GPP (Composition of Ganoderma lucidum Poly-saccharides and Polyporus umbellatus Poly-saccharides) Enhances Innate Immune Function in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. RAW264.7 Cells Culture and Sample Preparation
2.3. Macrophage Phagocytosis Assay
2.4. NO Assay
2.5. Real-time Quantitative PCR
2.6. Animals and GPP Treatment
2.7. Measurement of Immune Organ Indexes
2.8. Measurement of Phagocytotic Function of Peripheral Phagocytes in Mice
2.9. Measurement of NK Cell Activity in Mice
2.10. Statistical Analysis
3. Results
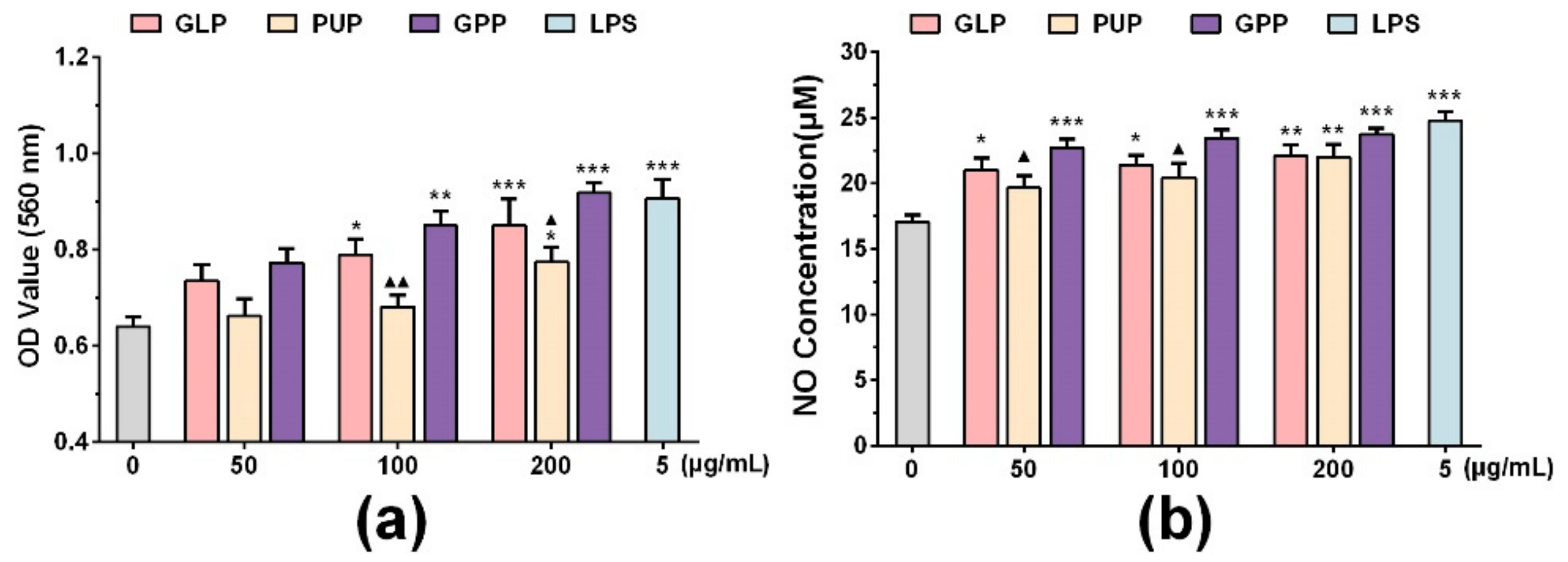
3.1. GPP Increased Phagocytosis and NO Production in RAW264.7 Cells
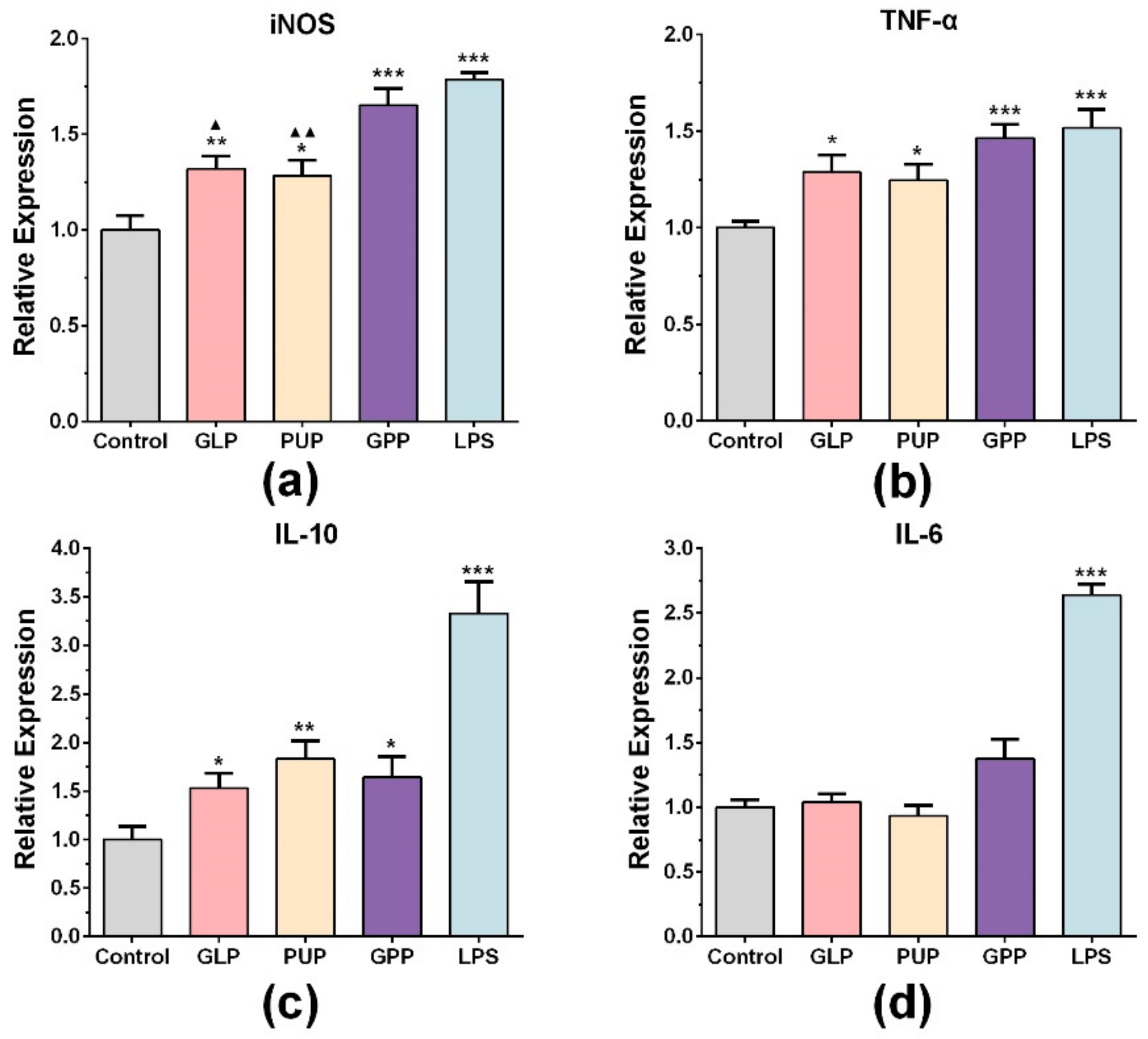
3.2. GPP Increased the mRNA Expression of INOS, TNF-α and IL-10
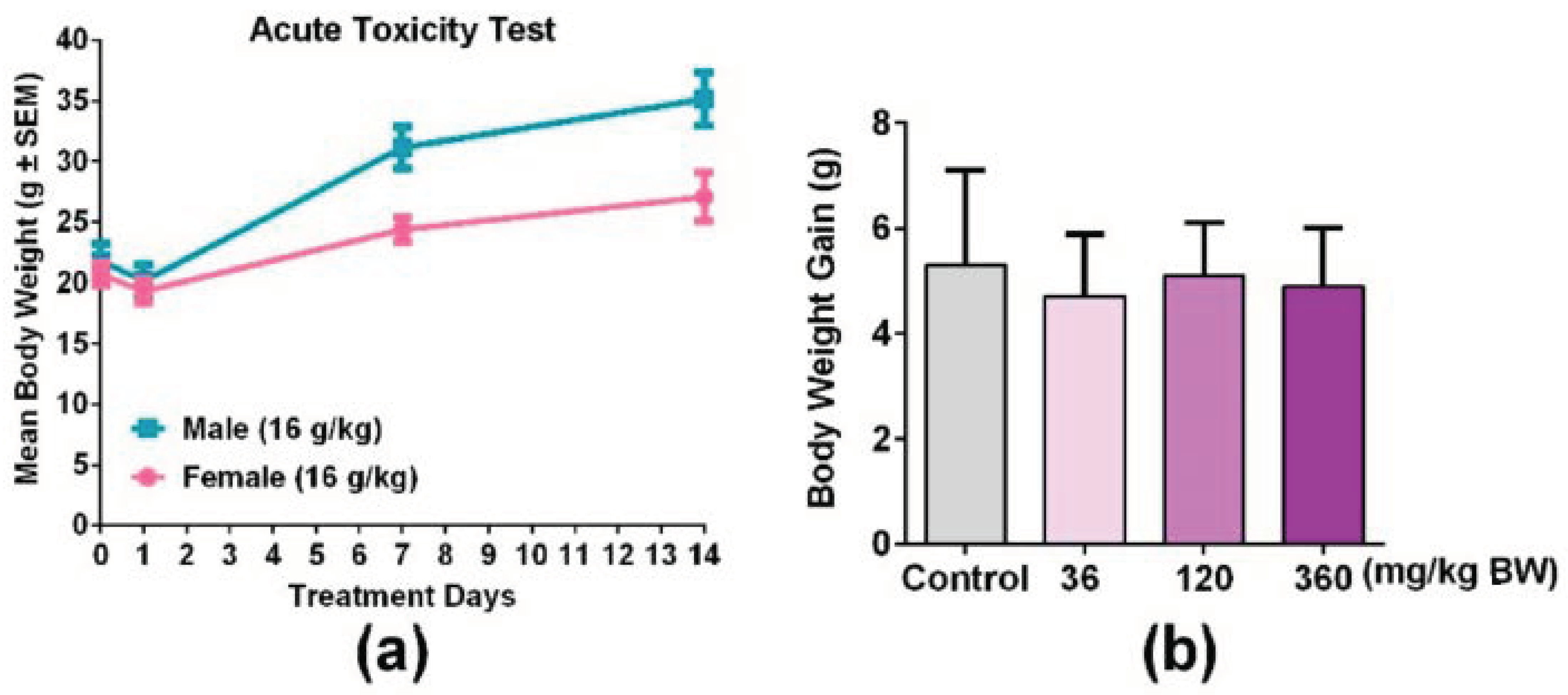
3.3. An Overdose of GPP Oral Administration was Safe in Mice
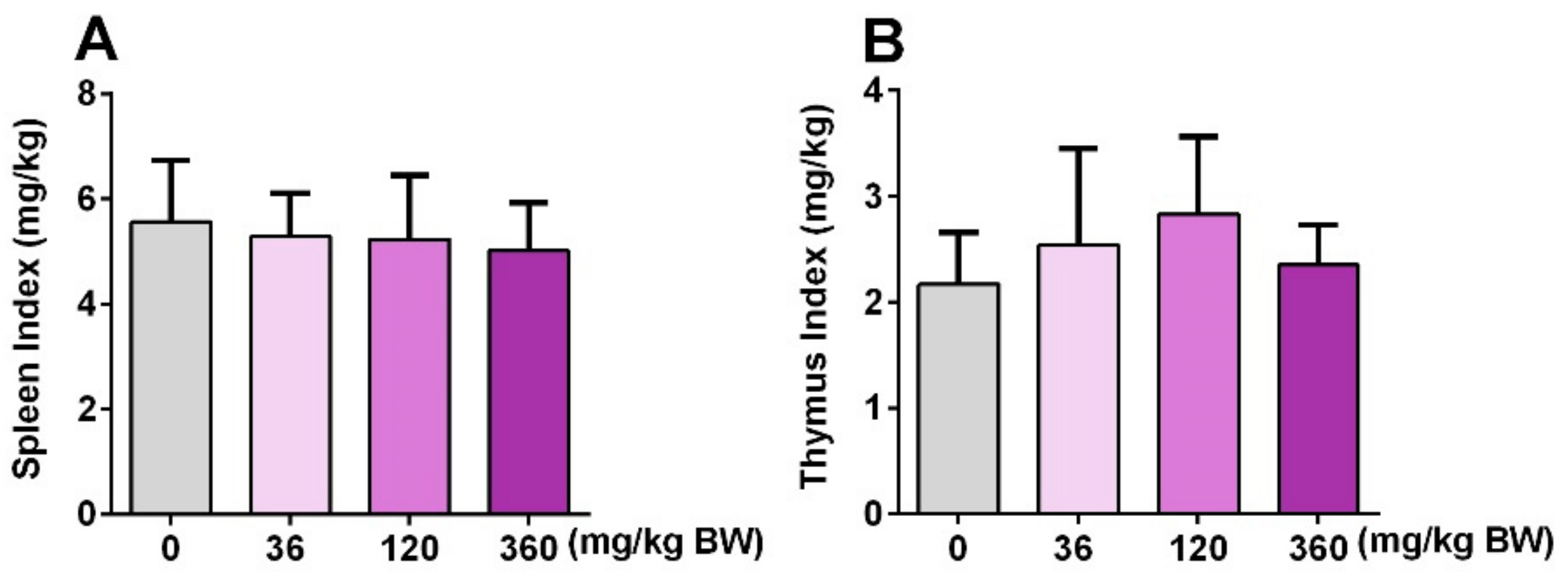
3.4. GPP Did Not Affect the Spleen Index and Thymus Index in Mice
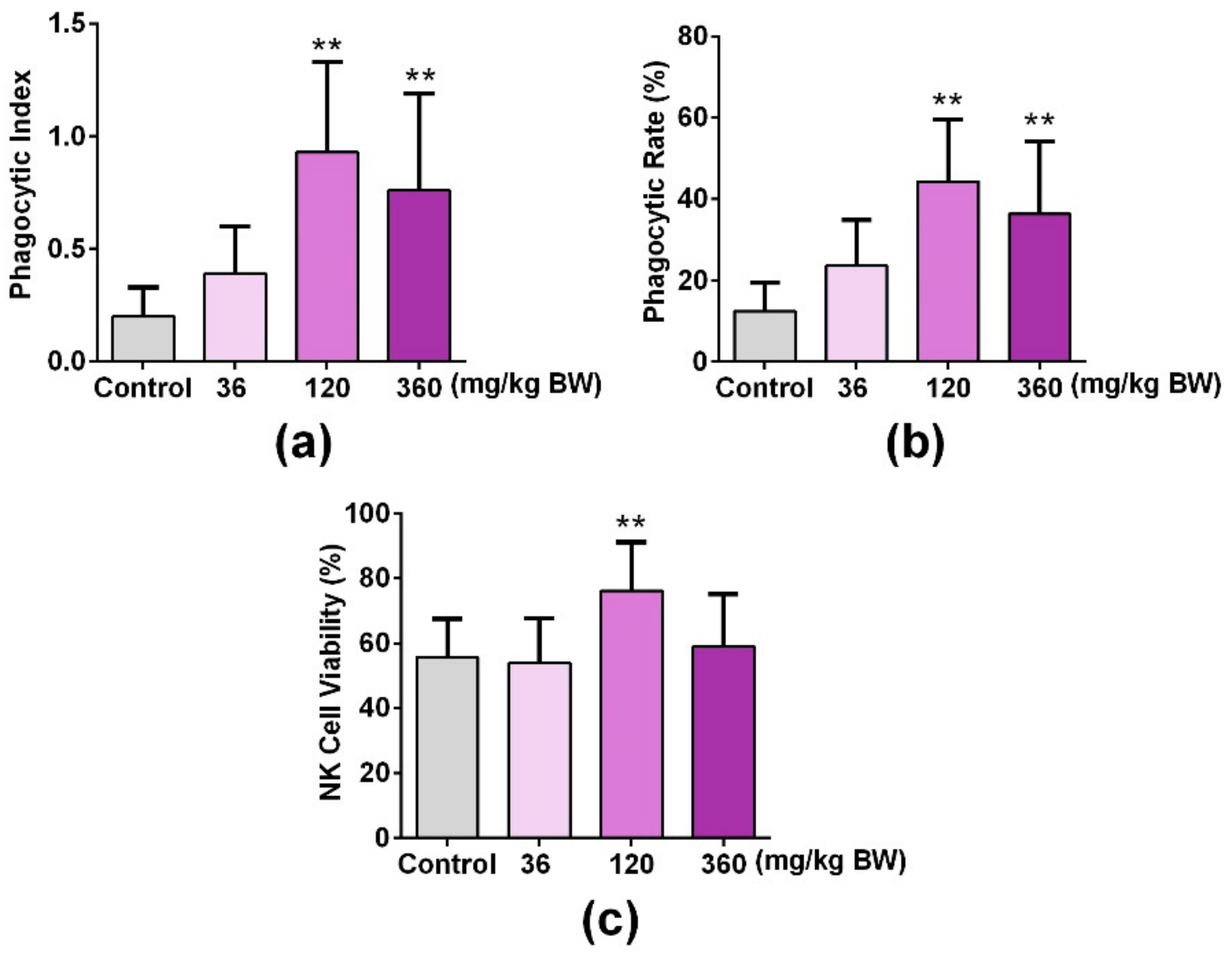
3.5. GPP Increased Peripheral Phagocytes and NK Cell Activity
4. Discussion
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chen, F.; Huang, G. Preparation and immunological activity of polysaccharides and their derivatives. Int. J. Biol. Macromol. 2018, 112, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Giavasis, I. Bioactive fungal polysaccharides as potential functional ingredients in food and nutraceuticals. Curr. Opin. Biotechnol. 2014, 26, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Chen, D.; Yang, L.; Zhu, N.; Li, J.; Zhao, J.; Hu, Z.; Wang, F.J.; Zhang, L.W. Comparative studies on the immunoregulatory effects of three polysaccharides using high content imaging system. Int. J. Biol. Macromol. 2016, 86, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Singdevsachan, S.K.; Auroshree, P.; Mishra, J.; Baliyarsingh, B.; Tayung, K.; Thatoi, H. Mushroom polysaccharides as potential prebiotics with their antitumor and immunomodulating properties: A review. Bioact. Carbohydr. Diet. Fibre 2016, 7, 1–14. [Google Scholar] [CrossRef]
- Zhang, Q.; Cong, R.; Hu, M.; Zhu, Y.; Yang, X. Immunoenhancement of edible fungal polysaccharides (lentinan, tremellan, and pachymaran) on cyclophosphamide-induced immunosuppression in mouse model. Evid. Based Complement. Alternat. Med. 2017, 2017, 9459156. [Google Scholar] [CrossRef]
- Jiang, M.H.; Zhu, L.; Jiang, J.G. Immunoregulatory actions of polysaccharides from Chinese herbal medicine. Expert Opin. Ther. Targets 2010, 14, 1367–1402. [Google Scholar] [CrossRef]
- Ahmad, M.F. Ganoderma lucidum: Persuasive biologically active constituents and their health endorsement. Biomed. Pharmacother. 2018, 107, 507–519. [Google Scholar] [CrossRef]
- Zhao, Y.Y. Traditional uses, phytochemistry, pharmacology, pharmacokinetics and quality control of Polyporus umbellatus (Pers.) Fries: A review. J. Ethnopharmacol. 2013, 149, 35–48. [Google Scholar] [CrossRef]
- Li, X.; Xu, W. TLR4-mediated activation of macrophages by the polysaccharide fraction from Polyporus umbellatus (Pers.) fries. J. Ethnopharmacol. 2011, 135, 1–6. [Google Scholar] [CrossRef]
- Lin, Z.B.; Zhang, H.N. Anti-tumor and immunoregulatory activities of Ganoderma lucidum and its possible mechanisms. Acta. Pharmacol. Sin. 2004, 25, 1387–1395. [Google Scholar]
- Yuan, D.; Mori, J.; Komatsu, K.I.; Makino, T.; Kano, Y. An anti-aldosteronic diuretic component (drain dampness) in Polyporus sclerotium. Biol. Pharm. Bull. 2004, 27, 867–870. [Google Scholar] [CrossRef] [PubMed]
- Batra, P.; Sharma, A.K.; Khajuria, R. Probing Lingzhi or Reishi medicinal mushroom Ganoderma lucidum (higher Basidiomycetes): A bitter mushroom with amazing health benefits. Int. J. Med. Mushrooms 2013, 15, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, X.; Zhong, Z.; Chen, L.; Wang, Y. Ganoderma lucidum polysaccharides: Immunomodulation and potential anti-tumor activities. Am. J. Chin. Med. 2011, 39, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Chang, Y.; Liu, Y.; Zhang, M.; Luo, H.; Hao, C.; Zeng, P.; Sun, Y.; Wang, H.; Zhang, L. Overview of Ganoderma sinense polysaccharide-an adjunctive drug used during concurrent chemo/radiation therapy for cancer treatment in China. Biomed. Pharmacother. 2017, 96, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.W.; Qin, G.F.; Han, B.; Li, C.X.; Yang, H.G.; Nie, P.H.; Zeng, X. Efficacy of Zhuling polyporus polysaccharide with BCG to inhibit bladder carcinoma. Carbohydr. Polym. 2015, 118, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Hume, D.A.; Ross, I.L.; Himes, S.R.; Sasmono, R.T.; Wells, C.A.; Ravasi, T. The mononuclear phagocyte system revisited. J. Leukoc. Biol. 2002, 72, 621–627. [Google Scholar] [PubMed]
- Locati, M.; Mantovani, A.; Sica, A. Macrophage activation and polarization as an adaptive component of innate immunity. Adv. Immunol. 2013, 120, 163–184. [Google Scholar] [PubMed]
- Hume, D.A. The mononuclear phagocyte system. Curr. Opin. Immunol. 2006, 18, 49–53. [Google Scholar] [CrossRef]
- Bonnardel, J.; Guilliams, M. Developmental control of macrophage function. Curr. Opin. Immunol. 2018, 50, 64–74. [Google Scholar] [CrossRef]
- Gordon, S.; Pluddemann, A. Tissue macrophages: Heterogeneity and functions. BMC Biol. 2017, 15, 53. [Google Scholar] [CrossRef]
- Sun, L.X.; Lin, Z.B.; Lu, J.; Li, W.D.; Niu, Y.D.; Sun, Y.; Hu, C.Y.; Zhang, G.Q.; Duan, X.S. The improvement of M1 polarization in macrophages by glycopeptide derived from Ganoderma lucidum. Immunol. Res. 2017, 65, 658–665. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.P.; Zhao, S.W.; Zheng, W.J.; Hua, Z.C.; Shi, Q.; Liu, Z.T. Effects of cyanobacteria bloom extract on some parameters of immune function in mice. Toxicol. Lett. 2003, 143, 27–36. [Google Scholar] [CrossRef]
- Huang, R.; Zhao, M.; Yang, X.; Huang, J.; Yang, Y.; Chen, B.; Tan, J.; Huang, J.; Li, Z.; Lv, Y.; et al. Effects of Prunella vulgaris on the mice immune function. PLoS ONE 2013, 8, e77355. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, Y.J.; Kim, H.J.; Kim, Y.S.; Park, W. Immunostimulatory effect of laminarin on RAW 264.7 mouse macrophages. Molecules 2012, 17, 5404–5411. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Qiu, L.; Li, Y.; Li, L.; Wang, X.; Liu, Z.; Guo, Y.; Wang, H. Effects of Marsdenia tenacissima polysaccharide on the immune regulation and tumor growth in H22 tumor-bearing mice. Carbohydr. Polym. 2016, 137, 52–58. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Xu, Z.; Ding, Z. Bioactive mushroom polysaccharides: A review on monosaccharide composition, biosynthesis and regulation. Molecules 2017, 22, e955. [Google Scholar] [CrossRef]
- Chaturvedi, V.K.; Agarwal, S.; Gupta, K.K.; Ramteke, P.W.; Singh, M.P. Medicinal mushroom: Boon for therapeutic applications. 3 Biotech. 2018, 8, 334. [Google Scholar] [CrossRef]
- Reis, F.S.; Barros, L.; Martins, A.; Ferreira, I.C. Chemical composition and nutritional value of the most widely appreciated cultivated mushrooms: An inter-species comparative study. Food Chem. Toxicol. 2012, 50, 191–197. [Google Scholar] [CrossRef]
- Cor, D.; Knez, Z.; Knez Hrncic, M. Antitumour, antimicrobial, antioxidant and antiacetylcholinesterase effect of Ganoderma lucidum Terpenoids and polysaccharides: A Review. Molecules 2018, 23, e649. [Google Scholar] [CrossRef]
- Xu, X.; Yan, H.; Tang, J.; Chen, J.; Zhang, X. Polysaccharides in Lentinus edodes: Isolation, structure, immunomodulating activity and future prospective. Crit. Rev. Food Sci. Nutr. 2014, 54, 474–487. [Google Scholar] [CrossRef]
- Soccol, C.R.; Bissoqui, L.Y.; Rodrigues, C.; Rubel, R.; Sella, S.R.; Leifa, F.; de Souza Vandenberghe, L.P.; Soccol, V.T. Pharmacological properties of biocompounds from spores of the lingzhi or reishi medicinal mushroom Ganoderma lucidum (Agaricomycetes): A review. Int. J. Med. Mushrooms 2016, 18, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, D.; Iida, T.; Nakase, H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int. J. Mol. Sci. 2017, 19, e92. [Google Scholar] [CrossRef] [PubMed]
- MacMicking, J.; Xie, Q.W.; Nathan, C. Nitric oxide and macrophage function. Annu. Rev. Immunol. 1997, 15, 323–350. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Zhao, Q.; Yang, T.; Ding, W.; Zhao, Y. Cellular metabolism and macrophage functional polarization. Int. Rev. Immunol. 2015, 34, 82–100. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Myasoedova, V.A.; Revin, V.V.; Orekhov, A.N.; Bobryshev, Y.V. The impact of interferon-regulatory factors to macrophage differentiation and polarization into M1 and M2. Immunobiology 2018, 223, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-associated macrophages as major players in the tumor microenvironment. Cancers 2014, 6, 1670–1690. [Google Scholar] [CrossRef]
- Gong, T.; Wang, C.F.; Yuan, J.R.; Li, Y.; Gu, J.F.; Zhao, B.J.; Zhang, L.; Jia, X.B.; Feng, L.; Liu, S.L. Inhibition of tumor growth and immunomodulatory effects of flavonoids and scutebarbatines of Scutellaria barbata D. Don in Lewis-Bearing C57BL/6 Mice. Evid. Based Complement. Alternat. Med. 2015, 2015, 630760. [Google Scholar] [CrossRef]
- Mononuclear Phagocyte System. In Dictionary of Rheumatology; Rovenský, J., Payer, J., Eds.; Springer: Vienna, Austria, 2009; p. 134. [Google Scholar]
- Paul, S.; Lal, G. The molecular mechanism of natural killer cells function and its importance in cancer immunotherapy. Front Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef]
- Sun, H.-Q.; Zhu, Z.-Y.; Yang, X.-Y.; Meng, M.; Dai, L.-C.; Zhang, Y.-M. Preliminary characterization and immunostimulatory activity of a novel functional polysaccharide from Astragalus residue fermented by Paecilomyces sinensis. RSC Adv. 2017, 7, 23875–23881. [Google Scholar] [CrossRef]
- Xu, J.; Chen, H.B.; Li, S.L. Understanding the molecular mechanisms of the interplay between herbal medicines and gut microbiota. Med. Res. Rev. 2017, 37, 1140–1185. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, M.; Xiao, J.; Xu, B. A critical review on health promoting benefits of edible mushrooms through gut microbiota. Int. J. Mol. Sci. 2017, 18, e1934. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Q.; Li, L.; Chen, H.; Liu, Q.; Wang, Z. GPP (Composition of Ganoderma lucidum Poly-saccharides and Polyporus umbellatus Poly-saccharides) Enhances Innate Immune Function in Mice. Nutrients 2019, 11, 1480. https://doi.org/10.3390/nu11071480
Huang Q, Li L, Chen H, Liu Q, Wang Z. GPP (Composition of Ganoderma lucidum Poly-saccharides and Polyporus umbellatus Poly-saccharides) Enhances Innate Immune Function in Mice. Nutrients. 2019; 11(7):1480. https://doi.org/10.3390/nu11071480
Chicago/Turabian StyleHuang, Qing, Liyuan Li, Huiling Chen, Qingfei Liu, and Zhao Wang. 2019. "GPP (Composition of Ganoderma lucidum Poly-saccharides and Polyporus umbellatus Poly-saccharides) Enhances Innate Immune Function in Mice" Nutrients 11, no. 7: 1480. https://doi.org/10.3390/nu11071480
APA StyleHuang, Q., Li, L., Chen, H., Liu, Q., & Wang, Z. (2019). GPP (Composition of Ganoderma lucidum Poly-saccharides and Polyporus umbellatus Poly-saccharides) Enhances Innate Immune Function in Mice. Nutrients, 11(7), 1480. https://doi.org/10.3390/nu11071480




