The Nutritional Value and Biological Activity of Concentrated Protein Fraction of Potato Juice
Abstract
1. Introduction
2. Materials and Methods
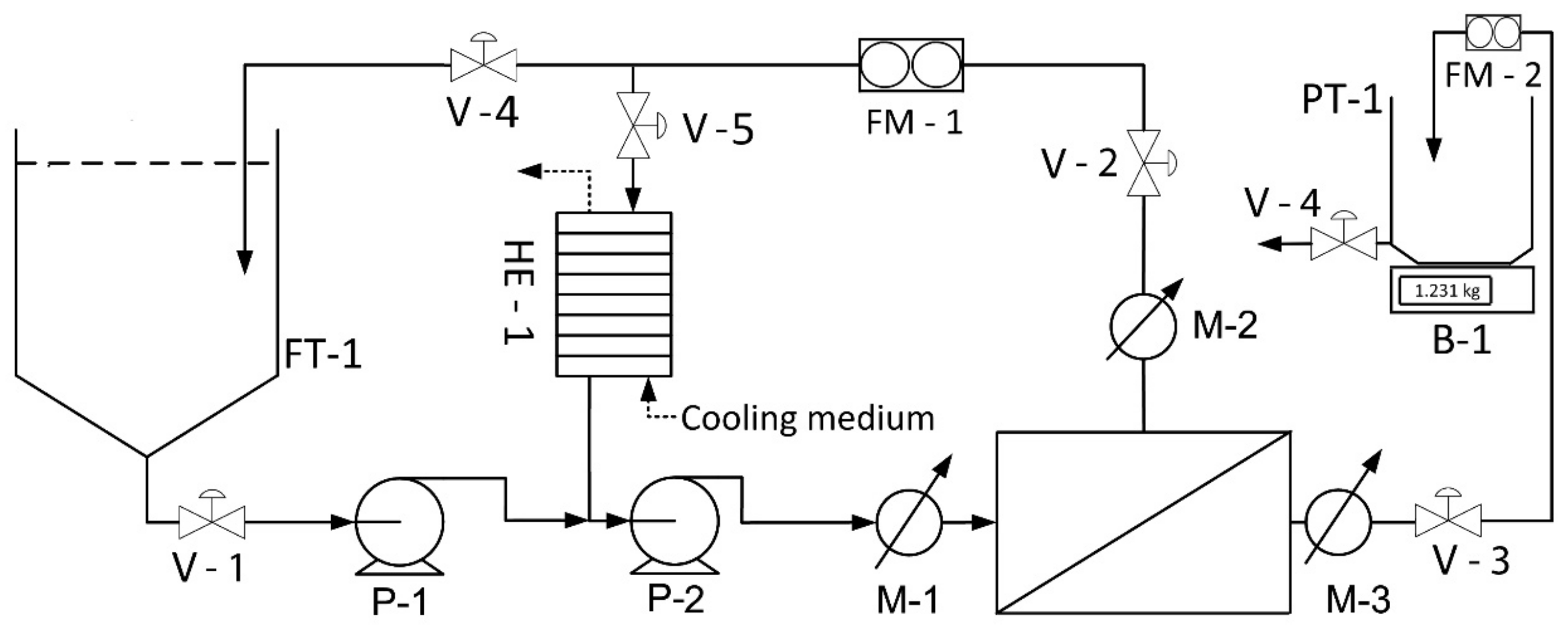
2.1. Experimental Material and Production of Protein Concentrate
2.2. Chemical Analysis
2.3. Analysis of Amino Acid Composition and Nutritional Profile
2.3.1. Amino Acid Composition
2.3.2. Protein Efficiency Ratio (PER)
2.3.3. Scoring of Amino Acids
2.3.4. Digestibility of PJPC
2.4. Determination of Mineral Content
2.5. Antioxidant Activity and Total Phenolic Compound Content
2.5.1. PJPC Ethanolic Extract Preparation
2.5.2. Determination of Antioxidant Activity
2.5.3. Determination of Total Phenolic Compounds (TPCs)
2.5.4. Determination of Total Flavonoid Compounds (TFCs)
2.6. Cell Cultures
2.7. In Vitro Cytotoxicity Assay
2.8. Statistical Analysis
3. Results and Discussion
3.1. Nutritional Value of PJPC
3.2. Antioxidant Activity of PJPC
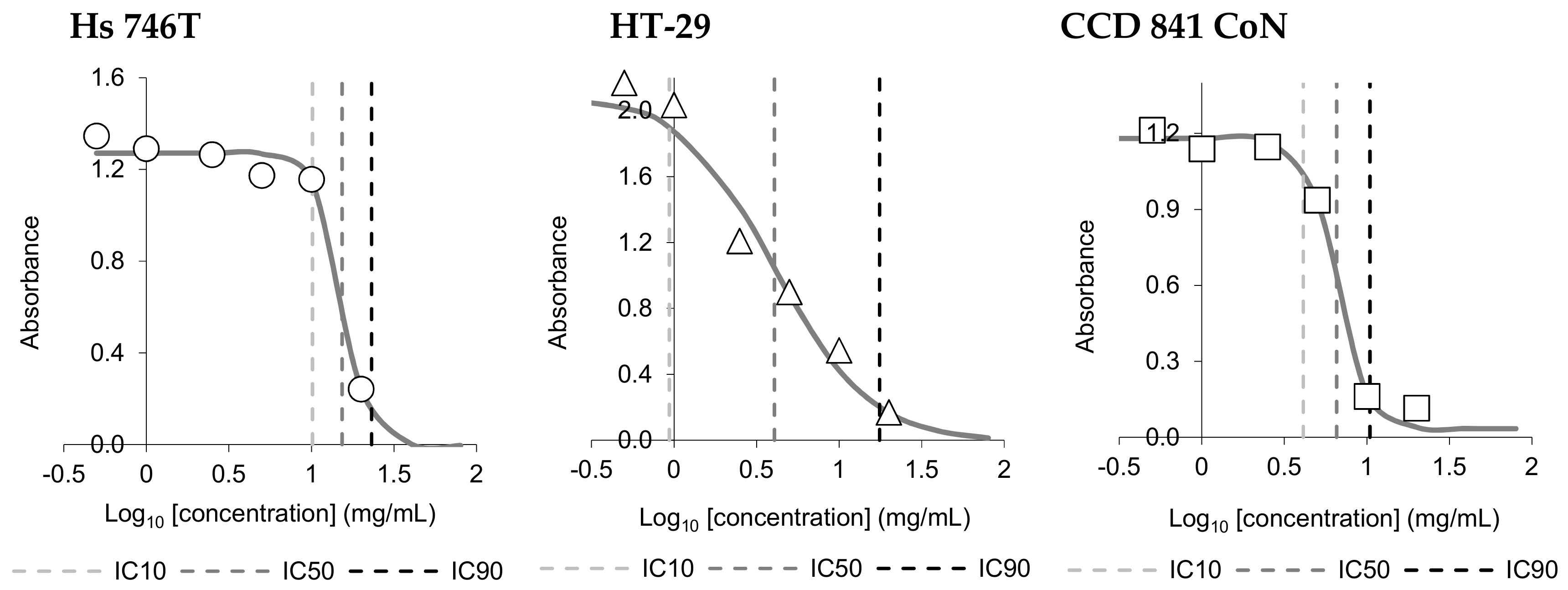
3.3. In Vitro Cytotoxic Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tuśnio, A.; Pastuszewska, B.; Święch, E.; Taciak, M. Response of young pigs to feeding potato protein and potato fibre—Nutritional, physiological and biochemical parameters. J. Anim. Feed Sci. 2011, 20, 361–378. [Google Scholar] [CrossRef]
- Lasik, M.; Nowak, J.; Kent, C.; Czarnecki, Z. Assessment of Metabolic activity of single and mixed microorganism population assigned for potato wastewater biodegradation. Pol. J. Environ. Stud. 2002, 11, 719–725. [Google Scholar]
- Ralet, M.-C.; Guéguen, J. Fractionation of Potato Proteins: Solubility, Thermal Coagulation and Emulsifying Properties. LWT-Food Sci. Technol. 2000, 33, 380–387. [Google Scholar] [CrossRef]
- Van Koningsveld, G.A.; Gruppen, H.; de Jongh, H.H.J.; Wijngaards, G.; van Boekel, M.A.J.S.; Walstra, P.; Voragen, A.G.J. Effects of pH and Heat Treatments on the Structure and Solubility of Potato Proteins in Different Preparations. J. Agric. Food Chem. 2001, 49, 4889–4897. [Google Scholar] [CrossRef] [PubMed]
- Pastuszewska, B.; Tuśnio, A.; Taciak, M.; Mazurczyk, W. Variability in the composition of potato protein concentrate produced in different starch factories—A preliminary survey. Anim. Feed Sci. Technol. 2009, 154, 260–264. [Google Scholar] [CrossRef]
- Burlingame, B.; Mouillé, B.; Charrondière, R. Nutrients, bioactive non-nutrients and anti-nutrients in potatoes. J. Food Compos. Anal. 2009, 22, 494–502. [Google Scholar] [CrossRef]
- Singh, J.; Colussi, R.; McCarthy, O.J.; Kaur, L. Potato Starch and Its Modification. In Advances in Potato Chemistry and Technology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 195–247. ISBN 9780128000021. [Google Scholar]
- Yang, L.; Xia, Y.; Junejo, S.A.; Zhou, Y. Composition, structure and physicochemical properties of three coloured potato starches. Int. J. Food Sci. Technol. 2018, 53, 2325–2334. [Google Scholar] [CrossRef]
- Guo, L.; Cui, B. The relationship between entanglement concentration and physicochemical properties of potato and sweet potato starch dispersions. Int. J. Food Sci. Technol. 2018, 53, 337–346. [Google Scholar] [CrossRef]
- Jørgensen, M.; Bauw, G.; Welinder, K.G. Molecular Properties and Activities of Tuber Proteins from Starch Potato cv. Kuras. J. Agric. Food Chem. 2006, 54, 9389–9397. [Google Scholar] [CrossRef]
- Park, Y.; Choi, B.H.; Kwak, J.-S.; Kang, C.-W.; Lim, H.-T.; Cheong, H.-S.; Hahm, K.-S. Kunitz-Type Serine Protease Inhibitor from Potato (Solanum tuberosum L. cv. Jopung). J. Agric. Food Chem. 2005, 53, 6491–6496. [Google Scholar] [CrossRef] [PubMed]
- Białas, W.; Kowalczewski, P.; Lewandowicz, G.; Olejnik, A.; Siger, A.; Dwiecki, K. Method for Obtaining a Pro-Healthy Protein Preparation. Polish Patent Aplication No. P.426837, 28 8 2018. [Google Scholar]
- Edens, L.; Van Der Lee, J.A.B.; Plijter, J.J. Novel Food Compositions. Patent No. WO1997042834A1, 10 11 1997. [Google Scholar]
- Giuseppin, M.L.F.; van der Sluis, C.; Laus Marc, C. Native Potato Protein Isolates. European Patent No. EP 1920662, 14 5 2008. [Google Scholar]
- Marchal, J.L.M.; Nijssen, H.M.J.; Knott, E.R.; Krol, F.A.R. Method for Improving a Protein. US Patent USA No. 7972647, 5 7 2011. [Google Scholar]
- Dabestani, S.; Arcot, J.; Chen, V. Protein recovery from potato processing water: Pre-treatment and membrane fouling minimization. J. Food Eng. 2017, 195, 85–96. [Google Scholar] [CrossRef]
- Waglay, A.; Karboune, S. A novel enzymatic approach based on the use of multi-enzymatic systems for the recovery of enriched protein extracts from potato pulp. Food Chem. 2017, 220, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Waglay, A.; Karboune, S.; Alli, I. Potato protein isolates: Recovery and characterization of their properties. Food Chem. 2014, 142, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Xiong, Y.L.; Chen, J. Antioxidant and emulsifying properties of potato protein hydrolysate in soybean oil-in-water emulsions. Food Chem. 2010, 120, 101–108. [Google Scholar] [CrossRef]
- Vlachojannis, J.E.; Cameron, M.; Chrubasik, S. Medicinal use of potato-derived products: A systematic review. Phyther. Res. 2010, 24, 159–162. [Google Scholar] [CrossRef] [PubMed]
- Mohamed Saleem, T.S.; Chetty, C.M.; Ramkanth, S.; Alagusundaram, M.; Gnanaprakash, K.; Thiruvengada Rajan, V.S.; Angalaparameswari, S. Solanum nigrum Linn—A review. Pharmacogn. Rev. 2009, 3, 342–345. [Google Scholar]
- Kujawska, M.; Olejnik, A.; Lewandowicz, G.; Kowalczewski, P.; Forjasz, R.; Jodynis-Liebert, J. Spray-Dried Potato Juice as a Potential Functional Food Component with Gastrointestinal Protective Effects. Nutrients 2018, 10, 259. [Google Scholar] [CrossRef]
- Kowalczewski, P.Ł.; Lewandowicz, G.; Krzywdzińska-Bartkowiak, M.; Piątek, M.; Baranowska, H.M.; Białas, W.; Jeziorna, M.; Kubiak, P. Finely comminuted frankfurters fortified with potato juice—Quality and structure. J. Food Eng. 2015, 167, 183–188. [Google Scholar] [CrossRef]
- Kowalczewski, P.; Lewandowicz, G.; Makowska, A.; Knoll, I.; Błaszczak, W.; Białas, W.; Kubiak, P. Pasta Fortified with Potato Juice: Structure, Quality, and Consumer Acceptance. J. Food Sci. 2015, 80, S1377–S1382. [Google Scholar] [CrossRef]
- Kowalczewski, P.; Różańska, M.; Makowska, A.; Jeżowski, P.; Kubiak, P. Production of wheat bread with spray-dried potato juice: Influence on dough and bread characteristics. Food Sci. Technol. Int. 2019, 25, 223–232. [Google Scholar] [CrossRef]
- Baranowska, H.M.; Masewicz, Ł.; Kowalczewski, P.Ł.; Lewandowicz, G.; Piątek, M.; Kubiak, P. Water properties in pâtés enriched with potato juice. Eur. Food Res. Technol. 2018, 244, 387–393. [Google Scholar] [CrossRef]
- Ruseler-van Embden, J.G.H.; van Lieshout, L.M.C.; Smits, S.A.; van Kessel, I.; Laman, J.D. Potato tuber proteins efficiently inhibit human faecal proteolytic activity: Implications for treatment of peri-anal dermatitis. Eur. J. Clin. Investig. 2004, 34, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Ledoigt, G.; Griffaut, B.; Debiton, E.; Vian, C.; Mustel, A.; Evray, G.; Maurizis, J.-C.; Madelmont, J.-C. Analysis of secreted protease inhibitors after water stress in potato tubers. Int. J. Biol. Macromol. 2006, 38, 268–271. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Park, S.-C.; Kim, M.-H.; Lim, H.-T.; Park, Y.; Hahm, K.-S. Antimicrobial activity studies on a trypsin–chymotrypsin protease inhibitor obtained from potato. Biochem. Biophys. Res. Commun. 2005, 330, 921–927. [Google Scholar] [CrossRef]
- Fischer, M.; Kuckenberg, M.; Kastilan, R.; Muth, J.; Gebhardt, C. Novel in vitro inhibitory functions of potato tuber proteinaceous inhibitors. Mol. Genet. Genom. 2015, 290, 387–398. [Google Scholar] [CrossRef]
- Pouvreau, L.; Gruppen, H.; Piersma, S.R.; van den Broek, L.A.M.; van Koningsveld, G.A.; Voragen, A.G.J. Relative Abundance and Inhibitory Distribution of Protease Inhibitors in Potato Juice from cv. Elkana. J. Agric. Food Chem. 2001, 49, 2864–2874. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.-H.; Park, S.-C.; Kim, J.-Y.; Lee, S.Y.; Lim, H.-T.; Cheong, H.; Hahm, K.-S.; Park, Y. Purification and characterization of a heat-stable serine protease inhibitor from the tubers of new potato variety “Golden Valley”. Biochem. Biophys. Res. Commun. 2006, 346, 681–686. [Google Scholar] [CrossRef]
- ISO. ISO 1871:2009 Food and Feed Products—General Guidelines for the Determination of Nitrogen by the Kjeldahl Method; ISO: Geneva, Switzerland, 2009. [Google Scholar]
- ISO. ISO 763:2003 Fruit and Vegetable Products—Determination of Ash Insoluble in Hydrochloric Acid; ISO: Geneva, Switzerland, 2003. [Google Scholar]
- Kowalczewski, P.Ł.; Olejnik, A.; Białas, W.; Kubiak, P.; Siger, A.; Nowicki, M.; Lewandowicz, G. Effect of Thermal Processing on Antioxidant Activity and Cytotoxicity of Waste Potato Juice. Open Life Sci. 2019, 14, 150–157. [Google Scholar] [CrossRef]
- Tomczak, A.; Zielińska-Dawidziak, M.; Piasecka-Kwiatkowska, D.; Lampart-Szczapa, E. Blue lupine seeds protein content and amino acids composition. Plant Soil Environ. 2018, 64, 147–155. [Google Scholar]
- AOAC. Official Method 994.12 Amino Acids in Feeds; AOAC International: Rockville, MD, USA, 2000. [Google Scholar]
- ISO. ISO 13904:2016 Animal Feeding Stuffs—Determination of Tryptophan Content; ISO: Geneva, Switzerland, 2016. [Google Scholar]
- Alsmeyer, R.H.; Cunningham, A.E.; Happich, M.L. Equations predicting PER from amino acid analysis. Food Technol. 1974, 28, 34–40. [Google Scholar]
- FAO. Dietary Protein Quality Evaluation in Human Nutrition; Report of an FAO Expert Consultation; FAO Food and Nutrition Paper 92; FAO: Rome, Italy, 2013; ISBN 978-92-5-107417-6. [Google Scholar]
- Butts, C.A.; Monro, J.A.; Moughan, P.J. In vitro determination of dietary protein and amino acid digestibility for humans. Br. J. Nutr. 2012, 108, S282–S287. [Google Scholar] [CrossRef]
- Rybicka, I.; Gliszczyńska-Świgło, A. Minerals in grain gluten-free products. The content of calcium, potassium, magnesium, sodium, copper, iron, manganese, and zinc. J. Food Compos. Anal. 2017, 59, 61–67. [Google Scholar] [CrossRef]
- European Food Safety Authority. Dietary Reference Values for nutrients Summary report. EFSA Support. Publ. 2017, 14, e15121. [Google Scholar]
- Król, A.; Amarowicz, R.; Weidner, S. Content of Phenolic Compounds and Antioxidant Properties in Seeds of Sweet and Bitter Cultivars of Lupine (Lupinus angustifolius L.). Nat. Prod. Commun. 2018, 13, 1235–1418. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. ISBN 9780121822002. [Google Scholar]
- Karadeniz, F.; Burdurlu, H.S.; Koca, N.; Soyer, Y. Antioxidant activity of selected fruits and vegetables grown in Turkey. Turk. J. Agric. For. 2005, 29, 297–303. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Olejnik, A.; Rychlik, J.; Kidoń, M.; Czapski, J.; Kowalska, K.; Juzwa, W.; Olkowicz, M.; Dembczyński, R.; Moyer, M.P. Antioxidant effects of gastrointestinal digested purple carrot extract on the human cells of colonic mucosa. Food Chem. 2016, 190, 1069–1077. [Google Scholar] [CrossRef]
- Barta, J.; Curn, V. Potato (Solanum tuberosum L.) Tuber Proteins—Classification, Characterization, Importance. Chem. Listy 2004, 98, 373–378. [Google Scholar]
- Galdón, B.R.; Mesa, D.R.; Rodríguez, E.M.R.; Romero, C.D. Amino acid content in traditional potato cultivars from the Canary Islands. J. Food Compos. Anal. 2010, 23, 148–153. [Google Scholar] [CrossRef]
- Friedman, M. Nutritional Value of Proteins from Different Food Sources. A Review. J. Agric. Food Chem. 1996, 44, 6–29. [Google Scholar] [CrossRef]
- Miedzianka, J.; Pęksa, A.; Aniołowska, M. Properties of acetylated potato protein preparations. Food Chem. 2012, 133, 1283–1291. [Google Scholar] [CrossRef]
- Pęksa, A.; Miedzianka, J.; Nemś, A. Amino acid composition of flesh-coloured potatoes as affected by storage conditions. Food Chem. 2018, 266, 335–342. [Google Scholar] [CrossRef]
- Sibian, M.S.; Saxena, D.C.; Riar, C.S. Effect of germination on chemical, functional and nutritional characteristics of wheat, brown rice and triticale: A comparative study. J. Sci. Food Agric. 2017, 97, 4643–4651. [Google Scholar] [CrossRef]
- Mir, N.A.; Riar, C.S.; Singh, S. Effect of pH and holding time on the characteristics of protein isolates from Chenopodium seeds and study of their amino acid profile and scoring. Food Chem. 2019, 272, 165–173. [Google Scholar] [CrossRef]
- Boye, J.; Wijesinha-Bettoni, R.; Burlingame, B. Protein quality evaluation twenty years after the introduction of the protein digestibility corrected amino acid score method. Br. J. Nutr. 2012, 108, S183–S211. [Google Scholar] [CrossRef]
- Rutherfurd, S.M.; Fanning, A.C.; Miller, B.J.; Moughan, P.J. Protein Digestibility-Corrected Amino Acid Scores and Digestible Indispensable Amino Acid Scores Differentially Describe Protein Quality in Growing Male Rats. J. Nutr. 2015, 145, 372–379. [Google Scholar] [CrossRef]
- Aletor, O. Protein quality evaluation and in vitro multi-enzyme digestibility of some plant protein isolates and concentrates. Archiva Zootechnica 2012, 15, 5–16. [Google Scholar]
- Pęksa, A.; Rytel, E.; Kita, A.; Lisińska, G.; Tajner-Czopek, A. The properties of potato protein. In Food; Global Science Books: Tokyo, Japan, 2009; Volume 3, pp. 79–87. [Google Scholar]
- Schmitz-Schug, I.; Foerst, P.; Kulozik, U. Impact of the spray drying conditions and residence time distribution on lysine loss in spray dried infant formula. Dairy Sci. Technol. 2013, 93, 443–462. [Google Scholar] [CrossRef]
- Cirkovic Velickovic, T.D.; Stanic-Vucinic, D.J. The Role of Dietary Phenolic Compounds in Protein Digestion and Processing Technologies to Improve Their Antinutritive Properties. Compr. Rev. Food Sci. Food Saf. 2018, 17, 82–103. [Google Scholar] [CrossRef]
- Pruska-Kędzior, A.; Kędzior, Z.; Gorący, M.; Pietrowska, K.; Przybylska, A.; Spychalska, K. Comparison of rheological, fermentative and baking properties of gluten-free dough formulations. Eur. Food Res. Technol. 2008, 227, 1523–1536. [Google Scholar] [CrossRef]
- Pellegrini, N.; Agostoni, C. Nutritional aspects of gluten-free products. J. Sci. Food Agric. 2015, 95, 2380–2385. [Google Scholar] [CrossRef]
- Rybicka, I.; Doba, K.; Bińczak, O. Improving the sensory and nutritional value of gluten-free bread. Int. J. Food Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Rybicka, I. The Handbook of Minerals on a Gluten-Free Diet. Nutrients 2018, 10, 1683. [Google Scholar] [CrossRef]
- Barceloux, D.G. Potatoes, Tomatoes, and Solanine Toxicity (Solanum tuberosum L., Solanum lycopersicum L.). Dis. Mon. 2009, 55, 391–402. [Google Scholar] [CrossRef]
- Slanina, P. Solanine (glycoalkaloids) in potatoes: Toxicological evaluation. Food Chem. Toxicol. 1990, 28, 759–761. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef]
- Li, H.-B.; Wong, C.-C.; Cheng, K.-W.; Chen, F. Antioxidant properties in vitro and total phenolic contents in methanol extracts from medicinal plants. LWT-Food Sci. Technol. 2008, 41, 385–390. [Google Scholar] [CrossRef]
- Kumari, B.; Tiwari, B.K.; Hossain, M.B.; Rai, D.K.; Brunton, N.P. Ultrasound-assisted extraction of polyphenols from potato peels: Profiling and kinetic modelling. Int. J. Food Sci. Technol. 2017, 52, 1432–1439. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Wojdylo, A.; Oszmianski, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef] [PubMed]
- Camire, M.E.; Kubow, S.; Donnelly, D.J. Potatoes and Human Health. Crit. Rev. Food Sci. Nutr. 2009, 49, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, K.L.; Park, J.S.; Brown, C.R.; Mathison, B.D.; Navarre, D.A.; Chew, B.P. Pigmented Potato Consumption Alters Oxidative Stress and Inflammatory Damage in Men. J. Nutr. 2011, 141, 108–111. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, L.M.; Cheon, K.; Nansel, T.R.; Albert, P.S. Candidate measures of whole plant food intake are related to biomarkers of nutrition and health in the US population (National Health and Nutrition Examination Survey 1999–2002). Nutr. Res. 2012, 32, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Marjan, Z.; Foong, C. Total antioxidant activity and phenolic content in selected vegetables. Food Chem. 2004, 87, 581–586. [Google Scholar] [CrossRef]
- Liu, Y.-W.; Han, C.-H.; Lee, M.-H.; Hsu, F.-L.; Hou, W.-C. Patatin, the Tuber Storage Protein of Potato (Solanum tuberosum L.), Exhibits Antioxidant Activity In Vitro. J. Agric. Food Chem. 2003, 51, 4389–4393. [Google Scholar] [CrossRef] [PubMed]
- Pihlanto, A.; Akkanen, S.; Korhonen, H.J. ACE-inhibitory and antioxidant properties of potato (Solanum tuberosum). Food Chem. 2008, 109, 104–112. [Google Scholar] [CrossRef]
- Elias, R.J.; Kellerby, S.S.; Decker, E.A. Antioxidant Activity of Proteins and Peptides. Crit. Rev. Food Sci. Nutr. 2008, 48, 430–441. [Google Scholar] [CrossRef]
- Galluzzi, L.; Buqué, A.; Kepp, O.; Zitvogel, L.; Kroemer, G. Immunological Effects of Conventional Chemotherapy and Targeted Anticancer Agents. Cancer Cell 2015, 28, 690–714. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Mu, T.-H.; Sun, M.-J. Purification and identification of antioxidant peptides from sweet potato protein hydrolysates by Alcalase. J. Funct. Foods 2014, 7, 191–200. [Google Scholar] [CrossRef]
- Ji, Y.; Gao, S. Antihepatocarcinoma Effect of Solanine and Its Mechanisms. Chin. Herb. Med. 2012, 4, 126–135. [Google Scholar]
- Ji, Y.B.; Gao, S.Y.; Ji, C.F.; Zou, X. Induction of apoptosis in HepG2 cells by solanine and Bcl-2 protein. J. Ethnopharmacol. 2008, 115, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Mandimika, T.; Baykus, H.; Poortman, J.; Garza, C.; Kuiper, H.; Peijnenburg, A. Induction of the cholesterol biosynthesis pathway in differentiated Caco-2 cells by the potato glycoalkaloid α-chaconine. Food Chem. Toxicol. 2007, 45, 1918–1927. [Google Scholar] [CrossRef] [PubMed]
| Parameter | FPJ | PJPC |
|---|---|---|
| Moisture (%) | 94.49 ± 3.21 a | 4.79 ± 0.32 b |
| Protein (g/100 g dm) | 2.37 ± 0.07 b | 63.40 ± 0.82 a |
| Ash (g/100 g dm) | 0.91 ± 0.03 b | 7.54 ± 0.27 a |
| α-chaconine (µg/100 g dm) | 990.06 ± 17.11 a | 837.81 ± 15.36 b |
| α-solanine (µg/100 g dm) | 601.24 ± 19.37 a | 438.40 ± 18.49 b |
| Amino Acid | FAO/WHO Standard (mg/g) | PJPC (g/16 g N) | AAS |
|---|---|---|---|
| Essential amino acids | |||
| Histidine | 16 | 1.95 ± 0.07 | 86.3 |
| Isoleucine | 30 | 4.31 ± 0.09 | 102.0 |
| Leucine | 61 | 9.06 ± 0.11 | 64.6 |
| Lysine | 48 | 8.33 ± 0.20 | 123.1 |
| Methionine + Cystine | 23 | 3.85 ± 0.18 | 118.7 |
| Phenylalanine + Tyrosine | 41 | 10.44 ± 0.27 | 161.1 |
| Threonine | 25 | 6.24 ± 0.17 | 177.2 |
| Tryptophan | 6.6 | 1.11 ± 0.09 | 120.3 |
| Valine | 40 | 5.64 ± 0.21 | 100.0 |
| Dispensable amino acids | |||
| Alanine | - | 5.10 ± 0.30 | - |
| Arginine | - | 4.69 ± 0.11 | - |
| Aspartic acid | - | 12.74 ± 0.52 | - |
| Glutamic acid | - | 11.22 ± 0.55 | - |
| Glycine | - | 5.20 ± 0.11 | - |
| Proline | - | 5.05 ± 0.19 | - |
| Serine | - | 5.06 ± 0.21 | - |
| Parameter | PJPC |
|---|---|
| PER1 | 3.21 ± 0.02 |
| PER2 | 3.13 ± 0.05 |
| PER3 | 1.87 ± 0.02 |
| Mineral | Content (mg/100 g) | % PRI/AI |
|---|---|---|
| Fe | 30.5 ± 4.2 | 191 |
| Mn | 3.79 ± 0.17 | 126 |
| K | 4341 ± 271 | 124 |
| Cu | 1.14 ± 0.07 | 114 |
| Mg | 241 ± 9 | 80 |
| Ca | 118 ± 8 | 12 |
| Zn | 6.04 ± 0.11 | 60 |
| Na | 84.5 ± 4.9 | - |
| Parameter | PJPC |
|---|---|
| FRAP (mmol g−1) | 1.41 ± 0.07 |
| TEAC (mmol g−1) | 1.53 ± 0.12 |
| TPC (mg g−1) | 5.82 ± 0.16 |
| TFC (mg g−1) | 2.10 ± 0.11 |
| Cell Line | IC10 | IC50 | IC90 |
|---|---|---|---|
| Hs 746T | 9.11 ± 0.96 a | 14.69 ± 0.57 a | 23.76 ± 0.65 a |
| HT-29 | 0.80 ± 0.14 c | 3.89 ± 0.21 c | 19.38 ± 2.24 b |
| CCD 841 CoN | 4.71 ± 0.57 b | 6.50 ± 0.20 b | 9.06 ± 1.19 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalczewski, P.Ł.; Olejnik, A.; Białas, W.; Rybicka, I.; Zielińska-Dawidziak, M.; Siger, A.; Kubiak, P.; Lewandowicz, G. The Nutritional Value and Biological Activity of Concentrated Protein Fraction of Potato Juice. Nutrients 2019, 11, 1523. https://doi.org/10.3390/nu11071523
Kowalczewski PŁ, Olejnik A, Białas W, Rybicka I, Zielińska-Dawidziak M, Siger A, Kubiak P, Lewandowicz G. The Nutritional Value and Biological Activity of Concentrated Protein Fraction of Potato Juice. Nutrients. 2019; 11(7):1523. https://doi.org/10.3390/nu11071523
Chicago/Turabian StyleKowalczewski, Przemysław Łukasz, Anna Olejnik, Wojciech Białas, Iga Rybicka, Magdalena Zielińska-Dawidziak, Aleksander Siger, Piotr Kubiak, and Grażyna Lewandowicz. 2019. "The Nutritional Value and Biological Activity of Concentrated Protein Fraction of Potato Juice" Nutrients 11, no. 7: 1523. https://doi.org/10.3390/nu11071523
APA StyleKowalczewski, P. Ł., Olejnik, A., Białas, W., Rybicka, I., Zielińska-Dawidziak, M., Siger, A., Kubiak, P., & Lewandowicz, G. (2019). The Nutritional Value and Biological Activity of Concentrated Protein Fraction of Potato Juice. Nutrients, 11(7), 1523. https://doi.org/10.3390/nu11071523








