Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value
Abstract
1. Introduction
2. Probiotics and Delivery Systems
3. Significance of Cell Viability
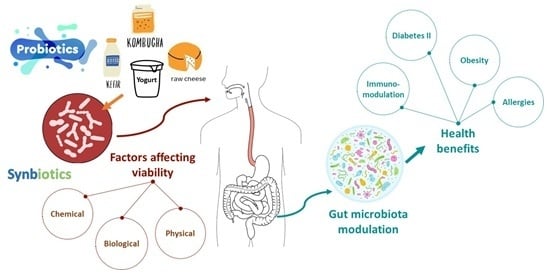
4. Factors Affecting Viability of Probiotics
4.1. Chemical Factors
4.2. Biological Factors
4.3. Physical Factors
5. Strategies for Enhanced Probiotic Viability
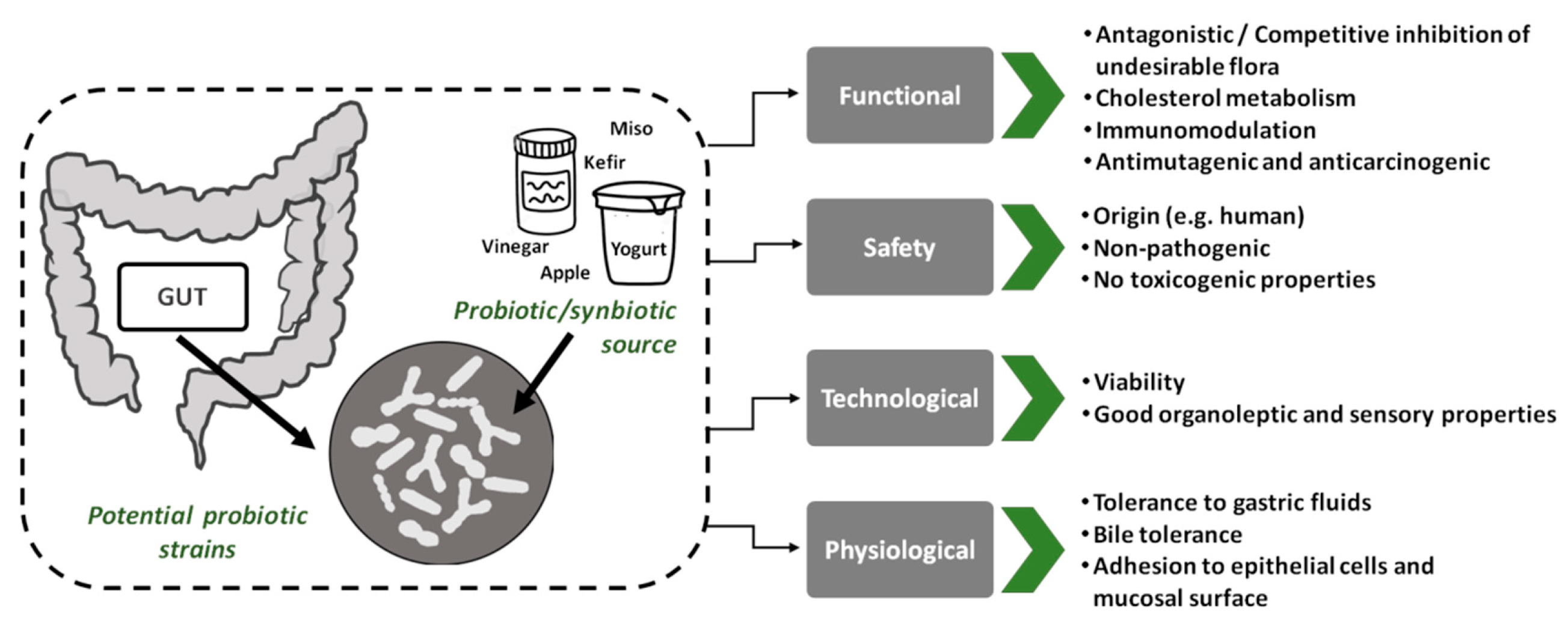
5.1. Selection of Probiotics
5.2. Strain Adaptation on Food Matrix and Human Microenvironment
5.3. Selection of Food Packaging Systems
5.4. Addition of Compounds as Probiotic Promoters
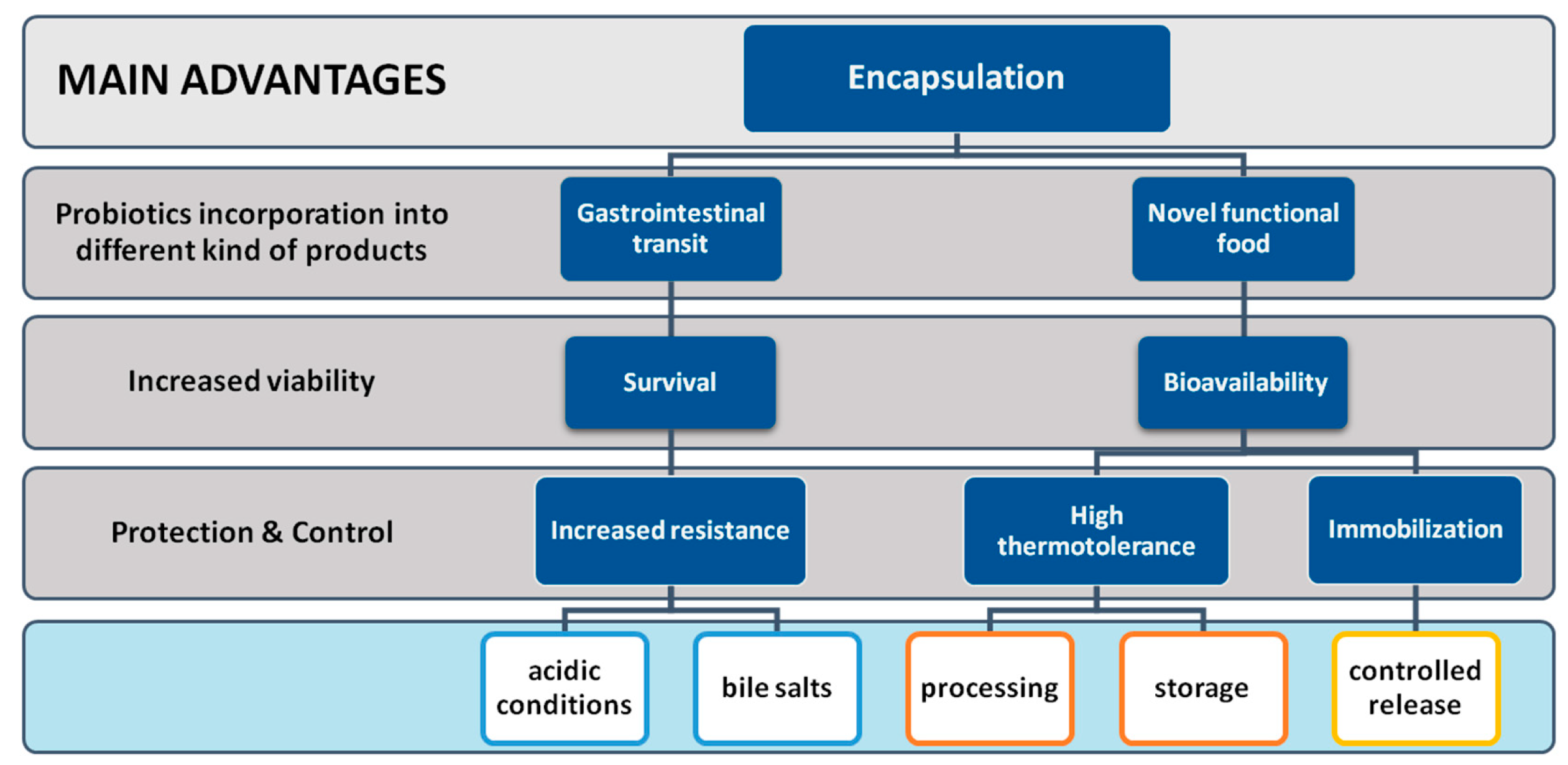
5.5. Encapsulation of Probiotics
5.5.1. Encapsulation Materials
5.5.2. Encapsulation Technologies
6. Development of Synbiotics
7. Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Savino, T.; Testa, S.; Messeni Petruzzelli, A. Researcher understanding of food innovations in Nordic and Southern European countries: A systematic literature review. Trends Food Sci. Technol. 2018, 77, 54–63. [Google Scholar] [CrossRef]
- Guerrero, L.; Claret, A.; Verbeke, W.; Sulmont-Rossé, C.; Hersleth, M. Chapter 5—Innovation in Traditional Food Products: Does It Make Sense? In Innovation Strategies in the Food Industry; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 77–89. [Google Scholar]
- Martin-Rios, C.; Demen-Meier, C.; Gössling, S.; Cornuz, C. Food waste management innovations in the foodservice industry. Waste Manag. 2018, 79, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Khedkar, S.; Carraresi, L.; Bröring, S. Food or pharmaceuticals? Consumers’ perception of health-related borderline products. PharmaNutrition 2017, 5, 133–140. [Google Scholar] [CrossRef]
- Brown, L.; Caligiuri, S.P.B.; Brown, D.; Pierce, G.N. Clinical trials using functional foods provide unique challenges. J. Funct. Foods 2018, 45, 233–238. [Google Scholar] [CrossRef]
- Statista. 2019. Available online: https://www.statista.com/ (accessed on 2 June 2019).
- FAO. Guidelines for the Evaluation of Probiotics in Food: Report of a Joint FAO; FAO: Rome, Italy, 2013. [Google Scholar]
- Meng, C.; Bai, C.; Brown, T.D.; Hood, L.E.; Tian, Q. Human Gut Microbiota and Gastrointestinal Cancer. Genom. Proteom. Bioinf. 2018, 16, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Introducing the concept of prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, M.; Kalkman, G.; Prevaes, S.; Tramper-Stranders, G.; Groot, K.D.W.-D.; Janssens, H.; Tiddens, H.; Van Westreenen, M.; Van Der Ent, C.; Sanders, E.; et al. WS07.5 Gut microbiome in healthy children and children with cystic fibrosis during the first 18 months of life. J. Cyst. Fibros. 2016, 15, S12. [Google Scholar] [CrossRef]
- Neffe-Skocińska, K.; Rzepkowska, A.; Szydłowska, A.; Kołożyn-Krajewska, D. Chapter 3—Trends and Possibilities of the Use of Probiotics in Food Production. In Alternative and Replacement Foods; Academic Press: Cambridge, MA, USA, 2018; pp. 65–94. [Google Scholar]
- Ambalam, P.; Raman, M.; Purama, R.K.; Doble, M. Probiotics, prebiotics and colorectal cancer prevention. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 119–131. [Google Scholar] [CrossRef]
- Bautista-Gallego, J.; Ferrocino, I.; Botta, C.; Ercolini, D.; Cocolin, L.; Rantsiou, K. Probiotic potential of a Lactobacillus rhamnosus cheese isolate and its effect on the fecal microbiota of healthy volunteers. Food Res. Int. 2019, 119, 305–314. [Google Scholar] [CrossRef]
- Tarrah, A.; de Castilhos, J.; Rossi, R.C.; da Duarte, V.S.; Ziegler, D.R.; Corich, V.; Giacomini, A. In vitro Probiotic Potential and Anti-cancer Activity of Newly Isolated Folate-Producing Streptococcus thermophilus Strains. Front. Microbiol. 2018, 9, 2214. [Google Scholar] [CrossRef]
- Bermúdez-Humarán, L.G.; Salinas, E.; Ortiz, G.G.; Ramirez-Jirano, L.J.; Morales, J.A.; Bitzer-Quintero, O.K. From Probiotics to Psychobiotics: Live Beneficial Bacteria Which Act on the Brain-Gut Axis. Nutrients 2019, 11, 890. [Google Scholar] [CrossRef] [PubMed]
- Bagga, D.; Reichert, J.L.; Koschutnig, K.; Aigner, C.S.; Holzer, P.; Koskinen, K.; Moissl-Eichinger, C.; Schopf, V. Probiotics drive gut microbiome triggering emotional brain signatures. Gut Microbes 2018, 9, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Yun, M.; Oh, Y.J.; Choi, H.J. Mind-altering with the gut: Modulation of the gut-brain axis with probiotics. J. Microbiol. 2018, 56, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhu, G. Gut-Brain Axis and Mood Disorder. Front. Psychiatry 2018, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Cryan, J.F. Gut-brain axis in 2016: Brain-gut-microbiota axis-mood, metabolism and behaviour. Nature reviews. Gastroenterol. Hepatol. 2017, 14, 69–70. [Google Scholar] [CrossRef]
- Sarkar, A.; Lehto, S.M.; Harty, S.; Dinan, T.G.; Cryan, J.F.; Burnet, P.W.J. Psychobiotics and the Manipulation of Bacteria-Gut-Brain Signals. Trends Neurosci. 2016, 39, 763–781. [Google Scholar] [CrossRef]
- Knorr, D. Technology aspects related to microorganisms in functional foods. Trends Food Sci. Technol. 1998, 9, 295–306. [Google Scholar] [CrossRef]
- Karimi, R.; Mortazavian, A.M.; Da Cruz, A.G. Viability of probiotic microorganisms in cheese during production and storage: A review. Dairy Sci. Technol. 2011, 91, 283–308. [Google Scholar] [CrossRef]
- Saxelin, M. Lactobacillus GG—A human probiotic strain with thorough clinical documentation. Food Rev. Intern. 1997, 13, 293–313. [Google Scholar] [CrossRef]
- Wilkinson, M.G. Flow cytometry as a potential method of measuring bacterial viability in probiotic products: A review. Trends Food Sci. Technol. 2018, 78, 1–10. [Google Scholar] [CrossRef]
- Mohammadi, R.; Mortazavian, A.M. Review article: Technological aspects of prebiotics in probiotic fermented milks. Food Rev. Int. 2011, 27, 192–212. [Google Scholar] [CrossRef]
- Stanton, C.; Desmond, C.; Coakley, M.; Collins, J.K.; Fitzgerald, G.; Ross, R.P. Challenges facing development of probiotic-containing functional foods. In Handbook of Fermented Functional Foods; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Vlasova, A.N.; Kandasamy, S.; Chattha, K.S.; Rajashekara, G.; Saif, L.J. Comparison of probiotic Lactobacilli and Bifidobacteria effects, immune responses and rotavirus vaccines and infection in different host species. Vet. Immunol. Immunopathol. 2016, 172, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, W.H. Introduction to prebiotics and probiotics. In Probiotics in Food Safety and Human Health; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Rivera-Espinoza, Y.; Gallardo-Navarro, Y. Non-dairy probiotic products. Food Microbiol. 2010, 27, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Holzapfel, W.H.; Haberer, P.; Snel, J.; Schillinger, U.; Huis In’T Veld, J.H.J. Overview of gut flora and probiotics. Int. J. Food Microbiol. 1998, 41, 85–101. [Google Scholar] [CrossRef]
- Holzapfel, W.H.; Haberer, P.; Geisen, R.; Björkroth, J.; Schillinger, U. Taxonomy and important features of probiotic microorganisms in food and nutrition. Am. J. Clin. Nutr. 2001, 73, 365s–373s. [Google Scholar] [CrossRef] [PubMed]
- Bruno Biavati, P.M. Bifidobacterium. In Bergey’s Manual of Systematics of Archaea and Bacteria; Whitman, W.B., Kämpfer, F.R.P., Trujillo, M., Chun, J., DeVos, P., Hedlund, B., Dedysh, S., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 1–57. [Google Scholar]
- Meena, G.S.; Kumar, N.; Majumdar, G.C.; Banerjee, R.; Meena, P.K.; Yadav, V. Growth characteristics modeling of Lactobacillus acidophilus using RSM and ANN. Braz. Arch. Boil. Technol. 2014, 57, 15–22. [Google Scholar] [CrossRef]
- Matejčeková, Z.; Liptáková, D.; Spodniaková, S.; Valík, Ľ. Characterization of the growth of Lactobacillus plantarum in milk in dependence on temperature. Acta Chim. Slovaca 2016, 9, 104–108. [Google Scholar] [CrossRef]
- Da Silva, A.P.R.; Longhi, D.A.; Dalcanton, F.; de Aragão, G.M.F. Modelling the growth of lactic acid bacteria at different temperatures. Braz. Arch. Biol. Technol. 2018, 61, 61. [Google Scholar] [CrossRef]
- Naito, Y.; Uchiyama, K.; Takagi, T. A next-generation beneficial microbe: Akkermansia muciniphila. J. Clin. Biochem. Nutr. 2018, 63, 33–35. [Google Scholar] [CrossRef]
- Martin, R.; Miquel, S.; Benevides, L.; Bridonneau, C.; Robert, V.; Hudault, S.; Chain, F.; Berteau, O.; Azevedo, V.; Chatel, J.M.; et al. Functional Characterization of Novel Faecalibacterium prausnitzii Strains Isolated from Healthy Volunteers: A Step Forward in the Use of F. prausnitzii as a Next-Generation Probiotic. Front. Microbiol. 2017, 8, 1226. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermudez-Humaran, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [PubMed]
- Putta, S.; Yarla, N.S.; Lakkappa, D.B.; Imandi, S.B.; Malla, R.R.; Chaitanya, A.K.; Chari, B.P.V.; Saka, S.; Vechalapu, R.R.; Kamal, M.A.; et al. Chapter 2—Probiotics: Supplements, Food, Pharmaceutical Industry. In Therapeutic, Probiotic, and Unconventional Foods; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Eor, J.Y.; Tan, P.L.; Lim, S.M.; Choi, D.H.; Yoon, S.M.; Yang, S.Y.; Kim, S.H. Laxative effect of probiotic chocolate on loperamide-induced constipation in rats. Food Res. Int. 2019, 116, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Mantzourani, I.; Kazakos, S.; Terpou, A.; Alexopoulos, A.; Bezirtzoglou, E.; Bekatorou, A.; Plessas, S. Potential of the Probiotic Lactobacillus Plantarum ATCC 14917 Strain to Produce Functional Fermented Pomegranate Juice. Foods 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.C.; Caruso, D.; Buonomo, G.; D’Avino, M.; Ciampaglia, R.; Maisto, M.; Schisano, C.; Bocchino, B.; Novellino, E. Lactofermented Annurca Apple Puree as a Functional Food Indicated for the Control of Plasma Lipid and Oxidative Amine Levels: Results from a Randomised Clinical Trial. Nutrients 2019, 11, 122. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, I.; Ben Slima, S.; Ktari, N.; Triki, M.; Abdehedi, R.; Abaza, W.; Moussa, H.; Abdeslam, A.; Ben Salah, R. Incorporation of probiotic strain in raw minced beef meat: Study of textural modification, lipid and protein oxidation and color parameters during refrigerated storage. Meat Sci. 2019, 154, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Soukoulis, C.; Yonekura, L.; Gan, H.-H.; Behboudi-Jobbehdar, S.; Parmenter, C.; Fisk, I. Probiotic edible films as a new strategy for developing functional bakery products: The case of pan bread. Food Hydrocoll. 2014, 39, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Lahtinen, S.J.; Gueimonde, M.; Ouwehand, A.C.; Reinikainen, J.P.; Salminen, S.J. Probiotic bacteria may become dormant during storage. Appl. Environ. Microbiol. 2005, 71, 1662–1663. [Google Scholar] [CrossRef]
- Galdeano, C.M.; Perdigon, G. Role of viability of probiotic strains in their persistence in the gut and in mucosal immune stimulation. J. Appl. Microbiol. 2004, 97, 673–681. [Google Scholar] [CrossRef]
- Pelletier, X.; Laure-Boussuge, S.; Donazzolo, Y. Hydrogen excretion upon ingestion of dairy products in lactose-intolerant male subjects: Importance of the live flora. Eur. J. Clin. Nutr. 2001, 55, 509–512. [Google Scholar] [CrossRef]
- Zou, J.; Dong, J.; Yu, X. Meta-analysis: Lactobacillus containing quadruple therapy versus standard triple first-line therapy for Helicobacter pylori eradication. Helicobacter 2009, 14, 97–107. [Google Scholar] [CrossRef]
- Adams, C.A. The probiotic paradox: Live and dead cells are biological response modifiers. Nutr. Res. Rev. 2010, 23, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Champagne, C.P.; Gomes da Cruz, A.; Daga, M. Strategies to improve the functionality of probiotics in supplements and foods. Curr. Opin. Food Sci. 2018, 22, 160–166. [Google Scholar] [CrossRef]
- Barer, M.R. Chapter 10—Bacterial Growth, Culturability and Viability. In Molecular Medical Microbiology, 2nd ed.; Tang, Y.-W., Sussman, M., Liu, D., Poxton, I., Schwartzman, J., Eds.; Academic Press: Boston, MA, USA, 2015. [Google Scholar]
- Grattepanche, F.; Lacroix, C. 13—Production of viable probiotic cells. In Microbial Production of Food Ingredients, Enzymes and Nutraceuticals; McNeil, B., Archer, D., Giavasis, I., Harvey, L., Eds.; Woodhead Publishing: Cambridge, UK, 2013. [Google Scholar]
- Dargahi, N.; Johnson, J.; Donkor, O.; Vasiljevic, T.; Apostolopoulos, V. Immunomodulatory effects of probiotics: Can they be used to treat allergies and autoimmune diseases? Maturitas 2019, 119, 25–38. [Google Scholar] [CrossRef] [PubMed]
- The Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2010, 486, 207. [Google Scholar] [CrossRef]
- Mills, S.; Stanton, C.; Lane, J.A.; Smith, G.J.; Ross, R.P. Precision Nutrition and the Microbiome, Part I: Current State of the Science. Nutrients 2019, 11, 923. [Google Scholar] [CrossRef]
- Sen, P.; Oresic, M. Metabolic Modeling of Human Gut Microbiota on a Genome Scale: An Overview. Metabolites 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Salminen, S.; Verhagen, H.; Rowland, I.; Heimbach, J.; Bañares, S.; Young, T.; Nomoto, K.; Lalonde, M. Novel probiotics and prebiotics: Road to the market. Curr. Opin. Biotechnol. 2015, 32, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Rastall, R.A.; Gibson, G.R. Recent developments in prebiotics to selectively impact beneficial microbes and promote intestinal health. Curr. Opin. Biotechnol. 2015, 32, 42–46. [Google Scholar] [CrossRef]
- Lee, Y.K.; Salminen, S. Handbook of Probiotics and Prebiotics 2009; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Tripathi, M.K.; Giri, S.K. Probiotic functional foods: Survival of probiotics during processing and storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Majid, I.; Ahmad Nayik, G.; Mohammad Dar, S.; Nanda, V. Novel food packaging technologies: Innovations and future prospective. J. Saudi Soc. Agric. Sci. 2018, 17, 454–462. [Google Scholar] [CrossRef]
- Jayamanne, V.S.; Adams, M.R. Determination of survival, identity and stress resistance of probiotic bifidobacteria in bio-yoghurts. Lett. Appl. Microbiol. 2006, 42, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.P.; Ding, W.K.; Fallourd, M.J.; Leyer, G. Improving the stability of probiotic bacteria in model fruit juices using vitamins and antioxidants. J. Food Sci. 2010, 75, M278–M282. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Sakaguchi, K.; Suzuki, T. Acquired tolerance to oxidative stress in Bifidobacterium longum 105-A via expression of a catalase gene. Appl. Environ. Microbiol. 2012, 78, 2988–2990. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.C.; Castro, M.H.; Malcata, F.X.; Kirby, R.M. Survival of Lactobacillus delbrueckii ssp. bulgaricus following spray-drying. J. Dairy Sci. 1995, 78, 1025–1031. [Google Scholar]
- Champagne, C.P.; Raymond, Y.; Gagnon, R. Viability of Lactobacillus rhamnosus R0011 in an apple-based fruit juice under simulated storage conditions at the consumer level. J. Food Sci. 2008, 73, M221–M226. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.K.; Shah, N.P. Survival of free and microencapsulated probiotic bacteria in orange and apple juices. Int. Food Res. J. 2008, 15, 219–232. [Google Scholar]
- Nualkaekul, S.; Salmeron, I.; Charalampopoulos, D. Investigation of the factors influencing the survival of Bifidobacterium longum in model acidic solutions and fruit juices. Food Chem. 2011, 129, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, V.M.; Ross, P.; Fitzgerald, G.F. Assessing the acid tolerance and the technological robustness of probiotic cultures for fortification in fruit juices. Innov. Food Sci. Emerg. Technol. 2007, 8, 279–284. [Google Scholar] [CrossRef]
- Hossain, M.I.; Sadekuzzaman, M.; Ha, S.-D. Probiotics as potential alternative biocontrol agents in the agriculture and food industries: A review. Food Res. Int. 2017, 100, 63–73. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; de Oliveira Coelho, B.; Magalhães Júnior, A.I.; Thomaz-Soccol, V.; Soccol, C.R. How to select a probiotic? A review and update of methods and criteria. Biotechnol. Adv. 2018, 36, 2060–2076. [Google Scholar] [CrossRef]
- Kołożyn-Krajewska, D.; Dolatowski, Z.J. Probiotic meat products and human nutrition. Process Biochem. 2012, 47, 1761–1772. [Google Scholar] [CrossRef]
- Speranza, B.; Racioppo, A.; Beneduce, L.; Bevilacqua, A.; Sinigaglia, M.; Corbo, M.R. Autochthonous lactic acid bacteria with probiotic aptitudes as starter cultures for fish-based products. Food Microbiol. 2017, 65, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Rouhi, M.; Sohrabvandi, S.; Mortazavian, A.M. Probiotic Fermented Sausage: Viability of Probiotic Microorganisms and Sensory Characteristics. Crit. Rev. Food Sci. 2013, 53, 331–348. [Google Scholar] [CrossRef] [PubMed]
- Vuyst, L.D.; Falony, G.; Leroy, F. Probiotics in fermented sausages. Meat Sci. 2008, 80, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.; Toner, M. Cryo-injury and biopreservation. Ann. N. Y. Acad. Sci. 2005, 1066, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Gill, C.O. Microbiology of frozen foods. In Handbook of Frozen Food Processing and Packaging; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Jay, J.M.; Loessner, M.J.; Golden, D.A. Modern Food Microbiology; Springer Science & Business Media: Berlin, Germany, 2005. [Google Scholar]
- Santivarangkna, C.; Kulozik, U.; Foerst, P. Effect of carbohydrates on the survival of Lactobacillus helveticus during vacuum drying. Lett. Appl. Microbiol. 2006, 42, 271–276. [Google Scholar] [CrossRef]
- Santivarangkna, C.; Kulozik, U.; Foerst, P. Inactivation mechanisms of lactic acid starter cultures preserved by drying processes. J. Appl. Microbiol. 2008, 105, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Schutyser, M.A.I.; Perdana, J.; Boom, R.M. Single droplet drying for optimal spray drying of enzymes and probiotics. Trends Food Sci. Technol. 2012, 27, 73–82. [Google Scholar] [CrossRef]
- Fonseca, F.; Cenard, S.; Passot, S. Freeze-Drying of Lactic Acid Bacteria. In Cryopreservation and Freeze-Drying Protocols; Wolkers, W.F., Oldenhof, H., Eds.; Springer: New York, NY, USA, 2015; Volume 1257, pp. 477–488. [Google Scholar]
- Fu, N.; Huang, S.; Xiao, J.; Chen, X.D. Chapter Six—Producing powders containing active dry probiotics with the aid of spray drying. In Advances in Food and Nutrition Research; Toldrá, F., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 85, pp. 211–262. [Google Scholar]
- Bosnea, L.A.; Kourkoutas, Y.; Albantaki, N.; Tzia, C.; Koutinas, A.A.; Kanellaki, M. Functionality of freeze-dried L. casei cells immobilized on wheat grains. LWT Food Sci. Technol. 2009, 42, 1696–1702. [Google Scholar] [CrossRef]
- Terpou, A.; Gialleli, A.-I.; Bekatorou, A.; Dimitrellou, D.; Ganatsios, V.; Barouni, E.; Koutinas, A.A.; Kanellaki, M. Sour milk production by wheat bran supported probiotic biocatalyst as starter culture. Food Bioprod. Process 2017, 101, 184–192. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Kirjavainen, P.V.; Shortt, C.; Salminen, S. Probiotics: Mechanisms and established effects. Int. Dairy J. 1999, 9, 43–52. [Google Scholar] [CrossRef]
- O’Brien, J.; Crittenden, R.; Ouwehand, A.C.; Salminen, S. Safety evaluation of probiotics. Trends Food Sci. Technol. 1999, 10, 418–424. [Google Scholar] [CrossRef]
- Sanders, M.E.; Klaenhammer, T.R.; Ouwehand, A.C.; Pot, B.; Johansen, E.; Heimbach, J.T.; Marco, M.L.; Tennilä, J.; Ross, R.P.; Franz, C.; et al. Effects of genetic, processing, or product formulation changes on efficacy and safety of probiotics. Ann. N. Y. Acad. Sci. 2014, 1309, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.T.; Allan-Wojtas, P.M.; Jin, Y.L.; Paulson, A.T. Survival of Ca-alginate microencapsulated Bifidobacterium spp. in milk and simulated gastrointestinal conditions. Food Microbiol. 2002, 19, 35–45. [Google Scholar] [CrossRef]
- Sabikhi, L.; Babu, R.; Thompkinson, D.K.; Kapila, S. Resistance of microencapsulated Lactobacillus acidophilus LA1 to processing treatments and simulated gut conditions. Food Bioprocess Technol. 2010, 3, 586–593. [Google Scholar] [CrossRef]
- Brinques, G.B.; Ayub, M.A.Z. Effect of microencapsulation on survival of Lactobacillus plantarum in simulated gastrointestinal conditions, refrigeration, and yogurt. J. Food Eng. 2011, 103, 123–128. [Google Scholar] [CrossRef]
- Vijayakumar, M.; Ilavenil, S.; Kim, D.H.; Arasu, M.V.; Priya, K.; Choi, K.C. In-vitro assessment of the probiotic potential of Lactobacillus plantarum KCC-24 isolated from Italian rye-grass (Lolium multiflorum) forage. Anaerobe 2015, 32, 90–97. [Google Scholar] [CrossRef]
- Turková, K.; Mavrič, A.; Narat, M.; Rittich, B.; Španová, A.; Rogelj, I.; Matijašić, B. Evaluation of Lactobacillus strains for selected probiotic properties. Folia Microbiol. 2013, 58, 261–267. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Evans, C.A.; Adams, M.C.; Baines, S.K. In vitro analysis of gastrointestinal tolerance and intestinal cell adhesion of probiotics in goat’s milk ice cream and yogurt. Food Res. Int. 2012, 49, 619–625. [Google Scholar] [CrossRef]
- Terpou, A.; Bekatorou, A.; Kanellaki, M.; Koutinas, A.A.; Nigam, P. Enhanced probiotic viability and aromatic profile of yogurts produced using wheat bran (Triticum aestivum) as cell immobilization carrier. Process Biochem. 2017, 55, 1–10. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Bosnea, L.; Kanellaki, M.; Kopsahelis, N. Novel frozen yogurt production fortified with sea buckthorn berries and probiotics. LWT Food Sci. Technol. 2019, 105, 242–249. [Google Scholar] [CrossRef]
- Clark, P.A.; Cotton, L.N.; Martin, J.H. Selection of bifidobacteria for use as dietary adjuncts in cultured dairy foods: II—Tolerance to simulated pH of human stomachs. Cult. Dairy Prod. J. 1993, 28, 11–14. [Google Scholar]
- Liong, M.T.; Shah, N.P. Acid and bile tolerance and cholesterol removal ability of lactobacilli strains. J. Dairy Sci. 2005, 88, 55–66. [Google Scholar] [CrossRef]
- Lo Curto, A.; Pitino, I.; Mandalari, G.; Dainty, J.R.; Faulks, R.M.; John Wickham, M.S. Survival of probiotic lactobacilli in the upper gastrointestinal tract using an in vitro gastric model of digestion. Food Microbiol. 2011, 28, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Muruzović, M.Ž.; Mladenović, K.G.; Čomić, L.R. In vitro evaluation of resistance to environmental stress by planktonic and biofilm form of lactic acid bacteria isolated from traditionally made cheese from Serbia. Food Biosci. 2018, 23, 54–59. [Google Scholar] [CrossRef]
- Beales, N. Adaptation of microorganisms to cold temperatures, weak acid preservatives, low pH, and osmotic stress: A review. Compr. Rev. Food Sci. Food Saf. 2004, 3, 1–20. [Google Scholar] [CrossRef]
- Upadrasta, A.; O’Sullivan, L.; O’Sullivan, O.; Sexton, N.; Lawlor, P.G.; Hill, C.; Fitzgerald, G.F.; Stanton, C.; Ross, R.P. The Effect of Dietary Supplementation with Spent Cider Yeast on the Swine Distal Gut Microbiome. PLoS ONE 2013, 8, e75714. [Google Scholar] [CrossRef]
- Saarela, M.; Rantala, M.; Hallamaa, K.; Nohynek, L.; Virkajärvi, I.; Mättö, J. Stationary-phase acid and heat treatments for improvement of the viability of probiotic lactobacilli and bifidobacteria. J. Appl. Microbiol. 2004, 96, 1205–1214. [Google Scholar] [CrossRef]
- Pénicaud, C.; Monclus, V.; Perret, B.; Passot, S.; Fonseca, F. Life cycle assessment of the production of stabilized lactic acid bacteria for the environmentally-friendly preservation of living cells. J. Clean. Prod. 2018, 184, 847–858. [Google Scholar] [CrossRef]
- Sauer, M.; Russmayer, H.; Grabherr, R.; Peterbauer, C.K.; Marx, H. The Efficient Clade: Lactic Acid Bacteria for Industrial Chemical Production. Trends Biotechnol. 2017, 35, 756–769. [Google Scholar] [CrossRef]
- Ruiz, L.; Ruas-Madiedo, P.; Gueimonde, M.; De Los Reyes-Gavilán, C.G.; Margolles, A.; Sánchez, B. How do bifidobacteria counteract environmental challenges? Mechanisms involved and physiological consequences. Genes Nutr. 2011, 6, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, R.; Sohrabvandi, S.; Mohammad Mortazavian, A. The starter culture characteristics of probiotic microorganisms in fermented milks. Eng. Life Sci. 2012, 12, 399–409. [Google Scholar] [CrossRef]
- Bron, P.A.; Marcelli, B.; Mulder, J.; van der Els, S.; Morawska, L.P.; Kuipers, O.P.; Kok, J.; Kleerebezem, M. Renaissance of traditional DNA transfer strategies for improvement of industrial lactic acid bacteria. Curr. Opin. Biotechnol. 2019, 56, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Panoff, J.M.; Thammavongs, B.; Guéguen, M. Cryoprotectants lead to phenotypic adaptation to freeze-thaw stress in Lactobacillus delbrueckii ssp. bulgaricus CIP 101027T. Cryobiology 2000, 40, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Gouesbet, G.; Jan, G.; Boyaval, P. Lactobacillus delbrueckii ssp. bulgaricus thermotolerance. Lait 2001, 81, 301–309. [Google Scholar] [CrossRef]
- Conrad, P.B.; Miller, D.P.; Cielenski, P.R.; De Pablo, J.J. Stabilization and preservation of Lactobacillus acidophilus in saccharide matrices. Cryobiology 2000, 41, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Parkar, S.G.; Redgate, E.L.; McGhie, T.K.; Hurst, R.D. In vitro studies of modulation of pathogenic and probiotic bacterial proliferation and adhesion to intestinal cells by blackcurrant juices. J. Funct. Foods 2014, 8, 35–44. [Google Scholar] [CrossRef]
- Van de Guchte, M.; Serror, P.; Chervaux, C.; Smokvina, T.; Ehrlich, S.D.; Maguin, E. Stress responses in lactic acid bacteria. Antonie van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2002, 82, 187–216. [Google Scholar] [CrossRef]
- De Angelis, M.; Gobbetti, M. Environmental stress responses in Lactobacillus: A review. Proteomics 2004, 4, 106–122. [Google Scholar] [CrossRef]
- Serrazanetti, D.I.; Guerzoni, M.E.; Corsetti, A.; Vogel, R. Metabolic impact and potential exploitation of the stress reactions in lactobacilli. Food Microbiol. 2009, 26, 700–711. [Google Scholar] [CrossRef]
- Alonso García, E.; Pérez Montoro, B.; Benomar, N.; Castillo-Gutiérrez, S.; Estudillo-Martínez, M.D.; Knapp, C.W.; Abriouel, H. New insights into the molecular effects and probiotic properties of Lactobacillus pentosus pre-adapted to edible oils. LWT Food Sci. Technol. 2019, 109, 153–162. [Google Scholar] [CrossRef]
- Capozzi, V.; Arena, M.P.; Russo, P.; Spano, G.; Fiocco, D. Chapter 16—Stressors and Food Environment: Toward Strategies to Improve Robustness and Stress Tolerance in Probiotics. In Probiotics, Prebiotics, and Synbiotics; Watson, R.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 245–256. [Google Scholar]
- Settachaimongkon, S.; van Valenberg, H.J.F.; Winata, V.; Wang, X.; Nout, M.J.R.; van Hooijdonk, T.C.M.; Zwietering, M.H.; Smid, E.J. Effect of sublethal preculturing on the survival of probiotics and metabolite formation in set-yoghurt. Food Microbiol. 2015, 49, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Sunny-Roberts, E.O.; Ananta, E.; Knorr, D. Flow cytometry assessment of Lactobacillus rhamnosus GG (ATCC 53103) response to non-electrolytes stress. Nutr. Food Sci. 2007, 37, 184–200. [Google Scholar] [CrossRef][Green Version]
- Gandhi, A.; Shah, N.P. Effect of salt on cell viability and membrane integrity of Lactobacillus acidophilus, Lactobacillus casei and Bifidobacterium longum as observed by flow cytometry. Food Microbiol. 2015, 49, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.P. Probiotic bacteria: Selective enumeration and survival in dairy foods. J. Dairy Sci. 2000, 83, 894–907. [Google Scholar] [CrossRef]
- Ahn, J.B.; Hwang, H.J.; Park, J.H. Physiological responses of oxygen-tolerant anaerobic Bifidobacterium longum under oxygen. J. Microbiol. Biotechnol. 2001, 11, 443–451. [Google Scholar]
- Talwalkar, A.; Kailasapathy, K. The role of oxygen in the viability of probiotic bacteria with reference to L. acidophilus and Bifidobacterium spp. Curr. Issues Intest. Microbiol. 2004, 5, 1–8. [Google Scholar]
- Chen, M.-J.; Tang, H.-Y.; Chiang, M.-L. Effects of heat, cold, acid and bile salt adaptations on the stress tolerance and protein expression of kefir-isolated probiotic Lactobacillus kefiranofaciens M1. Food Microbiol. 2017, 66, 20–27. [Google Scholar] [CrossRef]
- Pérez Montoro, B.; Benomar, N.; Caballero Gómez, N.; Ennahar, S.; Horvatovich, P.; Knapp, C.W.; Gálvez, A.; Abriouel, H. Proteomic analysis of Lactobacillus pentosus for the identification of potential markers involved in acid resistance and their influence on other probiotic features. Food Microbiol. 2018, 72, 31–38. [Google Scholar] [CrossRef]
- Desmond, C.; Stanton, C.; Fitzgerald, G.F.; Collins, K.; Paul Ross, R. Environmental adaptation of probiotic lactobacilli towards improvement of performance during spray drying. Int. Dairy J. 2001, 11, 801–808. [Google Scholar] [CrossRef]
- Desmond, C.; Ross, R.P.; O’Callaghan, E.; Fitzgerald, G.; Stanton, C. Improved survival of Lactobacillus paracasei NFBC 338 in spray-dried powders containing gum acacia. J. Appl. Microbiol. 2002, 93, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Ananta, E.; Knorr, D. Evidence on the role of protein biosynthesis in the induction of heat tolerance of Lactobacillus rhamnosus GG by pressure pre-treatment. Int. J. Food Microbiol. 2004, 96, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.; Castro, H.; Kirby, R. Inducible thermotolerance in Lactobacillus bulgaricus. Lett. Appl. Microbiol. 1994, 18, 218–221. [Google Scholar] [CrossRef]
- Korbekandi, H.; Mortazavian, A.M.; Iravani, S. Technology and stability of probiotic in fermented milks. In Probiotic and Prebiotic Foods: Technology, Stability and Benefits to the Human Health 2010; Nova Sciences Publishers: Hauppauge, NY, USA, 2010. [Google Scholar]
- da Cruz, A.G.; de Faria, J.A.F.; Van Dender, A.G.F. Packaging system and probiotic dairy foods. Food Res. Int. 2007, 40, 951–956. [Google Scholar] [CrossRef]
- Miller, C.W.; Nguyen, M.H.; Rooney, M.; Kailasapathy, K. The influence of packaging materials on the dissolved oxygen content of probiotic yoghurt. Packag. Technol. Sci. 2002, 15, 133–138. [Google Scholar] [CrossRef]
- Miller, C.W.; Nguyen, M.H.; Rooney, M.; Kailasapathy, K. The control of dissolved oxygen content in probiotic yoghurts by alternative packaging materials. Packag. Technol. Sci. 2003, 16, 61–67. [Google Scholar] [CrossRef]
- Cruz, A.G.; Castro, W.F.; Faria, J.A.F.; Bolini, H.M.A.; Celeghini, R.M.S.; Raices, R.S.L.; Oliveira, C.A.F.; Freitas, M.Q.; Conte Júnior, C.A.; Mársico, E.T. Stability of probiotic yogurt added with glucose oxidase in plastic materials with different permeability oxygen rates during the refrigerated storage. Food Res. Int. 2013, 51, 723–728. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nature reviews. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Tian, Q.; Wang, T.-T.; Tang, X.; Han, M.-Z.; Leng, X.-J.; Mao, X.-Y. Developing a potential prebiotic of yogurt: Growth of Bifidobacterium and yogurt cultures with addition of glycomacropeptide hydrolysate. Int. J. Food Sci. Technol. 2015, 50, 120–127. [Google Scholar] [CrossRef]
- Shin, H.S.; Lee, J.H.; Pestka, J.J.; Ustunol, Z. Growth and viability of commercial Bifidobacterium spp in skim milk containing oligosaccharides and inulin. J. Food Sci. 2000, 65, 884–887. [Google Scholar] [CrossRef]
- Capela, P.; Hay, T.K.C.; Shah, N.P. Effect of cryoprotectants, prebiotics and microencapsulation on survival of probiotic organisms in yoghurt and freeze-dried yoghurt. Food Res. Int. 2006, 39, 203–211. [Google Scholar] [CrossRef]
- McComas, K.A.; Gilliland, S.E. Growth of Probiotic and Traditional Yogurt Cultures in Milk Supplemented with Whey Protein Hydrolysate. J. Food Sci. 2003, 68, 2090–2095. [Google Scholar] [CrossRef]
- Carvalho, A.S.; Silva, J.; Ho, P.; Teixeira, P.; Malcata, F.X.; Gibbs, P. Effects of Various Sugars Added to Growth and Drying Media upon Thermotolerance and Survival throughout Storage of Freeze-Dried Lactobacillus delbrueckii ssp. bulgaricus. Biotechnol. Prog. 2004, 20, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Dave, R.I.; Shah, N.P. Ingredient Supplementation Effects on Viability of Probiotic Bacteria in Yogurt. J. Dairy Sci. 1998, 81, 2804–2816. [Google Scholar] [CrossRef]
- Akalin, A.S.; Fenderya, S.; Akbulut, N. Viability and activity of bifidobacteria in yoghurt containing fructooligosaccharide during refrigerated storage. Int. J. Food Sci. Technol. 2004, 39, 613–621. [Google Scholar] [CrossRef]
- Ananta, E.; Volkert, M.; Knorr, D. Cellular injuries and storage stability of spray-dried Lactobacillus rhamnosus GG. Int. Dairy J. 2005, 15, 399–409. [Google Scholar] [CrossRef]
- Cordeiro, B.F.; Oliveira, E.R.; da Silva, S.H.; Savassi, B.M.; Acurcio, L.B.; Lemos, L.; Alves, J.L.; Carvalho Assis, H.; Vieira, A.T.; Faria, A.M.C.; et al. Whey Protein Isolate-Supplemented Beverage, Fermented by Lactobacillus casei BL23 and Propionibacterium freudenreichii 138, in the Prevention of Mucositis in Mice. Front. Microbiol. 2018, 9, 2035. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Wang, X.; Li, W.; Chen, L.; Zeng, X.; Huang, Q.; Hu, B. Enhancing the Viability of Lactobacillus plantarum as Probiotics through Encapsulation with High Internal Phase Emulsions Stabilized with Whey Protein Isolate Microgels. J. Agric. Food Chem. 2018, 66, 12335–12343. [Google Scholar] [CrossRef]
- Guo, M.; Yadav, M.P.; Jin, T.Z. Antimicrobial edible coatings and films from micro-emulsions and their food applications. Int. J. Food Microbiol. 2017, 263, 9–16. [Google Scholar] [CrossRef]
- Bambace, M.F.; Alvarez, M.V.; del Moreira, M.R. Novel functional blueberries: Fructo-oligosaccharides and probiotic lactobacilli incorporated into alginate edible coatings. Food Res. Int. 2019, 122, 653–660. [Google Scholar] [CrossRef]
- Ebrahimi, B.; Mohammadi, R.; Rouhi, M.; Mortazavian, A.M.; Shojaee-Aliabadi, S.; Koushki, M.R. Survival of probiotic bacteria in carboxymethyl cellulose-based edible film and assessment of quality parameters. LWT Food Sci. Technol. 2018, 87, 54–60. [Google Scholar] [CrossRef]
- Soukoulis, C.; Behboudi-Jobbehdar, S.; Macnaughtan, W.; Parmenter, C.; Fisk, I.D. Stability of Lactobacillus rhamnosus GG incorporated in edible films: Impact of anionic biopolymers and whey protein concentrate. Food Hydrocoll. 2017, 70, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Pavli, F.; Kovaiou, I.; Apostolakopoulou, G.; Kapetanakou, A.; Skandamis, P.; Nychas, G.E.; Tassou, C.; Chorianopoulos, N. Alginate-Based Edible Films Delivering Probiotic Bacteria to Sliced Ham Pretreated with High Pressure Processing. Int. J. Mol. Sci. 2017, 18, 1867. [Google Scholar] [CrossRef] [PubMed]
- Ningtyas, D.W.; Bhandari, B.; Bansal, N.; Prakash, S. The viability of probiotic Lactobacillus rhamnosus (non-encapsulated and encapsulated) in functional reduced-fat cream cheese and its textural properties during storage. Food Control 2019, 100, 8–16. [Google Scholar] [CrossRef]
- Kailasapathy, K. Protecting probiotic by microencapsulation. Microbial. Aust. 2003, 24, 30–31. [Google Scholar]
- Lee, K.Y.; Heo, T.R. Survival of Bifidobacterium longum immobilized in calcium alginate beads in simulated gastric juices and bile salt solution. Appl. Environ. Microbiol. 2000, 66, 869–873. [Google Scholar] [CrossRef] [PubMed]
- Muthukumarasamy, P.; Allan-Wojtas, P.; Holley, R.A. Stability of Lactobacillus reuteri in different types of microcapsules. J. Food Sci. 2006, 71, M20–M24. [Google Scholar] [CrossRef]
- Cabuk, B.; Harsa, S.T. Improved viability of Lactobacillus acidophilus NRRL-B 4495 during freeze-drying in whey protein-pullulan microcapsules. J. Microencapsul. 2015, 32, 300–307. [Google Scholar] [CrossRef][Green Version]
- Călinoiu, L.-F.; Ştefănescu, B.E.; Pop, I.D.; Muntean, L.; Vodnar, D.C. Chitosan Coating Applications in Probiotic Microencapsulation. Coatings 2019, 9, 194. [Google Scholar] [CrossRef]
- de Araújo Etchepare, M.; Raddatz, G.C.; de Moraes Flores, É.M.; Zepka, L.Q.; Jacob-Lopes, E.; Barin, J.S.; Ferreira Grosso, C.R.; de Menezes, C.R. Effect of resistant starch and chitosan on survival of Lactobacillus acidophilus microencapsulated with sodium alginate. LWT Food Sci. Technol. 2016, 65, 511–517. [Google Scholar] [CrossRef]
- Singh, P.; Medronho, B.; Alves, L.; da Silva, G.J.; Miguel, M.G.; Lindman, B. Development of carboxymethyl cellulose-chitosan hybrid micro-and macroparticles for encapsulation of probiotic bacteria. Carbohydr. Polym. 2017, 175, 87–95. [Google Scholar] [CrossRef]
- Colín-Cruz, M.A.; Pimentel-González, D.J.; Carrillo-Navas, H.; Alvarez-Ramírez, J.; Guadarrama-Lezama, A.Y. Co-encapsulation of bioactive compounds from blackberry juice and probiotic bacteria in biopolymeric matrices. LWT Food Sci. Technol. 2019, 110, 94–101. [Google Scholar] [CrossRef]
- Vega-Sagardía, M.; Rocha, J.; Sáez, K.; Smith, C.T.; Gutierrez-Zamorano, C.; García-Cancino, A. Encapsulation, with and without oil, of biofilm forming Lactobacillus fermentum UCO-979C strain in alginate-xanthan gum and its anti-Helicobacter pylori effect. J. Funct. Foods 2018, 46, 504–513. [Google Scholar] [CrossRef]
- Schoina, V.; Terpou, A.; Bosnea, L.; Kanellaki, M.; Nigam, P.S. Entrapment of Lactobacillus casei ATCC393 in the viscus matrix of Pistacia terebinthus resin for functional myzithra cheese manufacture. LWT Food Sci. Technol. 2018, 89, 441–448. [Google Scholar] [CrossRef]
- Terpou, A.; Nigam, P.S.; Bosnea, L.; Kanellaki, M. Evaluation of Chios mastic gum as antimicrobial agent and matrix forming material targeting probiotic cell encapsulation for functional fermented milk production. LWT Food Sci. Technol. 2018, 97, 109–116. [Google Scholar] [CrossRef]
- Heidebach, T.; Först, P.; Kulozik, U. Influence of casein-based microencapsulation on freeze-drying and storage of probiotic cells. J. Food Eng. 2010, 98, 309–316. [Google Scholar] [CrossRef]
- Phoem, A.N.; Chanthachum, S.; Voravuthikunchai, S.P. Preparation of eleutherine americana-alginate complex microcapsules and application in Bifidobacterium longum. Nutrients 2015, 7, 831–848. [Google Scholar] [CrossRef]
- Thantsha, M.S.; Labuschagne, P.W.; Mamvura, C.I. Supercritical CO2 interpolymer complex encapsulation improves heat stability of probiotic bifidobacteria. World J. Microbiol. Biotechnol. 2014, 30, 479–486. [Google Scholar] [CrossRef]
- Cook, M.T.; Tzortzis, G.; Charalampopoulos, D.; Khutoryanskiy, V.V. Microencapsulation of a synbiotic into PLGA/alginate multiparticulate gels. Int. J. Pharm. 2014, 466, 400–408. [Google Scholar] [CrossRef]
- Duongthingoc, D.; George, P.; Katopo, L.; Gorczyca, E.; Kasapis, S. Effect of whey protein agglomeration on spray dried microcapsules containing Saccharomyces boulardii. Food Chem. 2013, 141, 1782–1788. [Google Scholar] [CrossRef]
- Bosnea, L.A.; Moschakis, T.; Biliaderis, C.G. Complex Coacervation as a Novel Microencapsulation Technique to Improve Viability of Probiotics Under Different Stresses. Food Bioprocess Technol. 2014, 7, 2767–2781. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, F.; Han, D.; Zhao, Y.; Liu, Z.; Lei, H.; Song, Y.; Huang, X.; Li, X.; Ma, A.; et al. Preparation and optimization of soy protein isolate–high methoxy pectin microcapsules loaded with Lactobacillus delbrueckii. Int. J. Food Sci. Technol. 2014, 49, 1287–1293. [Google Scholar] [CrossRef]
- Singh, P.; Medronho, B.; dos Santos, T.; Nunes-Correia, I.; Granja, P.; Miguel, M.G.; Lindman, B. On the viability, cytotoxicity and stability of probiotic bacteria entrapped in cellulose-based particles. Food Hydrocoll. 2018, 82, 457–465. [Google Scholar] [CrossRef]
- Rodríguez-Huezo, M.E.; Estrada-Fernández, A.G.; García-Almendárez, B.E.; Ludeña-Urquizo, F.; Campos-Montiel, R.G.; Pimentel-González, D.J. Viability of Lactobacillus plantarum entrapped in double emulsion during Oaxaca cheese manufacture, melting and simulated intestinal conditions. LWT Food Sci. Technol. 2014, 59, 768–773. [Google Scholar] [CrossRef]
- Sohail, A.; Turner, M.S.; Coombes, A.; Bostrom, T.; Bhandari, B. Survivability of probiotics encapsulated in alginate gel microbeads using a novel impinging aerosols method. Int. J. Food Microbiol. 2011, 145, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Sohail, A.; Turner, M.; Coombes, A.; Bhandari, B. The Viability of Lactobacillus rhamnosus GG and Lactobacillus acidophilus NCFM Following Double Encapsulation in Alginate and Maltodextrin. Food Bioprocess Technol. 2013, 6, 2763–2769. [Google Scholar] [CrossRef]
- López-Rubio, A.; Sanchez, E.; Wilkanowicz, S.; Sanz, Y.; Lagaron, J.M. Electrospinning as a useful technique for the encapsulation of living bifidobacteria in food hydrocolloids. Food Hydrocoll. 2012, 28, 159–167. [Google Scholar] [CrossRef]
- Laelorspoen, N.; Wongsasulak, S.; Yoovidhya, T.; Devahastin, S. Microencapsulation of Lactobacillus acidophilus in zein–alginate core–shell microcapsules via electrospraying. J. Funct. Foods 2014, 7, 342–349. [Google Scholar] [CrossRef]
- Su, R.; Zhu, X.-L.; Fan, D.-D.; Mi, Y.; Yang, C.-Y.; Jia, X. Encapsulation of probiotic Bifidobacterium longum BIOMA 5920 with alginate–human-like collagen and evaluation of survival in simulated gastrointestinal conditions. Int. J. Biol. Macromol. 2011, 49, 979–984. [Google Scholar] [CrossRef]
- Yao, M.; Li, B.; Ye, H.; Huang, W.; Luo, Q.; Xiao, H.; McClements, D.J.; Li, L. Enhanced viability of probiotics (Pediococcus pentosaceus Li05) by encapsulation in microgels doped with inorganic nanoparticles. Food Hydrocoll. 2018, 83, 246–252. [Google Scholar] [CrossRef]
- Coghetto, C.C.; Flores, S.H.; Brinques, G.B.; Záchia Ayub, M.A. Viability and alternative uses of a dried powder, microencapsulated Lactobacillus plantarum without the use of cold chain or dairy products. LWT Food Sci. Technol. 2016, 71, 54–59. [Google Scholar] [CrossRef]
- Zaeim, D.; Sarabi-Jamab, M.; Ghorani, B.; Kadkhodaee, R.; Tromp, R.H. Electrospray assisted fabrication of hydrogel microcapsules by single-and double-stage procedures for encapsulation of probiotics. Food Bioprod. Process 2017, 102, 250–259. [Google Scholar] [CrossRef]
- Gomez-Mascaraque, L.G.; Morfin, R.C.; Pérez-Masiá, R.; Sanchez, G.; Lopez-Rubio, A. Optimization of electrospraying conditions for the microencapsulation of probiotics and evaluation of their resistance during storage and in-vitro digestion. LWT Food Sci. Technol. 2016, 69, 438–446. [Google Scholar] [CrossRef]
- Moayyedi, M.; Eskandari, M.H.; Rad, A.H.E.; Ziaee, E.; Khodaparast, M.H.H.; Golmakani, M.-T. Effect of drying methods (electrospraying, freeze drying and spray drying) on survival and viability of microencapsulated Lactobacillus rhamnosus ATCC 7469. J. Funct. Foods 2018, 40, 391–399. [Google Scholar] [CrossRef]
- Tee, W.F.; Nazaruddin, R.; Tan, Y.N.; Ayob, M.K. Effects of encapsulation on the viability of potential probiotic Lactobacillus plantarum exposed to high acidity condition and presence of bile salts. Food Sci. Technol. Int. 2014, 20, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Zhao, J.; Liu, X.; Tian, F.; Zhang, H.P.; Zhang, H.; Chen, W. Microencapsulation of Bifidobacterium bifidum F-35 in reinforced alginate microspheres prepared by emulsification/internal gelation. Int. J. Food Sci. Technol. 2011, 46, 1672–1678. [Google Scholar] [CrossRef]
- Vaziri, A.S.; Alemzadeh, I.; Vossoughi, M.; Khorasani, A.C. Co-microencapsulation of Lactobacillus plantarum and DHA fatty acid in alginate-pectin-gelatin biocomposites. Carbohydr. Polym. 2018, 199, 266–275. [Google Scholar] [CrossRef]
- Wang, J.; Korber, D.R.; Low, N.H.; Nickerson, M.T. Entrapment, survival and release of Bifidobacterium adolescentis within chickpea protein-based microcapsules. Food Res. Int. 2014, 55, 20–27. [Google Scholar] [CrossRef]
- Burgain, J.; Gaiani, C.; Cailliez-Grimal, C.; Jeandel, C.; Scher, J. Encapsulation of Lactobacillus rhamnosus GG in microparticles: Influence of casein to whey protein ratio on bacterial survival during digestion. Innov. Food Sci. Emerg. Technol. 2013, 19, 233–242. [Google Scholar] [CrossRef]
- Chitprasert, P.; Sudsai, P.; Rodklongtan, A. Aluminum carboxymethyl cellulose–rice bran microcapsules: Enhancing survival of Lactobacillus reuteri KUB-AC5. Carbohydr. Polym. 2012, 90, 78–86. [Google Scholar] [CrossRef]
- Chun, H.; Kim, C.H.; Cho, Y.H. Microencapsulation of Lactobacillus plantarum DKL 109 using External Ionic Gelation Method. Korean J. Food Sci. Anim. Resour. 2014, 34, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Sousa, S.; Gomes, A.M.; Pintado, M.M.; Silva, J.P.; Costa, P.; Amaral, M.H.; Duarte, A.C.; Rodrigues, D.; Rocha-Santos, T.A.P.; Freitas, A.C. Characterization of freezing effect upon stability of, probiotic loaded, calcium-alginate microparticles. Food Bioprod. Process 2015, 93, 90–97. [Google Scholar] [CrossRef]
- Kamalian, N.; Mirhosseini, H.; Mustafa, S.; Manap, M.Y.A. Effect of alginate and chitosan on viability and release behavior of Bifidobacterium pseudocatenulatum G4 in simulated gastrointestinal fluid. Carbohydr. Polym. 2014, 111, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.J.; Omura, M.H.; Cedran, M.F.; Dekker, R.F.H.; Barbosa-Dekker, A.M.; Garcia, S. Effect of natural polymers on the survival of Lactobacillus casei encapsulated in alginate microspheres. J. Microencapsul. 2017, 34, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.Y.; Zheng, W.; Dong, Q.Y.; Li, Z.H.; Shi, L.E.; Tang, Z.X. Activity of Encapsulated Lactobacillus bulgaricus in Alginate-whey Protein Microspheres. Braz. Arch. Biol. Technol. 2014, 57, 736–741. [Google Scholar] [CrossRef]
- Trabelsi, I.; Ayadi, D.; Bejar, W.; Bejar, S.; Chouayekh, H.; Ben Salah, R. Effects of Lactobacillus plantarum immobilization in alginate coated with chitosan and gelatin on antibacterial activity. Int. J. Biol. Macromol. 2014, 64, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.H.; Korber, D.R.; Low, N.H.; Nickerson, M.T. Development of extrusion-based legume protein isolate–alginate capsules for the protection and delivery of the acid sensitive probiotic, Bifidobacterium adolescentis. Food Res. Int. 2013, 54, 730–737. [Google Scholar] [CrossRef]
- Shi, L.-E.; Li, Z.-H.; Zhang, Z.-L.; Zhang, T.-T.; Yu, W.-M.; Zhou, M.-L.; Tang, Z.-X. Encapsulation of Lactobacillus bulgaricus in carrageenan-locust bean gum coated milk microspheres with double layer structure. LWT Food Sci. Technol. 2013, 54, 147–151. [Google Scholar] [CrossRef]
- Shi, L.-E.; Li, Z.-H.; Li, D.-T.; Xu, M.; Chen, H.-Y.; Zhang, Z.-L.; Tang, Z.-X. Encapsulation of probiotic Lactobacillus bulgaricus in alginate–milk microspheres and evaluation of the survival in simulated gastrointestinal conditions. J. Food Eng. 2013, 117, 99–104. [Google Scholar] [CrossRef]
- Lotfipour, F.; Mirzaeei, S.; Maghsoodi, M. Preparation and Characterization of Alginate and Psyllium Beads Containing Lactobacillus acidophilus. Sci. World J. 2012, 2012. [Google Scholar] [CrossRef]
- Bajracharya, P.; Islam, M.A.; Jiang, T.; Kang, S.-K.; Choi, Y.-J.; Cho, C.-S. Effect of microencapsulation of Lactobacillus salivarus 29 into alginate/chitosan/alginate microcapsules on viability and cytokine induction. J. Microencapsul. 2012, 29, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Mutukumira, A.; Lee, S.; Maddox, I.; Shu, Q. Functional properties of free and encapsulated Lactobacillus reuteri DPC16 during and after passage through a simulated gastrointestinal tract. World J. Microbiol. Biotechnol. 2012, 28, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Satish Kumar, R.; Yuvaraj, N.; Paari, K.A.; Pattukumar, V.; Arul, V. Effect of cryopreservation and microencapsulation of lactic acid bacterium Enterococcus faecium MC13 for long-term storage. Biochem. Eng. J. 2011, 58, 140–147. [Google Scholar] [CrossRef]
- Doherty, S.B.; Gee, V.L.; Ross, R.P.; Stanton, C.; Fitzgerald, G.F.; Brodkorb, A. Development and characterisation of whey protein micro-beads as potential matrices for probiotic protection. Food Hydrocoll. 2011, 25, 1604–1617. [Google Scholar] [CrossRef]
- Klemmer, K.J.; Korber, D.R.; Low, N.H.; Nickerson, M.T. Pea protein-based capsules for probiotic and prebiotic delivery. Int. J. Food Sci. Technol. 2011, 46, 2248–2256. [Google Scholar] [CrossRef]
- Schell, D.; Beermann, C. Fluidized bed microencapsulation of Lactobacillus reuteri with sweet whey and shellac for improved acid resistance and in-vitro gastro-intestinal survival. Food Res. Int. 2014, 62, 308–314. [Google Scholar] [CrossRef]
- Marques da Silva, T.; Jacob Lopes, E.; Codevilla, C.F.; Cichoski, A.J.; Flores, É.M.d.M.; Motta, M.H.; da Silva, C.B.; Grosso, C.R.F.; de Menezes, C.R. Development and characterization of microcapsules containing Bifidobacterium Bb-12 produced by complex coacervation followed by freeze drying. LWT Food Sci. Technol. 2018, 90, 412–417. [Google Scholar] [CrossRef]
- Wang, S.Y.; Ho, Y.F.; Chen, Y.P.; Chen, M.J. Effects of a novel encapsulating technique on the temperature tolerance and anti-colitis activity of the probiotic bacterium Lactobacillus kefiranofaciens M1. Food Microbiol. 2015, 46, 494–500. [Google Scholar] [CrossRef]
- Shaharuddin, S.; Muhamad, I.I. Microencapsulation of alginate-immobilized bagasse with Lactobacillus rhamnosus NRRL 442: Enhancement of survivability and thermotolerance. Carbohydr. Polym. 2015, 119, 173–181. [Google Scholar] [CrossRef]
- Gebara, C.; Chaves, K.S.; Ribeiro, M.C.E.; Souza, F.N.; Grosso, C.R.F.; Gigante, M.L. Viability of Lactobacillus acidophilus La5 in pectin–whey protein microparticles during exposure to simulated gastrointestinal conditions. Food Res. Int. 2013, 51, 872–878. [Google Scholar] [CrossRef]
- Priya, A.J.; Vijayalakshmi, S.P.; Raichur, A.M. Enhanced Survival of Probiotic Lactobacillus acidophilus by Encapsulation with Nanostructured Polyelectrolyte Layers through Layer-by-Layer Approach. J. Agric. Food Chem. 2011, 59, 11838–11845. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.B.; Vaidyanathan, M.; Radhakrishnan, K.; Raichur, A.M. Enhanced viability of probiotic Saccharomyces boulardii encapsulated by layer-by-layer approach in pH responsive chitosan-dextran sulfate polyelectrolytes. J. Food Eng. 2014, 136, 1–8. [Google Scholar] [CrossRef]
- Nag, A.; Han, K.-S.; Singh, H. Microencapsulation of probiotic bacteria using pH-induced gelation of sodium caseinate and gellan gum. Int. Dairy J. 2011, 21, 247–253. [Google Scholar] [CrossRef]
- Okuro, P.K.; Thomazini, M.; Balieiro, J.C.C.; Liberal, R.D.; Fávaro-Trindade, C.S. Co-encapsulation of Lactobacillus acidophilus with inulin or polydextrose in solid lipid microparticles provides protection and improves stability. Food Res. Int. 2013, 53, 96–103. [Google Scholar] [CrossRef]
- de Pedroso, D.L.; Thomazini, M.; Heinemann, R.J.B.; Favaro-Trindade, C.S. Protection of Bifidobacterium lactis and Lactobacillus acidophilus by microencapsulation using spray-chilling. Int. Dairy J. 2012, 26, 127–132. [Google Scholar] [CrossRef]
- Arslan-Tontul, S.; Erbas, M. Single and double layered microencapsulation of probiotics by spray drying and spray chilling. LWT Food Sci. Technol. 2017, 81, 160–169. [Google Scholar] [CrossRef]
- Rajam, R.; Anandharamakrishnan, C. Microencapsulation of Lactobacillus plantarum (MTCC 5422) with fructooligosaccharide as wall material by spray drying. LWT Food Sci. Technol. 2015, 60, 773–780. [Google Scholar] [CrossRef]
- Jantzen, M.; Göpel, A.; Beermann, C. Direct spray drying and microencapsulation of probiotic Lactobacillus reuteri from slurry fermentation with whey. J. Appl. Microbiol. 2013, 115, 1029–1036. [Google Scholar] [CrossRef]
- Bustos, P.; Bórquez, R. Influence of Osmotic Stress and Encapsulating Materials on the Stability of Autochthonous Lactobacillus plantarum after Spray Drying. Dry Technol. 2013, 31, 57–66. [Google Scholar] [CrossRef]
- Fritzen-Freire, C.B.; Prudêncio, E.S.; Pinto, S.S.; Muñoz, I.B.; Amboni, R.D.M.C. Effect of microencapsulation on survival of Bifidobacterium BB-12 exposed to simulated gastrointestinal conditions and heat treatments. LWT Food Sci. Technol. 2013, 50, 39–44. [Google Scholar] [CrossRef]
- De Castro-Cislaghi, F.P.; Silva, C.D.R.E.; Fritzen-Freire, C.B.; Lorenz, J.G.; Sant’Anna, E.S. Bifidobacterium Bb-12 microencapsulated by spray drying with whey: Survival under simulated gastrointestinal conditions, tolerance to NaCl, and viability during storage. J. Food Eng. 2012, 113, 186–193. [Google Scholar] [CrossRef]
- Rajam, R.; Karthik, P.; Parthasarathi, S.; Joseph, G.S.; Anandharamakrishnan, C. Effect of whey protein–alginate wall systems on survival of microencapsulated Lactobacillus plantarum in simulated gastrointestinal conditions. J. Funct. Foods 2012, 4, 891–898. [Google Scholar] [CrossRef]
- Maciel, G.M.; Chaves, K.S.; Grosso, C.R.F.; Gigante, M.L. Microencapsulation of Lactobacillus acidophilus La-5 by spray-drying using sweet whey and skim milk as encapsulating materials. J. Dairy Sci. 2014, 97, 1991–1998. [Google Scholar] [CrossRef] [PubMed]
- Avila-Reyes, S.V.; Garcia-Suarez, F.J.; Jiménez, M.T.; San Martín-Gonzalez, M.F.; Bello-Perez, L.A. Protection of L. rhamnosus by spray-drying using two prebiotics colloids to enhance the viability. Carbohydr. Polym. 2014, 102, 423–430. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, R.C.S.; Finkler, L.; Finkler, C.L.L. Microencapsulation of Lactobacillus casei by spray drying. J. Microencapsul. 2014, 31, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.; Sousa, S.; Rocha-Santos, T.; Silva, J.P.; Sousa Lobo, J.M.; Costa, P.; Amaral, M.H.; Pintado, M.M.; Gomes, A.M.; Malcata, F.X.; et al. Influence of l-cysteine, oxygen and relative humidity upon survival throughout storage of probiotic bacteria in whey protein-based microcapsules. Int. Dairy J. 2011, 21, 869–876. [Google Scholar] [CrossRef]
- Thantsha, M.S.; Guest, J.; Mputle, I. Comparison of different methods for release of Bifidobacterium longum Bb46 from the poly(vinylpyrrolidone)-poly(vinylacetate-co-crotonic acid) interpolymer complex matrix, and the effect of grinding on the microparticles. World J. Microbiol. Biotechnol. 2011, 27, 2443–2448. [Google Scholar] [CrossRef][Green Version]
- De Prisco, A.; Maresca, D.; Ongeng, D.; Mauriello, G. Microencapsulation by vibrating technology of the probiotic strain Lactobacillus reuteri DSM 17938 to enhance its survival in foods and in gastrointestinal environment. LWT Food Sci. Technol. 2015, 61, 452–462. [Google Scholar] [CrossRef]
- Praepanitchai, O.-A.; Noomhorm, A.; Anal, A.K. Survival and Behavior of Encapsulated Probiotics (Lactobacillus plantarum) in Calcium-Alginate-Soy Protein Isolate-Based Hydrogel Beads in Different Processing Conditions (pH and Temperature) and in Pasteurized Mango Juice. Biomed. Res. Int. 2019, 2019, 8. [Google Scholar] [CrossRef]
- Mortazavian, A.; Razavi, S.H.; Ehsani, M.R.; Sohrabvandi, S. Principles and methods of microencapsulation of probiotic microorganisms. Iran. J. Biotechnol. 2007, 5, 1–18. [Google Scholar]
- Champagne, C.P.; Fustier, P. Microencapsulation for the improved delivery of bioactive compounds into foods. Curr. Opin. Biotechnol. 2007, 18, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Behboudi-Jobbehdar, S.; Soukoulis, C.; Yonekura, L.; Fisk, I. Optimization of Spray-Drying Process Conditions for the Production of Maximally Viable Microencapsulated L. acidophilus NCIMB 701748. Dry Technol. 2013, 31, 1274–1283. [Google Scholar] [CrossRef]
- Fritzen-Freire, C.B.; Prudêncio, E.S.; Amboni, R.D.M.C.; Pinto, S.S.; Negrão-Murakami, A.N.; Murakami, F.S. Microencapsulation of bifidobacteria by spray drying in the presence of prebiotics. Food Res. Int. 2012, 45, 306–312. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, L.; Lobato-Calleros, C.; Pimentel-González, D.J.; Vernon-Carter, E.J. Lactobacillus plantarum protection by entrapment in whey protein isolate: κ-carrageenan complex coacervates. Food Hydrocoll. 2014, 36, 181–188. [Google Scholar] [CrossRef]
- Ranadheera, C.S.; Evans, C.A.; Adams, M.C.; Baines, S.K. Microencapsulation of Lactobacillus acidophilus LA-5, Bifidobacterium animalis subsp. lactis BB-12 and Propionibacterium jensenii 702 by spray drying in goat’s milk. Small Rumin. Res. 2015, 123, 155–159. [Google Scholar] [CrossRef]
- Ying, D.; Schwander, S.; Weerakkody, R.; Sanguansri, L.; Gantenbein-Demarchi, C.; Augustin, M.A. Microencapsulated Lactobacillus rhamnosus GG in whey protein and resistant starch matrices: Probiotic survival in fruit juice. J. Funct. Foods 2013, 5, 98–105. [Google Scholar] [CrossRef]
- Ivanovska, T.P.; Petrushevska-Tozi, L.; Grozdanov, A.; Petkovska, R.; Hadjieva, J.; Popovski, E.; Stafilov, T.; Mladenovska, K. From optimization of synbiotic microparticles prepared by spray-drying to development of new functional carrot juice. Chem. Ind. Chem. Eng. Q. 2014, 20, 549–564. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Sada, A.; Orlando, P. Fermentative ability of alginate-prebiotic encapsulated Lactobacillus acidophilus and survival under simulated gastrointestinal conditions. J. Funct. Foods 2009, 1, 319–323. [Google Scholar] [CrossRef]
- Amine, K.M.; Champagne, C.P.; Raymond, Y.; St-Gelais, D.; Britten, M.; Fustier, P.; Salmieri, S.; Lacroix, M. Survival of microencapsulated Bifidobacterium longum in Cheddar cheese during production and storage. Food Control 2014, 37, 193–199. [Google Scholar] [CrossRef]
- Muthukumarasamy, P.; Holley, R.A. Microbiological and sensory quality of dry fermented sausages containing alginate-microencapsulated Lactobacillus reuteri. Int. J. Food Microbiol. 2006, 111, 164–169. [Google Scholar] [CrossRef]
- Rodrigues, D.; Sousa, S.; Gomes, A.; Pintado, M.; Silva, J.; Costa, P.; Amaral, M.; Rocha-Santos, T.; Freitas, A. Storage Stability of Lactobacillus paracasei as Free Cells or Encapsulated in Alginate-Based Microcapsules in Low pH Fruit Juices. Food Bioprocess Technol. 2012, 5, 2748–2757. [Google Scholar] [CrossRef]
- Doherty, S.B.; Auty, M.A.; Stanton, C.; Ross, R.P.; Fitzgerald, G.F.; Brodkorb, A. Application of whey protein micro-bead coatings for enhanced strength and probiotic protection during fruit juice storage and gastric incubation. J. Microencapsul. 2012, 29, 713–728. [Google Scholar] [CrossRef] [PubMed]
- Bhat, A.R.; Irorere, V.U.; Bartlett, T.; Hill, D.; Kedia, G.; Charalampopoulos, D.; Nualkaekul, S.; Radecka, I. Improving survival of probiotic bacteria using bacterial poly-gamma-glutamic acid. Int. J. Food Microbiol. 2015, 196, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Nualkaekul, S.; Cook, M.T.; Khutoryanskiy, V.V.; Charalampopoulos, D. Influence of encapsulation and coating materials on the survival of Lactobacillus plantarum and Bifidobacterium longum in fruit juices. Food Res. Int. 2013, 53, 304–311. [Google Scholar] [CrossRef]
- Homayouni, A.; Azizi, A.; Ehsani, M.R.; Yarmand, M.S.; Razavi, S.H. Effect of microencapsulation and resistant starch on the probiotic survival and sensory properties of synbiotic ice cream. Food Chem. 2008, 111, 50–55. [Google Scholar] [CrossRef]
- Mortazavian, A.M.; Ehsani, M.R.; Azizi, A.; Razavi, S.H.; Mousavi, S.M.; Sohrabvandi, S.; Reinheimer, J.A. Viability of calcium-alginate-microencapsulated probiotic bacteria in Iranian yogurt drink (Doogh) during refrigerated storage and under simulated gastrointestinal conditions. Aust. J. Dairy Technol. 2008, 63, 25–30. [Google Scholar]
- ÖZer, B.; Uzun, Y.S.; Kirmaci, H.A. Effect of Microencapsulation on Viability of Lactobacillus acidophilus LA-5 and Bifidobacterium bifidum BB-12 During Kasar Cheese Ripening. Int. J. Dairy Technol. 2008, 61, 237–244. [Google Scholar] [CrossRef]
- GonzÁLez-SÁNchez, F.; Azaola, A.; GutiÉRrez-LÓPez, G.F.; HernÁNdez-SÁNchez, H. Viability of microencapsulated Bifidobacterium animalis ssp. lactis BB12 in kefir during refrigerated storage. Int. J. Dairy Technol. 2010, 63, 431–436. [Google Scholar] [CrossRef]
- Ortakci, F.; Sert, S. Stability of free and encapsulated Lactobacillus acidophilus ATCC 4356 in yogurt and in an artificial human gastric digestion system. J. Dairy Sci. 2012, 95, 6918–6925. [Google Scholar] [CrossRef]
- Santillo, A.; Bevilacqua, A.; Corbo, M.R.; Sevi, A.; Sinigaglia, M.; Albenzio, M. Functional Pecorino cheese production by using innovative lamb rennet paste. Innov. Food Sci. Emerg. Technol. 2014, 26, 389–396. [Google Scholar] [CrossRef]
- Santillo, A.; Albenzio, M.; Bevilacqua, A.; Corbo, M.R.; Sevi, A. Encapsulation of probiotic bacteria in lamb rennet paste: Effects on the quality of Pecorino cheese. J. Dairy Sci. 2012, 95, 3489–3500. [Google Scholar] [CrossRef] [PubMed]
- Nualkaekul, S.; Lenton, D.; Cook, M.T.; Khutoryanskiy, V.V.; Charalampopoulos, D. Chitosan coated alginate beads for the survival of microencapsulated Lactobacillus plantarum in pomegranate juice. Carbohydr. Polym. 2012, 90, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Özer, B.; Kirmaci, H.A.; Şenel, E.; Atamer, M.; Hayaloğlu, A. Improving the viability of Bifidobacterium bifidum BB-12 and Lactobacillus acidophilus LA-5 in white-brined cheese by microencapsulation. Int. Dairy J. 2009, 19, 22–29. [Google Scholar] [CrossRef]
- Ribeiro, M.C.E.; Chaves, K.S.; Gebara, C.; Infante, F.N.S.; Grosso, C.R.F.; Gigante, M.L. Effect of microencapsulation of Lactobacillus acidophilus LA-5 on physicochemical, sensory and microbiological characteristics of stirred probiotic yoghurt. Food Res. Int. 2014, 66, 424–431. [Google Scholar] [CrossRef]
- Mousa, A.; Liu, X.M.; Chen, Y.Q.; Zhang, H.; Chen, W. Evaluation of physiochemical, textural, microbiological and sensory characteristics in set yogurt reinforced by microencapsulated Bifidobacterium bifidum F-35. Int. J. Food Sci. Technol. 2014, 49, 1673–1679. [Google Scholar] [CrossRef]
- Ziar, H.; Gérard, P.; Riazi, A. Calcium alginate-resistant starch mixed gel improved the survival of Bifidobacterium animalis subsp. lactis Bb12 and Lactobacillus rhamnosus LBRE-LSAS in yogurt and simulated gastrointestinal conditions. Int. J. Food Sci. Technol. 2012, 47, 1421–1429. [Google Scholar] [CrossRef]
- Sandoval-Castilla, O.; Lobato-Calleros, C.; García-Galindo, H.S.; Alvarez-Ramírez, J.; Vernon-Carter, E.J. Textural properties of alginate–pectin beads and survivability of entrapped Lb. casei in simulated gastrointestinal conditions and in yoghurt. Food Res. Int. 2010, 43, 111–117. [Google Scholar] [CrossRef]
- Urbanska, A.M.; Bhathena, J.; Prakash, S. Live encapsulated Lactobacillus acidophilus cells in yogurt for therapeutic oral delivery: Preparation and in vitro analysis of alginate–chitosan microcapsules. This article is one of a selection of papers published in this special issue (part 1 of 2) on the Safety and Efficacy of Natural Health Products. Can. J. Physiol. Pharm. 2007, 85, 884–893. [Google Scholar] [CrossRef]
- Ahmadi, A.; Milani, E.; Madadlou, A.; Mortazavi, S.; Mokarram, R.; Salarbashi, D. Synbiotic yogurt-ice cream produced via incorporation of microencapsulated Lactobacillus acidophilus (la-5) and fructooligosaccharide. J. Food Sci. Technol. 2014, 51, 1568–1574. [Google Scholar] [CrossRef]
- Duenas, M.; Munoz-Gonzalez, I.; Cueva, C.; Jimenez-Giron, A.; Sanchez-Patan, F.; Santos-Buelga, C.; Moreno-Arribas, M.V.; Bartolome, B. A survey of modulation of gut microbiota by dietary polyphenols. Biomed. Res. Int. 2015, 2015, 850902. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Moreno, M.J.; Muñoz-Redondo, J.M.; Cuevas, F.J.; Marrufo-Curtido, A.; León, J.M.; Ramírez, P.; Moreno-Rojas, J.M. The influence of pre-fermentative maceration and ageing factors on ester profile and marker determination of Pedro Ximenez sparkling wines. Food Chem. 2017, 230, 697–704. [Google Scholar] [CrossRef] [PubMed]
- Quigley, E.M.M. Prebiotics and Probiotics in Digestive Health. Clin. Gastroenterol. Hepatol. 2019, 17, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Choque Delgado, G.T.; Tamashiro, W. Role of prebiotics in regulation of microbiota and prevention of obesity. Food Res. Int. 2018, 113, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Kolida, S.; Gibson, G.R. Synbiotics in health and disease. Annu. Rev. Food Sci. Technol. 2011, 2, 373–393. [Google Scholar] [CrossRef] [PubMed]
- Asto, E.; Mendez, I.; Audivert, S.; Farran-Codina, A.; Espadaler, J. The Efficacy of Probiotics, Prebiotic Inulin-Type Fructans, and Synbiotics in Human Ulcerative Colitis: A Systematic Review and Meta-Analysis. Nutrients 2019, 11, 293. [Google Scholar] [CrossRef] [PubMed]
- Notay, M.; Foolad, N.; Vaughn, A.R.; Sivamani, R.K. Probiotics, Prebiotics, and Synbiotics for the Treatment and Prevention of Adult Dermatological Diseases. Am. J. Clin. Dermatol. 2017, 18, 721–732. [Google Scholar] [CrossRef]
- Tian, X.; Pi, Y.-P.; Liu, X.-L.; Chen, H.; Chen, W.-Q. Supplemented Use of Pre-, Pro-, and Synbiotics in Severe Acute Pancreatitis: An Updated Systematic Review and Meta-Analysis of 13 Randomized Controlled Trials. Front. Pharmacol. 2018, 9, 690. [Google Scholar] [CrossRef]
- Mandal, S.; Hati, S.; Puniya, A.K.; Singh, R.; Singh, K. Development of synbiotic milk chocolate using encapsulated Lactobacillus casei NCDC 298. J. Food Process Preserv. 2013, 37, 1031–1037. [Google Scholar] [CrossRef]
- Di Criscio, T.; Fratianni, A.; Mignogna, R.; Cinquanta, L.; Coppola, R.; Sorrentino, E.; Panfili, G. Production of functional probiotic, prebiotic, and synbiotic ice creams. J. Dairy Sci. 2010, 93, 4555–4564. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, G. Synbiotic encapsulation of probiotic Latobacillus plantarum by alginate-arabinoxylan composite microspheres. LWT Food Sci. Technol. 2018, 93, 135–141. [Google Scholar] [CrossRef]
- Nakkarach, A.; Withayagiat, U. Comparison of synbiotic beverages produced from riceberry malt extract using selected free and encapsulated probiotic lactic acid bacteria. Agric. Nat. Resour. 2018, 52, 467–476. [Google Scholar] [CrossRef]
- Sathyabama, S.; Ranjith kumar, M.; Bruntha devi, P.; Vijayabharathi, R.; Brindha priyadharisini, V. Co-encapsulation of probiotics with prebiotics on alginate matrix and its effect on viability in simulated gastric environment. LWT Food Sci. Technol. 2014, 57, 419–425. [Google Scholar] [CrossRef]
- Atia, A.; Gomma, A.I.; Fliss, I.; Beyssac, E.; Garrait, G.; Subirade, M. Molecular and biopharmaceutical investigation of alginate-inulin synbiotic coencapsulation of probiotic to target the colon. J. Microencapsul. 2017, 34, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.; Matthias, T.; Aminov, R. Potential Effects of Horizontal Gene Exchange in the Human Gut. Front. Immunol. 2017, 8, 1630. [Google Scholar] [CrossRef] [PubMed]


| Wall Materials | Encapsulation Technology | Microorganism | References |
|---|---|---|---|
| Whey | Agglomeration/Spray-drying | Saccharomyces boulardii | [167] |
| Whey Protein isolate/Gum Arabic | Complex coacervation | Lactobacillus paraplantarum, Lactobacillus paracasei | [168] |
| Soy protein isolate (SPI) and high methoxy pectin (HMP) | Complexation | Lactobacillus delbrueckii | [169] |
| Carboxymethyl-cellulose and chitosan | Crosslinking | Lactobacillus rhamnosus GG | [158] |
| Cellulose and chitosan | Crosslinking | Lactobacillus rhamnosus GG | [170] |
| Aguamiel, Ag, or sweet whey, SW, as inner aqueous phase | Double emulsion | Lactobacillus plantarum | [171] |
| Alginate | Dual aerosol | Lactobacillus rhamnosus GG and Lactobacillus acidophilus NCFM | [172] |
| Alginate and Maltodextrin | Dual aerosol/freeze drying or spray drying | Lactobacillus rhamnosus GG and Lactobacillus acidophilus NCFM | [173] |
| Whey protein concentrate and pullulan | Electrospinning | Bifidobacterium animalis subsp. lactis Bb12 | [174] |
| Alginate and acidified zein | Electrospraying | Lactobacillus acidophilus | [175] |
| Alginate–human-like collagen | Electrostatic droplet generation | Bifidobacterium longum BIOMA 5920 | [176] |
| Alginate-gelatin and MgO | Electrospraying | Pediococcus pentosaceus | [177] |
| Ca-alginate | Electrospraying | Lactobacillus plantrarum | [178] |
| Ca-alginate and chitosan | Electrospraying | Lactobacillus plantrarum | [179] |
| Whey protein isolate | Electrospraying | Lactobacillus plantrarum | [180] |
| Whey protein isolate/whey protein isolate and inulin/whey protein isolate and inulin and persian gum | Εlectrospraying/freeze drying/spray drying | Lactobacillus rhamnosus ATCC 7469 | [181] |
| κ-carrageenan | Emulsification, freeze-drying or extrusion | Lactobacillus plantarum | [182] |
| Alginate | Emulsification/internal gelation | Bifidobacterium bifidum F-35 | [183] |
| Alginate and pectin and gelatin | Emulsion | Lactobacillus plantarum | [184] |
| Chickpea protein–alginate | Emulsion | Bifidobacterium adolescentis | [185] |
| Casein, native whey and/or denatured whey proteins | Emulsion | Lactobacillus rhamnosus GG | [186] |
| Aluminum carboxymethyl cellulose–rice bran | Emulsion | Lactobacillus reuteri | [187] |
| Na- alginate (Al), alginate/1% gellan gum alginate/gum Arabic | External ionic gelation | Lactobacillus plantarum DKL 109 | [188] |
| Na-alginate | Extrusion | Lactobacillus paracasei LAFTI® L26, Lactobacillus acidophilus Ki, Bifidobacterium animalis BB-12, Lactobacillus casei -01 | [189] |
| Na-alginate and chitosan | Extrusion | Bifidobacterium pseudocatenulatum G4 | [190] |
| Na-alginate and fructo-oligosaccharides | Extrusion | Lactobacillus casei LC-01 and Lactobacillus casei BGP 93 | [191] |
| Alginate-whey protein | Extrusion | Lactobacillus delbrueckii subsp. Bulgaricus | [192] |
| Alginate coated with chitosan and gelatin | Extrusion | Lactobacillus plantarum TN9Lactobacillus plantarum TN9 | [193] |
| Legume protein isolate–alginate | Extrusion | Bifidobacterium. adolescentis | [194] |
| Carrageenan-locust bean gum coated milk microspheres | Extrusion | Lactobacillus bulgaricus | [195] |
| Alginate–milk | Extrusion | Lactobacillus bulgaricus | [196] |
| Alginate (ALG) and alginate-psyllium (ALG-PSL) | Extrusion | Lactobacillus acidophilus | [197] |
| Alginate/chitosan/alginate | Extrusion | Lactobacillus salivarus | [198] |
| Alginate-skim milk | Extrusion | Lactobacillus reuteri DPC16 | [199] |
| Alginate–chitosan | Extrusion | Enterococcus faecium MC13 | [200] |
| Whey protein isolate | Extrusion | Lactobacillus rhamnosus GG | [201] |
| Pea protein isolate–alginate | Extrusion | Bifidobacterium adolescentis | [202] |
| Na-alginate | Extrusion/emulsion | Bifidobacterium. longum | [164] |
| Na-alginate coated with starch and chitosan | Extrusion | Lactobacillus acidophilus | [157] |
| Sweet whey and shellac | Fluidized bed microencapsulation | Lactobacillus reuteri | [203] |
| Gelatin and gum Arabic | Freeze drying | Bifidobacterium lactis | [204] |
| Na-alginate, gellan gum and skim milk powder | Freeze drying | Lactobacillus kefiranofaciens M1 | [205] |
| Sugarcane bagasse (SB) and sodium alginate (naa) | Immobilization/extrusion | Lactobacillus rhamnosus NRRL 442 | [206] |
| Pectin coated with whey protein heat treated or without heat treatment | Ionotropic gelation and electrostatic interactions | Lactobacillus acidophilus La5 | [207] |
| Chitosan and carboxymethyl cellulose | Layer by layer | Lactobacillus acidophilus | [208] |
| Chitosan and dextran sulfate | Layer-by-layer technique (lbl) using oppositely charged polyelectrolytes | Saccharomyces boulardii | [209] |
| Sodium caseinate and gellan gum | Ph- induced gelation | Lactobacillus casei | [210] |
| Solid lipid microparticles With prebiotics (inulin, polydextrose) | Spray chilling | Lactobacillus acidophilus | [211] |
| Vegetable fat with lecithin | Spray chilling | Bifidobacterium lactis, Lactobacillus acidophilus | [212] |
| Gum Arabic and β-cyclodextrin | Spey chilling and spray drying | Lactobacillys acidophilus | [213] |
| Fructo-oligosaccharide (FOS) and whey proteins | Spray drying | Lactobacillus plantarum MTCC 5422 | [214] |
| Gum Arabic/maltodextrin/whey protein concentrate | Spray drying | Lactobacillus acidophilus | [159] |
| Slurry fermentation with whey | Spray drying | Lactobacillus reuteri | [215] |
| Skim milk and whey, maltodextrin, pectin, and arabic gum | Spray drying | Lactobacillus plantarum | [216] |
| Reconstituted skim milk (RSM) with prebiotics (inulin, oligofructose-enriched inulin, and oligofructose | Spray drying | Bifidobacterium BB-12 | [217] |
| Whey | Spray drying | Bifidobacterium Bb-12 | [218] |
| Whey protein isolate with sodium alginate and denatured whey protein isolate with sodium alginate | Spray drying and freeze drying | Lactobacillus plantarum | [219] |
| Sweet whey or skim milk | Spray drying | Lactobacillus acidophilus La-5 | [220] |
| Native rice starch and inulin | Spray drying | Lactobacillus rhamnosus | [221] |
| Maltodextrin | Spray drying | Lactobacillus casei | [222] |
| Whey protein | Spray drying | Lactobacilus acidophilus, Lactobacillus paracasei L26 and Bifidobacterium animalis BB-12 | [223] |
| Poly(vinylpyrrolidone)-poly(vinylacetate-co-crotonic acid) | Supercritical carbon dioxide | Bifidobacterium longum Bb46 | [224] |
| Alginates-chitosan | Vibrating technology/extrusion | Lactobacillus reuteri DSM 17938 | [225] |
| Food | Microorganism | Coating Materials | Method | References |
|---|---|---|---|---|
| Apple juice | Lactobacillus rhamnosus GG | WPI alone and in combination with a modified resistant starch (RS) | Spray drying | [233] |
| Carrot Juice | Lactobacillus casei | Chitosan-Ca-alginate | Extrusion | [234] |
| Carrot juice | Lactobacillus acidophilus | Alginate-inulin-xanthan gum | Extrusion | [235] |
| Cheddar cheese | Bifidobacterium longum | Na-alginate and palmitoylated alginate | (i) droplet extrusion method (ADE) and (ii) emulsion method | [236] |
| Dry fermented sausages | Lactobacillus reuteri | Alginate | Extrusion | [237] |
| Fermented milk | Lactobacillus casei ATCC393 | Chios mastic gum | Freeze drying | [162] |
| Fruit juice | Lactobacillus paracasei L26 | Alginate | Extrusion | [238] |
| Fruit juice | Lactobacillus rhamnosus GG | Whey/alginate | Droplet extrusion with coating via electrostatic deposition | [239] |
| Fruit juices | Bifidobacterium longum, Bifidobacterium breve | poly-γ-glutamic acid | Freeze drying | [240] |
| Fruit juices | Lactobacillus plantarum and Bifidobacterium longum | Alginate or pectin coated with chitosan, gelatin or glucomannan | Extrusion | [241] |
| Ice cream | Lactobacillus casei Lc-01 and Bifidobacterium lactis Bb-12 | Alginate and Hi-maize resistant starch | Emulsion | [242] |
| Iranian yogurt drink (Doogh) | Lactobacillus acidophilus LA-5 and Bifidobacterium lactis Bb-12 | Alginate | Extrusion | [243] |
| Kasar cheese | Lactobacillus acidophilus LA-5 and Bifidobacterium bifidum BB-12 | Alginate | Emulsion or extrusion | [244] |
| kefir | Bifidobacterium animals | Sodium alginate | Extrusion | [245] |
| Mango juice | Lactobacillus plantarum | Calcium-Alginate-Soy Protein Isolate | Gelation | [226] |
| Mozzarella cheese | Lactobacillus paracasei ssp. paracasei LBC-1 | Alginate | Extrusion | [246] |
| Oaxaca cheese | Lactobacillus plantarum | Aguamiel, Ag, or sweet whey, SW, as inner aqueous phase | Double emulsion | [171] |
| Pecorino cheese | L. acidophilus, B. longum and B. lactis | Na-alginate | Extrusion | [247] |
| Pecorino cheese | Lactobacillus acidophilus and a mix of Bifidobacterium longum and Bifidobacterium lactis | Alginate | Extrusion | [248] |
| Pomegranate juice | Lactobacillusplantarum | Alginate beads coating with double layer Chitosan | Extrusion | [249] |
| White-brined cheese | Bifidobacterium bifidum BB-12 and Lactobacillus acidophilus LA-5 | Alginate | Emulsion or extrusion | [250] |
| Yogurt | Lactobacillus acidophilus LA-5 | Pectin – Whey protein | Ionic gelation and complexation | [251] |
| Yogurt | Bifidobacterium bifidum F-35 | Whey/alginate | Extrusion | [252] |
| Yogurt | Lactobacillus acidophilus ATCC 4356 | Alginates | Extrusion | [246] |
| Yogurt | Bifidobacterium animalis subsp. lactis Bb12 and Lactobacillus rhamnosus | Alginate | Extrusion | [253] |
| Yogurt | Lactobacillus plantarum | Sodium alginate or pectin, coated with sodium alginate or chitosan | Extrusion | [91] |
| Yogurt | Lactobacillus casei | Sodium alginate (A), amidated low-methoxyl pectin (P), and blends | Extrusion | [254] |
| Yogurt | Lactobacillus acidophilus | alginate and chitosan | Extrusion | [255] |
| Yogurt—Ice cream | Lactobacillus acidophilus La-5 | Na-alginate | Extrusion | [256] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. https://doi.org/10.3390/nu11071591
Terpou A, Papadaki A, Lappa IK, Kachrimanidou V, Bosnea LA, Kopsahelis N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients. 2019; 11(7):1591. https://doi.org/10.3390/nu11071591
Chicago/Turabian StyleTerpou, Antonia, Aikaterini Papadaki, Iliada K. Lappa, Vasiliki Kachrimanidou, Loulouda A. Bosnea, and Nikolaos Kopsahelis. 2019. "Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value" Nutrients 11, no. 7: 1591. https://doi.org/10.3390/nu11071591
APA StyleTerpou, A., Papadaki, A., Lappa, I. K., Kachrimanidou, V., Bosnea, L. A., & Kopsahelis, N. (2019). Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients, 11(7), 1591. https://doi.org/10.3390/nu11071591