Pregnant Women in Four Low-Middle Income Countries Have a High Prevalence of Inadequate Dietary Intakes That Are Improved by Dietary Diversity
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Ethical Approval
2.3. Assessment of Food Intakes and Their Nutrient Adequacy
2.4. Statistical Analyses
3. Results
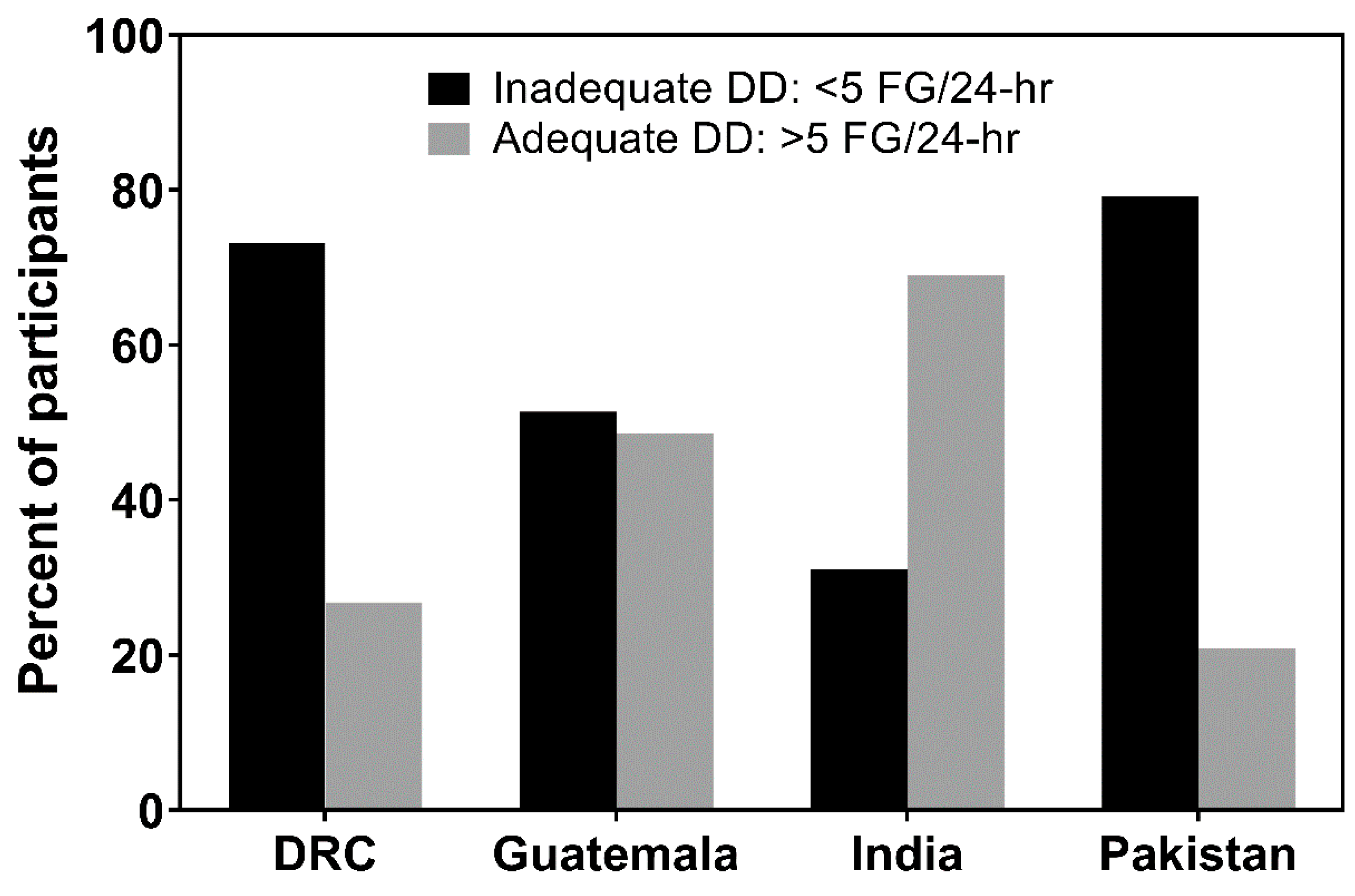
3.1. Dietary Diversity (DD)
3.2. Median Energy and Nutrient Intakes
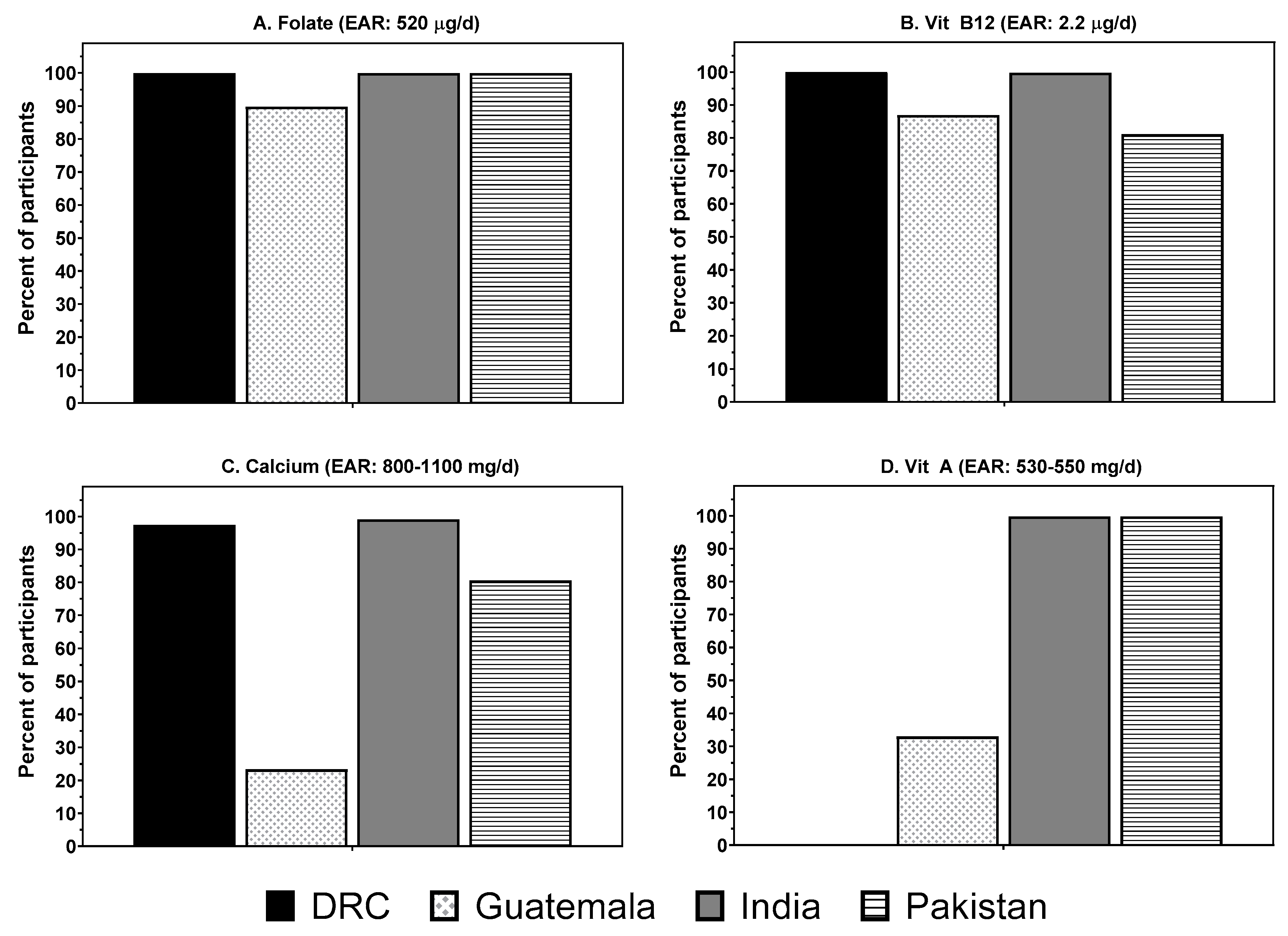
3.3. Estimated Prevalence of the Study Population “At Risk” of Inadequate Intakes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, G.; Imhoff-Kunsch, B.; Girard, A.W. Biological mechanisms for nutritional regulation of maternal health and fetal development. Paediatr. Perinat. Epidemiol. 2012, 26, 4–26. [Google Scholar] [CrossRef] [PubMed]
- Torheim, L.E.; Ferguson, E.L.; Penrose, K.; Arimond, M. Women in resource-poor settings are at risk of inadequate intakes of multiple micronutrients. J. Nutr. 2010, 140, 2051s–2058s. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Salas, P.; Moore, S.E.; Cole, D.; da Costa, K.A.; Cox, S.E.; Dyer, R.A.; Fulford, A.J.; Innis, S.M.; Waterland, R.A.; Zeisel, S.H.; et al. DNA methylation potential: Dietary intake and blood concentrations of one-carbon metabolites and cofactors in rural African women. Am. J. Clin. Nutr. 2013, 97, 1217–1227. [Google Scholar] [CrossRef]
- Ramakrishnan, U.; Grant, F.; Goldenberg, T.; Zongrone, A.; Martorell, R. Effect of women’s nutrition before and during early pregnancy on maternal and infant outcomes: A systematic review. Paediatr. Perinat. Epidemiol. 2012, 26, 285–301. [Google Scholar] [CrossRef] [PubMed]
- Nyaradi, A.; Li, J.; Hickling, S.; Foster, J.; Oddy, W.H. The role of nutrition in children’s neurocognitive development, from pregnancy through childhood. Front. Hum. Neurosci. 2013, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, A.J.; Humphrey, J.H. The stunting syndrome in developing countries. Paediatr. Int. Child Health 2014, 34, 250–265. [Google Scholar] [CrossRef] [PubMed]
- Adu-Afarwuah, S.; Lartey, A.; Okronipa, H.; Ashorn, P.; Ashorn, U.; Zeilani, M.; Arimond, M.; Vosti, S.A.; Dewey, K.G. Maternal Supplementation with Small-Quantity Lipid-Based Nutrient Supplements Compared with Multiple Micronutrients, but Not with Iron and Folic Acid, Reduces the Prevalence of Low Gestational Weight Gain in Semi-Urban Ghana: A Randomized Controlled Trial. J. Nutr. 2017, 147, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Hambidge, K.M.; Westcott, J.E.; Garces, A.; Figueroa, L.; Goudar, S.; Dhaded, S.M.; Pasha, O.; Ali, S.; Tshefu, A.; Lokangaka, A.; et al. A multi-country randomized controlled trial of comprehensive maternal nutrition supplementation initiated prior to conception: The Women First trial. Am. J. Clin. Nutr. 2019, 109, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Christian, P.; Mullany, L.C.; Hurley, K.M.; Katz, J.; Black, R.E. Nutrition and maternal, neonatal, and child health. Semin. Perinatol. 2015, 39, 361–372. [Google Scholar] [CrossRef]
- Althabe, F.; Moore, J.L.; Gibbons, L.; Berrueta, M.; Goudar, S.S.; Chomba, E.; Derman, R.J.; Patel, A.; Saleem, S.; Pasha, O.; et al. Adverse maternal and perinatal outcomes in adolescent pregnancies: The Global Network’s Maternal Newborn Health Registry study. Reprod. Health 2015, 12, S8. [Google Scholar] [CrossRef]
- Gibson, R.S.; Ferguson, E.L. An Interactive 24-Hour Recall for Assessing the Adequacy of Iron and Zinc Intakes in Developing Countries; LSI Press: Washington, DC, USA, 1999. [Google Scholar]
- Food and Agricultural Organization of the United Nations. Minimum Dietary Diversity for Women: A Guide for Measurement; FAO: Rome, Italy, 2016. [Google Scholar]
- Hambidge, K.M.; Krebs, N.F.; Westcott, J.E.; Garces, A.; Goudar, S.S.; Kodkany, B.S.; Pasha, O.; Tshefu, A.; Bose, C.L.; Figueroa, L.; et al. Preconception maternal nutrition: A multi-site randomized controlled trial. BMC Pregnancy Child. 2014, 14, 111. [Google Scholar] [CrossRef] [PubMed]
- Koso-Thomas, M.; McClure, E.M. The Global Network for Women’s and Children’s Health Research: A model of capacity-building research. Semin. Fetal Neonatal. Med. 2015, 20, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Lander, R.L.; Hambidge, K.M.; Krebs, N.F.; Westcott, J.E.; Garces, A.; Figueroa, L.; Tejeda, G.; Lokangaka, A.; Diba, T.S.; Somannavar, M.S.; et al. Repeat 24-hour recalls and locally developed food composition databases: A feasible method to estimate dietary adequacy in a multi-site preconception maternal nutrition RCT. Food Nutr. Res. 2017, 61, 1311185. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture. USDA National Nutrient Database for Standard Reference, Release 28; Agricultural Research Service: Beltsville, MD, USA, 2010.
- Stadlmayr, B.; Charrondiere, U.R.; Enujiugha, V.; Bayili, R.G.; Fagbohoun, E.G.; Samb, B.; Addy, P.; Barikmo, I.; Ouattara, F.; Oshuag, A. West African Food Composition Table; FAO of the United Nations: Rome, Italy, 2012. [Google Scholar]
- Bunch, S.; Murhpy, S.P. Users Guide to the Operation of the Worldfood Dietary Assessment System, Version 2.0; Office of Technology Licensing, University of California, Berkeley: Berkeley, CA, USA, 1997. [Google Scholar]
- Shaheen, N.; Rahim, A.; Mohiduzzaman, M.; Banu, C.; Bari, L.; Tukun, A.; Mannan, M.; Bhattacharjee, L.; Stadlmayr, B. Food composition table for Bangladesh. Final Res. Results 2013, 3, 187–209. [Google Scholar]
- Institute of Nutrition of Central America and Panama (INCAP). Table of Food Composition of Central America; INCAP/Pan-American Health Organization: Guatemala City, Guatemala, 2007. [Google Scholar]
- Food and Agriculture Organization/International Network of Food Data Systems (FAO/INFOODS). FAO/INFOODS Guidelines for Food Matching, Version 12; Food and Agriculture Organization Rome: Rome, Italy, 2012. [Google Scholar]
- Bognár, A. Tables on Weight Yield of Food and Retention Factors of Food Constituents for the Calculation of Nutrient Composition of Cooked Foods (Dishes); BFE: Karlsruhe, Germany, 2002. [Google Scholar]
- Rahim, A. Food and Foodways of Bangladesh: A Note on Recipe Composition and Eating Principle; Institute of Nutrition and Food Science, University of Dhaka: Dhaka, Bangladesh, 2013. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes. In The Essential Guide to Nutrient Requirements; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Gibson, R.S. Principles of Nutritional Assessment, 2nd ed.; Oxford University Press: New York, NY, USA, 2005. [Google Scholar]
- Institute of Medicine. Dietary Reference Intakes: Applications in Dietary Assessment; National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Lee, S.E.; Talegawkar, S.A.; Merialdi, M.; Caulfield, L.E. Dietary intakes of women during pregnancy in low-and middle-income countries. Public Health Nutr. 2013, 16, 1340–1353. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Huffman, S.L. Nutrition in pregnancy and early childhood and associations with obesity in developing countries. Matern. Child Nutr. 2013, 9, 105–119. [Google Scholar] [CrossRef] [PubMed]
- Yajnik, C.S. Transmission of obesity-adiposity and related disorders from the mother to the baby. Ann. Nutr. Metab. 2014, 64, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Baig-Ansari, N.; Badruddin, S.H.; Karmaliani, R.; Harris, H.; Jehan, I.; Pasha, O.; Moss, N.; McClure, E.M.; Goldenberg, R.L. Anemia prevalence and risk factors in pregnant women in an urban area of Pakistan. Food Nutr. Bull. 2008, 29, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Samuel, T.M.; Thomas, T.; Finkelstein, J.; Bosch, R.; Rajendran, R.; Virtanen, S.M.; Srinivasan, K.; Kurpad, A.V.; Duggan, C. Correlates of anaemia in pregnant urban South Indian women: A possible role of dietary intake of nutrients that inhibit iron absorption. Public Health Nutr. 2013, 16, 316–324. [Google Scholar] [CrossRef]
- Arsenault, J.E.; Yakes, E.A.; Islam, M.M.; Hossain, M.B.; Ahmed, T.; Hotz, C.; Lewis, B.; Rahman, A.S.; Jamil, K.M.; Brown, K.H. Very low adequacy of micronutrient intakes by young children and women in rural Bangladesh is primarily explained by low food intake and limited diversity. J. Nutr. 2013, 143, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Coffey, D. Prepregnancy body mass and weight gain during pregnancy in India and sub-Saharan Africa. Proc. Natl. Acad. Sci. USA 2015, 112, 3302–3307. [Google Scholar] [CrossRef]
- Thankachan, P.; Muthayya, S.; Walczyk, T.; Kurpad, A.V.; Hurrell, R.F. An analysis of the etiology of anemia and iron deficiency in young women of low socioeconomic status in Bangalore, India. Food Nutr. Bull. 2007, 28, 328–336. [Google Scholar] [CrossRef]
- Bloch, M.; Althabe, F.; Onyamboko, M.; Kaseba-Sata, C.; Castilla, E.E.; Freire, S.; Garcés, A.L.; Parida, S.; Goudar, S.S.; Kadir, M.M.; et al. Tobacco use and secondhand smoke exposure during pregnancy: An investigative survey of women in 9 developing nations. Am. J. Public Health 2008, 98, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.C.; Subramoney, S. Smokeless tobacco use, birth weight, and gestational age: Population based, prospective cohort study of 1217 women in Mumbai, India. BMJ 2004, 328, 1538. [Google Scholar] [CrossRef] [PubMed]
- Ashfaq, M.; Channa, M.A.; Malik, M.A.; Khan, D. Morphological changes in human placenta of wet snuff users. J. Ayub Med. Coll. Abbottabad JAMC 2008, 20, 110–113. [Google Scholar] [PubMed]
- Willis, D.N.; Popovech, M.A.; Gany, F.; Hoffman, C.; Blum, J.L.; Zelikoff, J.T. Toxicity of gutkha, a smokeless tobacco product gone global: Is there more to the toxicity than nicotine? Int. J. Environ. Res. Public Health 2014, 11, 919–933. [Google Scholar] [CrossRef] [PubMed]
- Hambidge, K.M.; Krebs, N.F.; Garces, A.; Westcott, J.E.; Figueroa, L.; Goudar, S.S.; Dhaded, S.; Pasha, O.; Aziz Ali, S.; Tshefu, A.; et al. Anthropometric indices for non-pregnant women of childbearing age differ widely among four low-middle income populations. BMC Public Health 2017, 18, 45. [Google Scholar] [CrossRef]
- Termote, C.; Bwama Meyi, M.; Dhed’a Djailo, B.; Huybregts, L.; Lachat, C.; Kolsteren, P.; Van Damme, P. A biodiverse rich environment does not contribute to a better diet: A case study from DR Congo. PLoS ONE 2012, 7, e30533. [Google Scholar] [CrossRef]
- Jaacks, L.M.; Slining, M.M.; Popkin, B.M. Recent underweight and overweight trends by rural-urban residence among women in low-and middle-income countries. J. Nutr. 2015, 145, 352–357. [Google Scholar] [CrossRef]
- Bielderman, I.; Vossenaar, M.; Melse-Boonstra, A.; Solomons, N.W. The potential double-burden of vitamin A malnutrition: Under-and overconsumption of fortified table sugar in the Guatemalan highlands. Eur. J. Clin. Nutr. 2016, 70, 947–953. [Google Scholar] [CrossRef]
- De Graaf, C. Why liquid energy results in overconsumption. Proc. Nutr. Soc. 2011, 70, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Popkin, B.M. Contemporary nutritional transition: Determinants of diet and its impact on body composition. Proc. Nutr. Soc. 2011, 70, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.M.; Micha, R.; Khatibzadeh, S.; Shi, P.; Lim, S.; Andrews, K.G.; Engell, R.E.; Ezzati, M.; Mozaffarian, D. Global, Regional, and National Consumption of Sugar-Sweetened Beverages, Fruit Juices, and Milk: A Systematic Assessment of Beverage Intake in 187 Countries. PLoS ONE 2015, 10, e0124845. [Google Scholar] [CrossRef] [PubMed]
- Levin, B.E. Metabolic imprinting: Critical impact of the perinatal environment on the regulation of energy homeostasis. Philos. Trans. R. Soc. Lond B Biol. Sci. 2006, 361, 1107–1121. [Google Scholar] [CrossRef] [PubMed]
- Hacker, A.N.; Fung, E.B.; King, J.C. Role of calcium during pregnancy: Maternal and fetal needs. Nutr. Rev. 2012, 70, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Hambidge, K.M.; Miller, L.V.; Mazariegos, M.; Westcott, J.; Solomons, N.W.; Raboy, V.; Kemp, J.F.; Das, A.; Goco, N.; Hartwell, T.; et al. Upregulation of zinc absorption matches increases in physiologic requirements for zinc in women consuming high-or moderate-phytate diets during late pregnancy and early lactation. J. Nutr. 2017. [Google Scholar] [CrossRef]
- Hambidge, K.M.; Krebs, N.F. Strategies for optimizing maternal nutrition to promote infant development. Reprod. Health 2018, 15, 87. [Google Scholar] [CrossRef]
- Arimond, M.; Wiesmann, D.; Becquey, E.; Carriquiry, A.; Daniels, M.C.; Deitchler, M.; Fanou-Fogny, N.; Joseph, M.L.; Kennedy, G.; Martin-Prevel, Y.; et al. Simple food group diversity indicators predict micronutrient adequacy of women’s diets in 5 diverse, resource-poor settings. J. Nutr. 2010, 140, 2059S–2069S. [Google Scholar] [CrossRef]
- Martin-Prével, Y.; Allemand, P.; Wiesmann, D.; Arimond, M.; Ballard, T.; Deitchler, M.; Dop, M.C.; Kennedy, G.; Lee, W.T.; Moursi, M. Moving Forward on Choosing a Standard Operational Indicator of Women’s Dietary Diversity. Available online: http://www.fao.org/3/a-i4942e.pdf (accessed on 13 April 2019).
- Iannotti, L.L.; Lutter, C.K.; Waters, W.F.; Gallegos Riofrio, C.A.; Malo, C.; Reinhart, G.; Palacios, A.; Karp, C.; Chapnick, M.; Cox, K.; et al. Eggs early in complementary feeding increase choline pathway biomarkers and DHA: A randomized controlled trial in Ecuador. Am. J. Clin. Nutr. 2017, 106, 1482–1489. [Google Scholar] [CrossRef]
- Rao, S.; Joshi, S.; Bhide, P.; Puranik, B.; Asawari, K. Dietary diversification for prevention of anaemia among women of childbearing age from rural India. Public Health Nutr. 2014, 17, 939–947. [Google Scholar] [CrossRef]
- Food and Agriculture Organization/International Network of Food Data Systems (FAO/INFOODS). FAO/INFOODS Food Composition Database For Biodiversity; Food and Agriculture Organization Rome: Rome, Italy, 2013. [Google Scholar]
- Savy, M.; Martin-Prevel, Y.; Traissac, P.; Eymard-Duvernay, S.; Delpeuch, F. Dietary diversity scores and nutritional status of women change during the seasonal food shortage in rural Burkina Faso. J. Nutr. 2006, 136, 2625–2632. [Google Scholar] [CrossRef] [PubMed]
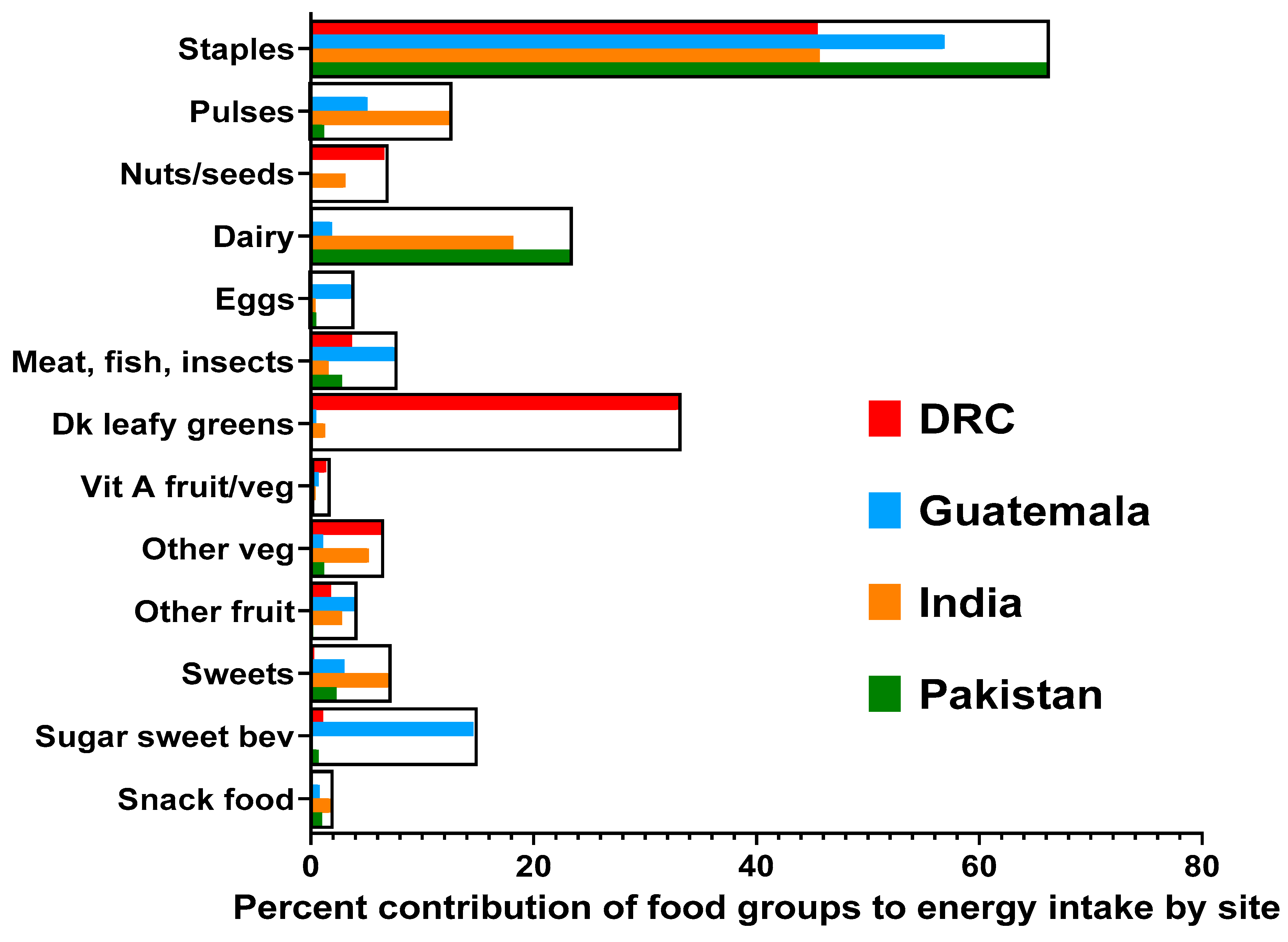
| DRC | Guatemala | India | Pakistan | |
|---|---|---|---|---|
| n | 218 | 230 | 245 | 273 |
| Maternal demographics 1 | ||||
| Age, years | 24.2 ± 4.8 | 25.3 ± 4.1 | 22.5 ± 3.2 | 23.8 ± 4.3 |
| <19 years (%) | 13.7 | 5.3 | 6.3 | 15.0 |
| ≥19 years (%) | 86.3 | 94.7 | 93.7 | 85.0 |
| Weight, kg | 51.0 ± 6.7 | 53.4 ± 9.2 | 46.2 ± 8.9 | 46.2 ± 7.3 |
| Height, cm | 156.1 ± 6.2 | 145.5 ± 4.9 | 151.4 ± 5.7 | 152.4 ± 6.3 |
| BMI | 20.8 ± 2.2 | 25.2 ± 4.0 | 20.3 ± 3.6 | 19.9 ± 3.0 |
| Dietary Recalls (n) | ||||
| Arm 1—Day 1 | 99 | 107 | 117 | 136 |
| Arm 1—Day 2 | 79 | 107 | 112 | 122 |
| Arm 1 Total | 178 | 214 | 229 | 258 |
| Arm 2—Day 1 | 119 | 123 | 128 | 137 |
| Arm 2—Day 2 | 98 | 118 | 120 | 120 |
| Arm 2 Total | 217 | 241 | 248 | 257 |
| Total Recalls | 395 | 455 | 477 | 515 |
| Description of recall day, n (%) | ||||
| Usual | 389 (98.5) | 403 (88.6) | 445 (93.3) | 445 (86.4) |
| Fasting | 0 (0) | 0 (0) | 11 (2.3) | 3 (0.6) |
| Feasting | 4 (1.0) | 47 (10.3) | 13 (2.7) | 49 (9.5) |
| Market | 2 (0.5) | 5 (1.1) | 8 (1.7) | 18 (3.5) |
| Reported health of recall day, n (%) | ||||
| Vomiting | 19 (4.8) | 11 (2.4) | 75 (15.7) | 58 (11.2) |
| Nausea | 20 (5.1) | 206 (48.1) | 99 (26.2) | 26 (5.1) |
| Well | 356 (90.1) | 238 (49.5) | 303 (58.1) | 431 (83.7) |
| DRC | Guatemala | India | Pakistan | |
|---|---|---|---|---|
| Energy, kcal/day | 1821, 1258, 2449 | 1875, 1449, 2456 | 1281, 1047, 1600 | 1443, 1120, 1858 |
| Energy, kcal/kg/day | 37, 24, 51 | 34, 26, 47 | 29, 23, 36 | 33, 24, 42 |
| Protein, g/day | 36.6, 24.2, 51.4 | 54.6, 41.0, 74.1 | 30.5, 23.5, 39.6 | 36.9, 28.7, 47.9 |
| Protein, g/kg/day | 0.74, 0.48, 1.02 | 1.04, 0.73, 1.38 | 0.67, 0.49, 0.88 | 0.82, 0.62, 1.08 |
| Total fat, g/day | 83.4, 53.6, 122.0 | 37.1, 23.9, 55.3 | 51.7, 40.2, 65.4 | 33.7, 24.9, 44.8 |
| CHO, g/day | 215.5, 157.0, 292.9 | 342.6, 256.6, 441.6 | 179.1, 143.1, 220.7 | 244.2, 179.2, 306.7 |
| Macronutrient distribution | ||||
| Protein (10–35%) 2 | 8% | 11% | 9% | 10% |
| Fat (20–35%) 2 | 43% | 17% | 36% | 21% |
| CHO (45–65%) 2 | 49% | 71% | 55% | 68% |
| Nutrient | EAR | DRC | Guatemala | India | Pakistan |
|---|---|---|---|---|---|
| Protein, g/kg/day | 0.88 g/kg | 0.74, 0.48, 1.02 | 1.04, 0.73, 1.38 | 0.67, 0.49, 0.88 | 0.82, 0.62, 1.08 |
| Calcium, mg/day | 1100 mg 2 | 498, 266, 720 | 1114, 606, 1320 | 395, 298, 603 | 524, 371, 658 |
| 800 mg 3 | 433, 239, 640 | 956, 667, 1212 | 386, 251, 549 | 558, 359, 838 | |
| Total iron, mg/day | 23 mg 2 | 9.18, 7.20, 13.81 | 14.15, 9.76, 18.89 | 8.20, 5.19, 10.65 | 10.12, 7.45, |
| 22 mg 3 | 8.74, 5.85, 13.28 | 13.31, 9.86, 17.71 | 7.62, 6.04, 9.70 | 9.58, 6.86, 15.69 | |
| Heme iron, mg/day 4,5 | - | 1.39 ± 2.52 | 2.77 ± 3.69 | 0.35 ± 1.21 | 1.80 ± 2.83 |
| Zinc, mg/day | 10.5 mg 2 | 3.42, 2.15, 5.95 | 9.24, 7.01, 12.40 | 4.81, 3.95, 6.83 | 8.63, 6.43, 11.07 |
| 9.5 mg 3 | 3.30, 2.01, 5.19 | 7.91, 5.91, 10.55 | 4.71, 3.79, 6.03 | 8.88, 6.46, 11.43 | |
| Vit A (RAE), µg/day | 530 µg 2 | 4876, 2956, 6353 | 1069, 848, 1374 | 227, 161, 350 | 152, 104, 202 |
| 550 µg 3 | 3672, 2246, 5401 | 1050, 688, 1469 | 220, 155, 303 | 159, 110, 228 | |
| Thiamine, mg/day | 1.2 mg | 0.61, 0.43, 0.95 | 1.11, 0.79, 1.53 | 0.49, 0.39, 0.65 | 1.15, 0.79, 1.43 |
| Riboflavin, mg/day | 1.2 mg | 0.68, 0.48, 0.98 | 1.25, 0.93, 1.63 | 0.75, 0.55, 0.98 | 1.00, 0.74, 1.39 |
| Vit B6, mg/day | 1.6 mg | 0.82, 0.55, 1.31 | 1.65, 1.18, 2.29 | 0.97, 0.73, 1.24 | 1.79, 1.24, 2.48 |
| Folate (DFE), µg/day | 520 µg | 184, 112, 276 | 348, 222, 489 | 122, 86, 177 | 90, 68, 123 |
| Vit B12, µg/day | 2.2 µg | 0.01, 0.003, 0.44 | 0.85, 0.45, 1.62 | 0.73, 0.32, 1.24 | 1.19, 0.61, 2.03 |
| Vit C, mg/day | 66 mg 2 | 74, 39, 125 | 103, 56, 148 | 28, 22, 43 | 12, 8, 22 |
| 70 mg 3 | 56, 28, 107 | 57, 25, 129 | 32, 19, 50 | 14, 9, 20 | |
| Choline, mg/day | 450 mg 4 | 203, 142, 279 | 203, 110, 329 | 111, 84, 144 | 117, 85, 166 |
| Betaine, mg/day | - | 308, 155, 478 | 61, 23, 132 | 70, 48, 97 | 34, 11, 117 |
| Phytate, mg/day | - | 1807, 1057, 2941 | 2486, 1594, 3140 | 1090, 788, 1475 | 2350, 1339, 2553 |
| Phytate:Zn molar ratio 5 | 59.38 ± 34.68 | 30.31 ± 10.24 | 23.25 ± 7.69 | 22.91 ± 4.70 |
| DRC | Guatemala | India | Pakistan | |||||
|---|---|---|---|---|---|---|---|---|
| Nutrient | Inadeq | Adeq | Inadeq | Adeq | Inadeq | Adeq | Inadeq | Adeq |
| n (%) | 289 | 106 | 234 | 221 | 148 | 329 | 408 | 107 |
| (73.2%) | (26.8%) | (51.4%) | (48.6%) | (31.0%) | (69.0%) | (79.2%) | (20.8%) | |
| Energy, kcal | 1780 | 1990 * | 1760 | 2010 * | 1120 | 1320 * | 1420 | 1550 |
| 1140, 2420 | 1490, 2750 | 1300, 2290 | 1580, 2680 | 900, 1440 | 1090, 1650 | 110, 1850 | 1210, 1910 | |
| Protein, g | 34.3 | 40.1 * | 50.6 | 59.1 * | 27.2 | 31.9 * | 36.1 | 40.8 *** |
| 23.0, 49.5 | 30.7, 57.3 | 36.1, 67.3 | 45.1, 80.5 | 19.7, 35.2 | 24.6, 40.5 | 27.2, 47.3 | 31.6, 49.8 | |
| Fat, g | 82.0 | 93.5 ** | 31.1 | 42.8 * | 46.1 | 53.8 * | 32.7 | 36.4 *** |
| 48.7, 114.5 | 65.5, 131.2 | 20.7, 49.9 | 31.1, 61.6 | 31.0, 57.7 | 42.4, 67.8 | 24.6, 44.3 | 27.9, 48.0 | |
| Calcium, mg | 399 | 493 ** | 933 | 979 | 337 | 406 *** | 540 | 594 |
| 222, 618 | 282, 755 | 624, 1208 | 685, 1222 | 219, 526 | 276, 556 | 352, 808 | 401, 862 | |
| Iron, mg | 8.42 | 9.89 ** | 12.75 | 14.21 ** | 6.96 | 8.08 * | 9.36 | 10.23 |
| 5.46, 12.52 | 6.70, 14.63 | 8.95, 16.63 | 10.39, 18.86 | 5.14, 8.68 | 6.35, 10.10 | 6.81, 15.52 | 7.42, 16.28 | |
| Zinc, mg | 2.94 | 4.42 * | 7.66 | 8.51 ** | 4.48 | 4.90 * | 8.77 | 9.09 |
| 1.88, 4.82 | 2.67, 6.20 | 5.65, 9.86 | 6.41, 11.32 | 3.36, 5.55 | 3.99, 6.35 | 6.34, 11.25 | 6.73, 11.73 | |
| Vit A, µg | 3540 | 4130 ** | 931 | 1150 * | 192 | 227 ** | 152 | 174 *** |
| 2050, 5280 | 2660, 6350 | 605, 1350 | 795, 1600 | 135, 268 | 163, 320 | 105, 217 | 116, 269 | |
| Folate, µg | 173 | 227 * | 303 | 377 ** | 97 | 134 ** | 88 | 97 *** |
| 107, 263 | 148, 344 | 210, 471 | 248, 516 | 68, 138 | 97, 191 | 64, 119 | 74, 133 | |
| Vit B12, µg | 0.01 | 0.26 * | 0.71 | 1.04 * | 0.66 | 0.74 | 1.19 | 1.21 |
| 0.002, 0.08 | 0.01, 1.04 | 0.22, 1.27 | 0.69, 1.86 | 0.27, 1.24 | 0.35, 1.24 | 0.60, 2.05 | 0.73, 1.93 | |
| Choline, mg | 193 | 220 ** | 162 | 234 * | 100 | 115 * | 112 | 134 ** |
| 126, 266 | 168, 307 | 89, 296 | 136, 353 | 69, 127 | 89, 150 | 82, 163 | 104, 180 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lander, R.L.; Hambidge, K.M.; Westcott, J.E.; Tejeda, G.; Diba, T.S.; Mastiholi, S.C.; Khan, U.S.; Garcés, A.; Figueroa, L.; Tshefu, A.; et al. Pregnant Women in Four Low-Middle Income Countries Have a High Prevalence of Inadequate Dietary Intakes That Are Improved by Dietary Diversity. Nutrients 2019, 11, 1560. https://doi.org/10.3390/nu11071560
Lander RL, Hambidge KM, Westcott JE, Tejeda G, Diba TS, Mastiholi SC, Khan US, Garcés A, Figueroa L, Tshefu A, et al. Pregnant Women in Four Low-Middle Income Countries Have a High Prevalence of Inadequate Dietary Intakes That Are Improved by Dietary Diversity. Nutrients. 2019; 11(7):1560. https://doi.org/10.3390/nu11071560
Chicago/Turabian StyleLander, Rebecca L., K. Michael Hambidge, Jamie E. Westcott, Gabriela Tejeda, Tshilenge S. Diba, Shivanand C. Mastiholi, Umber S. Khan, Ana Garcés, Lester Figueroa, Antoinette Tshefu, and et al. 2019. "Pregnant Women in Four Low-Middle Income Countries Have a High Prevalence of Inadequate Dietary Intakes That Are Improved by Dietary Diversity" Nutrients 11, no. 7: 1560. https://doi.org/10.3390/nu11071560
APA StyleLander, R. L., Hambidge, K. M., Westcott, J. E., Tejeda, G., Diba, T. S., Mastiholi, S. C., Khan, U. S., Garcés, A., Figueroa, L., Tshefu, A., Lokangaka, A., Goudar, S. S., Somannavar, M. S., Ali, S. A., Saleem, S., McClure, E. M., Krebs, N. F., & on behalf of the Women First Preconception Nutrition Trial Group. (2019). Pregnant Women in Four Low-Middle Income Countries Have a High Prevalence of Inadequate Dietary Intakes That Are Improved by Dietary Diversity. Nutrients, 11(7), 1560. https://doi.org/10.3390/nu11071560





