Allomyrina dichotoma Larva Extract Ameliorates the Hepatic Insulin Resistance of High-Fat Diet-Induced Diabetic Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of A. Dichotoma Larva Extract (ADLE)
2.2. Cell Culture
2.3. Preparation of Palmitate and MTT Assay
2.4. Animals
2.5. Intraperitoneal Glucose Tolerance Test and Intraperitoneal Insulin Tolerance Test
2.6. Biochemical Analysis in Blood
2.7. Assessments of Liver TG and TC
2.8. Western Blot Analysis
2.9. Quantitative Real-Time Polymerase Chain Reaction
2.10. Biochemical Staining
2.11. Statistical Analysis
3. Results
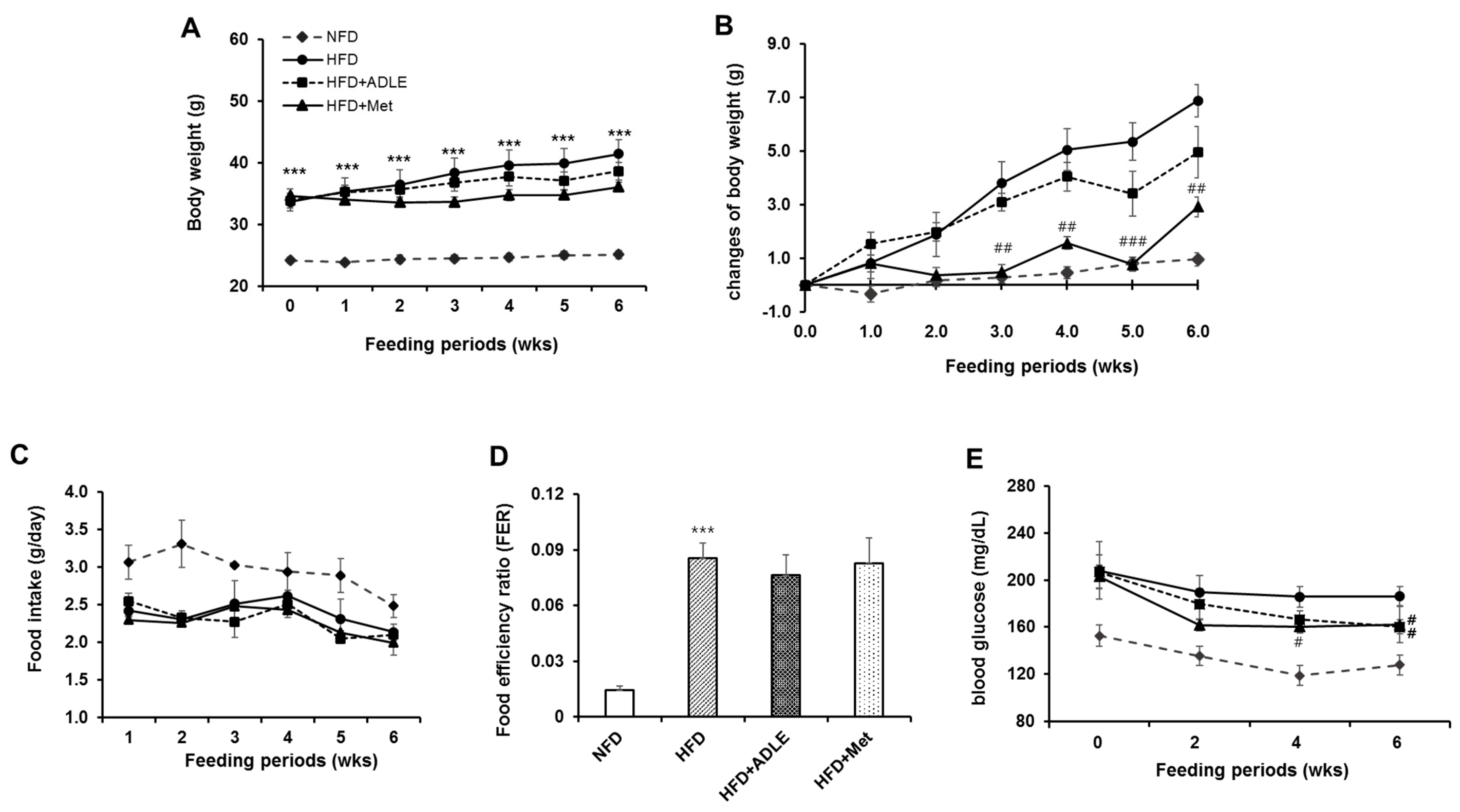
3.1. Changes of Body Weight, Food Intake, and Fasting Blood Glucose Level by HFD Administration in Mice
3.2. Treatment of ADLE Reduced Body Weight and Blood Glucose Level of HFD-Induced Diabetic Mice
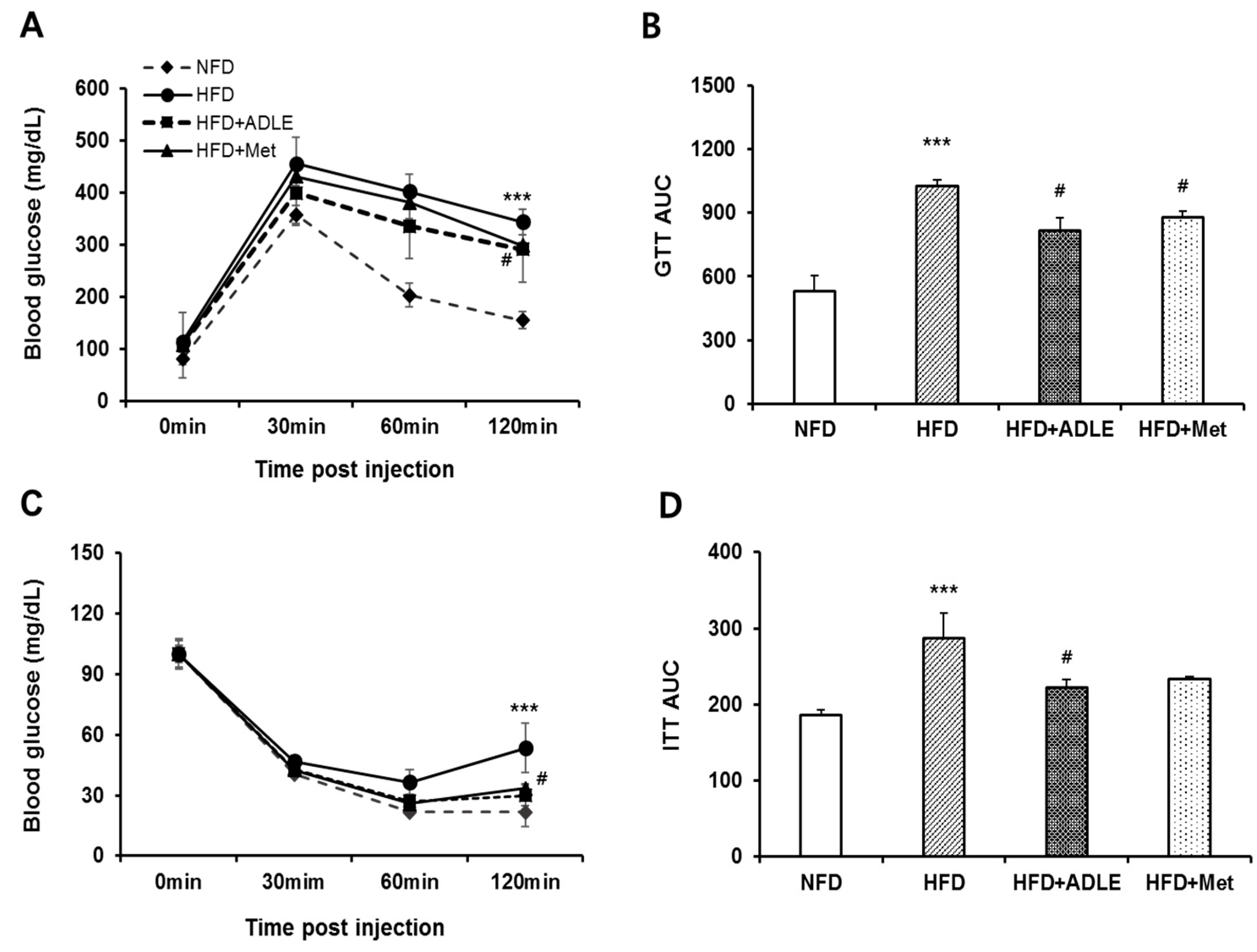
3.3. Treatment of ADLE Improved Glucose and Insulin Tolerance of HFD-Induced Diabetic Mice
3.4. ADLE Altered Lipid Profiles, AST and ALT Levels in Serum of HFD-Induced Diabetic Mice
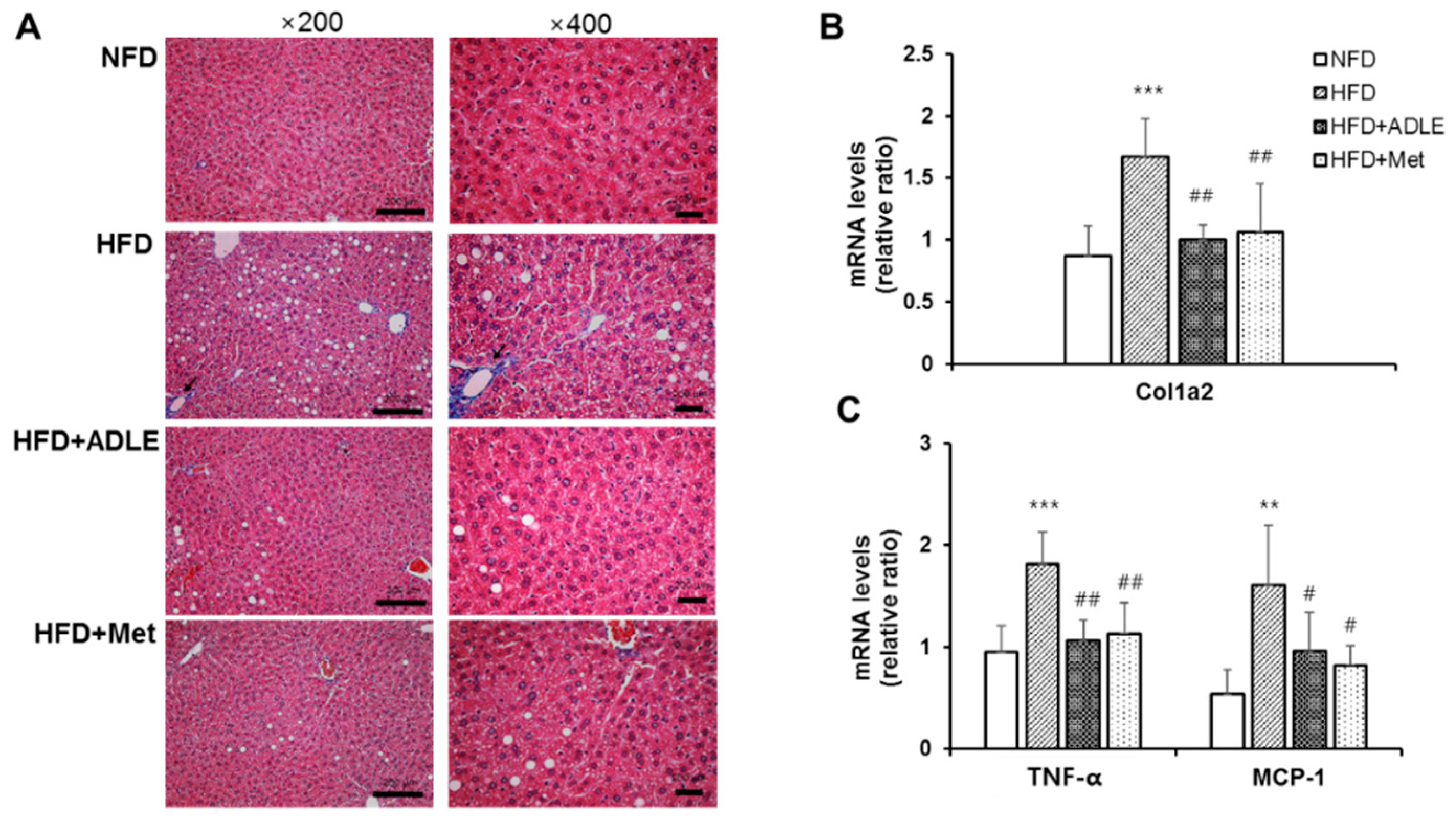
3.5. ADLE Reduced Hepatic Fibrosis and Expression of Inflammatory Genes in the Liver of HFD-Induced Diabetic Mice
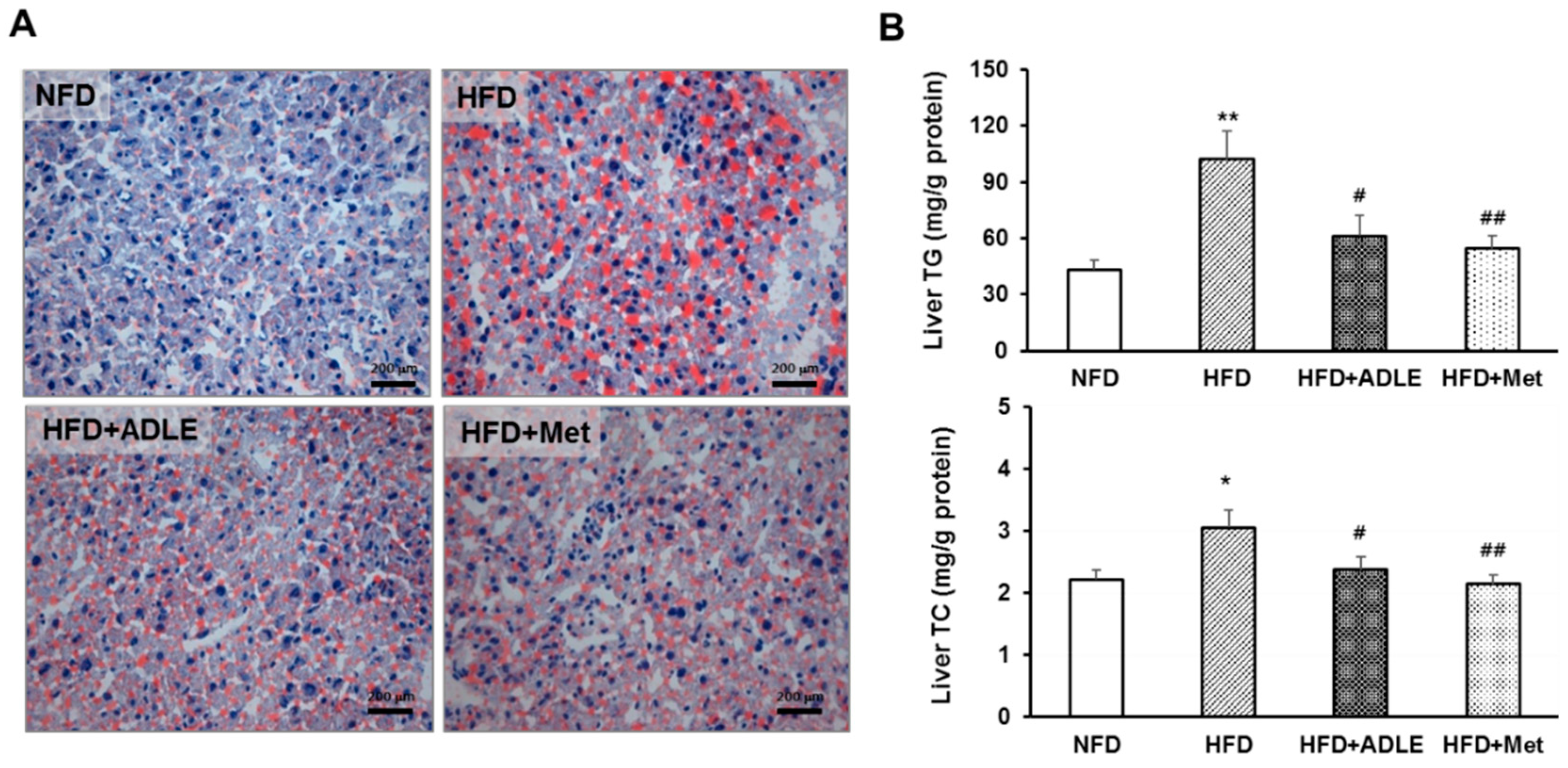
3.6. ADLE Reduced Lipid Accumulation in the Liver of HFD-Induced Diabetic Mice
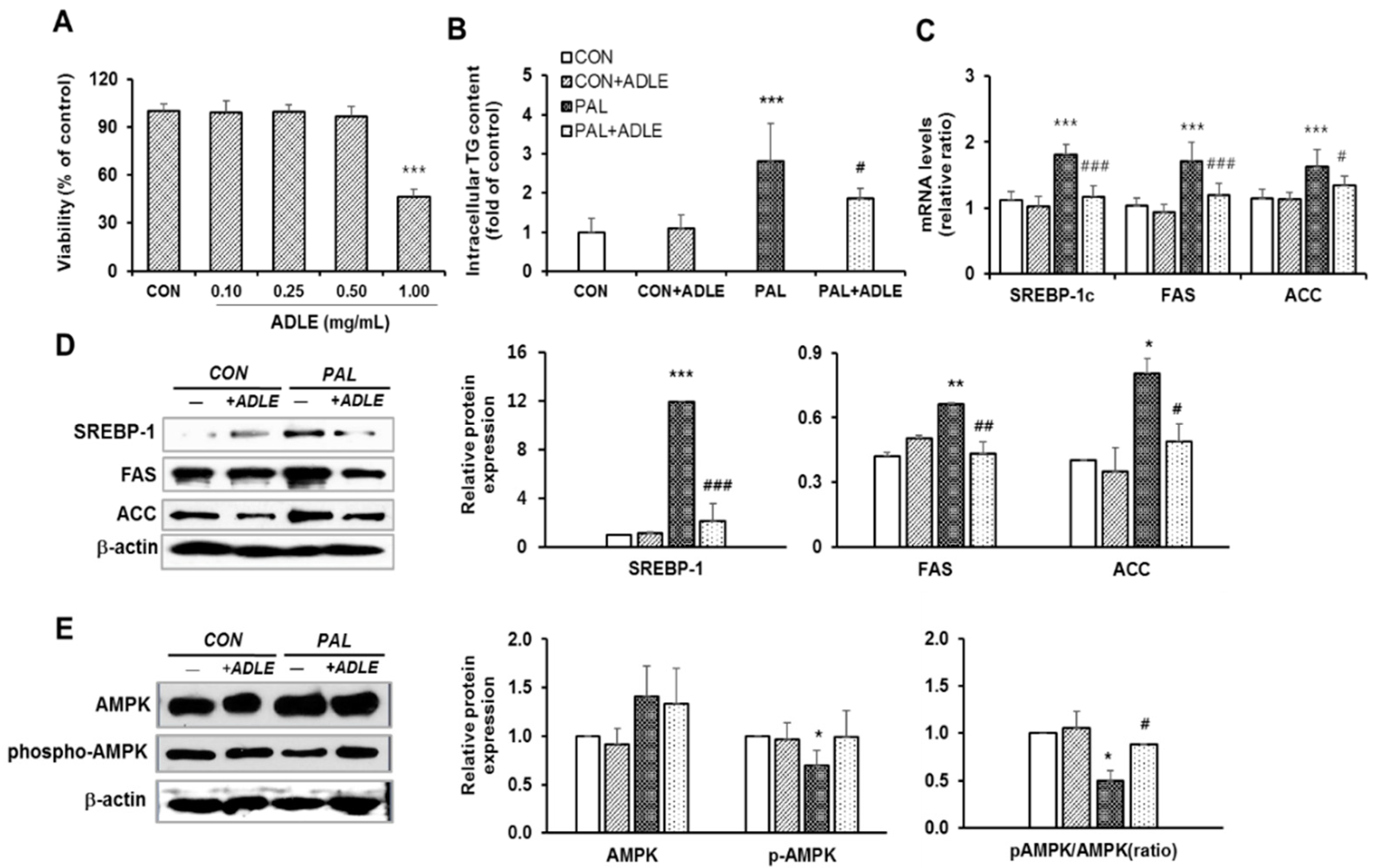
3.7. ADLE Reduced Lipogenic Gene Expression in Palmitate-Treated HepG2 Cells
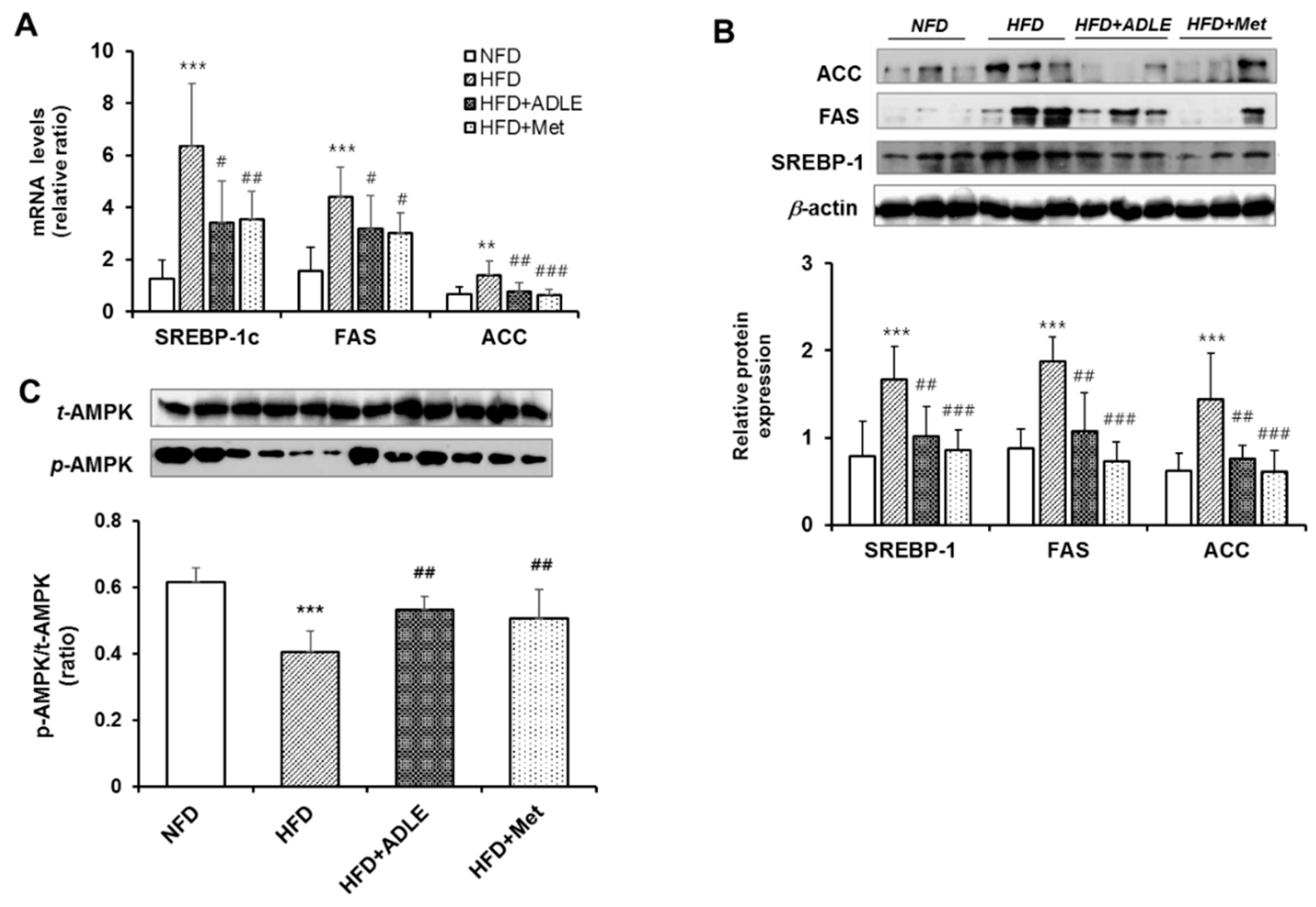
3.8. ADLE Reduced the Expression of Lipogenic Genes and Dephosphorylation of AMPK in the Liver of HFD-Induced Diabetic Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Angulo, P. Nonalcoholic fatty liver disease. N Engl. J. Med. 2002, 346, 1221–1231. [Google Scholar] [CrossRef]
- Loomba, R.; Sanyal, A.J. The global nafld epidemic. Nat. Rev. Gastroenterol Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef]
- Adams, L.A.; Anstee, Q.M.; Tilg, H.; Targher, G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017, 66, 1138–1153. [Google Scholar] [CrossRef] [Green Version]
- Neuschwander-Tetri, B.A. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: The central role of nontriglyceride fatty acid metabolites. Hepatology 2010, 52, 774–788. [Google Scholar] [CrossRef]
- Takahashi, Y.; Sugimoto, K.; Inui, H.; Fukusato, T. Current pharmacological therapies for nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J. Gastroenterol. 2015, 21, 3777–3785. [Google Scholar] [CrossRef]
- Sah, L.; Jung, C. Global perspective of edible insects as human food. Korean J. Soil Zool. 2012, 16, 1–8. [Google Scholar]
- Yoo, J.M.; Huang, J.S.; Goo, T.W.; Yun, E.Y. Comparative analysis of nutritional and harmful components in korean and chinese mealworms (tenebrio molitor). J. Korean Soc. Food Sci. Nutr. 2013, 42, 249–254. [Google Scholar] [CrossRef]
- Chung, M.Y.; Gwon, E.Y.; Hwang, J.S.; Goo, T.W.; Yun, E.Y. Analysis of general composition and harmful material of protaetia brevitarsis. J. Life Sci. 2013, 23, 664–668. [Google Scholar] [CrossRef]
- Miyanoshita, A.; Hara, S.; Sugiyama, M.; Asaoka, A.; Taniai, K.; Yukuhiro, F.; Yamakawa, M. Isolation and characterization of a new member of the insect defensin family from a beetle, allomyrina dichotoma. Biochem. Biophys. Res. Commun. 1996, 220, 526–531. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Umetsu, K.; Shinzawa, H.; Yuasa, I.; Maruyama, K.; Ohkura, T.; Yamashita, K.; Suzuki, T. Determination of carbohydrate-deficient transferrin separated by lectin affinity chromatography for detecting chronic alcohol abuse. FEBS Lett. 1999, 458, 112–116. [Google Scholar] [CrossRef] [Green Version]
- Sagisaka, A.; Miyanoshita, A.; Ishibashi, J.; Yamakawa, M. Purification, characterization and gene expression of a glycine and proline-rich antibacterial protein family from larvae of a beetle, allomyrina dichotoma. Insect Mol. Biol. 2001, 10, 293–302. [Google Scholar] [CrossRef]
- Kim, D.-S.; Huh, J.; You, G.-C.; Chae, S.-C.; Lee, O.-S.; Lee, H.B.; Lee, J.-B.; Kim, J.S. Allomyrina dichotoma larva extracts protect streptozotocin-induced oxidative cytotoxicity. J. Environ. Toxicol. 2007, 22, 349–355. [Google Scholar]
- Choi, S.E.; Jung, I.R.; Lee, Y.J.; Lee, S.J.; Lee, J.H.; Kim, Y.; Jun, H.S.; Lee, K.W.; Park, C.B.; Kang, Y. Stimulation of lipogenesis as well as fatty acid oxidation protects against palmitate-induced ins-1 beta-cell death. Endocrinology 2011, 152, 816–827. [Google Scholar] [CrossRef]
- Im, A.R.; Ji, K.Y.; Park, I.; Lee, J.Y.; Kim, K.M.; Na, M.; Chae, S. Anti-photoaging effects of four insect extracts by downregulating matrix metalloproteinase expression via mitogen-activated protein kinase-dependent signaling. Nutrients 2019, 11, 1159. [Google Scholar] [CrossRef]
- Yoon, Y.I.; Chung, M.Y.; Hwang, J.S.; Han, M.S.; Goo, T.W.; Yun, E.Y. Allomyrina dichotoma (arthropoda: Insecta) larvae confer resistance to obesity in mice fed a high-fat diet. Nutrients 2015, 7, 1978–1991. [Google Scholar] [CrossRef]
- Atamni, H.J.; Mott, R.; Soller, M.; Iraqi, F.A. High-fat-diet induced development of increased fasting glucose levels and impaired response to intraperitoneal glucose challenge in the collaborative cross mouse genetic reference population. BMC Genet. 2016, 17, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Weiskirchen, R.; Weiskirchen, S.; Tacke, F. Recent advances in understanding liver fibrosis: Bridging basic science and individualized treatment concepts. F1000Res 2018, 7. [Google Scholar] [CrossRef]
- Lee, J.H.; Seo, M.; Lee, H.J.; Baek, M.; Kim, I.W.; Kim, S.Y.; Kim, M.A.; Kim, S.H.; Hwang, J.S. Anti-inflammatory activity of antimicrobial peptide allomyrinasin derived from the dynastid beetle, allomyrina dichotoma. J. Microbiol. Biotechnol. 2019, 29, 687–695. [Google Scholar] [CrossRef]
- Gomez-Lechon, M.J.; Donato, M.T.; Martinez-Romero, A.; Jimenez, N.; Castell, J.V.; O’Connor, J.E. A human hepatocellular in vitro model to investigate steatosis. Chem. Biol. Interact. 2007, 165, 106–116. [Google Scholar] [CrossRef]
- Kanuri, G.; Bergheim, I. In vitro and in vivo models of non-alcoholic fatty liver disease (nafld). Int. J. Mol. Sci. 2013, 14, 11963–11980. [Google Scholar] [CrossRef]
- Ishii, M.; Maeda, A.; Tani, S.; Akagawa, M. Palmitate induces insulin resistance in human hepg2 hepatocytes by enhancing ubiquitination and proteasomal degradation of key insulin signaling molecules. Arch. Biochem. Biophys. 2015, 566, 26–35. [Google Scholar] [CrossRef]
- Samuel, V.T.; Liu, Z.X.; Wang, A.; Beddow, S.A.; Geisler, J.G.; Kahn, M.; Zhang, X.M.; Monia, B.P.; Bhanot, S.; Shulman, G.I. Inhibition of protein kinase cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J. Clin. Investig. 2007, 117, 739–745. [Google Scholar] [CrossRef]
- Samuel, V.T.; Liu, Z.X.; Qu, X.; Elder, B.D.; Bilz, S.; Befroy, D.; Romanelli, A.J.; Shulman, G.I. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J. Biol. Chem. 2004, 279, 32345–32353. [Google Scholar] [CrossRef]
- Xu, J.Y.; Zhang, L.; Li, Z.P.; Ji, G. Natural products on nonalcoholic fatty liver disease. Curr. Drug Targets 2015, 16, 1347–1355. [Google Scholar] [CrossRef]
- Aghazadeh, S.; Amini, R.; Yazdanparast, R.; Ghaffari, S.H. Anti-apoptotic and anti-inflammatory effects of silybum marianum in treatment of experimental steatohepatitis. Exp. Toxicol. Pathol. 2011, 63, 569–574. [Google Scholar] [CrossRef]
- Kim, J.; Yun, E.Y.; Park, S.W.; Goo, T.W.; Seo, M. Allomyrina dichotoma larvae regulate food intake and body weight in high fat diet-induced obese mice through mtor and mapk signaling pathways. Nutrients 2016, 8, 100. [Google Scholar] [CrossRef]
- Walenbergh, S.M.; Shiri-Sverdlov, R. Cholesterol is a significant risk factor for non-alcoholic steatohepatitis. Expert Rev. Gastroenterol Hepatol. 2015, 9, 1343–1346. [Google Scholar] [CrossRef]
- Arguello, G.; Balboa, E.; Arrese, M.; Zanlungo, S. Recent insights on the role of cholesterol in non-alcoholic fatty liver disease. Biochim. Biophys. Acta 2015, 1852, 1765–1778. [Google Scholar] [CrossRef] [Green Version]
- Scherer, T.; Lindtner, C.; O’Hare, J.; Hackl, M.; Zielinski, E.; Freudenthaler, A.; Baumgartner-Parzer, S.; Todter, K.; Heeren, J.; Krssak, M.; et al. Insulin regulates hepatic triglyceride secretion and lipid content via signaling in the brain. Diabetes 2016, 65, 1511–1520. [Google Scholar] [CrossRef]
- Park, E.Y.; Choi, H.; Yoon, J.Y.; Lee, I.Y.; Seo, Y.; Moon, H.S.; Hwang, J.H.; Jun, H.S. Polyphenol-rich fraction of ecklonia cava improves nonalcoholic fatty liver disease in high fat diet-fed mice. Mar. Drugs 2015, 13, 6866–6883. [Google Scholar] [CrossRef]
- Hayek, T.; Ito, Y.; Azrolan, N.; Verdery, R.B.; Aalto-Setala, K.; Walsh, A.; Breslow, J.L. Dietary fat increases high density lipoprotein (hdl) levels both by increasing the transport rates and decreasing the fractional catabolic rates of hdl cholesterol ester and apolipoprotein (apo) a-i. Presentation of a new animal model and mechanistic studies in human apo a-i transgenic and control mice. J. Clin. Investig. 1993, 91, 1665–1671. [Google Scholar]
- Hadizadeh, F.; Faghihimani, E.; Adibi, P. Nonalcoholic fatty liver disease: Diagnostic biomarkers. World J. Gastrointest Pathophysiol. 2017, 8, 11–26. [Google Scholar] [CrossRef]
- Unger, R.H.; Clark, G.O.; Scherer, P.E.; Orci, L. Lipid homeostasis, lipotoxicity and the metabolic syndrome. Biochim. Biophys. Acta 2010, 1801, 209–214. [Google Scholar] [CrossRef]
- Koyama, Y.; Brenner, D.A. Liver inflammation and fibrosis. J. Clin. Investig. 2017, 127, 55–64. [Google Scholar] [CrossRef]
- Ha, J.; Daniel, S.; Broyles, S.S.; Kim, K.H. Critical phosphorylation sites for acetyl-coa carboxylase activity. J. Biol. Chem. 1994, 269, 22162–22168. [Google Scholar]
- Li, Y.; Xu, S.; Mihaylova, M.M.; Zheng, B.; Hou, X.; Jiang, B.; Park, O.; Luo, Z.; Lefai, E.; Shyy, J.Y.; et al. Ampk phosphorylates and inhibits srebp activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011, 13, 376–388. [Google Scholar] [CrossRef]
- Osborne, T.F. Sterol regulatory element-binding proteins (srebps): Key regulators of nutritional homeostasis and insulin action. J. Biol. Chem. 2000, 275, 32379–32382. [Google Scholar] [CrossRef]
- You, M.; Matsumoto, M.; Pacold, C.M.; Cho, W.K.; Crabb, D.W. The role of amp-activated protein kinase in the action of ethanol in the liver. Gastroenterology 2004, 127, 1798–1808. [Google Scholar] [CrossRef]
- Kohjima, M.; Higuchi, N.; Kato, M.; Kotoh, K.; Yoshimoto, T.; Fujino, T.; Yada, M.; Yada, R.; Harada, N.; Enjoji, M.; et al. Srebp-1c, regulated by the insulin and ampk signaling pathways, plays a role in nonalcoholic fatty liver disease. Int. J. Mol. Med. 2008, 21, 507–511. [Google Scholar] [CrossRef]
- Garcia, D.; Hellberg, K.; Chaix, A.; Wallace, M.; Herzig, S.; Badur, M.G.; Lin, T.; Shokhirev, M.N.; Pinto, A.F.M.; Ross, D.S.; et al. Genetic liver-specific ampk activation protects against diet-induced obesity and nafld. Cell Rep. 2019, 26, 192–208.e6. [Google Scholar] [CrossRef]
- Mazza, A.; Fruci, B.; Garinis, G.A.; Giuliano, S.; Malaguarnera, R.; Belfiore, A. The role of metformin in the management of nafld. Exp. Diabetes Res. 2012, 2012, 716404. [Google Scholar] [CrossRef]
- Lenka Kouřimská, A.A. Nutritional and sensory quality of edible insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef] [Green Version]
- Shin, C.S.; Kim, D.Y.; Shin, W.S. Characterization of chitosan extracted from mealworm beetle (tenebrio molitor, zophobas morio) and rhinoceros beetle (allomyrina dichotoma) and their antibacterial activities. Int. J. Biol. Macromol. 2019, 125, 72–77. [Google Scholar] [CrossRef]

| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| SREBP-1c | 5′-CTTCTGGAGACATCGCAAAC-3′ | 5′-GGTAGACAACAGCCGCATC-3′ |
| ACC | 5′-AGGAAGATGGCGTCCGCTCTG-3′ | 5′-GGTGAGATGTGCTGGGTCAT-3′ |
| FAS | 5′-CTTGGGTGCTGACTACAACC-3′ | 5’-GCCCTCCCGTACACTCACTC-3′ |
| Col1a2 | 5’-CCGTGCTTCTCAGAACATCA-3’ | 5’-CTTGCCCCATTCATTTGTCT-3’ |
| MCP-1 | 5’-GCAGTTAACGCCCCACTCA-3’ | 5’-CCAGCCTACTCATTGGGATCA-3’ |
| TNF-α | 5’-CCAACGGCATGGATCTCAAAGACA-3’ | 5’-AGATAGCAAATCGGCTGACGGTGT-3’ |
| Cyclophilin | 5′-TGGAGAGCACCAAGACAGACA-3′ | 5′-TGCCGGAGTCGACAATGAT-3′ |
| Groups | Body Weight (g) | Gained Body Weight (g) | Food Intake (g/Day) | Blood Glucose (mg/dL) | |
|---|---|---|---|---|---|
| Initial | Final | ||||
| NFD (n = 6) | 16.32 ± 1.28 | 24.17 ± 0.44 | 7.85 ± 0.48 | 3.09 ± 0.26 | 152.50 ± 9.12 |
| HFD (n = 33) | 16.11 ± 1.71 | 34.15 ± 1.39 *** | 18.04 ± 0.61 *** | 2.44 ± 0.16 *** | 208.28 ± 8.30 *** |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Bae, G.D.; Lee, M.; Park, E.-Y.; Baek, D.J.; Kim, C.Y.; Jun, H.-S.; Oh, Y.S. Allomyrina dichotoma Larva Extract Ameliorates the Hepatic Insulin Resistance of High-Fat Diet-Induced Diabetic Mice. Nutrients 2019, 11, 1522. https://doi.org/10.3390/nu11071522
Kim K, Bae GD, Lee M, Park E-Y, Baek DJ, Kim CY, Jun H-S, Oh YS. Allomyrina dichotoma Larva Extract Ameliorates the Hepatic Insulin Resistance of High-Fat Diet-Induced Diabetic Mice. Nutrients. 2019; 11(7):1522. https://doi.org/10.3390/nu11071522
Chicago/Turabian StyleKim, Kyong, Gong Deuk Bae, Minho Lee, Eun-Young Park, Dong Jae Baek, Chul Young Kim, Hee-Sook Jun, and Yoon Sin Oh. 2019. "Allomyrina dichotoma Larva Extract Ameliorates the Hepatic Insulin Resistance of High-Fat Diet-Induced Diabetic Mice" Nutrients 11, no. 7: 1522. https://doi.org/10.3390/nu11071522
APA StyleKim, K., Bae, G. D., Lee, M., Park, E.-Y., Baek, D. J., Kim, C. Y., Jun, H.-S., & Oh, Y. S. (2019). Allomyrina dichotoma Larva Extract Ameliorates the Hepatic Insulin Resistance of High-Fat Diet-Induced Diabetic Mice. Nutrients, 11(7), 1522. https://doi.org/10.3390/nu11071522






