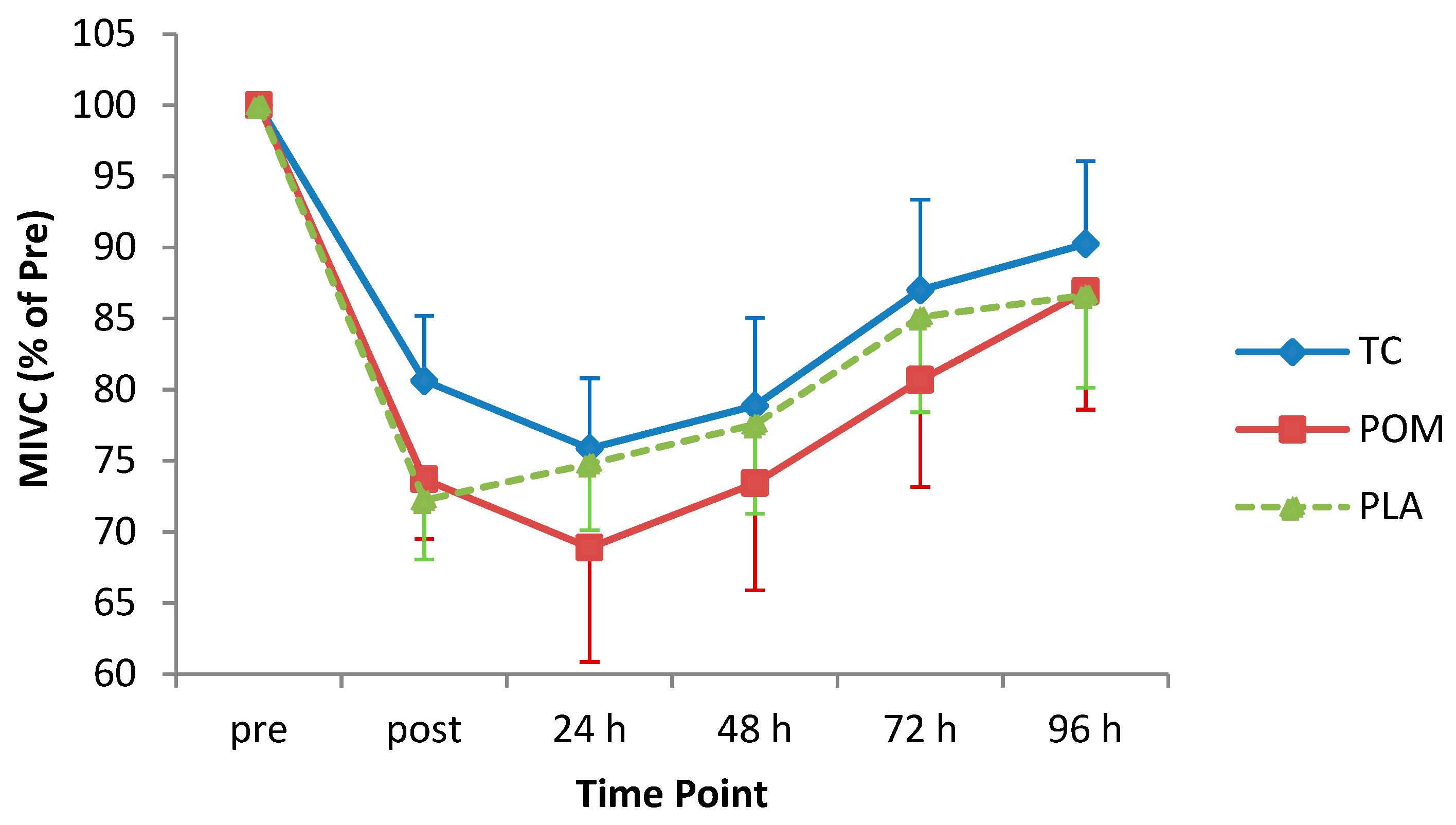
3.4. MIVC
3.5. Elbow Flexor Soreness
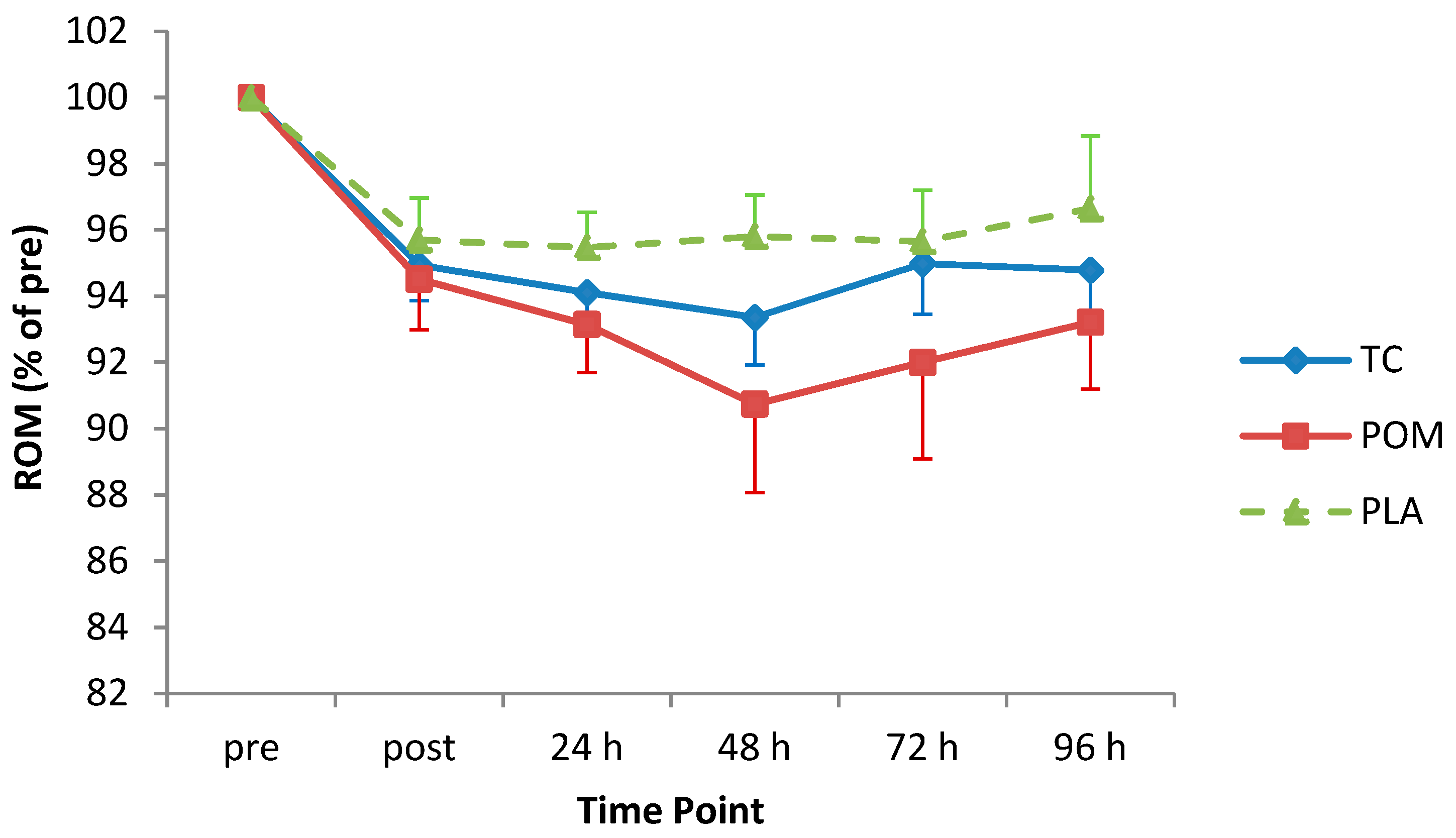
3.6. ROM
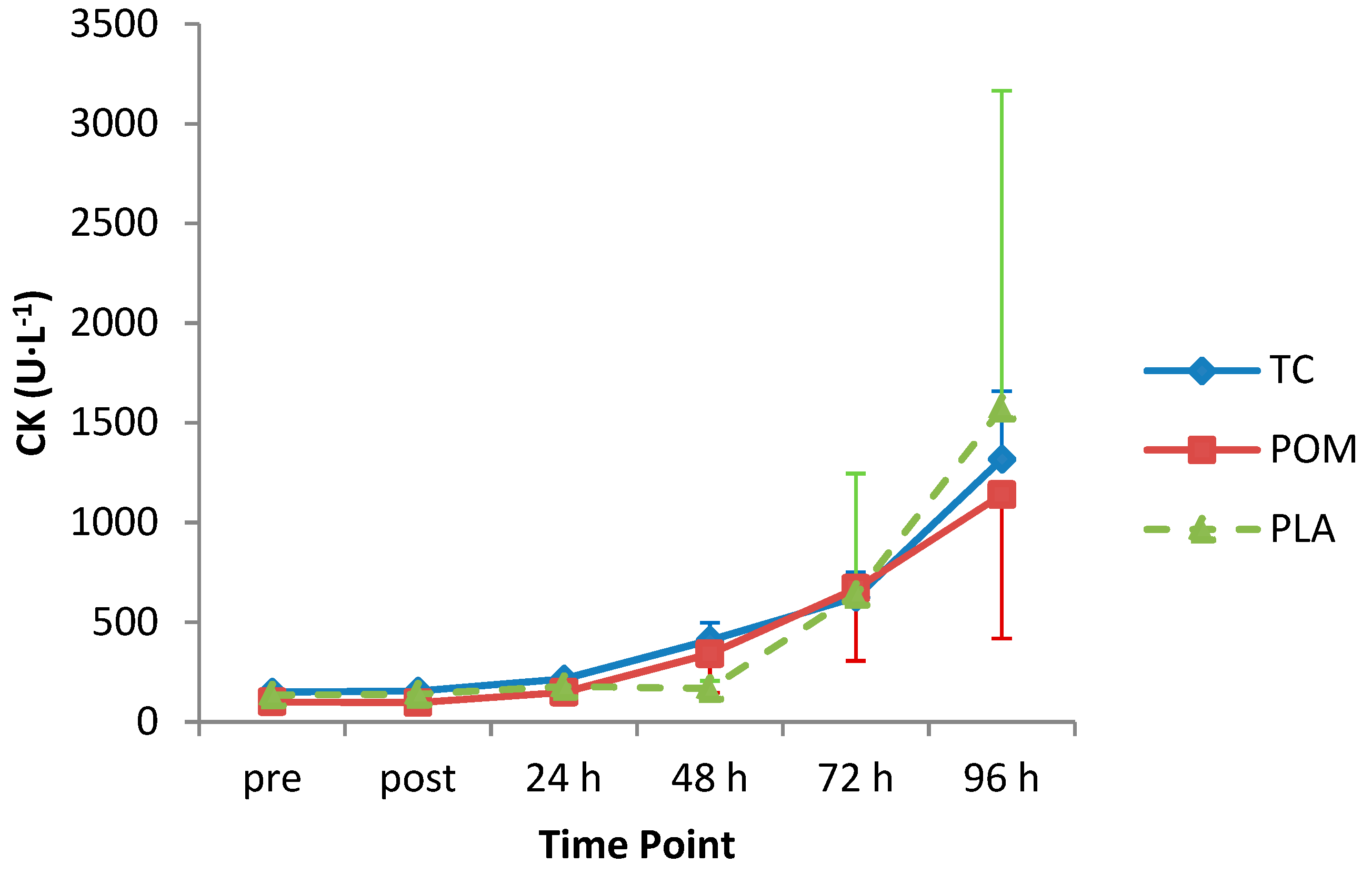
3.7. Creatine Kinase Concentration
3.8. Exploratory Analysis with Hyper-Responders Removed
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bowtell, J.; Kelly, V. Fruit-Derived polyphenol supplementation for athlete recovery and performance. Sports Med. 2019, 49, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Howatson, G.; van Someren, K.A. The prevention and treatment of exercise-induced muscle damage. Sports Med. 2008, 38, 483–503. [Google Scholar] [CrossRef] [PubMed]
- Owens, D.J.; Twist, C.; Cobley, J.N.; Howatson, G.; Close, G.L. Exercise-induced muscle damage: What is it, what causes it and what are the nutritional solutions? Eur. J. Sport Sci. 2019, 19, 71–85. [Google Scholar] [CrossRef] [PubMed]
- Proske, U.; Morgan, D.L. Muscle damage from eccentric exercise: Mechanism, mechanical signs, adaptation and clinical applications. J. Physiol. 2001, 537, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Hyldahl, R.D.; Hubal, M.J. Lengthening our perspective: Morphological, cellular, and molecular responses to eccentric exercise. Muscle Nerve 2014, 49, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Gissel, H.; Clausen, T. Excitation-induced Ca2+ influx and skeletal muscle cell damage. Acta Physiol. Scand. 2001, 171, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, T.A.; Best, T.M.; Merrick, M.A. The dual roles of neutrophils and macrophages in inflammation: A critical balance between tissue damage and repair. J. Athl. Train. 2006, 41, 457–465. [Google Scholar] [PubMed]
- Nguyen, H.X.; Tidball, J.G. Interactions between neutrophils and macrophages promote macrophage killing of rat muscle cells in vitro. J. Physiol. 2003, 547, 125–132. [Google Scholar] [CrossRef]
- Bazzucchi, I.; Patrizio, F.; Ceci, R.; Duranti, G.; Sgrò, P.; Sabatini, S.; Di Luigi, L.; Sacchetti, M.; Felici, F. The effects of quercetin supplementation on eccentric exercise-induced muscle damage. Nutrients 2019, 11, 205. [Google Scholar] [CrossRef]
- Clifford, T.; Bell, O.; West, D.J.; Howatson, G.; Stevenson, E.J.; Clifford, T. The effects of beetroot juice supplementation on indices of muscle damage following eccentric exercise. Eur. J. Appl. Physiol. 2016, 116, 353–362. [Google Scholar] [CrossRef]
- Lynn, A.; Garner, S.; Nelson, N.; Simper, T.N.; Hall, A.C.; Ranchordas, M.K. Effect of bilberry juice on indices of muscle damage and inflammation in runners completing a half-marathon: A randomised, placebo-controlled trial. J. Int. Soc. Sports Nutr. 2018, 15, 22. [Google Scholar] [CrossRef] [PubMed]
- McLeay, Y.; Barnes, M.J.; Mundel, T.; Hurst, S.M.; Hurst, R.D.; Stannard, S.R. Effect of New Zealand blueberry consumption on recovery from eccentric exercise-induced muscle damage. J. Int. Soc. Sports Nutr. 2012, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Peschek, K.; Pritchett, R.; Bergman, E.; Pritchett, K. The effects of acute post exercise consumption of two cocoa-based beverages with varying flavanol content on indices of muscle recovery following downhill treadmill running. Nutrients 2013, 6, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, W.; Machado, Á.S.; Souza, M.A.; Mello-Carpes, P.B.; Carpes, F.P. Effect of green tea extract supplementation on exercise-induced delayed onset muscle soreness and muscular damage. Physiol. Behav. 2018, 194, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Trombold, J.R.; Barnes, J.N.; Critchley, L.; Coyle, E.F. Ellagitannin consumption improves strength recovery 2-3 d after eccentric exercise. Med. Sci. Sports Exerc. 2010, 42, 493–498. [Google Scholar] [CrossRef]
- Connolly, D.A.J.; McHugh, M.P.; Padilla-Zakour, O.I.; Carlson, L.; Sayers, S.P. Efficacy of a tart cherry juice blend in preventing the symptoms of muscle damage. Br. J. Sports Med. 2006, 40, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.P.; Aviram, M.; Zhang, Y.; Henning, S.M.; Feng, L.; Dreher, M.; Heber, D. Comparison of antioxidant potency of commonly consumed polyphenol-rich beverages in the United States. J. Agric. Food Chem. 2008, 56, 1415–1422. [Google Scholar] [CrossRef]
- Bassiri-Jahromi, S.; Doostkam, A. Comparative evaluation of bioactive compounds of various cultivars of pomegranate (Punica granatum) in different world regions. AIMS Agric. Food 2018, 4, 41–55. [Google Scholar] [CrossRef]
- Vallejo, F.; García-Viguera, C.; Tomás-Barberán, F.A. Changes in broccoli (Brassica oleracea L. Var. italica) health-promoting compounds with inflorescence development. J. Agric. Food Chem. 2003, 51, 3776–3782. [Google Scholar] [CrossRef]
- Howatson, G.; McHugh, M.P.; Hill, J.A.; Brouner, J.; Jewell, A.P.; Van Someren, K.A.; Shave, R.E.; Howatson, S.A. Influence of tart cherry juice on indices of recovery following marathon running. Scand. J. Med. Sci. Sport 2009, 20, 843–852. [Google Scholar] [CrossRef]
- Bowtell, J.L.; Sumners, D.P.; Dyer, A.; Fox, P.; Mileva, K.N. Montmorency cherry juice reduces muscle damage caused by intensive strength exercise. Med. Sci. Sports Exerc. 2011, 43, 1544–1551. [Google Scholar] [CrossRef] [PubMed]
- Bell, P.G.; Walshe, I.H.; Davison, G.W.; Stevenson, E.J.; Howatson, G. Recovery facilitation with Montmorency cherries following high-intensity, metabolically challenging exercise. Appl. Physiol. Nutr. Metab. 2014, 40, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Bell, P.G.; Stevenson, E.; Davison, G.W.; Howatson, G. The effects of montmorency tart cherry concentrate supplementation on recovery following prolonged, intermittent exercise. Nutrients 2016, 8, 441. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.A.; Stevenson, E.J.; Howatson, G. Montmorency tart cherry (Prunus cerasus L.) supplementation accelerates recovery from exercise-induced muscle damage in females. Eur. J. Sport Sci. 2019, 19, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Bell, P.G.; Walshe, I.H.; Davison, G.W.; Stevenson, E.; Howatson, G. Montmorency cherries reduce the oxidative stress and inflammatory responses to repeated days high-intensity stochastic cycling. Nutrients 2014, 6, 829–843. [Google Scholar] [CrossRef] [PubMed]
- Levers, K.; Dalton, R.; Galvan, E.; O’Connor, A.; Goodenough, C.; Simbo, S.; Mertens-Talcott, S.U.; Rasmussen, C.; Greenwood, M.; Riechman, S.; et al. Effects of powdered Montmorency tart cherry supplementation on acute endurance exercise performance in aerobically trained individuals. J. Int. Soc. Sports Nutr. 2016, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Levers, K.; Dalton, R.; Galvan, E.; Goodenough, C.; O ’connor, A.; Simbo, S.; Barringer, N.; Mertens-Talcott, S.U.; Rasmussen, C.; Greenwood, M.; et al. Effects of powdered Montmorency tart cherry supplementation on an acute bout of intense lower body strength exercise in resistance trained males. J. Int. Soc. Sports Nutr. 2015, 12, 41. [Google Scholar] [CrossRef] [PubMed]
- McCormick, R.; Peeling, P.; Binnie, M.; Dawson, B.; Sim, M. Effect of tart cherry juice on recovery and next day performance in well-trained water polo players. J. Int. Soc. Sports Nutr. 2016, 13, 4–11. [Google Scholar] [CrossRef]
- Beals, K.; Allison, K.F.; Darnell, M.; Lovalekar, M.; Baker, R.; Nieman, D.C.; Vodovotz, Y.; Lephart, S.M. The effects of a tart cherry beverage on reducing exercise-induced muscle soreness. Isokinet. Exerc. Sci. 2017, 25, 53–63. [Google Scholar] [CrossRef]
- Trombold, J.R.; Reinfeld, A.S.; Casler, J.R.; Coyle, E.F. The effect of pomegranate juice supplementation on strength and soreness after eccentric exercise. J. Strength Cond. Res. 2011, 25, 1782–1788. [Google Scholar] [CrossRef]
- Machin, D.; Christmas, K.; Chou, T.; Hill, S.; Van Pelt, D.; Trombold, J.; Coyle, E. Effects of differing dosages of pomegranate juice supplementation after eccentric exercise. Physiol. J. 2014, 2014, 1–7. [Google Scholar] [CrossRef]
- Ammar, A.; Turki, M.; Chtourou, H.; Hammouda, O.; Trabelsi, K.; Kallel, C.; Abdelkarim, O.; Hoekelmann, A.; Bouaziz, M.; Ayadi, F.; et al. Pomegranate supplementation accelerates recovery of muscle damage and soreness and inflammatory markers after a weightlifting training session. PLoS ONE 2016, 11, 0160305. [Google Scholar] [CrossRef] [PubMed]
- Kirakosyan, A.; Seymour, E.M.; Kaufman, P.B.; Bolling, S.F. Tart cherry fruits: Implications for human health. In Bioactive Food as Dietary Interventions for Arthritis and Related Inflammatory Diseases: Bioactive Food in Chronic; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 473–484. [Google Scholar]
- Borges, G.; Crozier, A. HPLC–PDA–MS fingerprinting to assess the authenticity of pomegranate beverages. Food Chem. 2012, 135, 1863–1867. [Google Scholar] [CrossRef] [PubMed]
- Piccolella, S.; Fiorentino, A.; Pacifico, S.; D’Abrosca, B.; Uzzo, P.; Monaco, P. Antioxidant properties of sour cherries (Prunus cerasus L.): Role of colorless phytochemicals from the methanolic extract of ripe fruits. J. Agric. Food Chem. 2008, 56, 1928–1935. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.C. Variability in muscle damage after eccentric exercise and the repeated bout effect. Res. Q. Exerc. Sport 2013, 77, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar]
- Biodex Medical Systems Inc. Biodex System 3 Pro: Application/Operation Manual; Biodex Medical Systems Inc.: Shirley, NY, USA; Available online: https://www.biodex.com/sites/default/files/835000man_06159.pdf (accessed on 13 July 2019).
- Clarkson, P.M.; Nosaka, K.; Braun, B. Muscle function after exercise-induced muscle damage and rapid adaptation. Med. Sci. Sports Exerc. 1992, 24, 512–520. [Google Scholar] [CrossRef]
- Kuehl, K.; Perrier, E. Efficacy of Tart Cherry Juice in Reducing Muscle Pain During Running. J. Int. Soc. Sports Nutr. 2010, 7, 1–6. [Google Scholar] [CrossRef]
- Rawson, E.S.; Gunn, B.; Clarkson, P.M. The Effects of Creatine Supplementation on Exercise-Induced Muscle Damage. J. Strength Cond. Res. 2001, 15, 178–184. [Google Scholar]
- O’Fallon, K.S.; Kaushik, D.; Michniak-Kohn, B.; Dunne, C.P.; Zambraski, E.J.; Clarkson, P.M. Effects of quercetin supplementation on markers of muscle damage and inflammation after eccentric exercise. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.J.; Pereira, R.; Machado, M. The creatine kinase response to resistance exercise. J. Musculoskelet. Neuronal Interact. 2014, 14, 68–77. [Google Scholar] [PubMed]
- Warren, G.L.; Lowe, D.A.; Armstrong, R.B. Measurement tools used in the study of eccentric contraction–induced injury. Sports Med. 1999, 27, 43–59. [Google Scholar] [CrossRef] [PubMed]
- Cook, M.D.; Elisabeth, M.; Willems, T. Dietary Anthocyanin: A review of the exercise performance effects and related physiological responses. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 322–330. [Google Scholar] [CrossRef]
- Sanchez, V.; Baeza, R.; Chirife, J. Comparison of monomeric anthocyanins and colour stability of fresh, concentrate and freeze-dried encapsulated cherry juice stored at 38◦C. J. Berry Res. 2015, 5, 243–251. [Google Scholar] [CrossRef]
- Piljac-Žegarac, J.; Valek, L.; Martinez, S.; Belščak, A. Fluctuations in the phenolic content and antioxidant capacity of dark fruit juices in refrigerated storage. Food Chem. 2009, 113, 394–400. [Google Scholar] [CrossRef]
- Howatson, G.; van Someren, K.A. Evidence of a contralateral repeated bout effect after maximal eccentric contractions. Eur. J. Appl. Physiol. 2007, 101, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Hyldahl, R.D.; Chipkin, S.R.; Clarkson, P.M. A contralateral repeated bout effect attenuates induction of NF-κB DNA binding following eccentric exercise. J. Appl. Physiol. 2013, 116, 1473–1480. [Google Scholar] [CrossRef] [PubMed]
- Starbuck, C.; Eston, R.G.; Kraemer, W.J. Exercise-induced muscle damage and the repeated bout effect: Evidence for cross transfer. Eur. J. Appl. Physiol. 2012, 112, 1005–1013. [Google Scholar] [CrossRef]
- Powers, S.K.; Smuder, A.J.; Kavazis, A.N.; Hudson, M.B. Experimental guidelines for studies designed to investigate the impact of antioxidant supplementation on exercise performance. Int. J. Sport Nutr. Exerc. Metab. 2010, 20, 2–14. [Google Scholar] [CrossRef] [PubMed]

| Tart Cherry | Pomegranate | Placebo | |
|---|---|---|---|
| N | 12 | 12 | 12 |
| Age (years) | 24.0 (IQR 22.0, 33.0) | 24.0 (IQR 21.0, 32.5) | 24.0 (IQR 22.5, 32.0) |
| BMI (kg·m−2) | 26.2 (± 3.9) | 25.5 (± 3.9) | 25.2 (± 4.5) |
| LMM (kg) | 36.0 (± 5.2) | 35.0 (± 6.1) | 34.8 (± 6.2) |
| Baseline strength (N·cm−2) | 41.8 (IQR 34.3, 53.2) | 42.7 (IQR 33.6, 52.2) | 44.1 (IQR 37.2, 51.5) |
| Baseline CK level (U·L−1) | 148.9 (± 60.1) | 150.3 (± 44.6) | 130.9 (± 57.7) |
| Outcome Marker | Main Effect of Diet Group | Main Effect of Time | Diet × Time Interaction |
|---|---|---|---|
| MIVC | p = 0.78 | p < 0.001 | p = 0.97 |
| DOMS | p = 0.28 | p < 0.001 | p = 0.26 |
| ROM | p = 0.71 | p < 0.001 | p = 0.90 |
| CK | p = 0.87 | p = 0.01 | p = 0.35 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lamb, K.L.; Ranchordas, M.K.; Johnson, E.; Denning, J.; Downing, F.; Lynn, A. No Effect of Tart Cherry Juice or Pomegranate Juice on Recovery from Exercise-Induced Muscle Damage in Non-Resistance Trained Men. Nutrients 2019, 11, 1593. https://doi.org/10.3390/nu11071593
Lamb KL, Ranchordas MK, Johnson E, Denning J, Downing F, Lynn A. No Effect of Tart Cherry Juice or Pomegranate Juice on Recovery from Exercise-Induced Muscle Damage in Non-Resistance Trained Men. Nutrients. 2019; 11(7):1593. https://doi.org/10.3390/nu11071593
Chicago/Turabian StyleLamb, Kirstie L., Mayur K. Ranchordas, Elizabeth Johnson, Jessica Denning, Faye Downing, and Anthony Lynn. 2019. "No Effect of Tart Cherry Juice or Pomegranate Juice on Recovery from Exercise-Induced Muscle Damage in Non-Resistance Trained Men" Nutrients 11, no. 7: 1593. https://doi.org/10.3390/nu11071593
APA StyleLamb, K. L., Ranchordas, M. K., Johnson, E., Denning, J., Downing, F., & Lynn, A. (2019). No Effect of Tart Cherry Juice or Pomegranate Juice on Recovery from Exercise-Induced Muscle Damage in Non-Resistance Trained Men. Nutrients, 11(7), 1593. https://doi.org/10.3390/nu11071593




