Anti-Irritant and Anti-Inflammatory Effects of DHA Encapsulated in Resveratrol-Based Solid Lipid Nanoparticles in Human Keratinocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Solvents
2.2. RV-SLNs Preparation
2.3. Cell Lines
2.4. Reagents and Treatments
2.5. Analysis of Cell Viability
2.6. Analysis of Inflammatory Cytokine Production
2.7. Analysis of ROS Production
2.8. Analysis of NLRP3 Inflammasome Activation by Western Blotting
2.9. Statistical Analysis
3. Results and Discussion
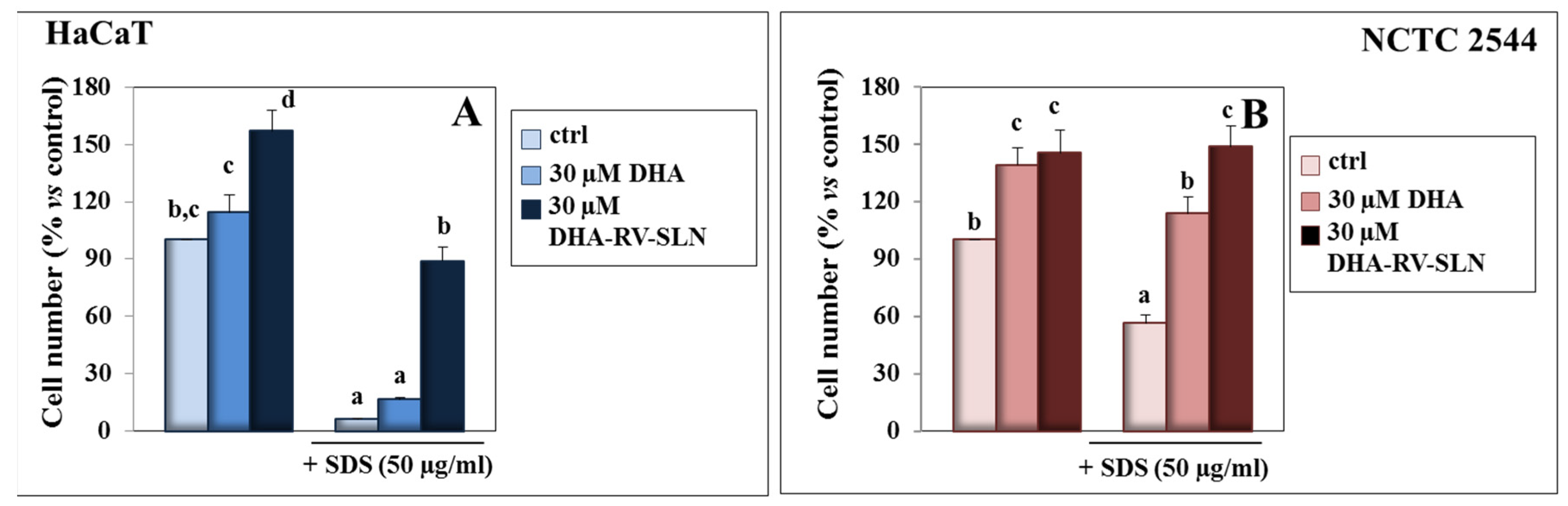
3.1. Effect of DHA and DHA-RV-SLNs on the SDS Growth Inhibiting Effect in Cultured Keratinocytes
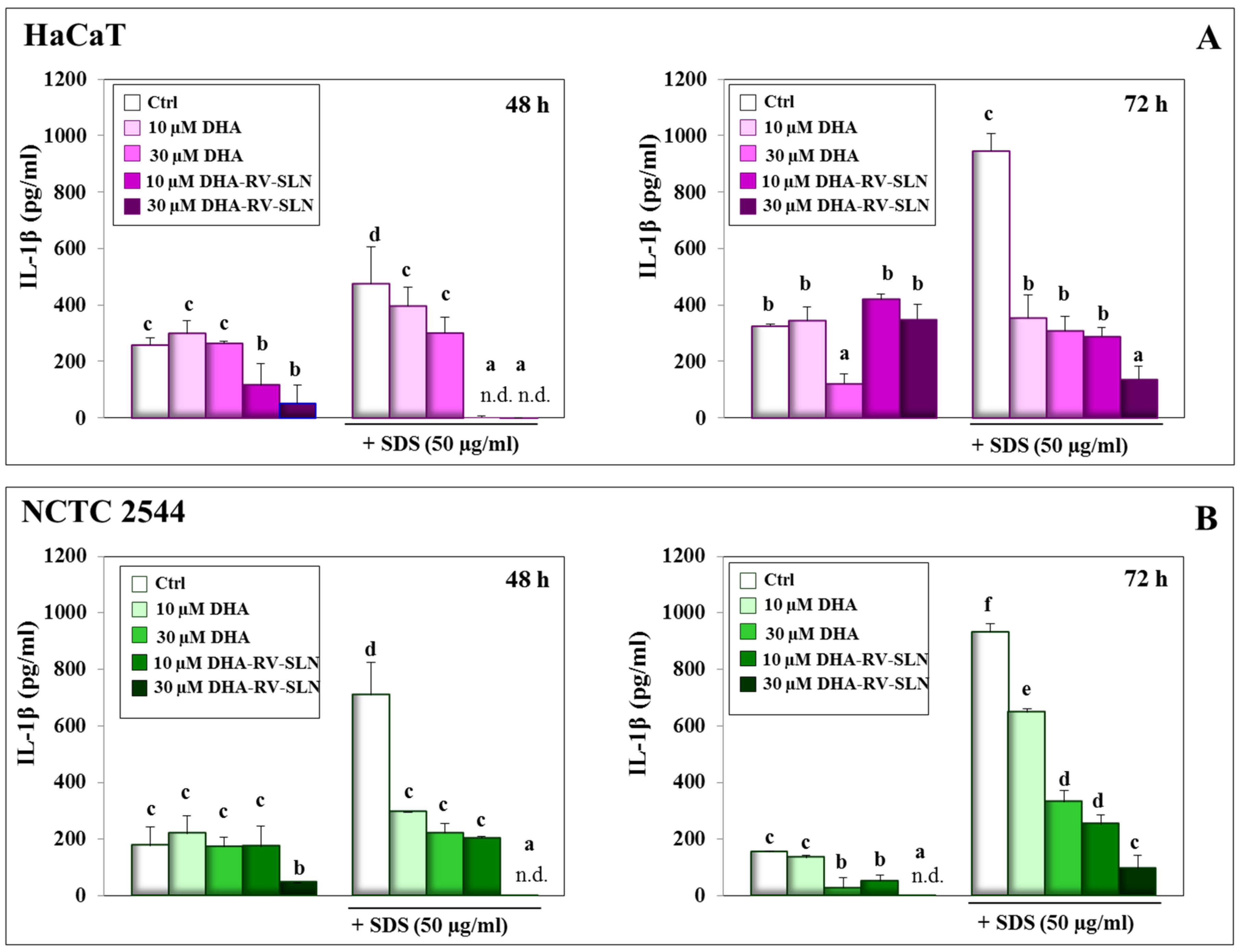
3.2. Effect of DHA and DHA-RV-SLNs on the Basal and SDS-Induced IL-1β Production in Cultured Keratinocytes
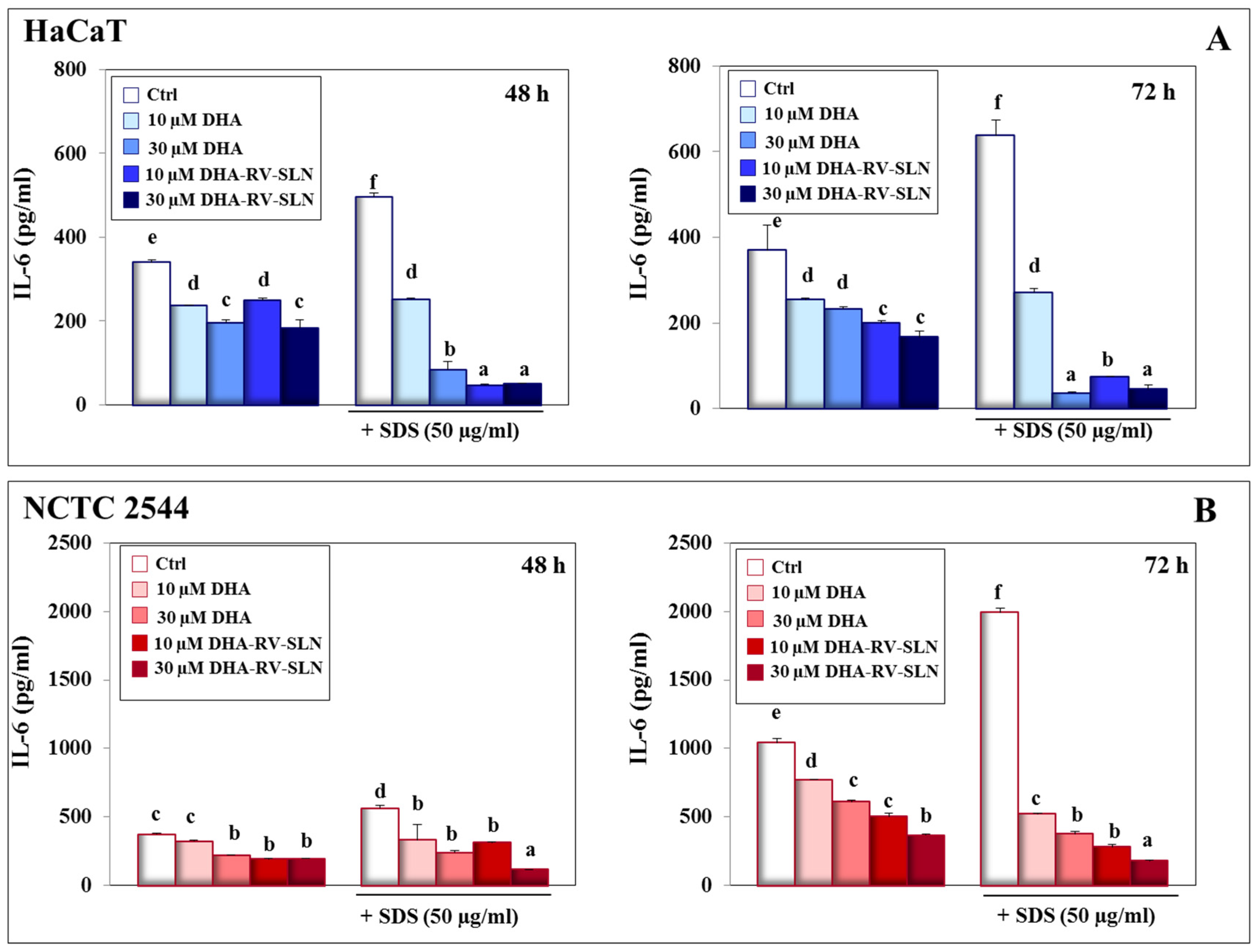
3.3. Effect of DHA and DHA-RV-SLNs on the Basal and SDS-Induced IL-6 Production in Cultured Keratinocytes
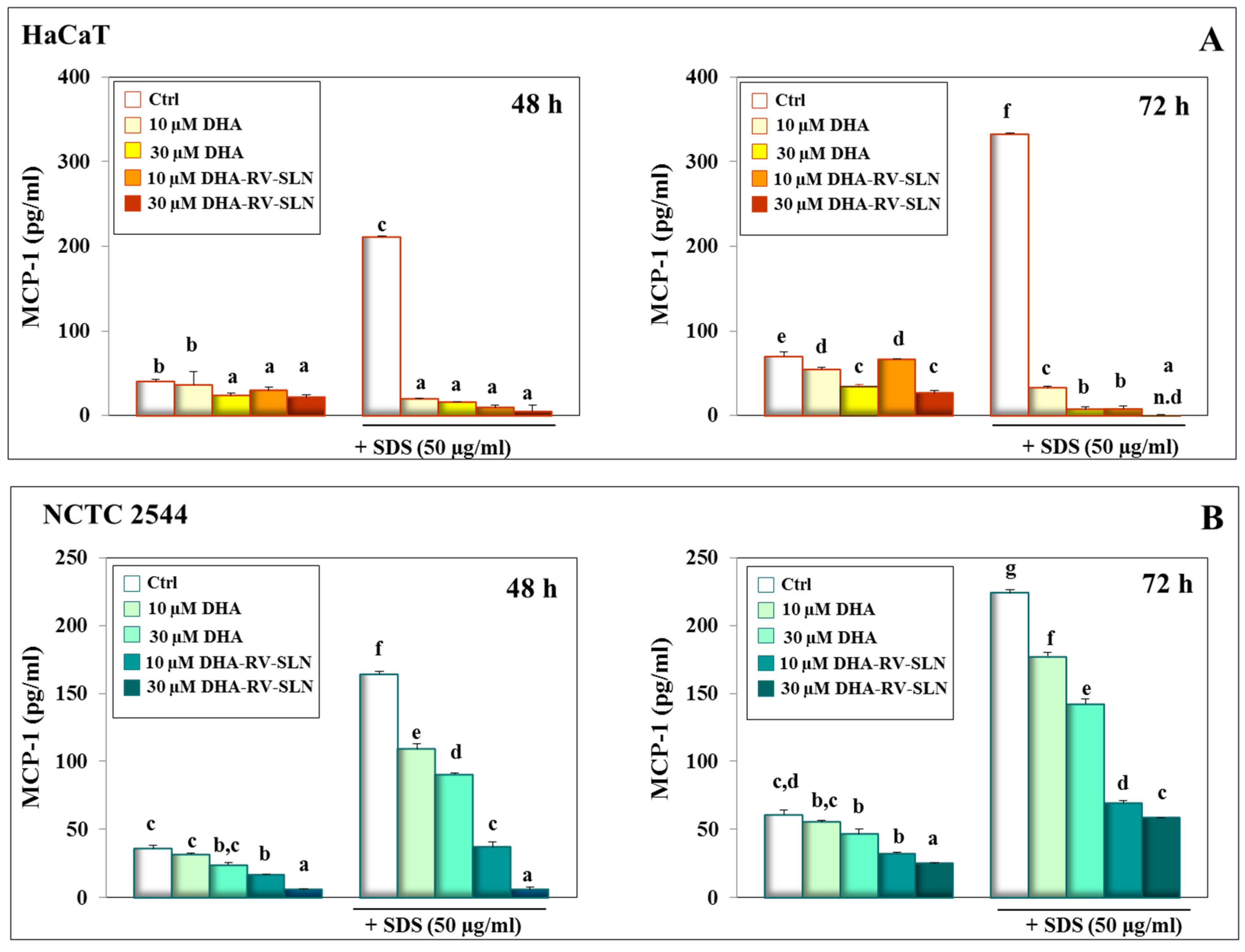
3.4. Effect of DHA and DHA-RV-SLNs on the Basal and SDS-Induced MCP-1 Production in Cultured Keratinocytes
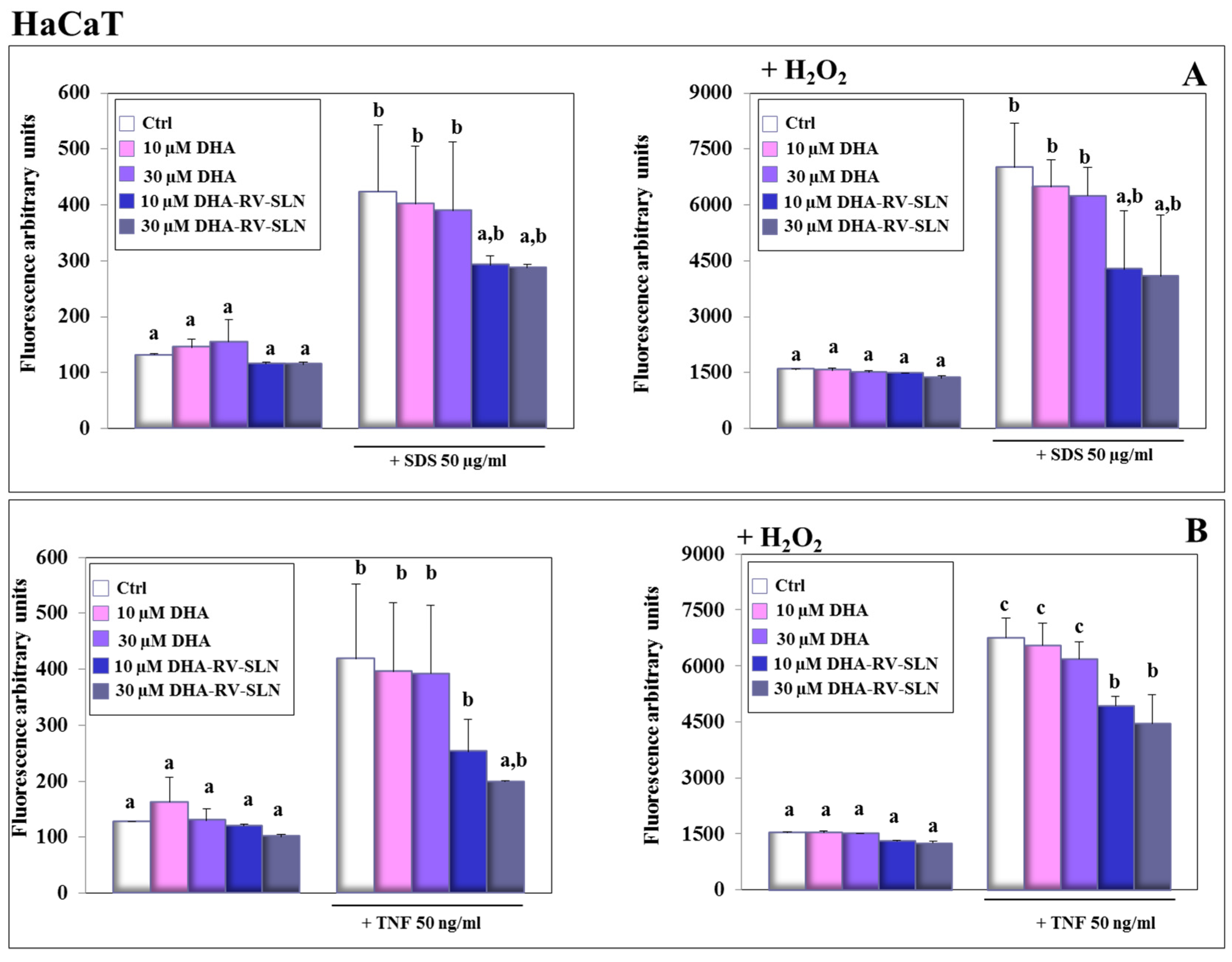
3.5. Effect of Free DHA and DHA-RV-SLNs on ROS Production Induced by SDS and TNF-α in Human Keratinocytes In Vitro
3.6. Effect of DHA and DHA-SLNs on the Activation of NLRP3 Inflammasome Induced by TNF-α in HaCaT Keratinocytes
3.7. Effect of DHA and DHA-RV-SLNs on the Basal and TNF-α-Induced IL-1β Production in Cultured Keratinocytes
3.8. Effect of DHA and DHA-RV-SLNs on the Basal and TNF-α-Induced IL-6 Production in Cultured Keratinocytes
3.9. Effect of DHA and DHA-RV-SLNs on the Basal and TNF-α-Induced MCP-1 Production in Cultured Keratinocytes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Tortosa-Caparrós, E.; Navas-Carrillo, D.; Marín, F.; Orenes-Piñero, E. Anti-inflammatory effects of omega 3 and omega 6 polyunsaturated fatty acids in cardiovascular disease and metabolic syndrome. Crit. Rev. Food Sci. Nutr. 2017, 57, 3421–3429. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Ottes Vasconcelos, R.; Fasano, E.; Calviello, G. Epigenetic regulation of gene expression and M2 macrophage polarization as new potential omega-3 polyunsaturated fatty acid targets in colon inflammation and cancer. Expert Opin. Ther. Targets 2016, 20, 843–858. [Google Scholar] [CrossRef]
- Devassy, J.G.; Leng, S.; Gabbs, M.; Monirujjaman, M.; Aukema, H.M. Omega-3 polyunsaturated fatty acids and oxylipins in neuroinflammation and management of Alzheimer disease. Adv. Nutr. 2016, 7, 905–916. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Bizzarro, A.; Piccioni, E.; Fasano, E.; Rossi, C.; Lauria, A.; Cittadini, A.R.; Masullo, C.; Calviello, G. EPA and DHA differentially affect in vitro inflammatory cytokine release by peripheral blood mononuclear cells from Alzheimer’s patients. Curr. Alzheimer Res. 2012, 9, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Dalli, J. The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin. Immunol. 2015, 27, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.C.; Carter, P.J. Therapeutic antibodies for autoimmunity and inflammation. Nat. Rev. Immunol. 2010, 10, 301–316. [Google Scholar] [CrossRef]
- Barnes, P.J. Pathophysiology of allergic inflammation. Immunol. Rev. 2011, 242, 31–50. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef]
- Han, H.; Roan, F.; Ziegler, S.F. The atopic march: Current insights into skin barrier dysfunction and epithelial cell-derived cytokines. Immunol. Rev. 2017, 278, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.E.; Leung, D.Y.M. Significance of skin barrier dysfunction in atopic dermatitis. Allergy Asthma Immunol. Res. 2018, 10, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Millsop, J.W.; Bhatia, B.K.; Debbaneh, M.; Koo, J.; Liao, W. Diet and psoriasis, part III: Role of nutritional supplements. J. Am. Acad. Dermatol. 2014, 71, 561–569. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.H.; Wang, P.W.; Yang, S.C.; Chou, W.L.; Fang, J.Y. Cosmetic and therapeutic applications of fish oil’s fatty acids on the skin. Mar. Drugs 2018, 16, 256. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Cassano, R.; Trombino, S.; Calviello, G. Nanomedicine-based formulations containing ω-3 polyunsaturated fatty acids: Potential application in cardiovascular and neoplastic diseases. Int. J. Nanomed. 2019, 14, 2809–2828. [Google Scholar] [CrossRef] [PubMed]
- Serini, S.; Cassano, R.; Corsetto, P.A.; Rizzo, A.M.; Calviello, G.; Trombino, S. Omega-3 PUFA loaded in resveratrol-based solid lipid nanoparticles: Physicochemical properties and antineoplastic activities in human colorectal cancer cells in vitro. Int. J. Mol. Sci. 2018, 19, 586. [Google Scholar] [CrossRef] [PubMed]
- Müller, R.H.; Lucks, J.S. Arzneistoffträger aus festen Lipidteilchen–Feste Lipid Nanosphären (SLN). European Patent 1996. [Google Scholar]
- Gasco, M.R. Method For Producing Solid Lipid Microspheres Having A Narrow Size Distribution. U.S. Patent 5250236, 5 October 1993. [Google Scholar]
- Serini, S.; Donato, V.; Piccioni, E.; Trombino, S.; Monego, G.; Toesca, A.; Innocenti, I.; Missori, M.; De Spirito, M.; Celleno, L.; et al. Docosahexaenoic acid reverts resistance to UV-induced apoptosis in human keratinocytes: Involvement of COX-2 and HuR. J. Nutr. Biochem. 2011, 22, 874–885. [Google Scholar] [CrossRef]
- Serini, S.; Fasano, E.; Piccioni, E.; Monego, G.; Cittadini, A.R.; Celleno, L.; Ranelletti, F.O.; Calviello, G. DHA induces apoptosis and differentiation in human melanoma cells in vitro: Involvement of HuR-mediated COX-2 mRNA stabilization and β-catenin nuclear translocation. Carcinogenesis 2012, 33, 164–173. [Google Scholar] [CrossRef]
- Serini, S.; Zinzi, A.; Ottes Vasconcelos, R.; Fasano, E.; Riillo, M.G.; Celleno, L.; Trombino, S.; Cassano, R.; Calviello, G. Role of β-catenin signaling in the anti-invasive effect of the omega-3 fatty acid DHA in human melanoma cells. J. Dermatol. Sci. 2016, 84, 149–159. [Google Scholar] [CrossRef]
- Ottes Vasconcelos, R.; Serini, S.; de Souza Votto, A.P.; Santos Trindade, G.; Fanali, C.; Sgambato, A.; Calviello, G. Combination of ω-3 fatty acids and cisplatin as a potential alternative strategy for personalized therapy of metastatic melanoma: An in-vitro study. Melanoma Res. 2019, 29, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Wang, K. Chronic inflammation in skin malignancies. J. Mol. Signal. 2016, 11, 2. [Google Scholar] [PubMed]
- Tan, C.H.; Rasool, S.; Johnston, G.A. Contact dermatitis: Allergic and irritant. Clin. Dermatol. 2014, 32, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Moll, R.; Divo, M.; Langbein, L. The human keratins: Biology and pathology. Histochem. Cell Biol. 2008, 129, 705–733. [Google Scholar] [CrossRef] [PubMed]
- Abais, J.M.; Xia, M.; Zhang, Y.; Boini, K.M.; Li, P.L. Redox regulation of NLRP3 inflammasomes: ROS as trigger or effector? Antioxid. Redox Signal. 2015, 22, 1111–1129. [Google Scholar] [CrossRef] [PubMed]
- Cassano, R.; Mellace, S.; Marrelli, M.; Conforti, F.; Trombino, S. α-Tocopheryl linolenate solid lipid nanoparticles for the encapsulation, protection, and release of the omega-3 polyunsaturated fatty acid: In vitro anti-melanoma activity evaluation. Colloids Surf. B Biointerfaces 2017, 151, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Trombino, S.; Mellace, S.; Cassano, R. Solid lipid nanoparticles for antifungal drugs delivery for topical applications. Ther. Deliv. 2016, 7, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Cassano, R.; Ferrarelli, T.; Mauro, M.V.; Cavalcanti, P.; Picci, N.; Trombino, S. Preparation, characterization and in vitro activities evaluation of solid lipid nanoparticles based on PEG-40 stearate for antifungal drugs vaginal delivery. Drug Deliv. 2016, 23, 1047–1056. [Google Scholar] [CrossRef]
- Trombino, S.; Cassano, R.; Ferrarelli, T.; Barone, E.; Picci, N.; Mancuso, C. Trans-ferulic acid-based solid lipid nanoparticles and their antioxidant effect in rat brain microsomes. Colloids Surf. B Biointerfaces 2013, 109, 273–279. [Google Scholar] [CrossRef]
- Trombino, S.; Cassano, R.; Muzzalupo, R.; Pingitore, A.; Cione, E.; Picci, N. Stearyl ferulate-based solid lipid nanoparticles for the encapsulation and stabilization of beta-carotene and alpha-tocopherol. Colloids Surf. B Biointerfaces 2009, 72, 181–187. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue exclusion test of cell viability. Curr. Protoc. Immunol. 2015, 111, 1–3. [Google Scholar] [PubMed]
- Ananthapadmanabhan, K.P.; Moore, D.J.; Subramanyan, K.; Misra, M.; Meyer, F. Cleansing without compromise: The impact of cleansers on the skin barrier and the technology of mild cleansing. Dermatol. Ther. 2004, 17, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Imokawa, G.; Akasaki, S.; Minematsu, Y.; Kawai, M. Importance of intercellular lipids in water-retention properties of the stratum corneum: Induction and recovery study of surfactant dry skin. Arch. Dermatol. Res. 1989, 281, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Flach, C.R.; Mendelsohn, R.; Walters, R.M. Imaging the distribution of sodium dodecyl sulfate in skin by confocal Raman and infrared microspectroscopy. Pharm. Res. 2012, 29, 2189–2201. [Google Scholar] [CrossRef] [PubMed]
- De Jongh, C.M.; Verberk, M.M.; Spiekstra, S.W.; Gibbs, S.; Kezic, S. Cytokines at different stratum corneum levels in normal and sodium lauryl sulphate-irritated skin. Skin Res. Technol. 2007, 13, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Saad, P.; Flach, C.R.; Walters, R.M.; Mendelsohn, R. Infrared spectroscopic studies of sodium dodecyl sulphate permeation and interaction with stratum corneum lipids in skin. Int. J. Cosmet. Sci. 2012, 34, 36–43. [Google Scholar] [CrossRef]
- Abbas, S.; Goldberg, J.W.; Massaro, M. Personal cleanser technology and clinical performance. Dermatol. Ther. 2004, 17, 35–42. [Google Scholar] [CrossRef]
- Kim, C.W.; Kim, C.D.; Choi, K.C. Establishment and evaluation of immortalized human epidermal keratinocytes for an alternative skin irritation test. J. Pharmacol. Toxicol. Methods 2017, 88, 130–139. [Google Scholar] [CrossRef]
- Corsini, E.; Viviani, B.; Zancanella, O.; Lucchi, L.; Visioli, F.; Serrero, G.; Bartesaghi, S.; Galli, C.L.; Marinovich, M. Induction of adipose differentiation related protein and neutral lipid droplet accumulation in keratinocytes by skin irritants. J. Investig. Dermatol. 2003, 121, 337–344. [Google Scholar] [CrossRef]
- Burlando, B.; Parodi, A.; Volante, A.; Bassi, A.M. Comparison of the irritation potentials of Boswellia serrata gum resin and of acetyl-11-keto-beta-boswellic acid by in vitro cytotoxicity tests on human skin-derived cell lines. Toxicol. Lett. 2008, 177, 144–149. [Google Scholar] [CrossRef]
- Sanchez, L.; Mitjans, M.; Infante, M.R.; Vinardell, M.P. Determination of interleukin-1alpha in human NCTC 2544 keratinocyte cells as a predictor of skin irritation from lysine-based surfactants. Toxicol. Lett. 2006, 167, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Parodi, A.; Sanguineti, R.; Catalano, M.; Penco, S.; Pronzato, M.A.; Scanarotti, C.; Bassi, A.M. A comparative study of leukaemia inhibitory factor and interleukin-1alpha intracellular content in a human keratinocyte cell line after exposure to cosmetic fragrances and sodium dodecyl sulphate. Toxicol. Lett. 2010, 192, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Maibach, H.I. The sodium lauryl sulfate model: An overview. Contact Dermat. 1995, 33, 1–7. [Google Scholar] [CrossRef]
- Lawrence, J.N.; Dally, J.J.; Benford, D.J. Measurement of eicosanoid release in keratinocyte cultures to investigate skin irritation and tumour promoting activity. Toxicol. In Vitro 1995, 9, 285–290. [Google Scholar] [CrossRef]
- Zhang, Y.; Newton, B.; Lewis, E.; Fu, P.P.; Kafoury, R.; Ray, P.C.; Yu, H. Cytotoxicity of organic surface coating agents used for nanoparticles synthesis and stability. Toxicol. In Vitro 2015, 29, 762–768. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Rhodes, L.E.; Al-Aasswad, N.M.; Massey, K.A.; Nicolaou, A. Impact of EPA ingestion on COX- and LOX-mediated eicosanoid synthesis in skin with and without a pro-inflammatory UVR challenge--report of a randomized controlled study in humans. Mol. Nutr. Food Res. 2014, 58, 580–590. [Google Scholar] [CrossRef]
- Barcelos, R.C.; de Mello-Sampayo, C.; Antoniazzi, C.T.; Segat, H.J.; Silva, H.; Veit, J.C.; Piccolo, J.; Emanuelli, T.; Bürger, M.E.; Silva-Lima, B.; et al. Oral supplementation with fish oil reduces dryness and pruritus in the acetone-induced dry skin rat model. J. Dermatol. Sci. 2015, 79, 298–304. [Google Scholar] [CrossRef]
- Seweryn, A. Interactions between surfactants and the skin-Theory and practice. Adv. Colloid Interface Sci. 2018, 256, 242–255. [Google Scholar] [CrossRef]
- Nicolaou, A. Eicosanoids in skin inflammation. Prostaglandins Leukot. Essent. Fat. Acids 2013, 88, 131–138. [Google Scholar] [CrossRef]
- Bou-Dargham, M.J.; Khamis, Z.I.; Cognetta, A.B.; Sang, Q.A. The role of interleukin-1 in inflammatory and malignant human skin diseases and the rationale for targeting interleukin-1 alpha. Med. Res. Rev. 2017, 37, 180–216. [Google Scholar] [CrossRef]
- Pauloin, T.; Dutot, M.; Liang, H.; Chavinier, E.; Warnet, J.M.; Rat, P. Corneal protection with high-molecular-weight hyaluronan against in vitro and in vivo sodium lauryl sulfate-induced toxic effects. Cornea 2009, 28, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Szél, E.; Polyánka, H.; Szabó, K.; Hartmann, P.; Degovics, D.; Balázs, B.; Németh, I.B.; Korponyai, C.; Csányi, E.; Kaszaki, J.; et al. Anti-irritant and anti-inflammatory effects of glycerol and xylitol in sodium lauryl sulphate-induced acute irritation. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 2333–2341. [Google Scholar] [CrossRef] [PubMed]
- Özyurt, K.; Ertaş, R.; Atasoy, M. Biologics for psoriasis: What is new? Dermatol. Ther. 2019, e12916. [Google Scholar] [CrossRef] [PubMed]
- Toshitani, A.; Ansel, J.C.; Chan, S.C.; Li, S.-H.; Hanifin, J.M. Increased interleukin 6 production by T cells derived from patients with atopic dermatitis. J. Investig. Dermatol. 1993, 100, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ramot, Y.; Torrelo, A.; Paller, A.S.; Si, N.; Babay, S.; Kim, P.W.; Sheikh, A.; Lee, C.C.; Chen, Y.; et al. Mutations in proteasome subunit β type 8 cause chronic atypical neutrophilic dermatosis with lipodystrophy and elevated temperature with evidence of genetic and phenotypic heterogeneity. Arthritis Rheum. 2012, 64, 895–907. [Google Scholar] [CrossRef]
- Svobodová, A.; Zdarilová, A.; Vostálová, J. Lonicera caerulea and Vaccinium myrtillus fruit polyphenols protect HaCaT keratinocytes against UVB-induced phototoxic stress and DNA damage. J. Dermatol. Sci. 2009, 56, 196–204. [Google Scholar] [CrossRef]
- Fasano, E.; Serini, S.; Mondella, N.; Trombino, S.; Celleno, L.; Lanza, P.; Cittadini, A.; Calviello, G. Antioxidant and anti-inflammatory effects of selected natural compounds contained in a dietary supplement on two human immortalized keratinocyte lines. Biomed. Res. Int. 2014, 2014, 327452. [Google Scholar] [CrossRef]
- Schempp, C.M.; Meinke, M.C.; Lademann, J.; Ferrari, Y.; Brecht, T.; Gehring, W. Topical antioxidants protect the skin from chemical-induced irritation in the repetitive washing test: A placebo-controlled, double-blind study. Contact Dermat. 2012, 67, 234–237. [Google Scholar] [CrossRef]
- Serini, S.; Fasano, E.; Piccioni, E.; Cittadini, A.R.; Calviello, G. Dietary n-3 polyunsaturated fatty acids and the paradox of their health benefits and potential harmful effects. Chem. Res. Toxicol. 2011, 24, 2093–2105. [Google Scholar] [CrossRef]
- Diaz-Gerevini, G.T.; Repossi, G.; Dain, A.; Tarres, M.C.; Das, U.N.; Eynard, A.R. Beneficial action of resveratrol: How and why? Nutrition 2016, 32, 174–178. [Google Scholar] [CrossRef]
- Tschopp, J.; Schroder, K. NLRP3 inflammasome activation: The convergence of multiple signalling pathways on ROS production? Nat. Rev. Immunol. 2010, 10, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Kitessa, S.M.; Abeywardena, M.; Wijesundera, C.; Nichols, P.D. DHA containing oilseed: A timely solution for the sustainability issues surrounding fish oil sources of the health-benefitting long-chain omega-3 oils. Nutrients 2014, 6, 2035–2058. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serini, S.; Cassano, R.; Facchinetti, E.; Amendola, G.; Trombino, S.; Calviello, G. Anti-Irritant and Anti-Inflammatory Effects of DHA Encapsulated in Resveratrol-Based Solid Lipid Nanoparticles in Human Keratinocytes. Nutrients 2019, 11, 1400. https://doi.org/10.3390/nu11061400
Serini S, Cassano R, Facchinetti E, Amendola G, Trombino S, Calviello G. Anti-Irritant and Anti-Inflammatory Effects of DHA Encapsulated in Resveratrol-Based Solid Lipid Nanoparticles in Human Keratinocytes. Nutrients. 2019; 11(6):1400. https://doi.org/10.3390/nu11061400
Chicago/Turabian StyleSerini, Simona, Roberta Cassano, Enrica Facchinetti, Gaia Amendola, Sonia Trombino, and Gabriella Calviello. 2019. "Anti-Irritant and Anti-Inflammatory Effects of DHA Encapsulated in Resveratrol-Based Solid Lipid Nanoparticles in Human Keratinocytes" Nutrients 11, no. 6: 1400. https://doi.org/10.3390/nu11061400
APA StyleSerini, S., Cassano, R., Facchinetti, E., Amendola, G., Trombino, S., & Calviello, G. (2019). Anti-Irritant and Anti-Inflammatory Effects of DHA Encapsulated in Resveratrol-Based Solid Lipid Nanoparticles in Human Keratinocytes. Nutrients, 11(6), 1400. https://doi.org/10.3390/nu11061400








