Dietary Factors and Modulation of Bacteria Strains of Akkermansia muciniphila and Faecalibacterium prausnitzii: A Systematic Review
Abstract
1. Introduction
2. Material and Methods
2.1. Literature Search
2.2. Study Selection Criteria
2.3. Data Extraction
2.4. Assessing the Quality of Trials
3. Results
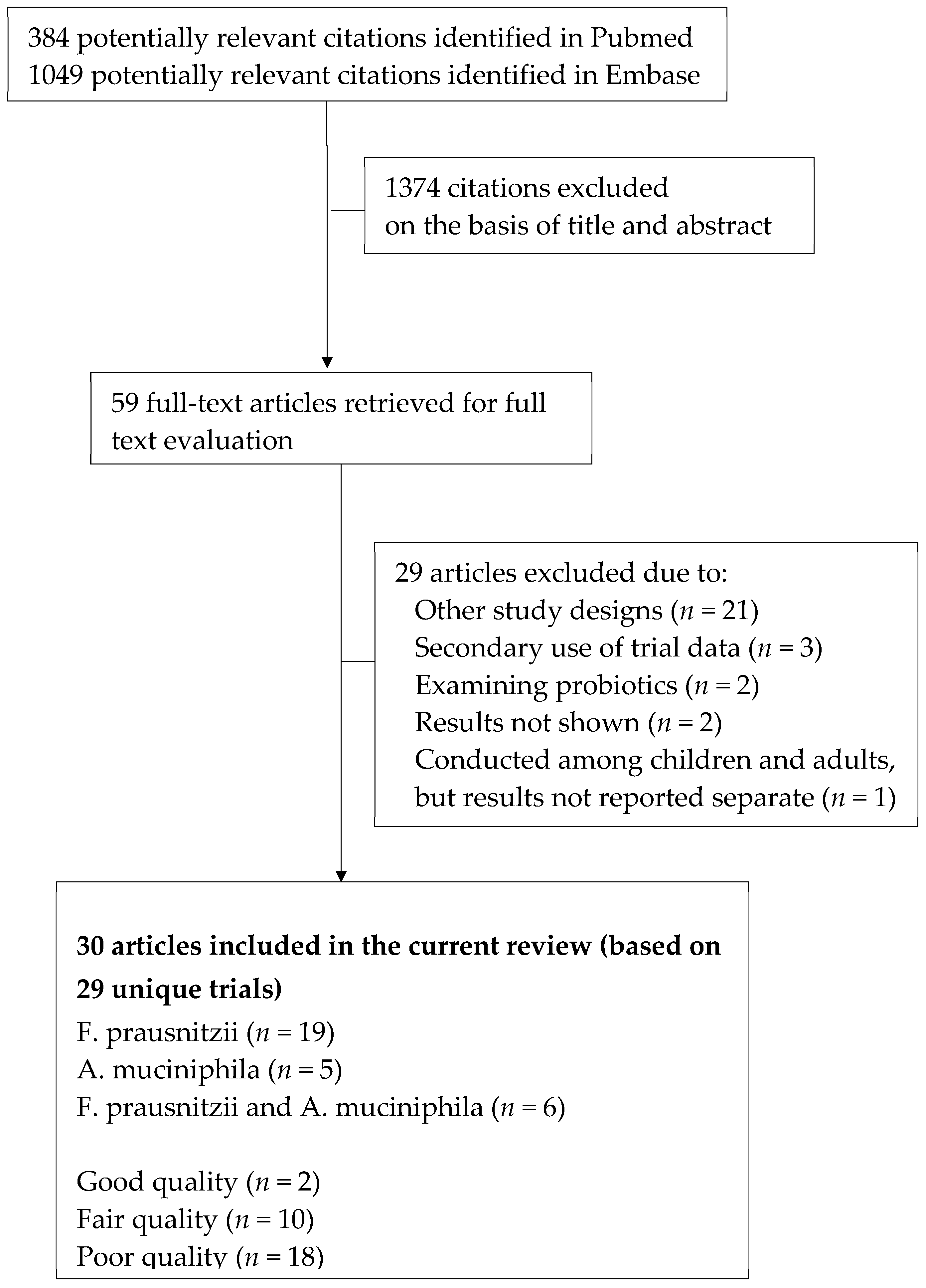
3.1. Study Identification and Selection
3.2. Characteristics of the Included Trials
3.3. Trials Examining A. muciniphila
3.4. Trials Examining F. prausnitzii
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
Search Strategy
PUBMED 384
EMBASE 1049
References
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Shen, J.; Ran, Z.H. Association between Faecalibacterium prausnitzii Reduction and Inflammatory Bowel Disease: A Meta-Analysis and Systematic Review of the Literature. Gastroenterol. Res. Pract. 2014, 2014, 872725. [Google Scholar] [CrossRef] [PubMed]
- Davies, N.K.; O’Sullivan, J.M.; Plank, L.D.; Murphy, R. Altered gut microbiome after bariatric surgery and its association with metabolic benefits: A systematic review. Surg. Obes. Relat. Dis. Off. J. Am. Soc. Bariatr. Surg. 2019, 15, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Halder, C.V.; Faria, A.V.S.; Andrade, S.S. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract. Res. Clin. Gastroenterol. 2017, 31, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Geerlings, S.Y.; Kostopoulos, I.; de Vos, W.M.; Belzer, C. Akkermansia muciniphila in the Human Gastrointestinal Tract: When, Where, and How? Microorganisms 2018, 6, 75. [Google Scholar] [CrossRef]
- Lopez-Siles, M.; Martinez-Medina, M.; Surís-Valls, R.; Aldeguer, X.; Sabat-Mir, M.; Duncan, S.H.; Flint, H.J.; Garcia-Gil, L.J. Changes in the Abundance of Faecalibacterium prausnitzii Phylogroups I and II in the Intestinal Mucosa of Inflammatory Bowel Disease and Patients with Colorectal Cancer. Inflamm. Bowel Dis. 2016, 22, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- de Goffau, M.C.; Luopajarvi, K.; Knip, M.; Ilonen, J.; Ruohtula, T.; Härkönen, T.; Orivuori, L.; Hakala, S.; Welling, G.W.; Harmsen, H.J.; et al. Fecal microbiota composition differs between children with beta-cell autoimmunity and those without. Diabetes 2013, 62, 1238–1244. [Google Scholar] [CrossRef]
- Li, J.; Lin, S.; Vanhoutte, P.P.; Woo, C.C.; Xu, A. Akkermansia Muciniphila Protects Against Atherosclerosis by Preventing Metabolic Endotoxemia-Induced Inflammation in Apoe-/- Mice. Circulation 2016, 133, 2434–2446. [Google Scholar] [CrossRef]
- Derrien, M.; Vaughan, E.E.; Plugge, C.C.; de Vos, W.M. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54 Pt 5, 1469–1476. [Google Scholar] [CrossRef]
- Derrien, M.; Van Baarlen, P.; Hooiveld, G.; Norin, E.; Muller, M.; de Vos, W.M. Modulation of Mucosal Immune Response, Tolerance, and Proliferation in Mice Colonized by the Mucin-Degrader Akkermansia muciniphila. Front. Microbiol. 2011, 2, 166. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Louis, P.; Young, P.; Holtrop, G.; Flint, H.H. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ. Microbiol 2010, 12, 304–314. [Google Scholar] [CrossRef]
- Ganesan, K.; Chung, S.S.; Vanamala, J.; Xu, B. Causal Relationship between Diet-Induced Gut Microbiota Changes and Diabetes: A Novel Strategy to Transplant Faecalibacterium prausnitzii in Preventing Diabetes. Int. J. Mol. Sci. 2018, 19, 3720. [Google Scholar] [CrossRef]
- Schneeberger, M.; Everard, A.; Gomez-Valades, A.G.; Matamoros, S.; Ramírez, S.; Delzenne, N.M.; Gomis, R.; Claret, M.; Cani, P.D. Akkermansia muciniphila inversely correlates with the onset of inflammation, altered adipose tissue metabolism and metabolic disorders during obesity in mice. Sci. Rep. 2015, 5, 16643. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermúdez-Humarán, L.G.; Gratadoux, J.J.; Blugeon, S.; Bridonneau, C.; Furet, J.P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Ejtahed, H.H.; Soroush, A.A.; Siadat, S.S.; Hoseini-Tavassol, Z.; Larijani, B.; Hasani-Ranjbar, S. Targeting obesity management through gut microbiota modulation by herbal products: A systematic review. Complement. Ther. Med. 2019, 42, 184–204. [Google Scholar] [CrossRef]
- Anhe, F.F.; Pilon, G.; Roy, D.; Desjardins, Y.; Levy, E.; Marette, A. Triggering Akkermansia with dietary polyphenols: A new weapon to combat the metabolic syndrome? Gut Microbes 2016, 7, 146–153. [Google Scholar] [CrossRef]
- Jang, S.; Sun, J.; Chen, P.; Lakshman, S.; Molokin, A.; Harnly, J.M.; Vinyard, B.T.; Urban J.F., Jr.; Davis, C.D.; Solano-Aguilar, G. Flavanol-Enriched Cocoa Powder Alters the Intestinal Microbiota, Tissue and Fluid Metabolite Profiles, and Intestinal Gene Expression in Pigs. J. Nutr. 2016, 146, 673–680. [Google Scholar] [CrossRef]
- Magistrelli, D.; Zanchi, R.; Malagutti, L.; Galassi, G.; Canzi, E.; Rosi, F. Effects of Cocoa Husk Feeding on the Composition of Swine Intestinal Microbiota. J. Agric. Food Chem. 2016, 64, 2046–2052. [Google Scholar] [CrossRef]
- Anhê, F.F.; Varin, T.V.; Le Barz, M.; Desjardins, Y.; Levy, E.; Roy, D.; Marette, A. Gut Microbiota Dysbiosis in Obesity-Linked Metabolic Diseases and Prebiotic Potential of Polyphenol-Rich Extracts. Curr. Obes. Rep. 2015, 4, 389–400. [Google Scholar] [CrossRef]
- Walker, J.J.; Eckardt, P.; Aleman, J.O.; da Rosa, J.C.; Liang, Y.; Iizumi, T.; Etheve, S.; Blaser, M.J.; L Breslow, J.; Holt, P.R. The effects of trans-resveratrol on insulin resistance, inflammation, and microbiota in men with the metabolic syndrome: A pilot randomized, placebo-controlled clinical trial. J. Clin. Transl. Res. 2019, 4, 122–135. [Google Scholar]
- Halmos, E.E.; Christophersen, C.C.; Bird, A.A.; Shepherd, S.S.; Gibson, P.P.; Muir, J.J. Diets that differ in their FODMAP content alter the colonic luminal microEnvironironment. Gut 2015, 64, 93–100. [Google Scholar] [CrossRef]
- Halmos, E.E.; Christophersen, C.C.; Bird, A.A.; Shepherd, S.S.; Muir, J.J.; Gibson, P.P. Consistent Prebiotic Effect on Gut Microbiota With Altered FODMAP Intake in Patients with Crohn’s Disease: A Randomised, Controlled Cross-Over Trial of Well-Defined Diets. Clin. Transl. Gastroenterol. 2016, 7, e164. [Google Scholar] [CrossRef]
- Ramirez-Farias, C.; Slezak, K.; Fuller, Z.; Duncan, A.; Holtrop, G.; Louis, P. Effect of inulin on the human gut microbiota: Stimulation of Bifidobacterium adolescentis and Faecalibacterium prausnitzii. Br. J. Nutr. 2009, 101, 541–550. [Google Scholar] [CrossRef]
- Ramnani, P.; Gaudier, E.; Bingham, M.; van Bruggen, P.; Tuohy, K.K.; Gibson, G.G. Prebiotic effect of fruit and vegetable shots containing Jerusalem artichoke inulin: A human intervention study. Br. J. Nutr. 2010, 104, 233–240. [Google Scholar] [CrossRef]
- Roshanravan, N.; Mahdavi, R.; Alizadeh, E.; Ghavami, A.; Rahbar Saadat, Y.; Mesri Alamdari, N.; Alipour, S.; Dastouri, M.R.; Ostadrahimi, A. The effects of sodium butyrate and inulin supplementation on angiotensin signaling pathway via promotion of Akkermansia muciniphila abundance in type 2 diabetes; A randomized, double-blind, placebo-controlled trial. J. Cardiovasc. Thorac. Res. 2017, 9, 183–190. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.D.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.J.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2008, 283, 2008–2012. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Benjamin, J.L.; Hedin, C.R.; Koutsoumpas, A.; Ng, S.C.; McCarthy, N.E.; Hart, A.L.; Kamm, M.A.; Sanderson, J.D.; Knight, S.C.; Forbes, A.; et al. Randomised, double-blind, placebo-controlled trial of fructo-oligosaccharides in active Crohn’s disease. Gut 2011, 60, 923–929. [Google Scholar] [CrossRef]
- Blatchford, P.; Stoklosinski, H.; Eady, S.; Wallace, A.; Butts, C.; Gearry, R.; Gibson, G.; Ansell, J. Consumption of kiwifruit capsules increases Faecalibacterium prausnitzii abundance in functionally constipated individuals: A randomised controlled human trial. J. Nutr. Sci. 2017, 6, e52. [Google Scholar] [CrossRef]
- Clavel, T.; Fallani, M.; Lepage, P.; Levenez, F.; Mathey, J.; Rochet, V.; Sérézat, M.; Sutren, M.; Henderson, G.; Bennetau-Pelissero, C.; et al. Isoflavones and functional foods alter the dominant intestinal microbiota in postmenopausal women. J. Nutr. 2005, 135, 2786–2792. [Google Scholar] [CrossRef]
- Dao, M.M.; Everard, A.; Aron-Wisnewsky, J.; Sokolovska, N.; Prifti, E.; Verger, E.O.; Kayser, B.D.; Levenez, F.; Chilloux, J.; Hoyles, L.; et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: Relationship with gut microbiome richness and ecology. Gut 2016, 65, 426–436. [Google Scholar] [CrossRef]
- Dewulf, E.M.; Cani, P.D.; Claus, S.P.; Fuentes, S.; Puylaert, P.G.; Neyrinck, A.M.; Bindels, L.B.; de Vos, W.M.; Gibson, G.R.; Thissen, J.P.; et al. Insight into the prebiotic concept: Lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 2013, 62, 1112–1121. [Google Scholar] [CrossRef]
- Fava, F.; Gitau, R.; Griffin, B.B.; Gibson, G.G.; Tuohy, K.K.; Lovegrove, J.J. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome ’at-risk’ population. Int. J. Obes. (2005) 2013, 37, 216–223. [Google Scholar] [CrossRef]
- Fernando, W.W.; Hill, J.J.; Zello, G.G.; Tyler, R.R.; Dahl, W.W.; Van Kessel, A.A. Diets supplemented with chickpea or its main oligosaccharide component raffinose modify faecal microbial composition in healthy adults. Benef. Microbes 2010, 1, 197–207. [Google Scholar] [CrossRef]
- Guadamuro, L.; Delgado, S.; Redruello, B.; Flórez, A.B.; Suárez, A.; Martínez-Camblor, P.; Mayo, B. Equol status and changes in fecal microbiota in menopausal women receiving long-term treatment for menopause symptoms with a soy-isoflavone concentrate. Front. Microbiol. 2015, 6, 777. [Google Scholar] [CrossRef]
- Hooda, S.; Boler, B.M.; Serao, M.C.; Brulc, J.M.; Staeger, M.A.; Boileau, T.W.; Dowd, S.E.; Fahey, G.C., Jr.; Swanson, K.S. 454 pyrosequencing reveals a shift in fecal microbiota of healthy adult men consuming polydextrose or soluble corn fiber. J. Nutr. 2012, 142, 1259–1265. [Google Scholar] [CrossRef]
- Hustoft, T.N.; Hausken, T.; Ystad, S.O.; Valeur, J.; Brokstad, K.; Hatlebakk, J.G.; Lied, G.A. Effects of varying dietary content of fermentable short-chain carbohydrates on symptoms, fecal microEnvironironment, and cytokine profiles in patients with irritable bowel syndrome. Neurogastroenterol. Motil. Off. J. Eur. Gastrointest. Motil. Soc. 2017, 29, e12969. [Google Scholar] [CrossRef]
- Lee, T.; Clavel, T.; Smirnov, K.; Schmidt, A.; Lagkouvardos, I.; Walker, A.; Lucio, M.; Michalke, B.; Schmitt-Kopplin, P.; Fedorak, R.; et al. Oral versus intravenous iron replacement therapy distinctly alters the gut microbiota and metabolome in patients with IBD. Gut 2017, 66, 863–871. [Google Scholar] [CrossRef]
- Li, Z.; Henning, S.M.; Lee, R.P.; Lu, Q.Y.; Summanen, P.H.; Thames, G.; Corbett, K.; Downes, J.; Tseng, C.H.; Finegold, S.M.; et al. Pomegranate extract induces ellagitannin metabolite formation and changes stool microbiota in healthy volunteers. Food Funct. 2015, 6, 2487–2495. [Google Scholar] [CrossRef]
- Majid, H.H.; Cole, J.; Emery, P.P.; Whelan, K. Additional oligofructose/inulin does not increase faecal bifidobacteria in critically ill patients receiving enteral nutrition: A randomised controlled trial. Clin. Nutr. (Edinb. Scotl.) 2014, 33, 966–972. [Google Scholar] [CrossRef]
- Medina-Vera, I.; Sanchez-Tapia, M.; Noriega-Lopez, L.; Granados-Portillo, O.; Guevara-Cruz, M.; Flores-López, A.; Avila-Nava, A.; Fernández, M.L.; Tovar, A.R.; Torres, N. A dietary intervention with functional foods reduces metabolic endotoxaemia and attenuates biochemical abnormalities by modifying faecal microbiota in people with type 2 diabetes. Diabetes Metab. 2019, 45, 122–131. [Google Scholar] [CrossRef]
- Moreno-Indias, I.; Sanchez-Alcoholado, L.; Perez-Martinez, P.; Andrés-Lacueva, C.; Cardona, F.; Tinahones, F.; Queipo-Ortuño, M.I. Red wine polyphenols modulate fecal microbiota and reduce markers of the metabolic syndrome in obese patients. Food Funct. 2016, 7, 1775–1787. [Google Scholar] [CrossRef]
- Most, J.; Penders, J.; Lucchesi, M.; Goossens, G.G.; Blaak, E.E. Gut microbiota composition in relation to the metabolic response to 12-week combined polyphenol supplementation in overweight men and women. Eur. J. Clin. Nutr. 2017, 71, 1040–1045. [Google Scholar] [CrossRef]
- Pinheiro, I.; Robinson, L.; Verhelst, A.; Marzorati, M.; Winkens, B.; den Abbeele, P.V.; Possemiers, S. A yeast fermentate improves gastrointestinal discomfort and constipation by modulation of the gut microbiome: Results from a randomized double-blind placebo-controlled pilot trial. BMC Complement. Altern. Med. 2017, 17, 441. [Google Scholar] [CrossRef]
- Tagliabue, A.; Ferraris, C.; Uggeri, F.; Trentani, C.; Bertoli, S.; de Giorgis, V.; Veggiotti, P.; Elli, M. Short-term impact of a classical ketogenic diet on gut microbiota in GLUT1 Deficiency Syndrome: A 3-month prospective observational study. Clin. Nutr. ESPEN 2017, 17, 33–37. [Google Scholar] [CrossRef]
- Wijayabahu, A.A.; Waugh, S.S.; Ukhanova, M.; Mai, V. Dietary raisin intake has limited effect on gut microbiota composition in adult volunteers. Nutr. J. 2019, 18, 14. [Google Scholar] [CrossRef]
- Xu, J.; Lian, F.; Zhao, L.; Zhao, Y.; Chen, X.; Zhang, X.; Guo, Y.; Zhang, C.; Zhou, Q.; Xue, Z.; et al. Structural modulation of gut microbiota during alleviation of type 2 diabetes with a Chinese herbal formula. ISME J. 2015, 9, 552–562. [Google Scholar] [CrossRef]
- Benus, R.F.; van der Werf, T.S.; Welling, G.W.; Judd, P.A.; Taylor, M.A.; Harmsen, H.J.; Whelan, K. Association between Faecalibacterium prausnitzii and dietary fibre in colonic fermentation in healthy human subjects. Br. J. Nutr. 2010, 104, 693–700. [Google Scholar] [CrossRef]
- James, S.L.; Christophersen, C.T.; Bird, A.R.; Conlon, M.A.; Rosella, O.; Gibson, P.R.; Muir, J.G. Abnormal fibre usage in UC in remission. Gut 2015, 64, 562–570. [Google Scholar] [CrossRef]
- Vulevic, J.; Juric, A.; Tzortzis, G.; Gibson, G.G. A mixture of trans-galactooligosaccharides reduces markers of metabolic syndrome and modulates the fecal microbiota and immune function of overweight adults1–3. J. Nutr. 2013, 143, 324–331. [Google Scholar] [CrossRef]
- West, N.P.; Christophersen, C.T.; Pyne, D.B.; Cripps, A.W.; Conlon, M.A.; Topping, D.L.; Kang, S.; McSweeney, C.S.; Fricker, P.A.; Aguirre, D.; et al. Butyrylated starch increases colonic butyrate concentration but has limited effects on immunity in healthy physically active individuals. Exerc. Immunol. Rev. 2013, 19, 102–119. [Google Scholar]
- Bull, M.M.; Plummer, N.N. Part 1: The Human Gut Microbiome in Health and Disease. Integr. Med. 2014, 13, 17–22. [Google Scholar]
- Vyas, U.; Ranganathan, N. Probiotics, prebiotics, and synbiotics: Gut and beyond. Gastroenterol. Res. Pract. 2012, 2012, 872716. [Google Scholar] [CrossRef]
- Karlsson, F.H.; Tremaroli, V.; Nookaew, I.; Bergström, G.; Behre, C.J.; Fagerberg, B.; Nielsen, J.; Bäckhed, F. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature 2013, 498, 99–103. [Google Scholar] [CrossRef]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef]
- Belzer, C.; Chia, L.W.; Aalvink, S.; Chamlagain, B.; Piironen, V.; Knol, J.; de Vos, W.M. Microbial Metabolic Networks at the Mucus Layer Lead to Diet-Independent Butyrate and Vitamin B12 Production by Intestinal Symbionts. MBio 2017, 8. [Google Scholar] [CrossRef]
- Chang, P.P.; Hao, L.; Offermanns, S.; Medzhitov, R. The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 2247–2252. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef]
- Wang, H.; Hou, L.; Kwak, D.; Fassett, J.; Xu, X.; Chen, A.; Chen, W.; Blazar, B.R.; Xu, Y.; Hall, J.L.; et al. Increasing Regulatory T Cells With Interleukin-2 and Interleukin-2 Antibody Complexes Attenuates Lung Inflammation and Heart Failure Progression. Hypertension 2016, 68, 114–122. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef]
- Lukovac, S.; Belzer, C.; Pellis, L.; Keijser, B.J.; de Vos, W.M.; Montijn, R.C.; Roeselers, G. Differential modulation by Akkermansia muciniphila and Faecalibacterium prausnitzii of host peripheral lipid metabolism and histone acetylation in mouse gut organoids. MBio 2014, 5. [Google Scholar] [CrossRef]
- Lee, H.; Ko, G. Effect of metformin on metabolic improvement and gut microbiota. Appl. Environ. Microbiol. 2014, 80, 5935–5943. [Google Scholar] [CrossRef]
- Shin, N.R.; Lee, J.C.; Lee, H.Y.; Kim, M.S.; Whon, T.W.; Lee, M.S.; Bae, J.W. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut 2014, 63, 727–735. [Google Scholar] [CrossRef]
- Everard, A.; Belzer, C.; Geurts, L.; Ouwerkerk, J.P.; Druart, C.; Bindels, L.B.; Guiot, Y.; Derrien, M.; Muccioli, G.G.; Delzenne, N.M.; et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl. Acad. Sci. USA 2013, 110, 9066–9071. [Google Scholar] [CrossRef]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15. [Google Scholar] [CrossRef]
- Morrison, D.D.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Jardine, M. Nutrition Considerations for Microbiota Health in Diabetes. Diabetes Spectr. A Publ. Am. Diabetes Assoc. 2016, 29, 238–244. [Google Scholar] [CrossRef][Green Version]
- Liu, X.; Wu, Y.; Li, F.; Zhang, D. Dietary fiber intake reduces risk of inflammatory bowel disease: Result from a meta-analysis. Nutr. Res. 2015, 35, 753–758. [Google Scholar] [CrossRef]
- McRae, M.M. Dietary Fiber Intake and Type 2 Diabetes Mellitus: An Umbrella Review of Meta-analyses. J. Chiropr. Med. 2018, 17, 44–53. [Google Scholar] [CrossRef]
- Ismail, T.; Sestili, P.; Akhtar, S. Pomegranate peel and fruit extracts: A review of potential anti-inflammatory and anti-infective effects. J. Ethnopharmacol. 2012, 143, 397–405. [Google Scholar] [CrossRef]
- Medjakovic, S.; Jungbauer, A. Pomegranate: A fruit that ameliorates metabolic syndrome. Food Funct. 2013, 4, 19–39. [Google Scholar] [CrossRef]
- Franco, O.H.; Chowdhury, R.; Troup, J.; Voortman, T.; Kunutsor, S.; Kavousi, M.; Oliver-Williams, C.; Muka, T. Use of Plant-Based Therapies and Menopausal Symptoms: A Systematic Review and Meta-analysis. JAMA 2016, 315, 2554–2563. [Google Scholar] [CrossRef]
- Glisic, M.; Kastrati, N.; Gonzalez-Jaramillo, V.; Bramer, W.M.; Ahmadizar, F.; Chowdhury, R.; Danser, A.J.; Roks, A.J.; Voortman, T.; Franco, O.H.; et al. Associations between Phytoestrogens, Glucose Homeostasis, and Risk of Diabetes in Women: A Systematic Review and Meta-Analysis. Adv. Nutr. 2018, 9, 726–740. [Google Scholar] [CrossRef]
- Ozdal, T.; Sela, D.D.; Xiao, J.; Boyacioglu, D.; Chen, F.; Capanoglu, E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients 2016, 8, 78. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Gomez, A.; Luckey, D.; Taneja, V. The gut microbiome in autoimmunity: Sex matters. Clin. Immunol. 2015, 159, 154–162. [Google Scholar] [CrossRef]
- Kaplan, H.; Hill, K.; Lancaster, J.; Hurtado, A.A. A theory of human life history evolution: Diet, intelligence, and longevity. Evol. Anthropol. Issues News Rev. 2000, 9, 156–185. [Google Scholar] [CrossRef]
- Zeevi, D.; Korem, T.; Zmora, N.; Israeli, D.; Rothschild, D.; Weinberger, A.; Ben-Yacov, O.; Lador, D.; Avnit-Sagi, T.; Lotan-Pompan, M.; et al. Personalized Nutrition by Prediction of Glycemic Responses. Cell 2015, 163, 1079–1094. [Google Scholar] [CrossRef]
- Moen, B.; Berget, I.; Rud, I.; Hole, A.A.; Kjos, N.N.; Sahlstrom, S. Extrusion of barley and oat influence the fecal microbiota and SCFA profile of growing pigs. Food Funct. 2016, 7, 1024–1032. [Google Scholar] [CrossRef]
- Grundy, M.M.-L.; Lapsley, K.; Ellis, P.P. A review of the impact of processing on nutrient bioaccessibility and digestion of almonds. Int. J. Food Sci. Technol. 2016, 51, 1937–1946. [Google Scholar] [CrossRef]
- Zinöcker, M.M.; Lindseth, I.I. The Western Diet-Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef]
| Lead Author, Publication Year | Study Design | Location/Age Range | Individual Health Status | Total Participants | Sex | Period of Intervention | Dietary Treatment Characteristics | Main Findings | |
|---|---|---|---|---|---|---|---|---|---|
| Intervention Type | Control Type | ||||||||
| Blatchford P et al. 2017* [1] | Randomized double-blind placebo-controlled cross-over trial | New Zealand/23–56 | Healthy participants with no clinical symptoms of constipation and functionally constipated participants | 29 | W and M | 4 weeks each intervention (2 weeks washout period between each intervention) | ACTAZIN™ (600 mg/d) green kiwifruit extract low dose | Placebo (isomalt coloured green 2400 mg/d) | A. muciniphila was significantly more abundant in the functionally constipated group, but no effect of the interventions on A. muciniphila. |
| ACTAZIN™ (2400 mg/d) green kiwifruit extract high dose | |||||||||
| Livaux™ (2400 mg/d) gold kiwifruit extract | |||||||||
| Dao M et al. 2015 [13] | Single-arm cross-over trial | France/41.9 ± 12.3 | Overweight and obese participants | 49 | W and M | 12 weeks | Caloric restriction diet (1200 kcal/d for W and 1500 kcal/d for M) | Weight stabilization diet (prescribed individually by a dietitian) | Caloric restriction diet: Subjects with A. muciniphila at or above the median had a decrease in abundance of A. muciniphila while in the group with A. muciniphila lower than median there was an increase. The difference was statistical significant. Weight stabilization diet: In both subjects with A. muciniphila at or above and lower, there was a decrease in abundance of A. muciniphila with no difference between the groups. |
| Halmos EP et al. 2015* [16] | Single-arm blinded randomized cross-over trial | Australia/18+ | Healthy participants and participants with irritable bowel syndrome | 33 | W and M | 6 weeks | Diet low in FODMAPs | Diet containing FODMAP content of a typical Australian diet | Typical Australian diet increased absolute and relative abundance for mucus-associated A. muciniphila (p < 0.001). |
| Halmos EP et al. 2016* [17] | Single-arm blinded randomized cross-over trial | Australia/18+ | Patients with clinically quiescent Crohn’s disease | 9 | W and M | 6 weeks | Diet low in FODMAPs | Diet containing FODMAP content of a typical Australian diet | Relative abundance was higher for mucus-associated A. muciniphila during the Australian compared with low FODMAP diet (p = 0.016). |
| Hooda S et al. 2012* [6] | Randomized double-blind placebo-controlled cross-over trial | USA/27.5 ± 4.33 | Healthy participants | 25 | M | 9 weeks | Polydextrose (PDX) (7 g, 3 times per day) | Placebo (no supplemental fiber control (NFC) (0 g, 3 times per day)) | A. muciniphila was greater after PDX intake than after the NFC or SCF treatment (p < 0.05). |
| Soluble corn fiber (SCF) (7 g, 3 times per day) | |||||||||
| James SL et al. 2015* [54] | Randomized single-blind cross-over trial | Australia/18–72 | Patients with UC in remission and healthy subjects | 29 | W and M | 8 weeks | ‘Low resistant starch (RS)/wheat bran (WB)’ foods containing 2–5 g RS and 2–5 g WB fibre per day | NA | Patients with UC had a lower abundance of A. muciniphila. For both cohorts, increasing the intake of RS/WB gave no indication of changes in relative or absolute abundance. |
| ‘High RS/WB’ foods containing 15 g RS and 12 g WB fibre per day | |||||||||
| Li Z et al. 2015 [20] | Single-arm trial | USA/28.9 ± 8 | Healthy volunteers | 20 | W and M | 4 weeks | Pomegranate extract (1000 mg) | NA | The data were not shown for the overall population. A. muciniphila was 33 (at baseline) and 47 fold (after 4 weeks) higher in stool samples of Urolithin A producers compared to non-producers. |
| Medina-Vera I et al. 2019* [9] | Randomized, double-blind placebo-controlled trial | Mexico/30–60 | Patients with Type 2 Diabetes | 81 | W and M | 3 months | A reduced-energy diet with a dietary portfolio (DP) (14 g of dehydrated nopal, 4 g of chia seeds, 30 g of soy protein and 4 g of inulin) | Placebo (28 g of calcium caseinate and 15 g of maltodextrin) | DP consumption increased levels of A. muciniphila by approximately 125%. |
| Pinheiro I et al. 2017 [11] | Randomized, double-blind placebo-controlled trial | Belgium/20–69 | Healthy with reduced bowel movements and other symptoms of GI discomfort stratified in severe and moderate | 80 | W and M | 6 weeks | EpiCor fermentate (500 mg/d) | Placebo (maltodextrin (500 mg/d)) | Significant relative increase of A. muciniphila in the moderate GI discomfort symptoms group at visit week 3 (p = 0.0001) and visit week 6 (p = 0.036). |
| Roshanravan N et al. 2017 [25] | Randomized, double-blind placebo-controlled trial | Iran/30–55 | Overweight and obese diabetes patients | 60 | W and M | 6 weeks | Group A: Butyrate group (600 mg/d sodium butyrate + inulin placebo) | Butyrate + inulin placebo (6 starch capsules (100 mg) and 10 g of starch powder) | The percentage changes of A. muciniphila abundance indicated a significant increase in group taking sodium butyrate and inulin (group A and B) in comparison with the placebo group (p < 0.05). A non-significant rise in this bacterium concentration was seen after supplementation with both sodium butyrate and inulin (group C). |
| Group B: inulin group (10 g/d inulin powder + butyrate placebo) | |||||||||
| Group C: butyrate + inulin group (600 mg/d sodium butyrate + 10 g/d inulin powder) | |||||||||
| Walker JM et al. 2019 [27] | Randomized, double-blind placebo-controlled trial | USA/30–70 | Obese insulin resistant subjects with metabolic syndrome | 28a | M | 5 weeks | Resveratrol (500 mg Mega-RES 99% capsules twice daily) | Placebo (two 500 mg placebo capsules twice daily) | Overall, there was no difference. However, when split by ethnicity, resveratrol administration to Caucasian subjects led to an increase in A. muciniphila compared to the non-Caucasians. |
| Lead Author, Publication Year | Study Design | Location/Age Range | Individual Health Status | Total Participants | Sex | Period of Intervention | Dietary Treatment Characteristics | Main Findings | |
|---|---|---|---|---|---|---|---|---|---|
| Intervention Type | Control Type | ||||||||
| Benjamin JL et al. 2011 [12] | Randomized double-blinded placebo-controlled trial | UK/39.5 ± 14.4 | Patients with Crohn’s disease | 103 | M | 4 weeks | Normal diet supplemented with 15 g/day FOS, comprising fructose polymers of differing chain lengths | Placebo (maltodextrin 15 g/day) | No significant differences between patients in the FOS and placebo group at week 4 (p = 0.95). |
| Benus RFJ et al. 2010 [52] | Randomized double-blinded cross-over trial | UK/21–34 | Healthy | 14 | W and M | 4 weeks | A formula supplemented with dietary fibre (14 g/l) consisting of pea fibre and fructo-oligosaccharides | NA | There were large and statistically significant reductions in the numbers of the F. prausnitzii group during both the fibre-free and fibre-supplemented diets. No differences between the fibre-free and fibre-supplemented diet (p = 0.23). |
| A fibre-free enteral formula | |||||||||
| Blatchford P et al. 2017* [1] | Randomized double-blind placebo-controlled cross-over trial | New Zealand/23–56 | Healthy participants who had no clinical symptoms of constipation and functionally constipated participants | 29/W and M | W and M | 4 weeks each intervention (2 weeks washout period between each intervention) | ACTAZIN™ L (600 mg/d) | Placebo (isomalt coloured green 2400 mg/d) | F. prausnitzii abundance significantly increased from 3.4 to 7.0% following Livaux™ supplementation in the functionally constipated group (p = 0.024). |
| ACTAZIN™ H (2400 mg/d) | |||||||||
| Livaux™ (2400 mg/d) | |||||||||
| Clavel T et al. 2005 [2] | Randomized double-blind placebo-controlled trial | France/60.4 ± 7.1 | Postmenopausal women | 39 | W | 30 days | Probiotic group: isoflavones (100 mg/d) + B. animalis DN-173 010 | Placebo (isoflavones 100 mg/d) | Bacterial percentages for F. prausnitzii subgroup decreased significantly in control subjects compared to the probiotic and prebiotic group (p = 0.034). |
| Prebiotic group: isoflavones (100 mg/d) + FOS (7 g/d) | |||||||||
| Dewulf EM et al. 2012 [3] | Double-blind placebo-controlled trial | Belgium/ 47.5 ± 8.5 | Obese | 30 | W | 3 months | ITF prebiotics (Synergy 1, namely, inulin/oligofructose 50/50 mix) | Placebo (maltodextrin) | Treatment with ITF prebiotics, but not the placebo, led to an increase in F. prausnitzii. |
| Fava F et al. 2013 [14] | Five-arm parallel, placebo-controlled, single-blind study | UK/56.0 ± 9.5 | Individuals at increased risk of metabolic syndrome | 88 | W and M | 24 weeks | High SFA diet | NA | Numbers of F. prausnitzii increased after intervention with high CHO and low GI (p = 0.022) and high SFA (p = 0.018) diet compared to baseline. |
| High MUFA/high GI | |||||||||
| High MUFA/Low GI | |||||||||
| High CHO/High GI | |||||||||
| High CHO/Low GI | |||||||||
| Fernando WMU et al. 2010 [4] | Randomized cross-over trial | Canada/25.6 ± 8.7 | Healthy | 12 | W and M | 9 weeks | Control diet + 5 g/d raffinose | Control diet | F. prausnitzii was more abundant in the raffinose diet and the chickpea diet compared to the control diet. |
| Control diet + 200 g/d canned chickpea | |||||||||
| Guadamuro L et al. 2015 [15] | Single-arm trial | Spain/48–61 | Menopausal women with no chronic disease | 16 | W | 24 weeks | One tablet isoflavoneconcentrate (80 mg) per day | NA | There was an increase in the intensity of F. prausnitzii. |
| Halmos EP et al. 2015* [16] | Single-arm blinded randomized cross-over trial | Australia/18+ | Irritable bowel syndrome and healthy individuals | 33 | W and M | 6 weeks | Diet low in FODMAPs | Diet containing FODMAP content of a typical Australian diet | Low FODMAP diet reduced total bacterial abundance, but did not impact relative abundance of F. prausnitzii. |
| Halmos EP et al. 2016* [17] | Single-arm blinded randomized cross-over trial | Australia/18+ | Patients with clinically quiescent Crohn’s disease | 9 | W and M | 6 weeks | Diet low in FODMAPs | Diet containing FODMAP content of a typical Australian diet | No significant difference in F. prausnitzii between the two diets. |
| Hooda S et al. 2012* [6] | Randomized double-blind placebo-controlled cross-over trial | USA/27.5 ± 4.33 | Healthy | 25 | M | 9 weeks | Polydextrose (PDX) (7 g, 3 times per day) | Placebo: no supplemental fiber control (NFC) (0 g, 3 times per day) | F. prausnitzii was greater in participants when they consumed PDX or SCF than when they consumed NFC (p < 0.05). |
| Soluble corn fiber (SCF) (7 g, 3 times per day) | |||||||||
| Hustoft TN et al. 2016 [7] | Randomized double-blind placebo-controlled cross-over trial | Norway/18–52 | Diarrhea-predominant or mixed irritated bowel syndrome | 20 | W and M | 10 days each intervention (3 weeks washout period) | Fructo-oligosaccharides (FOS) 16 g/d | Placebo: Maltodextrin 16 g/d | Ten days of FOS supplementation increased the level of F. prausnitzii. |
| James SL et al. 2015* [54] | Randomized single-blind cross-over trial | Australia/18–72 | Patients with UC in remission and healthy subjects | 29 | W and M | 8 weeks | ‘Low resistant starch (RS)/wheat bran (WB)’ foods containing 2–5 g RS and 2–5 g WB fibre per day | NA | For both cohorts, increasing the intake of RS/WB gave no indication of changes in relative or absolute abundance in F. prausnitzii. |
| ‘High RS/WB’ foods containing 15 g RS and 12 g WB fibre per day | |||||||||
| Lee T et al. 2017 [19] | Randomized, double-blind placebo-controlled trial | Canada/18+ | Iron deficient Inflammatory bowel disease patients | 72 | W and M | 12 weeks | Oral iron sulfate 300 mg, tablet, twice a day | Iron sucrose, 300 mg, intravenous, three or four/day | Lower abundance of F. prausnitzii after oral iron therapy compared to intravenous iron therapy (p = 0.009). |
| Majid HA et al. 2014 [8] | Multi-centre, randomized double-blind controlled trial | UK/70.8 ± 9.7 | Patients from the ICU starting exclusive nasogastric enteral nutrition | 22 | W and M | Up to 14 days | Oligofructose/inulin 7 g/d | Placebo: maltodextrin 7 g/d | There were significantly lower concentrations of F. prausnitzii in patients receiving additional oligofructose/inulin (p = 0.01). |
| Medina-Vera I et al. 2019* [9] | Single-centre randomized, controlled, double-blind parallel-group trial | Mexico/30–60 | Patients with Type 2 Diabetes | 81 | W and M | 3 months | A reduced-energy diet with a dietary portfolio (DP) comprising 14 g of dehydrated nopal, 4 g of chia seeds, 30 g of soy protein and 4 g of inulin | Placebo, comprising of 28 g of calcium caseinate and 15 g of maltodextrin. | Dietary intervention with functional foods significantly modified faecal microbiota compared with placebo. DP consumption for 12 weeks increased levels of F. prausnitzii by approximately 34%. |
| Moreno-Indias I et al. 2016 [21] | Randomized, cross-over controlled trial | Spain/45–50 | Metabolic syndrome and healthy individuals | 20 | M | 10 weeks (75 days) | Red wine, 272 mL/day | De-alcoholized (no ethanol) red wine, 272 mL/dat | In metabolic syndrome patients, there was a significant increase of F. prausnitzii, after the red wine and de-alcoholized red wine intake periods compared to baseline. In the healthy group, a significant increase in the number of F. prausnitzii through the intervention period was observed. |
| Most J et al. 2017 [10] | Randomized double-blind placebo-controlled trial | The Netherlands/20–50 | Obese | 42 | W and M | 12 weeks | A combination of epigallocatechin-3-gallate (EGCG) and resveratrol (RES) supplements (EGCG + RES; 282 and 80 mg/day, respectively) | Placebo (partly hydrolyzed microcrystalline cellulose-filled supplements) | EGCG+RES supplementation significantly decreased Bacteroidetes and tended to reduce F. prausnitzii in men (p = 0.05 and p = 0.10, respectively) but not in women (P = 0.15 and P = 0.77, respectively). |
| Ramirez-Farias C et al. 2008 [23] | Randomized, cross-over trial | UK/38.1 ± 2.43 | Healthy adults | 12 | W and M | 3 weeks | Inulin–oligofructose, 5 g, twice daily | Did not consume any supplement | F. prausnitzii exhibited a significant increase after intervention (p = 0.019). |
| Ramnani P et al. 2010 [24] | Three-arm parallel, placebo-controlled, double-blind study | UK/18–50 | Healthy adults | 60 | W and M | 3 weeks intervention (3 weeks washout period) | Jerusalem artichoke (JA) inulin- predominantly made of pear-carrot-sea buckthorn and JA juices or purées (PCS); two 100 mL shots per day | Placebo: Water-based preparation with added sugar, thickened and flavoured with blood orange, carrot and raspberry extracts and flavours (but no juice or purees) | No significant differences during the intervention and washout period. |
| JA inulin- predominantly made of plum-pear-beetroot and JA juices or purées (PPB); Two 100 mL shots per day | |||||||||
| Tagliabue A et al. 2017 [26] | Single-arm trial | USA/18–34 | Glucose Transporter 1 Deficiency Disorder (GLUT1-DS) patients | 6 | W and M | 12 weeks | Ketogenic diet including a minimum of 0.8–1 gram per kilogram of body weight of protein from animal sources (e.g., eggs, milk, meat, poultry and fish) | NA | There was no statistical significant difference. |
| Vulevic J et al. 2013 [55] | Randomized double-blind placebo-controlled cross-over trial | UK/45.2 ± 11.9 | Overweight subjects predisposed to the development of metabolic syndrome | 45 | W and M | 12 weeks each intervention (4 week washout period) | Bi2muno (B-GOS) | Placebo (maltodextrin) | The two dietary interventions had no significant effects on counts of total bacteria and F. prausnitzii cluster during the study. |
| West NP et al. 2013 [56] | Randomized double-blind controlled trial | Australia/37.4 ± 8.4 | Healthy active cyclists | 41 | W and M | 28 days | Ingestion of 40 g/day of butyrylated high amylose maize starch (HAMSB) | Low amylose maize starch (LAMS) | There were relative greater increases in faecal F. prausnitzii (5.1-fold; p < 0.01) in the HAMSB group. |
| Wijayabahu AT et al. 2019 [28] | Single-arm trial | USA/18–59 | Healthy individuals | 13 | W and M | 2 weeks | Sun-dried raisins: Three servings per day; one serving contained 28.3 g raisins and 2 grams of dietary fiber | NA | F. prausnitzii significantly increased after the first week of raisin intake and this increase continued during the second week of raisin consumption (p < 0.05). |
| Xu J et al. 2015 [29] | Randomized, double-blind placebo-controlled clinical trial | China/8.5 ± 2.6 | Recently diagnosed type-2 diabetes patients | 187 | W and M | 12 weeks | Low dose of Gegen Qinlian Decoction | Placebo | All three doses of GQD treatment significantly enriched F. prausnitzii compared with baseline. |
| Medium dose of Gegen Qinlian Decoction | |||||||||
| High dose of Gegen Qinlian Decoction | |||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verhoog, S.; Taneri, P.E.; Roa Díaz, Z.M.; Marques-Vidal, P.; Troup, J.P.; Bally, L.; Franco, O.H.; Glisic, M.; Muka, T. Dietary Factors and Modulation of Bacteria Strains of Akkermansia muciniphila and Faecalibacterium prausnitzii: A Systematic Review. Nutrients 2019, 11, 1565. https://doi.org/10.3390/nu11071565
Verhoog S, Taneri PE, Roa Díaz ZM, Marques-Vidal P, Troup JP, Bally L, Franco OH, Glisic M, Muka T. Dietary Factors and Modulation of Bacteria Strains of Akkermansia muciniphila and Faecalibacterium prausnitzii: A Systematic Review. Nutrients. 2019; 11(7):1565. https://doi.org/10.3390/nu11071565
Chicago/Turabian StyleVerhoog, Sanne, Petek Eylul Taneri, Zayne M. Roa Díaz, Pedro Marques-Vidal, John P. Troup, Lia Bally, Oscar H. Franco, Marija Glisic, and Taulant Muka. 2019. "Dietary Factors and Modulation of Bacteria Strains of Akkermansia muciniphila and Faecalibacterium prausnitzii: A Systematic Review" Nutrients 11, no. 7: 1565. https://doi.org/10.3390/nu11071565
APA StyleVerhoog, S., Taneri, P. E., Roa Díaz, Z. M., Marques-Vidal, P., Troup, J. P., Bally, L., Franco, O. H., Glisic, M., & Muka, T. (2019). Dietary Factors and Modulation of Bacteria Strains of Akkermansia muciniphila and Faecalibacterium prausnitzii: A Systematic Review. Nutrients, 11(7), 1565. https://doi.org/10.3390/nu11071565








