Intra-Abdominal Fat Adipocyte Hypertrophy through a Progressive Alteration of Lipolysis and Lipogenesis in Metabolic Syndrome Rats
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diet
- ME = Maximum error.
- = # of the standard deviations of the mean estimator.
- = Variance of the SBP of the rats in our Institute.
- SS = sample size.
2.2. Serum Biochemical Variables
2.3. Adipocyte Isolation
2.4. Western Blotting for LPL, PKA, Perilipin A, and HSL
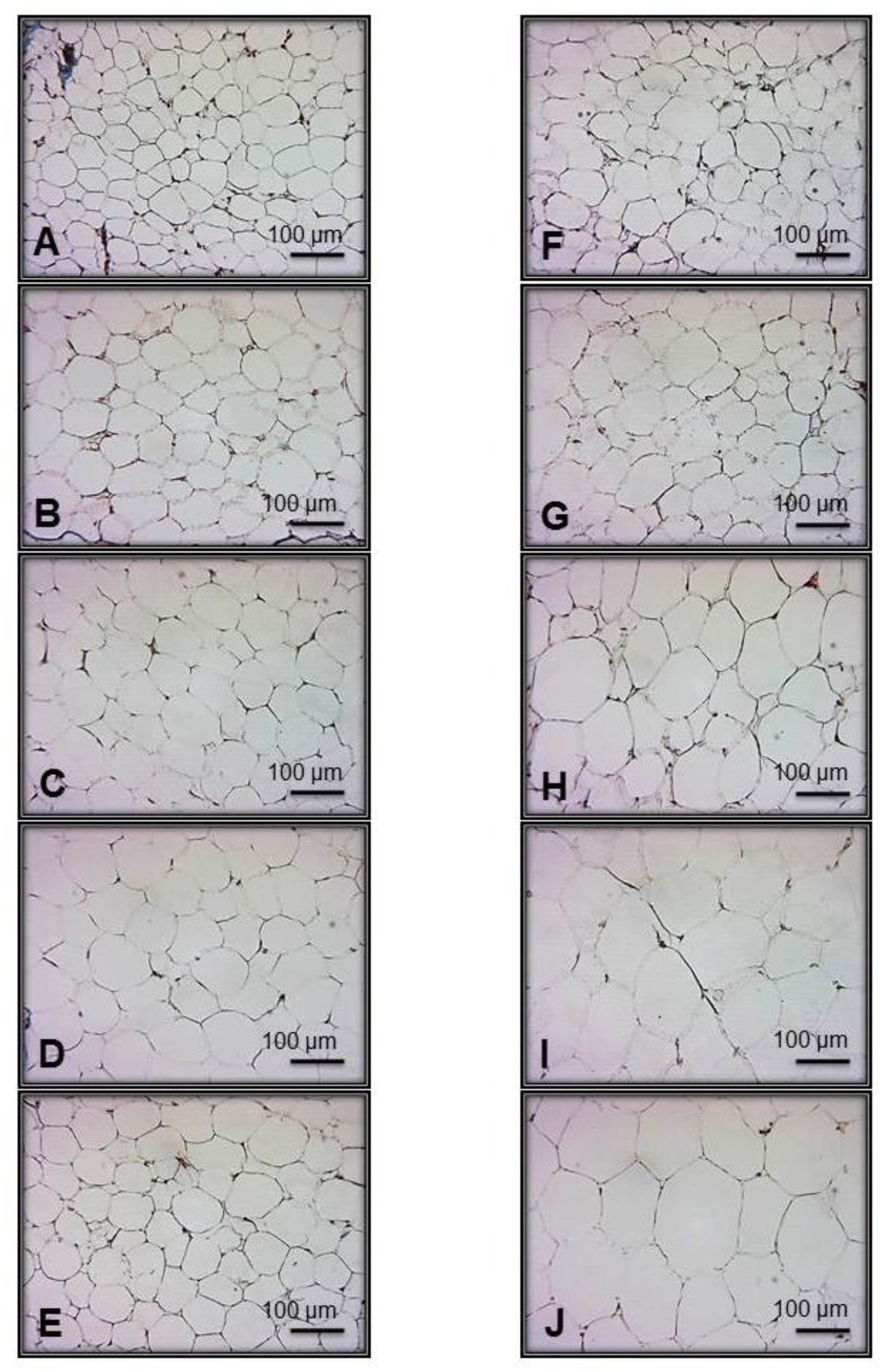
2.5. Retroperitoneal Fat Histology
2.6. LPL and HSL Activities
2.7. Extraction and Derivatization Non-Esterified Fatty Acid (NEFAs)
2.8. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. NEFAs Composition in Intra-Abdominal Adipocyte Homogenate
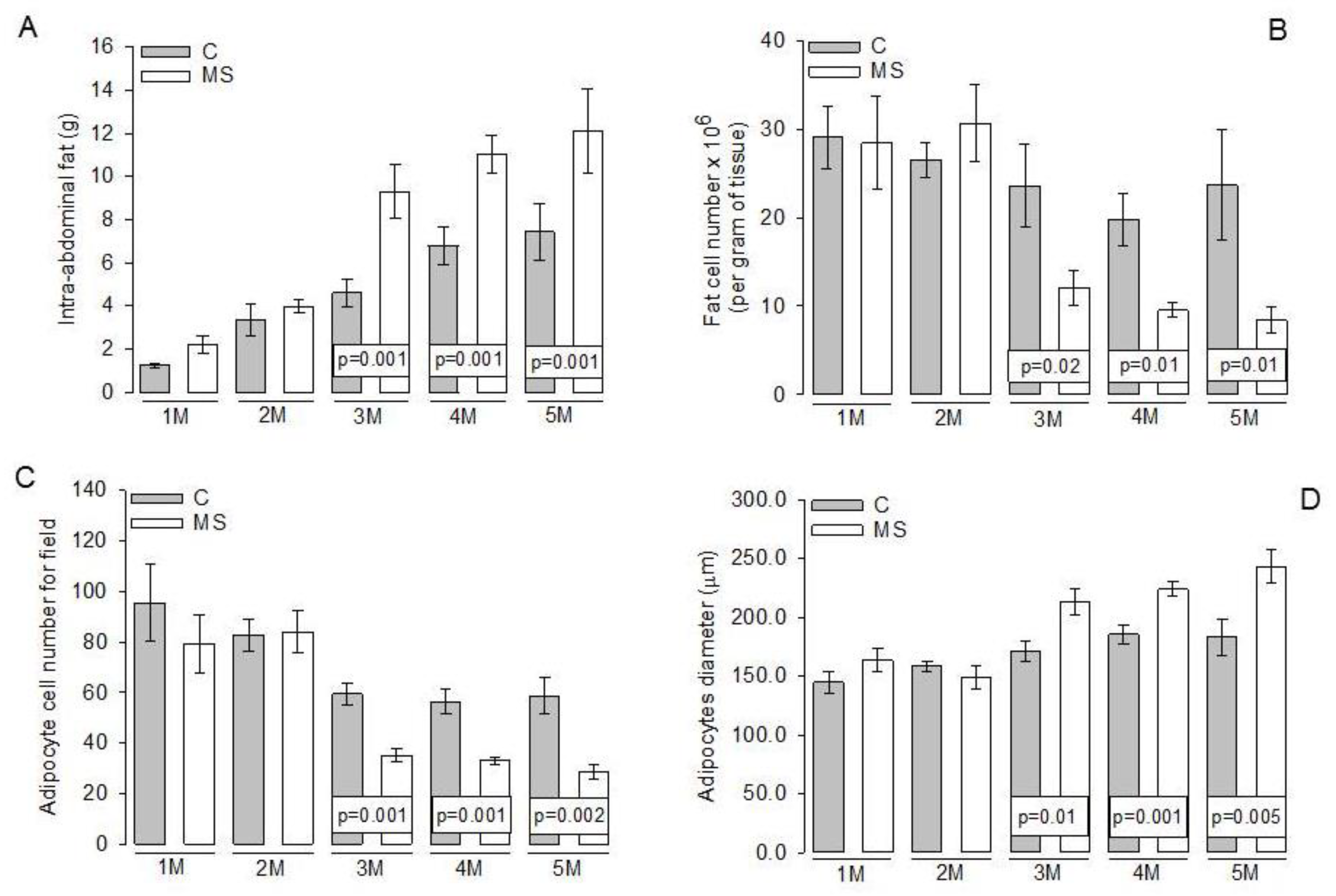
3.3. Intra-Abdominal Adipocytes
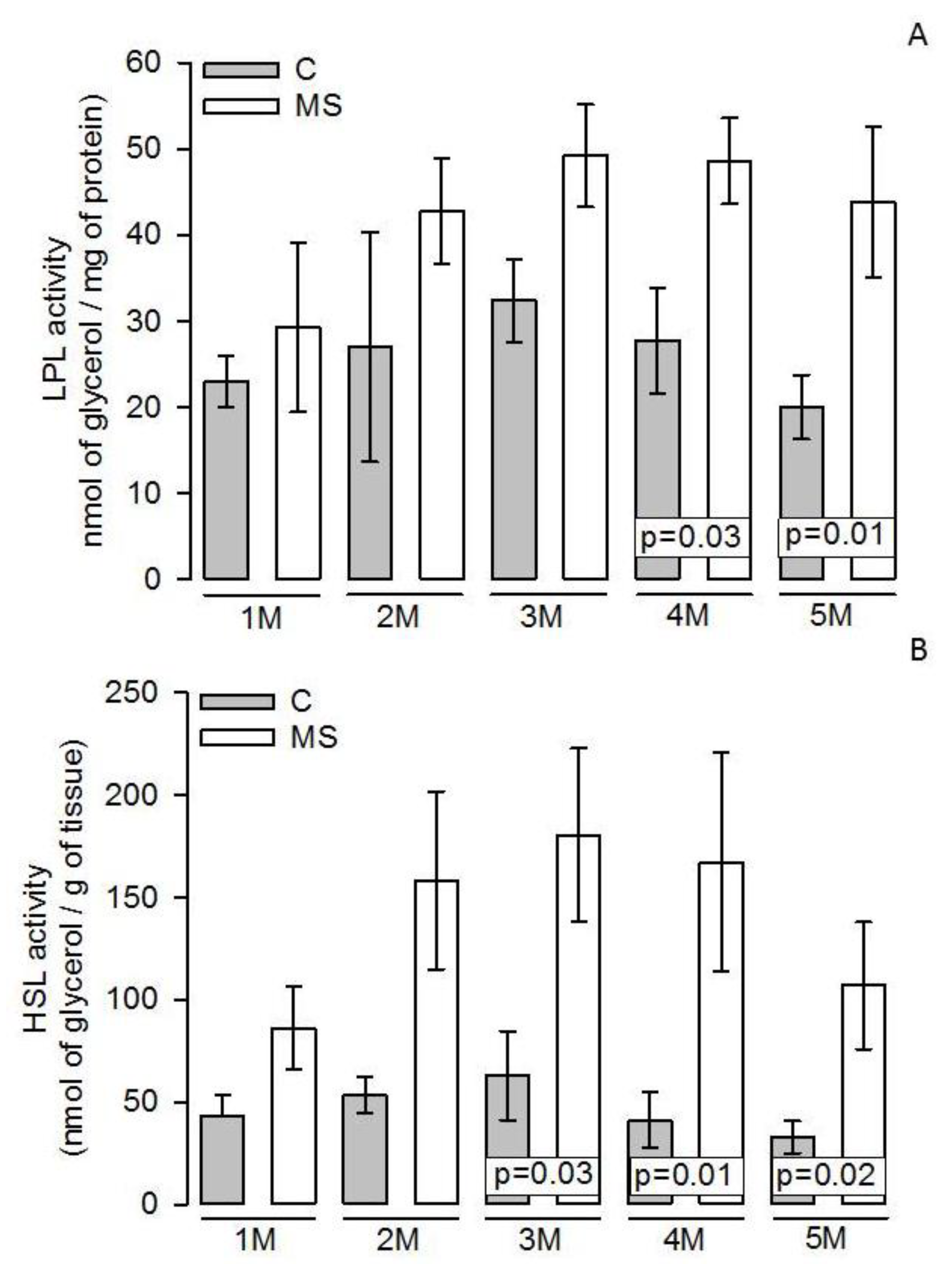
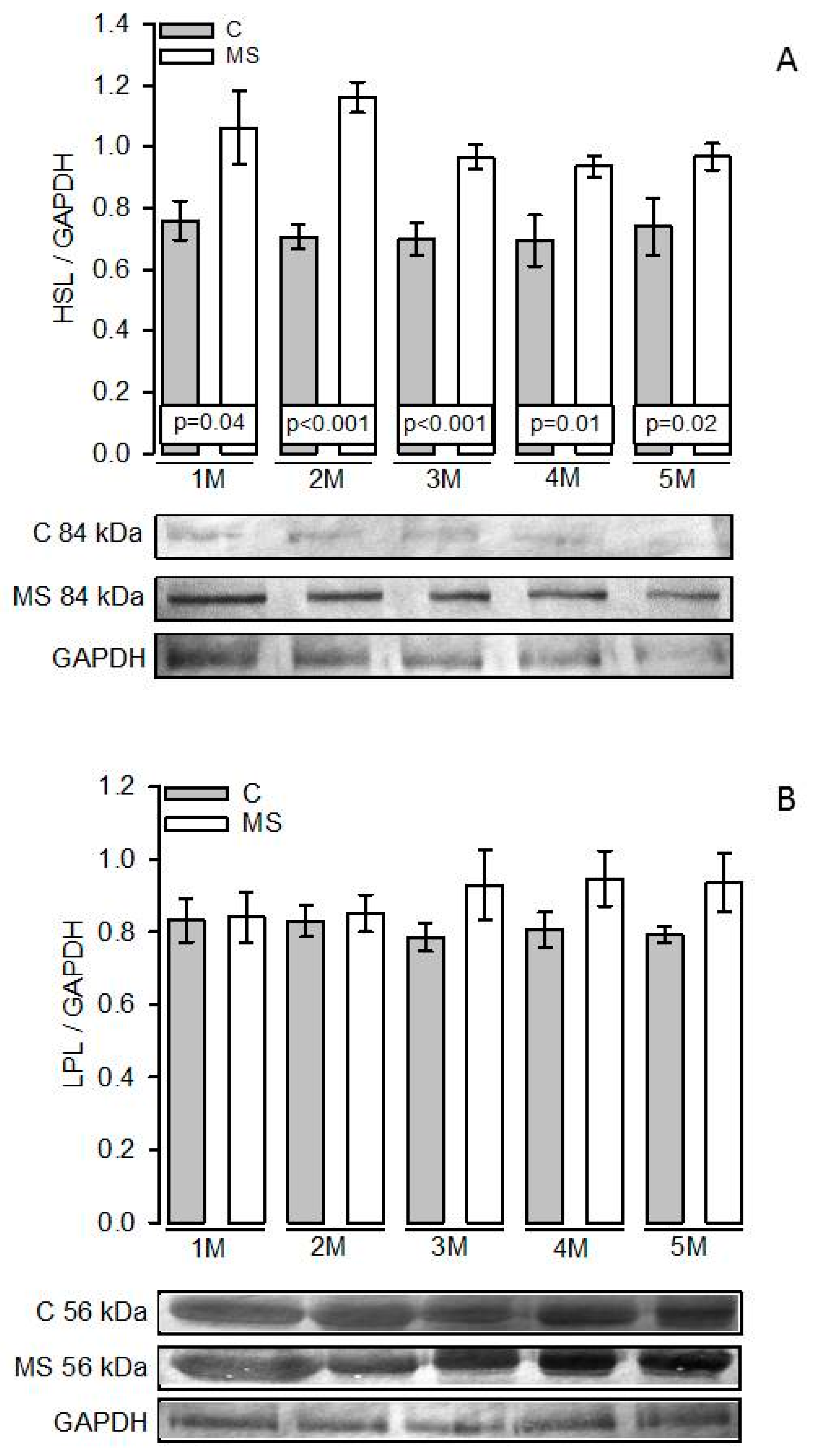
3.4. LPL and HSL Activities in Adipocyte Homogenate
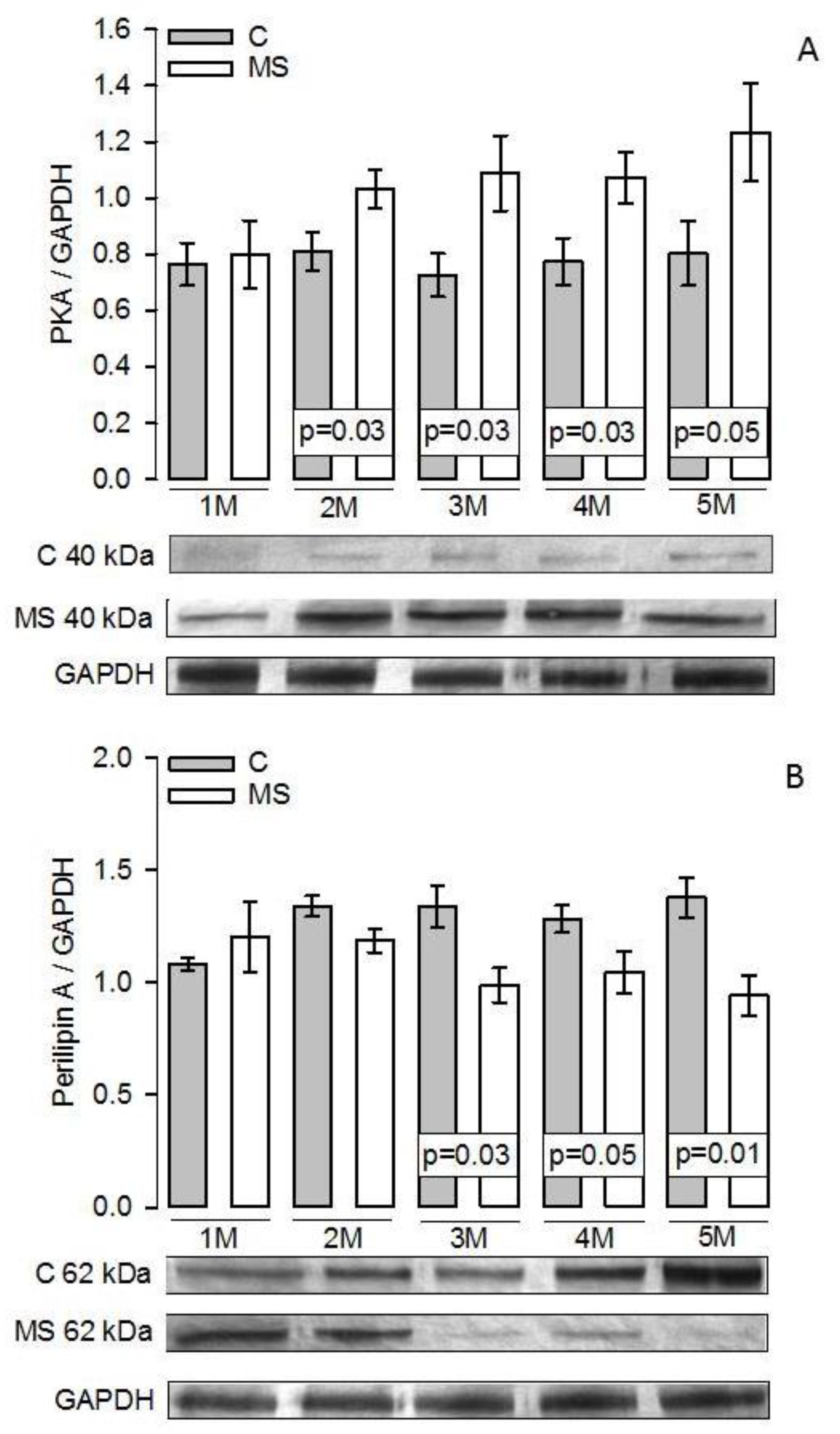
3.5. PKA, Perilipin A, HSL, and LPL Expressions
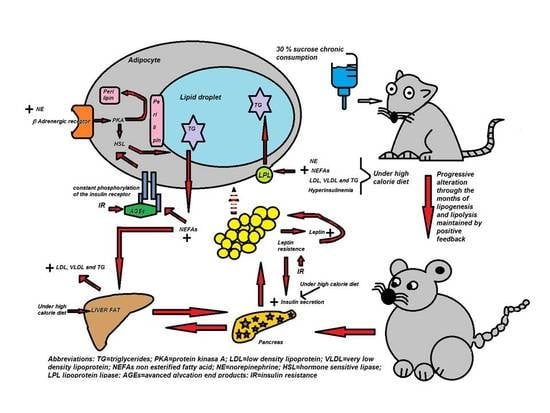
4. Discussion
4.1. Progressive Changes in Body and Biochemical Variables during the Establishment of the MS
4.2. Changes in Abdominal Tissue
4.3. Lipogenesis and LPL
4.4. Lipolysis and HSL
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lorenzo, C.; Williams, K.; Hunt, K.J.; Haffner, S.M. The National Cholesterol Education Program Adult Treatment Panel III, International Diabetes Federation, and World Health Organization definitions of the metabolic. syndrome as predictors of incident cardiovascular disease and diabetes. Diabetes Care 2007, 30, 8–13. [Google Scholar] [CrossRef]
- Lafontan, M. Adipose tissue and adipocyte dysregulation. Diabetes Metab. 2014, 40, 16–28. [Google Scholar] [CrossRef]
- Xie, B.; Waters, M.J.; Schirra, H.J. Investigating potential mechanisms of obesity by metabolomics. J. Biomed. Biotechnol. 2012, 2012, 805683. [Google Scholar] [CrossRef]
- Singla, P.; Bardoloi, A.; Parkash, A.A. Metabolic effects of obesity: A review. World J. Diabetes 2010, 1, 76–88. [Google Scholar] [CrossRef]
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef]
- Zechner, R.; Zimmermann, R.; Eichmann, T.O.; Kohlwein, S.D.; Haemmerle, G.; Lass, A.; Madeo, F. FAT SIGNALS-lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012, 15, 279–291. [Google Scholar] [CrossRef]
- Kersten, S. Mechanisms of nutritional and hormonal regulation of lipogenesis. EMBO Rep. 2001, 2, 282–286. [Google Scholar] [CrossRef]
- Gaidhu, M.P.; Anthony, N.M.; Patel, P.; Hawke, T.J.; Ceddia, R.B. Dysregulation of lipolysis and lipid metabolism in visceral and subcutaneous adipocytes by high-fat diet: role of ATGL, HSL, and AMPK. Am. J. Physiol. Cell Physiol. 2010, 298, C961–C971. [Google Scholar] [CrossRef]
- Duncan, R.E.; Ahmadian, M.; Jaworski, K.; Sarkadi-Nagy, E.; Sul, H.S. Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 2007, 27, 79–101. [Google Scholar] [CrossRef]
- Tansey, J.T.; Sztalryd, C.; Hlavin, E.M.; Kimmel, A.R.; Londos, C. The central role of perilipin a in lipid metabolism and adipocyte lipolysis. IUBMB Life 2004, 56, 379–385. [Google Scholar] [CrossRef]
- Lim, W.Y.; Chia, Y.Y.; Liong, S.Y.; Ton, S.H.; Kadir, K.A.; Husain, S.N. Lipoprotein lipase expression, serum lipid and tissue lipid deposition in orally-administered glycyrrhizic acid-treated rats. Lipids Health Dis. 2009, 29, 31. [Google Scholar] [CrossRef]
- Wang, H.; Eckel, R.H. Lipoprotein lipase: from gene to obesity. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E271–E288. [Google Scholar] [CrossRef]
- Mancuso, P.; Bouchard, B. The impact of aging on adipose function and adipokine synthesis. Front. Endocrinol. (Lausanne) 2019, 10, 137. [Google Scholar] [CrossRef]
- Baños, G.; Carvajal, K.; Cardoso, G.; Zamora, J.; Franco, M. Vascular reactivity and effect of serum in a rat model of hypertriglyceridemia and hypertension. Am. J. Hypertens. 1997, 10, 379–388. [Google Scholar] [CrossRef]
- Kohn, D.F.; Clifford, C.B. Laboratory Animal Medicine, 2nd ed.; Fox, J.G., Andreson, L.C., Loew, F.M., Quimby, F.W., Eds.; American College of Laboratory Animal Medicine Series: San Diego, CA, USA, 2002; p. 126. [Google Scholar]
- Pérez-Torres, I.; Torres-Narváez, J.C.; Guarner-Lans, V.; Díaz-Díaz, E.; Perezpeña-Diazconti, M.; Palacios, A.R.; Manzano-Pech, L. Myocardial protection from ischemia-reperfusion damage by the antioxidant effect of Hibiscus Sabdariffa Linnaeus on metabolic syndrome rats. Oxid. Med. Cell. Longev. 2019, 2019, 1724194. [Google Scholar] [CrossRef]
- Jiménez, I.U.; Díaz-Díaz, E.; Castro, J.S.; Ramos, J.P.; León, M.C.; Alvarado, J.A.; Auriostigue, J.C.; Correa-Rotter, R.; Aguilar, C.A.; Larrea, F. Circulating concentrations of advanced glycation end products, its association with the development of diabetes mellitus. Arch. Med. Res. 2017, 48, 360–369. [Google Scholar] [CrossRef]
- Rodbell, M. metabolism of isolated fat cells. i. effects of hormones on glucose metabolism and lipolysis. J. Biol. Chem. 1964, 239, 375–380. [Google Scholar]
- Guerra, R.C.; Zuñiga-Muñoz, A.; Guarner-Lans, V.; Díaz-Díaz, E.; Tena, C.A.; Pérez-Torres, I. Modulation of the activities of catalase, cu-zn, mn superoxide dismutase, and glutathione peroxidase in adipocyte from ovariectomised female rats with metabolic syndrome. Int. J. Endocrinol. 2014, 2014, 175080. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Commerford, S.R.; Pagliassotti, M.J.; Melby, C.L.; Wei, Y.; Gayles, E.C.; Hill, J.O. Fat oxidation, lipolysis, and free fatty acid cycling in obesity-prone and obesity-resistant rats. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E875–E885. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Tserng, K.Y.; Kliegman, R.M.; Miettinen, E.L.; Kalhan, S.C. A rapid, simple, and sensitive procedure for the determination of free fatty acids in plasma using glass capillary column gas-liquid chromatography. J. Lipid Res. 1981, 22, 852–858. [Google Scholar]
- López, Y.R.; Pérez-Torres, I.; Zúñiga-Muñoz, A.; Lans, V.G.; Díaz-Díaz, E.; Castro, E.S.; Espejel, R.V. Effect of glycine on adipocyte hypertrophy in a metabolic syndrome rat model. Curr. Drug. Deliv. 2016, 13, 158–169. [Google Scholar] [CrossRef]
- Reaven, G.M.; Ho, H. Sugar-induced hypertension in Sprague-Dawley rats. Am. J. Hypertens. 1991, 4, 610–614. [Google Scholar] [CrossRef]
- Muniyappa, R.; Lee, S.; Chen, H.; Quon, M.J. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am. J. Physiol. Endocrinol. Metab. 2008, 294, E15–E26. [Google Scholar] [CrossRef]
- Rubio-Ruiz, M.E.; Guarner-Lans, V.; Cano-Martínez, A.; Díaz-Díaz, E.; Manzano-Pech, L.; Gamas-Magaña, A.; Castrejón-Tellez, V.; Tapia-Cortina, C.; Pérez-Torres, I. Resveratrol and quercetin administration improves antioxidant DEFENSES and reduces fatty liver in metabolic syndrome rats. Molecules 2019, 24, 1297. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.Y.; Suhaimi, F.H.; Fairus, A.; Ima-Nirwana, S. Animal models of metabolic syndrome: A review. Nutr. Metab. (London) 2016, 13, 65. [Google Scholar] [CrossRef]
- Dupas, J.; Feray, A.; Goanvec, C.; Guernec, A.; Samson, N.; Bougaran, P.; Guerrero, F.; Mansourati, J. Metabolic syndrome and hypertension resulting from fructose enriched diet in wistar rats. Biomed. Res. Int. 2017, 2017, 2494067. [Google Scholar] [CrossRef]
- Brown, A.E.; Walker, M. Genetics of insulin resistance and the metabolic syndrome. Curr. Cardiol. Rep. 2016, 18, 75. [Google Scholar] [CrossRef]
- Song, F.; Schmidt, A.M. Glycation and insulin resistance: novel mechanisms and unique targets? Arter. Thromb. Vasc. Biol. 2012, 32, 1760–1765. [Google Scholar] [CrossRef]
- Mark, A.B.; Poulsen, M.W.; Andersen, S.; Andersen, J.M.; Bak, M.J.; Ritz, C.; Holst, J.J.; Nielsen, J.; de Courten, B.; Ove Dragsted, L.; et al. Consumption of a diet low in advanced glycation end products for 4 weeks improves insulin sensitivity in overweight women. Diabetes Care 2014, 37, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Rhinesmith, T.; Turkette, T.; Root-Bernstein, R. Rapid non-enzymatic glycation of the insulin receptor under hyperglycemic conditions inhibits insulin binding in vitro: implications for insulin resistance. Int. J. Mol. Sci. 2017, 18, 2602. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Ruiz, M.E.; Guarner-Lans, V.; Pérez-Torres, I.; Soto, M.E. Mechanisms underlying metabolic syndrome-related sarcopenia and possible therapeutic measures. Int. J. Mol. Sci. 2019, 20, 647. [Google Scholar] [CrossRef] [PubMed]
- Egan, J.J.; Greenberg, A.S.; Chang, M.K.; Wek, S.A.; Moos, M.C.; Londos, C. Mechanism of hormone stimulated lipolysis in adipocytes: translocation of hormone-sensitive lipase to the lipid storage droplet. Proc. Natl. Acad. Sci. USA 1992, 89, 8537–8541. [Google Scholar] [CrossRef] [PubMed]
- García-Jiménez, S.; Bernal, F.G.; Martínez, M.F.; Monroy, N.A.; Toledano, J.C.; Meneses, A.A.; Gonzalez, M.L.; Aveleyra, O.E.; Terrazas, M.A.; Boll, M.C.; et al. Serum leptin is associated with metabolic syndrome in obese Mexican subjects. J. Clin. Lab. Anal. 2015, 29, 5–9. [Google Scholar] [CrossRef] [PubMed]
- Hattori, T.; Murase, T.; Ohtake, M.; Inoue, T.; Tsukamoto, H.; Takatsu, M.; Kato, Y.; Hashimoto, K.; Murohara, T.; et al. Characterization of a new animal model of metabolic syndrome: The DahlS.Z-Lepr(fa)/Lepr(fa) rat. Nutr. Diabetes 2011, 1, e1. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Higareda-Almaraz, J.C.; Karbiener, M.; Giroud, M.; Pauler, F.M.; Gerhalter, T.; Herzig, S.M. Norepinephrine triggers an immediate-early regulatory network response in primary human white adipocytes. BMC. Genomics. 2018, 19, 794. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; López, M.; Rahmouni, K. The cellular and molecular bases of leptin and ghrelin resistance in obesity. Nat. Rev. Endocrinol. 2017, 13, 338–351. [Google Scholar] [CrossRef]
- Bjørbaek, C.; Kahn, B.B. Leptin signaling in the central nervous system and the periphery. Recent Prog. Horm. Res. 2004, 59, 305–331. [Google Scholar] [CrossRef]
- Bessesen, D.H.; Robertson, A.D.; Eckel, R.H. Weight reduction increases adipose but decreases cardiac LPL in reduced-obese Zucker rats. Am. J. Physiol. 1991, 261, E246–E251. [Google Scholar] [CrossRef]
- Berger, J.J.; Barnard, R.J. Effect of diet on fat cell size and hormone-sensitive lipase activity. J. Appl. Physiol. 1999, 87, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.S.; Ton, S.H.; Phang, S.C.W.; Tan, J.B.L.; Abdul, K.K. Increased susceptibility of post-weaning rats on high-fat diet to metabolic syndrome. J. Adv. Res. 2017, 8, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Castellano-Castillo, D.; Moreno-Indias, I.; Fernández-García, J.C.; Alcaide-Torres, J.; Moreno-Santos, I.; Ocaña, L.; Gluckman, E.; Tinahones, F.; Queipo-Ortuño, M.I.; et al. Adipose tissue LPL methylation is associated with triglyceride concentrations in the metabolic syndrome. Clin. Chem. 2018, 64, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Arner, P. Human fat cell lipolysis: Biochemistry, regulation and clinical role. Best. Pract. Res. Clin. Endocrinol. Metab. 2005, 19, 471–482. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; El Hafidi, M.; Zamora-González, J.; Infante, O.; Chavira, R.; Baños, G. Modulation of aortic vascular reactivity by sex hormones in a male rat model of metabolic syndrome. Life Sci. 2007, 80, 2170–2180. [Google Scholar] [CrossRef]
- Large, V.; Arner, P.; Reynisdottir, S.; Grober, J.; Van Harmelen, V.; Holm, C.; Langin, D. Hormone-sensitive lipase expression and activity in relation to lipolysis in human fat cells. J. Lipid Res. 1998, 39, 1688–1695. [Google Scholar]
- Deng, J.; Liu, S.; Zuo, L.; Xu, C.; Geng, B.; Xu, G. Lipolysis response to endoplasmic reticulum stress in adipose cells. J. Biol. Chem. 2012, 287, 6240–6249. [Google Scholar] [CrossRef]
- Diaz-Villasenor, A.; Granados, O.; Gonzalez-Palacios, B.; Tovar-Palacio, C.; Torre-Villalvazo, I.; Olivares-Garcia, V.; Torres, N.; Tovar, A.R. Differential modulation of the functionality of white adipose tissue of obese Zucker (fa/fa) rats by the type of protein and the amount and type of fat. J. Nutr. Biochem. 2013, 24, 1798–1809. [Google Scholar] [CrossRef]
- Botion, L.M.; Green, A. Long-term regulation of lipolysis and hormone-sensitive lipase by insulin and glucose. Diabetes 1999, 48, 1691–1697. [Google Scholar] [CrossRef]
- Winzell, M.S.; Svensson, H.; Arner, P.; Ahrén, B.; Holm, C. The expression of hormone-sensitive lipase in clonal beta-cells and rat islets is induced by long-term exposure to high glucose. Diabetes 2001, 50, 2225–2230. [Google Scholar] [CrossRef][Green Version]
- Lönnqvist, F.; Arner, P.; Nordfors, L.; Schalling, M. Overexpression of the obese (ob) gene in adipose tissue of human obese subjects. Nat. Med. 1995, 1, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Siegrist-Kaiser, C.A.; Pauli, V.; Juge-Aubry, C.E.; Boss, O.; Pernin, A.; Chin, W.W.; Cusin, I.; Rohner-Jeanrenaud, F.; Burger, A.G.; Zapf, J.; et al. Direct effects of leptin on brown and white adipose tissue. J. Clin. Investig. 1997, 100, 2858–2864. [Google Scholar] [CrossRef] [PubMed]
- Smitka, K.; Marešová, D. Adipose tissue as an endocrine organ: An update on pro-inflammatory and anti-inflammatory microenvironment. Prague. Med. Rep. 2015, 116, 87–111. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.W.; Chiang, M.T.; Yao, H.T.; Chiang, W. The effect of high-fat and high-fructose diets on glucose tolerance and plasma lipid and leptin levels in rats. Diabetes Obes. Metab. 2004, 6, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Perez, I.; Hafidi, M.E.; Sanchez, C.; Baños, G. COMMUNICATIONS-Effect of sugar-induced hypertension in rats on the pattern of serum arachidonic, dihomo-g-linoleic and linoleic acids. Med. Sci. Res. 1999, 27, 847–885. [Google Scholar]
- Pérez-Torres, I.; El Hafidi, M.; Infante, O.; Baños, G. Effects of sex hormone levels on aortic vascular reactivity and variables associated with the metabolic syndrome in sucrose-fed female rats. Can. J. Physiol. Pharm. 2008, 86, 25–35. [Google Scholar] [CrossRef]
- Large, V.; Peroni, O.; Letexier, D.; Ray, H.; Beylot, M. Metabolism of lipids in human white adipocyte. Diabetes Metab. 2004, 30, 294–309. [Google Scholar] [CrossRef]
- Xu, B.; Xie, X. Neurotrophic factor control of satiety and body weight. Nat. Rev. Neurosci. 2016, 17, 282–292. [Google Scholar] [CrossRef]
- Fu, Q.; Hu, Y.; Wang, Q.; Liu, Y.; Li, N.; Xu, B.; Kim, S.; Chiamvimonvat, N.; Xiang, Y.K. High-fat diet induces protein kinase A and G-protein receptor kinase phosphorylation of β2-adrenergic receptor and impairs cardiac adrenergic reserve in animal hearts. J. Physiol. 2017, 595, 1973–1986. [Google Scholar] [CrossRef]
- Kou, X.H.; Zhu, M.F.; Chen, D.; Lu, Y.; Song, H.Z.; Ye, J.L.; Yue, L.F. Bilobetin ameliorates insulin resistance by PKA-mediated phosphorylation of PPARα in rats fed a high-fat diet. Br. J. Pharm. 2012, 165, 2692–2706. [Google Scholar] [CrossRef]
- London, E.; Nesterova, M.; Sinaii, N.; Szarek, E.; Chanturiya, T.; Mastroyannis, S.A.; Gavrilova, O.; Stratakis, C.A. Differentially regulated protein kinase A (PKA) activity in adipose tissue and liver is associated with resistance to diet-induced obesity and glucose intolerance in mice that lack PKA regulatory subunit type IIα. Endocrinology 2014, 155, 3397–3408. [Google Scholar] [CrossRef] [PubMed]
- Brasaemle, D.L.; Rubin, B.; Harten, I.A.; Gruia-Gray, J.; Kimmel, A.R.; Londos, C. Perilipin A increases triacylglycerol storage by decreasing the rate of triacylglycerol hydrolysis. J. Biol. Chem. 2000, 275, 38486–38493. [Google Scholar] [CrossRef] [PubMed]
- Tansey, J.T.; Sztalryd, C.; Gruia-Gray, J.; Roush, D.L.; Zee, J.V.; Gavrilova, O.; Reitman, M.L.; Deng, C.X.; Li, C.; Kimmel, A.R.; et al. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc. Natl. Acad. Sci. USA 2001, 98, 6494–6499. [Google Scholar] [CrossRef] [PubMed]
- Ray, H.; Pinteur, C.; Frering, V.; Beylot, M.; Large, V. Depot-specific differences in perilipin and hormone-sensitive lipase expression in lean and obese. Lipids Health Dis. 2009, 8, 58. [Google Scholar] [CrossRef] [PubMed]
- Mottagui-Tabar, S.; Ryden, M.; Lofgren, P.; Faulds, G.; Hoffstedt, J.; Brookes, A.J.; Andersson, I.; Arner, P. Evidence for an important role of perilipin in the regulation of human adipocyte lipolysis. Diabetologia 2003, 46, 789–797. [Google Scholar] [CrossRef][Green Version]
- Tawfik, S.H.; Mahmoud, B.F.; Saad, M.I.; Shehata, M.; Kamel, M.A.; Helmy, M.H. Similar and additive effects of ovariectomy and diabetes on insulin resistance and lipid metabolism. Biochem. Res. Int. 2015, 2015, 567945. [Google Scholar] [CrossRef]
- Castrejón-Tellez, V.; Rodríguez-Pérez, J.M.; Pérez-Torres, I.; Pérez-Hernández, N.; Cruz-Lagunas, A.; Guarner-Lans, V.; Vargas-Alarcón, G.; Rubio-Ruiz, M.E. The effect of resveratrol and quercetin treatment on PPAR mediated uncoupling protein (UCP-) 1, 2, and 3 expression in visceral white adipose tissue from metabolic syndrome rats. Int. J. Mol. Sci. 2016, 17, 1069. [Google Scholar] [CrossRef]
- Torre-Villalvazo, I.; Tovar, A.R.; Ramos-Barragán, V.E.; Cerbón-Cervantes, M.A.; Torres, N. Soy protein ameliorates metabolic abnormalities in liver and adipose tissue of rats fed a high fat diet. J. Nutr. 2008, 138, 462–468. [Google Scholar] [CrossRef]
| Variables | 1 Month | 2 Months | 3 Months | 4 Months | 5 Months | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | MS | C | MS | C | MS | C | MS | C | MS | |
| Body weight (g) | 228.2 ± 11.1 | 228.2 ± 17.1 | 317.2 ± 12.3 | 302.1 ± 16.7 | 413.7 ± 16.3 | 422.0 ± 17.2 | 491.3 ± 22.4 | 477.1 ± 21.5 | 443.0 ± 23.6 | 492.6 ± 13.4 § |
| Water Intake (mL) | 27.5 ± 1.0 | 15.7 ± 0.4 | 24.7 ± 1.6 | 13.2 ± 1.2 | 21.7 ± 1.8 | 29.8 ± 0.8 | 29.0 ± 1.5 | 17.2 ± 4.8 | 11.4 ± 1.7 | 19.1 ± 1.6 |
| Ingested food (g) | 37.1 ± 1.4 | 26.4 ± 1.4 | 39.2 ± 1.7 | 41.0 ± 1.1 | 53.5 ± 4.4 | 46.4 ± 1.7 | 50.7 ± 2.7 | 50.7 ± 4.5 | 45.0 ± 4.2 | 78.5 ± 6.4 † |
| SBP (mmHg) | 120.4 ± 2.9 | 125.9 ± 1.3 | 128.1 ± 1.4 | 136.9 ± 1.5 † | 121.5 ± 0.4 | 135.6 ± 0.4 † | 125.2 ± 0.2 | 139.4 ± 0.5 † | 125.2 ± 0.8 | 136.3 ± 0.2 † |
| Variables | 1 Month | 2 Months | 3 Months | 4 Months | 5 Months | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | MS | C | MS | C | MS | C | MS | C | MS | |
| Glucose (mmol/L) | 5.3 ± 0.2 | 5.6 ± 0.5 | 6.1 ± 0.3 | 5.7 ± 0.3 | 6.0 ± 0.1 | 5.9 ± 0.2 | 5.5 ± 0.1 | 5.7 ± 0.1 | 5.8 ± 0.2 | 5.5 ± 0.1 |
| TC (mm/dL) | 55.0 ± 3.0 | 59.2 ± 2.2 | 55.5 ± 2.7 | 52.2 ± 1.9 | 53.3 ± 1.7 | 50.7 ± 2.9 | 52.0 ± 1.4 | 53.8 ± 2.3 | 56.0 ± 2.7 | 47.5 ± 3.3 |
| TG (mg/dL) | 51.7 ± 2.1 | 110.1 ± 16.6 † | 55.5 ± 3.7 | 127.2 ± 14.5 † | 52.6 ± 2.0 | 100.0 ± 9.6 † | 44.8 ± 2.2 | 114.3 ± 7.0 † | 62.7 ± 4.4 | 94.7 ± 14.5 † |
| Insulin (µU/mL) | 1.5 ± 0.1 | 1.3 ± 0.3 | 2.0 ± 0.3 | 3.0 ± 0.4 † | 2.6 ± 0.1 | 4.1 ± 0.2 † | 2.0 ± 0.2 | 3.5 ± 0.3 † | 1.7 ± 0.3 | 4.0 ± 0.5 † |
| HOMA IR | 0.3 ± 0.04 | 0.3 ± 0.1 | 0.5 ± 0.06 | 0.6 ± 0.1 | 0.6 ± 0.05 | 1.0 ± 0.06 † | 0.5 ± 0.06 | 0.9 ± 0.09 † | 0.4 ± 0.08 | 1.1 ± 0.2 † |
| Leptin (ng/mL) | 4.2 ± 0.4 | 4.7 ± 0.5 | 5.7 ± 0.4 | 8.0 ± 0.4 † | 4.4 ± 0.6 | 8.8 ± 1.1 † | 3.9 ± 0.9 | 9.7 ± 1.4 † | 6.7 ± 1.5 | 12.7 ± 2.0 † |
| AGEs (mU/mL) | 235.5 ± 5.0 | 282.4 ± 26.4 | 259.5 ± 22.3 | 262.5 ± 74.1 | 185.5 ± 11.3 | 227.8 ± 18.1 | 145.1 ± 13.3 | 218.4 ± 30.4 * | 162.4 ± 26.3 | 262.7 ± 30.7 * |
| NEFAs (%) | 1 Month | 2 Months | 3 Months | 4 Months | 5 Months | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | MS | C | MS | C | MS | C | MS | C | MS | |
| Palmitic | 28.4 ± 0.4 | 25.1 ± 3.4 | 28.2 ± 0.6 | 26.7 ± 0.9 | 24.5 ± 0.1 | 32.5 ± 1.1 † | 30.1 ± 0.8 | 34.2 ± 1.3 * | 27.8 ± 1.5 | 34.1 ± 1.7 * |
| Palmitoleic | 4.2 ± 0.2 | 4.9 ± 0.7 | 4.8 ± 0.5 | 5.8 ± 1.5 | 3.8 ± 0.3 | 13.7 ± 0.1 † | 5.8 ± 1.0 | 12.1 ± 0.9 † | 6.5 ± 1.3 | 12.6 ± 0.8 † |
| Stearic | 7.2 ± 0.1 | 4.8 ± 0.4 | 6.1 ± 0.4 | 4.6 ± 0.8 | 15.1 ± 7.5 | 6.8 ± 2.1 | 9.1 ± 2.0 | 12.4 ± 1.2 | 12.1 ± 1.8 | 10.1 ± 1.9 |
| Oleic | 38.7 ± 2.4 | 36.8 ± 3.8 | 37.1 ± 0.9 | 44.6 ± 0.9 | 30.1 ± 2.3 | 40.9 ± 2.3 * | 31.7 ± 2.8 | 37.4 ± 2.2 * | 29.0 ± 4.1 | 39.7 ± 2.4 * |
| Linoleic | 17.9 ± 2.3 | 11.6 ± 0.9 | 22.2 ± 0.5 | 16.5 ± 2.3 | 18.3 ± 6.1 | 7.7 ± 2.3 | 14.7 ± 2.0 | 8.2 ± 1.6 * | 19.1 ± 1.9 | 6.0 ± 1.5 † |
| Total | 93.4 ± 5.4 | 83.2 ± 9.2 | 98.4 ± 2.9 | 98.2 ± 6.4 | 91.8 ± 16.3 | 101.6 ± 7.9 | 91.4 ± 8.6 | 104.3 ± 7.2 | 94.5 ± 10.6 | 102.5 ± 8.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Torres, I.; Gutiérrez-Alvarez, Y.; Guarner-Lans, V.; Díaz-Díaz, E.; Manzano Pech, L.; Caballero-Chacón, S.d.C. Intra-Abdominal Fat Adipocyte Hypertrophy through a Progressive Alteration of Lipolysis and Lipogenesis in Metabolic Syndrome Rats. Nutrients 2019, 11, 1529. https://doi.org/10.3390/nu11071529
Pérez-Torres I, Gutiérrez-Alvarez Y, Guarner-Lans V, Díaz-Díaz E, Manzano Pech L, Caballero-Chacón SdC. Intra-Abdominal Fat Adipocyte Hypertrophy through a Progressive Alteration of Lipolysis and Lipogenesis in Metabolic Syndrome Rats. Nutrients. 2019; 11(7):1529. https://doi.org/10.3390/nu11071529
Chicago/Turabian StylePérez-Torres, Israel, Yolanda Gutiérrez-Alvarez, Verónica Guarner-Lans, Eulises Díaz-Díaz, Linaloe Manzano Pech, and Sara del Carmen Caballero-Chacón. 2019. "Intra-Abdominal Fat Adipocyte Hypertrophy through a Progressive Alteration of Lipolysis and Lipogenesis in Metabolic Syndrome Rats" Nutrients 11, no. 7: 1529. https://doi.org/10.3390/nu11071529
APA StylePérez-Torres, I., Gutiérrez-Alvarez, Y., Guarner-Lans, V., Díaz-Díaz, E., Manzano Pech, L., & Caballero-Chacón, S. d. C. (2019). Intra-Abdominal Fat Adipocyte Hypertrophy through a Progressive Alteration of Lipolysis and Lipogenesis in Metabolic Syndrome Rats. Nutrients, 11(7), 1529. https://doi.org/10.3390/nu11071529