Life after Harvest: Circadian Regulation in Photosynthetic Pigments of Rocket Leaves during Supermarket Storage Affects the Nutritional Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Material, Experimental Design, and Sampling
2.2. Visual/Freshness Quality and Chlorophyll a Fluorescence Determination
2.3. Leaf Optical Properties and Index Calculations
2.4. Analysis of Carotenoids and Chlorophylls
2.5. Tocopherol, Phytosterol and Fatty Acid Extraction and Analysis
2.6. Data Processing and Statistics
3. Results
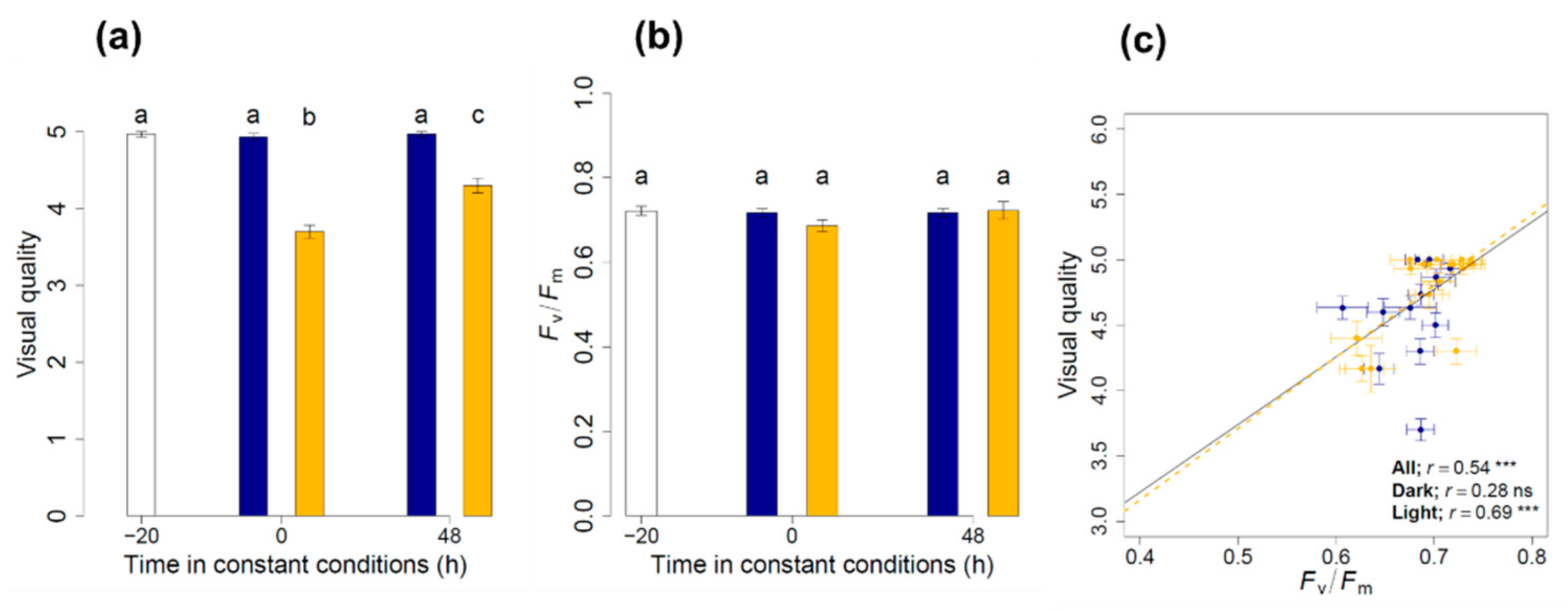
3.1. Maintenance of the Physiological Status and Visual Quality
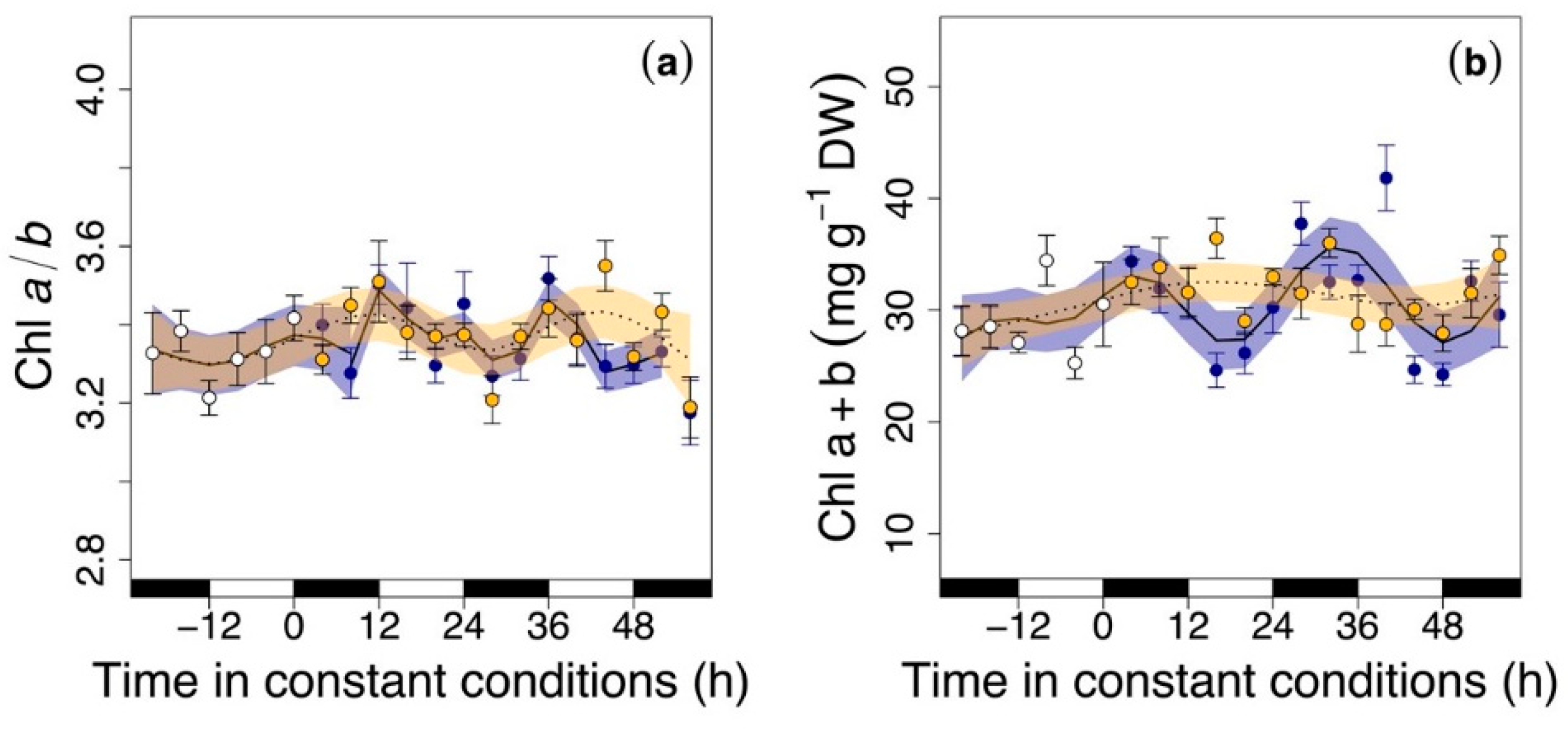
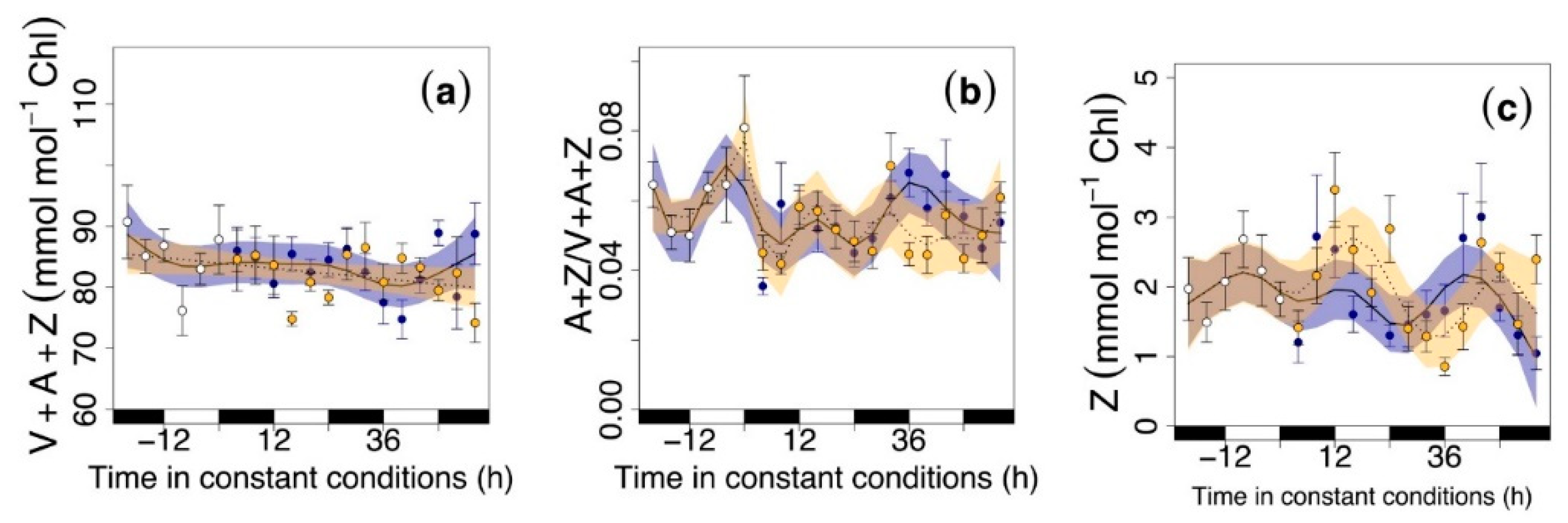
3.2. Circadian Regulation of Pigments
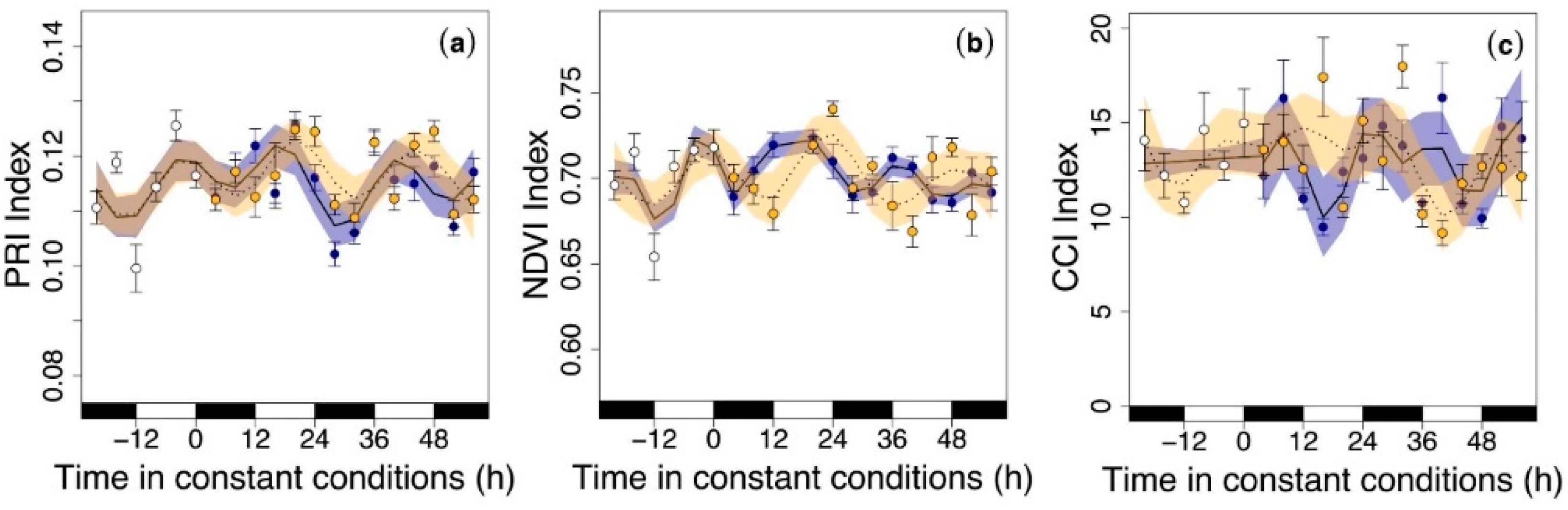
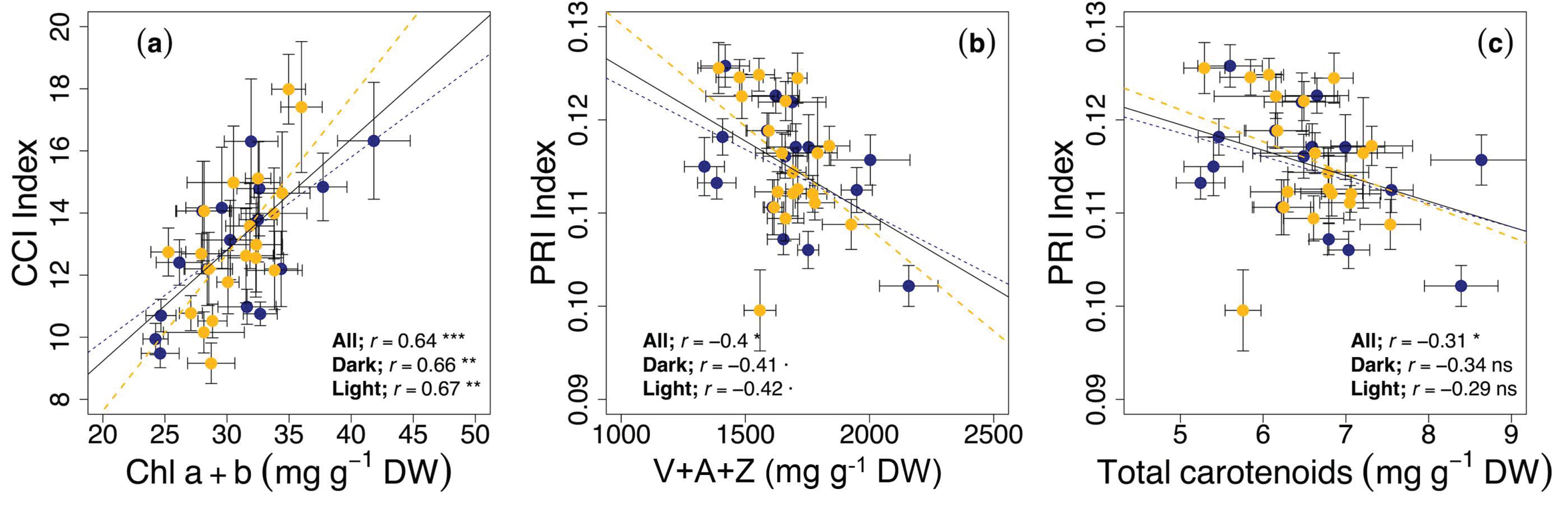
3.3. Circadian Regulation of Reflectance Indices
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ma, L.; Liu, R.; Du, J.H.; Liu, T.; Wu, S.S.; Liu, X.H. Lutein, zeaxanthin and meso-zeaxanthin supplementation associated with macular pigment optical density. Nutrients 2016, 8, 426. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Roger Illingworth, D.; Connor, S.L.; Barton Duell, P.; Connor, W.E. Competitive inhibition of carotenoid transport and tissue concentrations by high dose supplements of lutein, zeaxanthin and beta-carotene. Eur. J. Nutr. 2010, 49, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Stringham, J.M.; Bovier, E.R.; Wong, J.C.; Hammond, B.R. The influence of dietary lutein and zeaxanthin on visual performance. J. Food Sci. 2010, 75, R24–R29. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.P.; Perez-Bueno, M.L.; Zia, A.; Horton, P.; Ruban, A.V. The zeaxanthin-independent and zeaxanthin-dependent qE components of nonphotochemical quenching involve common conformational changes within the photosystem II antenna in Arabidopsis. Plant Physiol. 2008, 149, 1061–1075. [Google Scholar] [CrossRef]
- Fiedor, J.; Burda, K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Adams, W.W. Antioxidants in photosynthesis and human nutrition. Science 2002, 298, 2149–2153. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer chemoprevention by carotenoids. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Emami, S. β-Ionone and its analogs as promising anticancer agents. Eur. J. Med. Chem. 2016, 123, 141–154. [Google Scholar] [CrossRef]
- Voutilainen, S.; Nurmi, T.; Mursu, J.; Rissanen, T.H. Carotenoids and cardiovascular health. Am. J. Clin. Nutr. 2006, 83, 1265–1271. [Google Scholar] [CrossRef]
- Fanciullino, A.L.; Bidel, L.P.R.; Urban, L. Carotenoid responses to environmental stimuli: Integrating redox and carbon controls into a fruit model. Plant Cell Environ. 2014, 37, 273–289. [Google Scholar] [CrossRef]
- Kumar Saini, R.; Hariram Nile, S.; Won Park, S. Carotenoids from fruits and vegetables: Chemistry, analysis, occurrence, bioavailability and biological activities. FRIN 2015, 76, 735–750. [Google Scholar]
- Goodspeed, D.; Liu, J.D.; Chehab, E.W.; Sheng, Z.; Francisco, M.; Kliebenstein, D.J.; Braam, J. Report postharvest circadian entrainment enhances crop pest resistance and phytochemical cycling. Curr. Biol. 2013, 23, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Resco De Dios, V.; Gessler, A.; Ferrio, J.P.; Alday, J.G.; Bahn, M.; Del Castillo, J.; Devidal, S.; García-Muñoz, S.; Kayler, Z.; Landais, D.; et al. Circadian rhythms have significant effects on leaf-to-canopy scale gas exchange under field conditions. Gigascience 2016, 5, 43. [Google Scholar] [CrossRef] [PubMed]
- Hussien, D.; Dakhiya, Y.; Fridman, E.; Kiflawi, M.; Green, R.M. Correlations between circadian rhythms and growth in challenging environments. Plant Physiol. 2017, 173, 1724–1734. [Google Scholar]
- García-Plazaola, J.I.; Fernández-Marín, B.; Ferrio, J.P.; Alday, J.G.; Hoch, G.; Landais, D.; Milcu, A.; Tissue, D.T.; Voltas, J.; Gessler, A.; et al. Endogenous circadian rhythms in pigment composition induce changes in photochemical efficiency in plant canopies. Plant Cell Environ. 2017, 40, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.D.; Goodspeed, D.; Sheng, Z.; Li, B.; Yang, Y.; Kliebenstein, D.J.; Braam, J. Keeping the rhythm: Light/dark cycles during postharvest storage preserve the tissue integrity and nutritional content of leafy plants. BMC Plant Biol. 2015, 15, 92. [Google Scholar] [CrossRef] [PubMed]
- Bell, L.; Methven, L.; Signore, A.; Oruna-Concha, M.J.; Wagstaff, C. Analysis of seven salad rocket (Eruca sativa) accessions: The relationships between sensory attributes and volatile and non-volatile compounds. Food Chem. 2017, 218, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Sims, D.; Gamon, J. Relationship between leaf pigment content and spectral reflectance across a wide range species, leaf structures and development stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Wong, C.Y.S.; Gamon, J.A. The photochemical reflectance index provides an optical indicator of spring photosynthetic activation in evergreen conifers. New Phytol. 2015, 206, 196–208. [Google Scholar] [CrossRef]
- Mc Clung, C.R. You have access Plant Circadian Rhythms. Plant Cell 2006, 18, 792–803. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Rinderle, U. The role of chlorophyll fluorescence in the detection of stress conditions in plants. CRC Crit. Rev. Anal. Chem. 1988, 19, S29–S85. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Esteban, R.; Fleta-Soriano, E.; Buezo, J.; Míguez, F.; Becerril, J.M.; García-Plazaola, J.I. Enhancement of zeaxanthin in two-steps by environmental stress induction in rocket and spinach. Food Res. Int. 2014, 65, 207–214. [Google Scholar] [CrossRef]
- Garcia-Plazaola, J.I.; Becerril, J.M. A rapid high performance liquid chromatography method to measure lipophilic antioxidantsin stressed plants: Simultaneous determination of carotenoids and tocopherols. Phytochem. Anal. 1999, 10, 307–313. [Google Scholar] [CrossRef]
- Garcia-Plazaola, J.I.; Esteban, R. PrometheusWiki contributors Determination of chlorophylls and carotenoids by HPLC. Version 10. 2012. Available online: /tiki-pagehistory.php?page=Determination of chlorophylls and carotenoids by HPLC&preview=10 (accessed on 10 May 2019).
- Fernández-Marín, B.; García-Plazaola, J.I.; Hernández, A.; Esteban, R. Plant photosynthetic pigments: Methods and tricks for correct quantification and identification. In Advances in Plant Ecophysiology Techniques; Springer: Cham, Switzerland, 2018; pp. 29–50. [Google Scholar]
- García-Plazaola, J.I.; Becerril, J.M. Seasonal changes in photosynthetic pigments and antioxidants in beech (Fagus sylvatica) in a Mediterranean climate: Implications for tree decline diagnosis. Funct. Plant Biol. 2001, 28, 225. [Google Scholar] [CrossRef]
- Guil-Guerrero, J.L.; Gómez-Mercado, F.; Ramos-Bueno, R.P.; González-Fernández, M.J.; Urrestarazu, M.; Jiménez-Becker, S.; de Bélair, G. Fatty acid profiles and sn-2 fatty acid distribution of γ-linolenic acid-rich Borago species. J. Food Compos. Anal. 2018, 66, 74–80. [Google Scholar] [CrossRef]
- López Ortíz, C.M.; Prats Moya, M.S.; Berenguer Navarro, V. A rapid chromatographic method for simultaneous determination of β-sitosterol and tocopherol homologues in vegetable oils. J. Food Compos. Anal. 2006, 19, 141–149. [Google Scholar] [CrossRef]
- Pérez-Fernández, V.; Spagnoli, M.; Rocco, A.; Aturki, Z.; Sciubba, F.; De Salvador, F.R.; Engel, P.; Curini, R.; Gentili, A. Non-aqueous reversed-phase liquid-chromatography of tocopherols and tocotrienols and their mass spectrometric quantification in pecan nuts. J. Food Compos. Anal. 2017, 64, 171–180. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized additive models: An introduction with R. Texts Stat. Sci. 2006, xvii, 392. [Google Scholar]
- Yerushalmi, S.; Green, R.M. Evidence for the adaptive significance of circadian rhythms. Ecol. Lett. 2009, 12, 970–981. [Google Scholar] [CrossRef]
- Fernández-Marín, B.; Atherton, J.; Olascoaga, B.; Kolari, P.; Porcar-Castell, A.; García-Plazaola, J.I. When the sun never sets: Daily changes in pigment composition in three subarctic woody plants during the summer solstice. Trees Struct. Funct. 2018, 32, 615–630. [Google Scholar] [CrossRef]
- Velez-Ramirez, A.I.; van Ieperen, W.; Vreugdenhil, D.; Millenaar, F.F. Plants under continuous light. Trends Plant Sci. 2011, 16, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, S.; Li, X.; Huang, H.; Sui, X.; Zhang, Z. Molecular cloning and characterization of the light-regulation and circadian-rhythm of the VDE gene promoter from Zingiber officinale. Plant Cell Rep. 2012, 31, 1381–1392. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Michael, T.P.; Hudson, M.E.; Kay, S.A.; Chory, J.; Schuler, M.A. Cytochrome P450 Monooxygenases as Reporters for Circadian-Regulated Pathways. Plant Physiol. 2009, 150, 858–878. [Google Scholar] [CrossRef] [PubMed]
- Esteban, R.; Barrutia, O.; Artetxe, U.; Fernández-Marín, B.; Hernández, A.; García-Plazaola, J.I. Internal and external factors affecting photosynthetic pigment composition in plants: A meta-analytical approach. New Phytol. 2015, 206, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Cohu, C.M.; Muller, O.; Adams, W.W.; Demmig-Adams, B. Leaf anatomical and photosynthetic acclimation to cool temperature and high light in two winter versus two summer annuals. Physiol. Plant. 2014, 152, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Benke, K.; Tomkins, B. Future food-production systems: Vertical farming and controlled-environment agriculture. Sustain. Sci. Pract. Policy 2017, 13, 13–26. [Google Scholar] [CrossRef]

| Tocopherol Content (Tf) (mg/100 g DW) | |||||||||||
| Time in Constant Conditions (Hours) | α-Tf | β-Tf | γ-Tf | δ-Tf | Total Pool | ||||||
| −20 h | 8.41 ± 0.4 | - | - | - | 8.41 ± 0.4 | ||||||
| 48 h (dark) | 12.29 ± 2.0 | - | - | - | 12.29 ± 2.0 | ||||||
| 48 h (light) | 12.29 | - | - | - | 12.29 | ||||||
| Phytosterol Content (mg/100 g DW) | |||||||||||
| Time in Constant Conditions (hours) | Δ5-Avenasterol | Campesterol | β-Sitoesterol | Total Pool | |||||||
| −20 h | 32.26 ± 3.6 | 16.13 ± 3.1 | 66.45 ± 5.7 | 114.84 | |||||||
| 48 h (dark) | 30.86 ± 9.7 | 14.84 ± 4.8 | 52.39 ± 7.25 | 98.09 ± 2.3 | |||||||
| 48 h (light) | 22.04 ± 13.2 | 11.18 ± 2.1 | 57.45 ± 15.7 | 90.67 ± 3.3 | |||||||
| % of Fatty Acids with Respect to the Total | |||||||||||
| Time in Constant Conditions (hours) | Palmitic Acid (16:0) | Linoleic Acid (18:2n6) | Linolenic Acid (18:3n3) | ||||||||
| −20 h | 16.9 ± 0.2 | 15.5 ± 0.0 | 67.3 ± 0.2 | ||||||||
| 40 h (dark) | 16.9 ± 0.2 | 15.9 ± 0.0 | 67.1 ± 0.2 | ||||||||
| 40 h (light) | 18.0 ± 0.1 | 14.7 ± 0.1 | 67.2 ± 0.2 | ||||||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz de Larrinaga, L.; Resco de Dios, V.; Fabrikov, D.; Guil-Guerrero, J.L.; Becerril, J.M.; García-Plazaola, J.I.; Esteban, R. Life after Harvest: Circadian Regulation in Photosynthetic Pigments of Rocket Leaves during Supermarket Storage Affects the Nutritional Quality. Nutrients 2019, 11, 1519. https://doi.org/10.3390/nu11071519
Ruiz de Larrinaga L, Resco de Dios V, Fabrikov D, Guil-Guerrero JL, Becerril JM, García-Plazaola JI, Esteban R. Life after Harvest: Circadian Regulation in Photosynthetic Pigments of Rocket Leaves during Supermarket Storage Affects the Nutritional Quality. Nutrients. 2019; 11(7):1519. https://doi.org/10.3390/nu11071519
Chicago/Turabian StyleRuiz de Larrinaga, Lorena, Victor Resco de Dios, Dmitri Fabrikov, José Luis Guil-Guerrero, José María Becerril, José Ignacio García-Plazaola, and Raquel Esteban. 2019. "Life after Harvest: Circadian Regulation in Photosynthetic Pigments of Rocket Leaves during Supermarket Storage Affects the Nutritional Quality" Nutrients 11, no. 7: 1519. https://doi.org/10.3390/nu11071519
APA StyleRuiz de Larrinaga, L., Resco de Dios, V., Fabrikov, D., Guil-Guerrero, J. L., Becerril, J. M., García-Plazaola, J. I., & Esteban, R. (2019). Life after Harvest: Circadian Regulation in Photosynthetic Pigments of Rocket Leaves during Supermarket Storage Affects the Nutritional Quality. Nutrients, 11(7), 1519. https://doi.org/10.3390/nu11071519






