Influence of Equimolar Doses of Beetroot Juice and Sodium Nitrate on Time Trial Performance in Handcycling
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Physical Activity and Dietary Standardization
2.4. Supplementation
2.5. Exercise Testing
2.5.1. Maximal Oxygen Uptake and Familiarization
2.5.2. Time Trial
2.6. Plasma Nitrate and Nitrite Measurements
2.7. Data Analysis and Statistics
3. Results
3.1. Blood Pressure and Heart Rate at Rest
3.2. Plasma Nitrate and Nitrite Concentrations Pre and Post Supplement Ingestion
3.3. Performance Parameters during Warm-up and TT
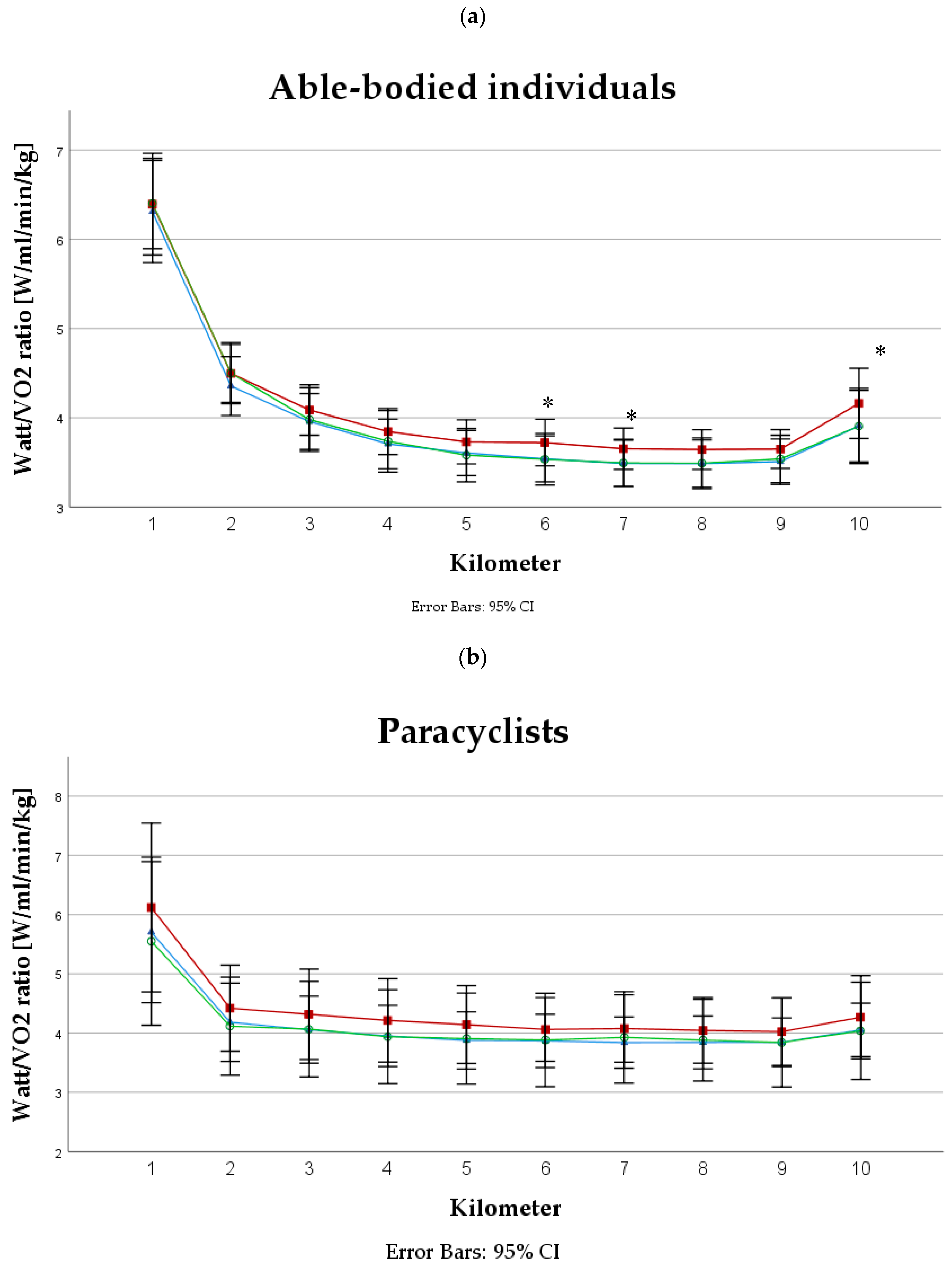
3.4. Oxygen Uptake and PO to VO2 Ratio
4. Discussion
4.1. Performance
4.2. Oxygen Consumption and PO to VO2 Ratio
4.3. Other Parameters
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahrendt, D.M. Ergogenic aids: Counseling the athlete. Am. Fam. Physician 2001, 63, 913–922. [Google Scholar] [PubMed]
- Santamaria, P. Nitrate in vegetables: Toxicity, content, intake and ec regulation. J. Sci. Food Agric. 2006, 86, 10–17. [Google Scholar] [CrossRef]
- Nyakayiru, J.; Kouw, I.W.K.; Cermak, N.M.; Senden, J.M.; van Loon, L.J.C.; Verdijk, L.B. Sodium nitrate ingestion increases skeletal muscle nitrate content in humans. J. Appl. Physiol. 2017, 123, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Weitzberg, E.; Gladwin, M.T. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nat. Rev. Drug Discov. 2008, 7, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed]
- Reid, M.B. Role of nitric oxide in skeletal muscle: Synthesis, distribution and functional importance. Acta Physiol. Scand. 1998, 162, 401–409. [Google Scholar] [CrossRef]
- Stamler, J.S.; Meissner, G. Physiology of nitric oxide in skeletal muscle. Physiol. Rev. 2001, 81, 209–237. [Google Scholar] [CrossRef]
- Moncada, S.; Palmer, R.M.; Higgs, E.A. Nitric oxide: Physiology, pathophysiology, and pharmacology. Pharmacol. Rev. 1991, 43, 109–142. [Google Scholar]
- Gilchrist, M.; Shore, A.C.; Benjamin, N. Inorganic nitrate and nitrite and control of blood pressure. Cardiovasc. Res. Cent. Bull. 2011, 89, 492–498. [Google Scholar] [CrossRef]
- Kapil, V.; Milsom, A.B.; Okorie, M.; Maleki-Toyserkani, S.; Akram, F.; Rehman, F.; Arghandawi, S.; Pearl, V.; Benjamin, N.; Loukogeorgakis, S.; et al. Inorganic nitrate supplementation lowers blood pressure in humans: Role for nitrite-derived no. Hypertension 2010, 56, 274–281. [Google Scholar] [CrossRef]
- Kapil, V.; Weitzberg, E.; Lundberg, J.O.; Ahluwalia, A. Clinical evidence demonstrating the utility of inorganic nitrate in cardiovascular health. Nitric Oxide 2014, 38, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Hoon, M.W.; Johnson, N.A.; Chapman, P.G.; Burke, L.M. The effect of nitrate supplementation on exercise performance in healthy individuals: A systematic review and meta-analysis. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M. Dietary nitrate supplementation and exercise performance. Sports Med. 2014, 44 (Suppl. 1), S35–S45. [Google Scholar] [CrossRef]
- McMahon, N.F.; Leveritt, M.D.; Pavey, T.G. The effect of dietary nitrate supplementation on endurance exercise performance in healthy adults: A systematic review and meta-analysis. Sports Med. 2017, 47, 735–756. [Google Scholar] [CrossRef] [PubMed]
- Cermak, N.M.; Gibala, M.J.; van Loon, L.J. Nitrate supplementation’s improvement of 10-km time-trial performance in trained cyclists. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Lansley, K.E.; Winyard, P.G.; Bailey, S.J.; Vanhatalo, A.; Wilkerson, D.P.; Blackwell, J.R.; Gilchrist, M.; Benjamin, N.; Jones, A.M. Acute dietary nitrate supplementation improves cycling time trial performance. Med. Sci. Sports Exerc. 2011, 43, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Vasconcellos, J.; Henrique Silvestre, D.; Dos Santos Baiao, D.; Werneck-de-Castro, J.P.; Silveira Alvares, T.; Paschoalin, V.M. A single dose of beetroot gel rich in nitrate does not improve performance but lowers blood glucose in physically active individuals. J. Nutr. Metab. 2017, 2017, 7853034. [Google Scholar] [CrossRef]
- Shannon, O.M.; Barlow, M.J.; Duckworth, L.; Williams, E.; Wort, G.; Woods, D.; Siervo, M.; O’Hara, J.P. Dietary nitrate supplementation enhances short but not longer duration running time-trial performance. Eur. J. Appl. Physiol. 2017, 117, 775–785. [Google Scholar] [CrossRef]
- Bailey, S.J.; Winyard, P.; Vanhatalo, A.; Blackwell, J.R.; Dimenna, F.J.; Wilkerson, D.P.; Tarr, J.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation reduces the o2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J. Appl. Physiol. 2009, 107, 1144–1155. [Google Scholar] [CrossRef]
- Lansley, K.E.; Winyard, P.G.; Fulford, J.; Vanhatalo, A.; Bailey, S.J.; Blackwell, J.R.; DiMenna, F.J.; Gilchrist, M.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation reduces the o2 cost of walking and running: A placebo-controlled study. J. Appl. Physiol. 2011, 110, 591–600. [Google Scholar] [CrossRef]
- Porcelli, S.; Ramaglia, M.; Bellistri, G.; Pavei, G.; Pugliese, L.; Montorsi, M.; Rasica, L.; Marzorati, M. Aerobic fitness affects the exercise performance responses to nitrate supplementation. Med. Sci. Sports Exerc. 2015, 47, 1643–1651. [Google Scholar] [CrossRef] [PubMed]
- Wylie, L.J.; Kelly, J.; Bailey, S.J.; Blackwell, J.R.; Skiba, P.F.; Winyard, P.G.; Jeukendrup, A.E.; Vanhatalo, A.; Jones, A.M. Beetroot juice and exercise: Pharmacodynamic and dose-response relationships. J. Appl. Physiol. 2013, 115, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.J.; Schiffer, T.A.; Borniquel, S.; Sahlin, K.; Ekblom, B.; Lundberg, J.O.; Weitzberg, E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metab. 2011, 13, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.J.; Fulford, J.; Vanhatalo, A.; Winyard, P.G.; Blackwell, J.R.; DiMenna, F.J.; Wilkerson, D.P.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J. Appl. Physiol. 2010, 109, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Haider, G.; Folland, J.P. Nitrate supplementation enhances the contractile properties of human skeletal muscle. Med. Sci. Sports Exerc. 2014, 46, 2234–2243. [Google Scholar] [CrossRef]
- Ferguson, S.K.; Hirai, D.M.; Copp, S.W.; Holdsworth, C.T.; Allen, J.D.; Jones, A.M.; Musch, T.I.; Poole, D.C. Impact of dietary nitrate supplementation via beetroot juice on exercising muscle vascular control in rats. J. Physiol. 2013, 591, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Lundby, C.; Boushel, R.; Robach, P.; Moller, K.; Saltin, B.; Calbet, J.A. During hypoxic exercise some vasoconstriction is needed to match o2 delivery with o2 demand at the microcirculatory level. J. Physiol. 2008, 586, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Calbet, J.A.; Lundby, C.; Sander, M.; Robach, P.; Saltin, B.; Boushel, R. Effects of atp-induced leg vasodilation on vo2 peak and leg o2 extraction during maximal exercise in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R447–R453. [Google Scholar] [CrossRef]
- Hernandez, A.; Schiffer, T.A.; Ivarsson, N.; Cheng, A.J.; Bruton, J.D.; Lundberg, J.O.; Weitzberg, E.; Westerblad, H. Dietary nitrate increases tetanic [ca2+]i and contractile force in mouse fast-twitch muscle. J. Physiol. 2012, 590 Pt 15, 3575–3583. [Google Scholar] [CrossRef]
- Jones, A.M.; Ferguson, S.K.; Bailey, S.J.; Vanhatalo, A.; Poole, D.C. Fiber type-specific effects of dietary nitrate. Exerc. Sport Sci. Rev. 2016, 44, 53–60. [Google Scholar] [CrossRef]
- Kiilerich, K.; Birk, J.B.; Damsgaard, R.; Wojtaszewski, J.F.; Pilegaard, H. Regulation of pdh in human arm and leg muscles at rest and during intense exercise. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E36–E42. [Google Scholar] [CrossRef] [PubMed]
- Schantz, P.; Randall-Fox, E.; Hutchison, W.; Tyden, A.; Astrand, P.O. Muscle fibre type distribution, muscle cross-sectional area and maximal voluntary strength in humans. Acta Physiol. Scand. 1983, 117, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Calbet, J.A.; Holmberg, H.C.; Rosdahl, H.; van Hall, G.; Jensen-Urstad, M.; Saltin, B. Why do arms extract less oxygen than legs during exercise? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R1448–R1458. [Google Scholar] [CrossRef] [PubMed]
- Calbet, J.A.; Gonzalez-Alonso, J.; Helge, J.W.; Sondergaard, H.; Munch-Andersen, T.; Saltin, B.; Boushel, R. Central and peripheral hemodynamics in exercising humans: Leg vs arm exercise. Scand J. Med. Sci. Sports. 2015, 25 (Suppl. 4), 144–157. [Google Scholar] [CrossRef] [PubMed]
- Van Hall, G.; Jensen-Urstad, M.; Rosdahl, H.; Holmberg, H.C.; Saltin, B.; Calbet, J.A. Leg and arm lactate and substrate kinetics during exercise. Am. J. Physiol. Endocrinol. Metab. 2003, 284, E193–E205. [Google Scholar] [CrossRef] [PubMed]
- Muggeridge, D.J.; Howe, C.C.; Spendiff, O.; Pedlar, C.; James, P.E.; Easton, C. The effects of a single dose of concentrated beetroot juice on performance in trained flatwater kayakers. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Peeling, P.; Cox, G.R.; Bullock, N.; Burke, L.M. Beetroot juice improves on-water 500 m time-trial performance, and laboratory-based paddling economy in national and international-level kayak athletes. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 278–284. [Google Scholar] [CrossRef]
- Bond, H.; Morton, L.; Braakhuis, A.J. Dietary nitrate supplementation improves rowing performance in well-trained rowers. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 251–256. [Google Scholar] [CrossRef]
- Hoon, M.W.; Jones, A.M.; Johnson, N.A.; Blackwell, J.R.; Broad, E.M.; Lundy, B.; Rice, A.J.; Burke, L.M. The effect of variable doses of inorganic nitrate-rich beetroot juice on simulated 2,000-m rowing performance in trained athletes. Int. J. Sports Physiol. Perform. 2014, 9, 615–620. [Google Scholar] [CrossRef]
- World Medical Association. Declaration of Helsinki—Ethical principles for medical research involving human subjects. J. Indian Med. Assoc. 2009, 107, 403–405. [Google Scholar]
- Govoni, M.; Jansson, E.A.; Weitzberg, E.; Lundberg, J.O. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric Oxide 2008, 19, 333–337. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Esen, O.; Nicholas, C.; Morris, M.; Bailey, S.J. No effect of beetroot juice supplementation on 100-m and 200-m swimming performance in moderately trained swimmers. Int. J. Sports Physiol. Perform. 2019. [Google Scholar] [CrossRef] [PubMed]
- Bruce, C.R.; Anderson, M.E.; Fraser, S.F.; Stepto, N.K.; Klein, R.; Hopkins, W.G.; Hawley, J.A. Enhancement of 2000-m rowing performance after caffeine ingestion. Med. Sci. Sports Exerc. 2000, 32, 1958–1963. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M. Influence of dietary nitrate on the physiological determinants of exercise performance: A critical review. Appl. Physiol. Nutr. Metab. 2014, 39, 1019–1028. [Google Scholar] [CrossRef]
- Schantz, P.; Sjoberg, B.; Widebeck, A.M.; Ekblom, B. Skeletal muscle of trained and untrained paraplegics and tetraplegics. Acta Physiol. Scand. 1997, 161, 31–39. [Google Scholar] [CrossRef]
- Vanhatalo, A.; Bailey, S.J.; Blackwell, J.R.; DiMenna, F.J.; Pavey, T.G.; Wilkerson, D.P.; Benjamin, N.; Winyard, P.G.; Jones, A.M. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R1121–R1131. [Google Scholar] [CrossRef] [PubMed]
- Cermak, N.M.; Res, P.; Stinkens, R.; Lundberg, J.O.; Gibala, M.J.; van Loon, L.J. No improvement in endurance performance after a single dose of beetroot juice. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 470–478. [Google Scholar] [CrossRef]
- Jo, E.; Fischer, M.; Auslander, A.T.; Beigarten, A.; Daggy, B.; Hansen, K.; Kessler, L.; Osmond, A.; Wang, H.; Wes, R. The effects of multi-day vs. Single pre-exercise nitrate supplement dosing on simulated cycling time trial performance and skeletal muscle oxygenation. J. Strength Cond. Res. 2019, 33, 217–224. [Google Scholar] [CrossRef]
- Bescos, R.; Ferrer-Roca, V.; Galilea, P.A.; Roig, A.; Drobnic, F.; Sureda, A.; Martorell, M.; Cordova, A.; Tur, J.A.; Pons, A. Sodium nitrate supplementation does not enhance performance of endurance athletes. Med. Sci. Sports Exerc. 2012, 44, 2400–2409. [Google Scholar] [CrossRef]
- Christensen, P.M.; Nyberg, M.; Bangsbo, J. Influence of nitrate supplementation on vo(2) kinetics and endurance of elite cyclists. Scand. J. Med. Sci. Sports 2013, 23, e21–e31. [Google Scholar] [CrossRef] [PubMed]
- Peacock, O.; Tjonna, A.E.; James, P.; Wisloff, U.; Welde, B.; Bohlke, N.; Smith, A.; Stokes, K.; Cook, C.; Sandbakk, O. Dietary nitrate does not enhance running performance in elite cross-country skiers. Med. Sci. Sports Exerc. 2012, 44, 2213–2219. [Google Scholar] [CrossRef] [PubMed]
- Lane, S.C.; Hawley, J.A.; Desbrow, B.; Jones, A.M.; Blackwell, J.R.; Ross, M.L.; Zemski, A.J.; Burke, L.M. Single and combined effects of beetroot juice and caffeine supplementation on cycling time trial performance. Appl. Physiol. Nutr. Metab. 2014, 39, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Cocks, M.; Shaw, C.S.; Shepherd, S.O.; Fisher, J.P.; Ranasinghe, A.M.; Barker, T.A.; Tipton, K.D.; Wagenmakers, A.J. Sprint interval and endurance training are equally effective in increasing muscle microvascular density and enos content in sedentary males. J. Physiol. 2013, 591, 641–656. [Google Scholar] [CrossRef] [PubMed]
- Totzeck, M.; Hendgen-Cotta, U.B.; Rammos, C.; Frommke, L.M.; Knackstedt, C.; Predel, H.G.; Kelm, M.; Rassaf, T. Higher endogenous nitrite levels are associated with superior exercise capacity in highly trained athletes. Nitric Oxide 2012, 27, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Gollnick, P.D.; Matoba, H. The muscle fiber composition of skeletal muscle as a predictor of athletic success. An overview. Am. J. Sports Med. 1984, 12, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Ade, C.J.; Broxterman, R.M.; Craig, J.C.; Schlup, S.J.; Wilcox, S.L.; Barstow, T.J. Upper body aerobic exercise as a possible predictor of lower body performance. Aerosp. Med. Hum. Perform. 2015, 86, 599–605. [Google Scholar] [CrossRef]
- Bhambhani, Y. Physiology of wheelchair racing in athletes with spinal cord injury. Sports Med. 2002, 32, 23–51. [Google Scholar] [CrossRef]
- Keil, M.; Totosy de Zepetnek, J.O.; Brooke-Wavell, K.; Goosey-Tolfrey, V.L. Measurement precision of body composition variables in elite wheelchair athletes, using dual-energy x-ray absorptiometry. Eur. J. Sport Sci. 2016, 16, 65–71. [Google Scholar] [CrossRef]
- Boorsma, R.K.; Whitfield, J.; Spriet, L.L. Beetroot juice supplementation does not improve performance of elite 1500-m runners. Med. Sci. Sports Exerc. 2014, 46, 2326–2334. [Google Scholar] [CrossRef]
- Wilkerson, D.P.; Hayward, G.M.; Bailey, S.J.; Vanhatalo, A.; Blackwell, J.R.; Jones, A.M. Influence of acute dietary nitrate supplementation on 50 mile time trial performance in well-trained cyclists. Eur. J. Appl. Physiol. 2012, 112, 4127–4134. [Google Scholar] [CrossRef] [PubMed]
- Bourdillon, N.; Fan, J.L.; Uva, B.; Muller, H.; Meyer, P.; Kayser, B. Effect of oral nitrate supplementation on pulmonary hemodynamics during exercise and time trial performance in normoxia and hypoxia: A randomized controlled trial. Front. Physiol. 2015, 6, 288. [Google Scholar] [CrossRef] [PubMed]
- Larsen, F.J.; Weitzberg, E.; Lundberg, J.O.; Ekblom, B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiol. 2007, 191, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Pawlak-Chaouch, M.; Boissiere, J.; Gamelin, F.X.; Cuvelier, G.; Berthoin, S.; Aucouturier, J. Effect of dietary nitrate supplementation on metabolic rate during rest and exercise in human: A systematic review and a meta-analysis. Nitric Oxide 2016, 53, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Flueck, J.L.; Bogdanova, A.; Mettler, S.; Perret, C. Is beetroot juice more effective than sodium nitrate? The effects of equimolar nitrate dosages of nitrate-rich beetroot juice and sodium nitrate on oxygen consumption during exercise. Appl. Physiol. Nutr. Metab. 2016, 41, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Bescos, R.; Rodriguez, F.A.; Iglesias, X.; Ferrer, M.D.; Iborra, E.; Pons, A. Acute administration of inorganic nitrate reduces vo(2peak) in endurance athletes. Med. Sci. Sports Exerc. 2011, 43, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- Pimental, N.A.; Sawka, M.N.; Billings, D.S.; Trad, L.A. Physiological responses to prolonged upper-body exercise. Med. Sci. Sports Exerc. 1984, 16, 360–365. [Google Scholar] [CrossRef]
- Pendergast, D.R. Cardiovascular, respiratory, and metabolic responses to upper body exercise. Med. Sci. Sports Exerc. 1989, 21 (Suppl. 5), S121–S125. [Google Scholar] [CrossRef]
- Braakhuis, A.J.; Hopkins, W.G. Impact of dietary antioxidants on sport performance: A review. Sports Med. 2015, 45, 939–955. [Google Scholar] [CrossRef]
- Webb, A.J.; Patel, N.; Loukogeorgakis, S.; Okorie, M.; Aboud, Z.; Misra, S.; Rashid, R.; Miall, P.; Deanfield, J.; Benjamin, N.; et al. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension 2008, 51, 784–790. [Google Scholar] [CrossRef]
- Siervo, M.; Lara, J.; Ogbonmwan, I.; Mathers, J.C. Inorganic nitrate and beetroot juice supplementation reduces blood pressure in adults: A systematic review and meta-analysis. J. Nutr. 2013, 143, 818–826. [Google Scholar] [CrossRef] [PubMed]
- Krogh, K.; Mosdal, C.; Laurberg, S. Gastrointestinal and segmental colonic transit times in patients with acute and chronic spinal cord lesions. Spinal Cord 2000, 38, 615–621. [Google Scholar] [CrossRef] [PubMed]
| ID | Age [year] | Height [cm] | Body Mass [kg] | VO2peak [ml/min/kg] | POmax [W] | Lesion Level | Category |
|---|---|---|---|---|---|---|---|
| 1 | 42 | 188 | 66 | 41.5 | 200 | Th5 | MH3 |
| 2 | 34 | 169 | 72 | 29.7 | 144 | L4 | MH4 |
| 3 | 29 | 170 | 47 | 42.8 | 151 | C4 | MH2 |
| 4 | 54 | 178 | 75 | 49.7 | 222 | Th12 | MH5 |
| 5 | 32 | 173 | 60 | 36.9 | 180 | Th3 | MH3 |
| 6 | 61 | 178 | 61 | 45.9 | 208 | Th4 | MH3 |
| 7 | 40 | 165 | 64 | 44.8 | 220 | Th10 | MH4 |
| 8 | 35 | 190 | 76 | 17.3 | 98 | C6 | MH1 |
| 40 ± 11 | 176 ± 9 | 65 ± 9 | 38.6 ± 10.5 | 178 ± 44 |
| Able-Bodied Individuals | Paracyclists | |||||
|---|---|---|---|---|---|---|
| PLA | NIT | BR | PLA | NIT | BR | |
| Systolic BP [mmHg] | ||||||
| Pre | 124 ± 9 | 125 ± 9 | 124 ± 10 | 129 ± 16 | 130 ± 19 | 128 ± 17 |
| Post | 125 ± 9 | 125 ± 13 | 125 ± 10 | 134 ± 14 | 131 ± 18 | 135 ± 17 |
| Diastolic BP [mmHg] | ||||||
| Pre | 77 ± 7 | 78 ± 10 | 79 ± 9 | 79 ± 15 | 78 ± 18 | 79 ± 15 |
| Post | 80 ± 8 | 80 ± 9 | 82 ± 9 | 84 ± 13 | 83 ± 16 | 84 ± 14 |
| HR [bpm] | ||||||
| Pre | 61 ± 6 | 62 ± 6 | 61 ±7 | 70 ± 17 | 70 ± 14 | 67 ± 17 |
| Post | 56 ± 7 | 55 ± 7 | 55 ± 9 | 61 ± 10 | 62 ± 10 | 59 ± 12 |
| Able-Bodied Individuals | Paracyclists | ||||
|---|---|---|---|---|---|
| Pre | Post | Pre | Post | ||
| Plasma [NO3−] in uM | PLA | 68.5 ± 8.4 | 67.9 ± 8.6 | 37.9 ± 18.8 | 38.1 ±18.9 |
| NIT | 64.8 ±10.9 | 278.7 ± 154.9 *† | 36.8 ± 19.9 | 85.9 ± 73.2 *† | |
| BR | 63.5 ± 8.9 | 273.7 ± 82.8 *† | 38.8 ± 17.9 | 125.8 ± 99.3 *† | |
| Plasma [NO2−] in nM | PLA | 44.7 ± 21.5 | 41.2 ± 28.7 | 66.9 ± 27.9 | 92.3 ± 109.9 |
| NIT | 56.7 ± 22.6 | 141.2 ± 75.7 *† | 57.7 ± 11.6 | 136.7 ± 69.6 *† | |
| BR | 61.5 ± 53.8 | 121.2 ± 57.3 *† | 108.7 ± 134.3 † | 263.3 ± 159.2 *† | |
| Able-Bodied Individuals | Paracyclists | |||||
|---|---|---|---|---|---|---|
| PLA | NIT | BR | PLA | NIT | BR | |
| POaverage [W] | 55 ± 19 | 58 ± 16 | 55 ± 18 | 90 ± 31 * | 95 ± 31 * | 88 ± 29 * |
| HRaverage [bpm] | 93 ± 19 | 95 ± 15 | 94 ± 16 | 119 ± 20 * | 115 ± 18 * | 111 ± 19 * |
| HRmax [bpm] | 105 ± 21 | 107 ± 15 | 107 ± 16 | 140 ± 25 * | 137 ± 23 * | 137 ± 26 * |
| RPE [6; 20] | 11 [7; 13] | 11 [7; 12] | 11 [7; 13] | 12 [8; 14] | 11 [7; 12] | 12 [8; 13] |
| [Lac] [mmol/L] | 2.57 ± 1.43 | 2.67 ± 1.34 | 2.68 ± 1.13 | 1.89 ± 0.86 * | 1.74 ± 0.77 * | 1.87 ± 0.73 * |
| Able-Bodied Individuals | Paracyclists | |||||
|---|---|---|---|---|---|---|
| PLA | NIT | BR | PLA | NIT | BR | |
| Time to complete [s] | 1182 ± 84 | 1194 ± 102 | 1195 ± 94 | 1106 ± 247 * | 1091 ± 235 * | 1071 ± 199 * |
| POaverage [W] | 114 ± 16 | 113 ± 20 | 112 ± 17 | 142 ± 49 | 144 ± 44 | 145 ± 42 |
| HRaverage [bpm] | 152 ± 13 | 153 ± 9 | 150 ± 11 | 162 ± 27 * | 158± 30 * | 158 ± 31 * |
| HRmax [bpm] | 174 ± 10 | 175± 8 | 175 ± 9 | 176 ± 24 | 170 ± 31 | 173 ± 28 |
| RPE [6; 20] | 18 [16; 20] | 19 [17; 20] | 18.5 [17; 20] | 19 [17; 20] | 19 [17; 20] | 19 [17; 20] |
| [Lac] [mmol/L] | 11.94 ± 2.40 | 12.28 ± 2.84 | 11.66 ± 2.15 | 10.13 ± 5.98 | 10.05 ± 6.37 | 9.92 ± 5.99 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flueck, J.L.; Gallo, A.; Moelijker, N.; Bogdanov, N.; Bogdanova, A.; Perret, C. Influence of Equimolar Doses of Beetroot Juice and Sodium Nitrate on Time Trial Performance in Handcycling. Nutrients 2019, 11, 1642. https://doi.org/10.3390/nu11071642
Flueck JL, Gallo A, Moelijker N, Bogdanov N, Bogdanova A, Perret C. Influence of Equimolar Doses of Beetroot Juice and Sodium Nitrate on Time Trial Performance in Handcycling. Nutrients. 2019; 11(7):1642. https://doi.org/10.3390/nu11071642
Chicago/Turabian StyleFlueck, Joelle Leonie, Alessandro Gallo, Nynke Moelijker, Nikolay Bogdanov, Anna Bogdanova, and Claudio Perret. 2019. "Influence of Equimolar Doses of Beetroot Juice and Sodium Nitrate on Time Trial Performance in Handcycling" Nutrients 11, no. 7: 1642. https://doi.org/10.3390/nu11071642
APA StyleFlueck, J. L., Gallo, A., Moelijker, N., Bogdanov, N., Bogdanova, A., & Perret, C. (2019). Influence of Equimolar Doses of Beetroot Juice and Sodium Nitrate on Time Trial Performance in Handcycling. Nutrients, 11(7), 1642. https://doi.org/10.3390/nu11071642







