Abstract
Dramatic changes in the environment and human lifestyle have been associated with the rise of various chronic complex diseases, such as inflammatory bowel disease (IBD). A dysbiotic gut microbiota has been proposed as a crucial pathogenic element, contributing to immune imbalances and fostering a proinflammatory milieu, which may be associated with disease relapses or even the initiation of IBD. In addition to representing important regulators of the mucosal immunity and the composition of the gut microbiota, food components have been shown to be potential environmental triggers of epigenetic modifications. In the context of chronic intestinal inflammation, dietary habits and specific food components have been implicated as important modulators of epigenetic mechanisms, including DNA methylation, which may predispose a person to the increased risk of the initiation and evolution of IBD. This review provides novel insights about how dietary factors may interact with the intestinal mucosa and modulate immune homeostasis by shaping the intestinal ecosystem, as well as the potential influence of diet in the etiopathogenesis and management of IBD.
1. Introduction
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), is regarded as a heterogeneous disease of unknown etiology, with an unpredictable clinical course and usually labeled as a chronic disabling disorder [1,2]. Although the mechanisms leading to the development of IBD are rather complex and have not yet been completely elucidated, the current information points to a multifactorial origin. The chronic inflammatory process underlying the pathogenesis of IBD reflects a dysregulated immune response, stemming from a conflicting interaction involving several environmental factors, an altered innate immunity, and a predisposing genetic background [3,4,5,6].
Information derived from epidemiological studies has revealed consistent associations between the increase in the occurrence in IBD and other autoimmune and chronic inflammatory disorders, and the decline in infectious diseases, accompanied by the use of antibiotics, immunizations, and overall improvements in sanitary conditions and the quality of water [7]. IBD has progressively become a global disease, affecting newly industrialized nations in Asia, South America, and the Middle East [8,9,10]. Although the prevalence of IBD in newly industrialized countries is still lower than in developed countries, the incidence rate is increasing rapidly [11]. Among all the environmental changes usually associated with IBD, dietary habits are one element, both from the patients’ perspective, including personal observations and common practical concerns, and due to scientific evidence implicating diet as a triggering factor in disease relapses [12]. In this article, we discuss the effects of diet on IBD, especially considering the basic physiological processes that underlie these associations.
2. Diet and Microbiota
Although the influence of dietary habits on the intestinal microbiota has long been speculated, only after the introduction of new technology, including next-generation DNA sequencing and metabolic profiling, was reliable information able to be obtained [13]. For example, the microbiota composition is currently thought of as a dynamic process that changes with age and fluctuates in consonance with environmental exposures, including diet, among other factors [14].
The results of studies analyzing the microbial communities of the gut obtained from mammalian species have endorsed the assumption that diet can influence microbial diversity. Such diversity has been shown to increase from carnivores to omnivores and then to herbivores [15]. Regarding humans, the intestinal microbiota reflects predominant omnivorous habits, but substantial inconsistencies have been reported. For example, opposing patterns of the intestinal microbiota composition were reported when comparing samples from African children living in rural communities with children from a European urban center, a phenomenon that has been attributed to dietary differences [16]. In this case, in terms of health issues, individuals from the European urban center are expected to be at increased risk for the development of several immune-mediated disorders. Such diseases, likely including IBD, are probably associated with the microbiome structure, often resulting from dietary incompatibilities between the current environments and those in which humans evolved.
The Western style of diet, defined by high caloric content due to large amounts of fat and carbohydrates, has been associated with a markedly reduced microbiome diversity [17]. In addition to the plethora of fat, Western diets usually have high concentrations of dietary omega-6 fatty acids, from vegetable oils, resulting in a high omega-6 to omega-3 ratio. Whereas omega-3 fatty acids, such as a-linolenic acid from vegetables and eicosapentaenoic acid and docosahexaenoic acid from fish, have anti-inflammatory properties, omega-6 fatty acids, particularly arachidonic acid, are considered pro-inflammatory. This may explain why the Western diet has been regarded as a key factor for producing intestinal inflammation [18]. Other relevant studies focusing on diet potentially affecting the intestinal microbiota are summarized in Table 1.

Table 1.
Relationship between gut microbiota alterations and dietary patterns.
Previous studies have analyzed the gut microbiota of infants and children within one population, but few have attempted to compare the microbial composition considering distinct socio-economic, geographic, and cultural aspects [16]. To analyze the potential effect of environmental factors on defining how and from where a gut microbiota may be acquired, investigators characterized bacterial species and gene contents of fecal samples from a cohort of healthy Amerindians from Venezuela, rural Malawian communities, and metropolitan USA inhabitants. The investigators found that bacterial diversity increases with age, and the degree of similarity among members of a family is consistent in all three populations. The fecal microbiota of USA adults was the least diverse compared to the others [19]. Therefore, it appears that differences in social structures and cultural traditions known to impact food and many other environmental factors may also condition the magnitude of the circulation of microbes among members of a family or community.
3. Diet and Immune Response
Western lifestyle factors have been associated with the progressive increase in various metabolic, autoimmune, and chronic inflammatory disorders, including IBD [32,33,34]. In addition to industrialization, urbanization, the improvement of sanitary conditions, antibiotic usage, and other factors, abundant evidence supports the involvement of dietary habits in shaping the gut microbiota and influencing the interaction with the immune system [35,36,37].
Dietary fiber has been shown to interfere in host immunity via multiple pathways. Fibers and starches present in fruits and vegetables represent a substrate for the production of butyrate and other short chain fatty acids (SCFAs) by intestinal microbiota. Butyrate, for example, is well-recognized as a critical element in epithelial homeostasis, but also plays an important role in downregulating the immune response by inhibiting the transcription of inflammatory cytokines and promoting the differentiation of lamina propria Tregs [5,38,39]. Low dietary fiber may precipitate the catabolism of the mucous layer, leading to increased permeability and compromising the epithelial barrier against potential pathogenic luminal bacteria [40].
Investigations based on experimental models have provided results regarding the effects of specific diets on the immune response. For instance, a high-fat (HF) and high-sugar diet generated a proinflammatory intestinal environment, associated with an overgrowth of Proteobacteria, reduction in butyrate production, and expression of the butyrate GPR43 receptor, also underexpressed in CD patients [41]. Results from another experimental model showed that HF diets led to the upregulation of tumor necrosis factor-α (TNF-α) and interferon-γ expression, and to the decrease in the densities of colonic Tregs [42]. A high intake of fat has been shown to have several effects on the mucosal immune system, revealing the complex interaction between the lamina propria immune cells and the intestinal microbiota. For example, the ingestion of high-fat meals increases the expression of interlukein-1β (IL-1β), TNF-α, IL-6, and necrosis factor-κB (NF-κB) in the colon [43,44]. Likewise, mice fed with cholesterol-enriched meals displayed acute inflammasome-dependent intestinal inflammation, increasing the production of IL-1β, and driving the accumulation of CD11b+ and CD11c+ cells in the lamina propria [45].
The increasing knowledge about the influence of the gut microbiome on the mucosal and systemic immune response has contributed to the understanding of the mechanisms underlying the pathogenesis of IBD and other chronic inflammatory conditions. Dysbiosis has been confirmed in samples from patients with CD, displaying an increase in Enterobacteriales such as Escherichia coli, and a decrease in Clostridiales such as Faecalibacterium prausnitzii [46,47,48]. In a study involving UC, the results supported a role for reduced microbial diversity, although not as prominent as the dysbiosis seen in CD [49]. A large cohort study analyzing fecal samples from four different European countries revealed that CD and UC are distinct in terms of the microbiome [50]. Notably, some specific intestinal microbes can shape the immune response. For example, Listeria monocytogenes, Clostridium difficile, and Toxoplasma gondii have been shown to elicit innate lymphoid cells-1 (ILC1) and Th1 cells, which orchestrate the secretion of cytokines, such as interferon-gamma and TNF-α, critically important in the immunity against those intestinal pathogens [51]. Clostridia strains have been shown to promote the accumulation of Foxp3 Treg cells in the gut [52], which in turn down-regulate inflammatory responses, including those in experimental colitis [53]. Some microorganisms induce the activation of subsets of immune cells responsible for promoting intestinal inflammation, such as Th17 cells [54]. For instance, segmented filamentous bacteria can activate Th17 cells, whereas a decrease in Th17 cell-inducing bacteria can attenuate the severity of colitis in mice [55]. In previous studies, adherent invasive E. coli (AIEC) and Bifidobacteria adolescentis [56,57], in addition to E. coli O157 and Citrobacter rodentium [54], have been shown to induce populations of Th17 cells.
Dietary habits can affect human health either directly or via changes in the gut microbiota. The gut microbiota is a highly complex and dynamic system that can be shaped by environmental factors, including the diet [58]. Regarding the dietary factors directly or indirectly associated with the immune response and intestinal homeostasis, diets with high concentrations of fat and sugar have been associated with intestinal colonization with potential IBD-related pathobionts. For instance, adherent invasive E. coli (AIEC) strains, induced by high-fat high-sugar diets, may become pathogenic when the ligand CEACAM 6 is presented by the intestinal epithelium. Under such circumstances, AIEC may form biofilms, adhere to, and translocate via M cells and the associated epithelium [59]. These findings reinforce the idea that changes in the local environment exert a selective pressure on several species, which in turn shape the microbiota according to the most adaptable microorganisms to the intestinal milieu [60]. A selection of dietary factors that may affect the host immunity is listed in Table 2.

Table 2.
Dietary patterns potentially affecting microbiota and immunity.
4. Diet and IBD
Advances resulting from clinical and experimental investigations have demonstrated that the microbiome might play a critical role in the pathogenesis of IBD [65,66]. In parallel, results from other studies have supported a link between dietary patterns and the structure of the intestinal microbiome in healthy individuals and in patients with gastrointestinal disorders, including IBD [17,27,67]. Given the potential effects that diet can have on the composition and function of the intestinal microbiome, diet may also be involved in the pathogenesis of IBD, either directly or indirectly. As a consequence, due to the associations with the microbiome and IBD, specific dietary interventions are promising novel therapies.
Data have confirmed that food plays a crucial role in the gut microenvironment, and modifications of dietary habits may have an important impact on the microbial composition and function, but also on the gut barrier and immunity [5]. Previous studies demonstrated that modifications in a single food group might produce various outcomes. For instance, changing the dietary composition from a predominant plant- to animal-based diet results in a change in the intestinal microbial environment and functionality, determining alterations in bile acids and sulfide metabolism, which have been implicated in IBD pathogenesis [27,68]. In an experimental study, investigators demonstrated that a low-fiber diet induces the expansion and activity of colonic mucous-degrading microorganisms, potentially favoring the development of severe colitis by enteric pathogens [40]. In another experimental study, mice fed a Western-style diet (rich in saturated fats and simple carbohydrates, but depleted in dietary fiber), developed an alteration in the colonic microbiota, resulting in increased permeability and a reduced inner mucus layer. Both defects could be prevented by transplanting microbiota from chow-fed mice, whereas the administration of Bifidobacterium longum was sufficient to restore the mucus layer [69]. These findings suggest that specific bacteria are crucial for normal mucus and barrier function and, in humans, this information could help us to understand important mechanisms underlying IBD pathogenesis.
Unveiling the association between diet and the development of IBD would ideally require knowledge on dietary details before the onset of the disease. In this regard, large longitudinal studies have been performed that have started to provide information to support specific dietary patterns as risk factors for IBD [17]. For instance, the metabolic activity of the microbiota may present important abnormalities in IBD. A reduced production of SCFAs, which are promoted by the presence of dietary fiber, has been shown in UC and CD. Particularly in patients with UC, a deficient production of SCFAs has been shown, and could not be corrected by increasing the intake of wheat bran-associated fiber and high amylose-associated-resistant starch [5,70].
Among the dietary factors potentially implicated in the development of IBD, dietary fiber has received special attention due to evidence showing associated protective mechanisms, including the conversion to SCFAs [71,72], interference in the structure of the gut microbiota [16], and maintenance of the barrier function [73]. However, a large prospective cohort study evaluating the impact of the intake of dietary fiber from several sources failed to identify any protective effect against the development of IBD [74].
Regarding the presumably harmful Western diet, in addition to its general composition characterized by high animal or dairy fat and animal protein, it is important to draw attention to the ubiquitous presence of emulsifiers, thickeners, and artificial sweeteners, many of which are associated with abnormal intestinal permeability, dysbiosis, and intestinal inflammation in animal models [75,76]. As an integral part of this type of diet, the common use of processed foods, usually rich in phosphate [77], but poor in micronutrients such zinc and other nutrients including omega-3 fatty acids, and vitamins D and E [78,79,80], may also be involved in the development or the predisposition to chronic intestinal inflammation.
5. Environmental Factors and IBD
The rise of IBD in the developing world has usually been attributed to the industrialization and urbanization of societies [11,81]. The observation of the coincident increase in immune-mediated and inflammatory diseases in the more developed and industrialized nations has long been associated with the hygiene hypothesis [82]. This hypothesis appears to be relatively reductionist, as it does not explain the dynamics of IBD evolution in long-modernized Asian countries, for example. Whereas improvements in sanitation and hygiene practices are usually related to the urban compared to the rural environment, several other concomitant factors affecting the rise of immune-mediated disorders need to be pondered. For example, areas with a higher prevalence of IBD also have fewer infections and are characterized by the widespread use of vaccines and antibiotics, in addition to the availability of clean food and water [83,84,85]. Other considerations associated with modern urban life in developed and developing nations include, for instance, population density, lifestyle modifications, level of education, and exposure to pollutants [86]. A positive association between ambient air pollution and IBD has been previously suggested [81], and the residential exposures to nitrogen dioxide and sulfur dioxide were shown to increase the risk of early-onset IBD [87].
The increased risk for developing IBD has also been associated with the exposure to different medications. Among them, the use of antibiotics has been shown to increase the risk of IBD, especially CD [88,89]. In a nested case-control analysis of the population-based database of prescription drugs, investigators found that patients diagnosed with IBD in childhood were more likely to have used antibiotics during the first year of life [90]. This positive association was further corroborated, for example, in a prospective study in which the use of antibiotics was strongly associated with the development of CD in childhood [91]. Although causality cannot be firmly established to date, exposure to antibiotics in childhood has been proposed to affect the normal development of tolerance to enteric bacteria, increasing the risk of IBD [92].
Other medications have been linked to IBD. For example, oral contraceptives have been shown to have a positive association with the development of IBD, in particular with CD [93]. In a case-control study, investigators found a positive association between nonsteroidal anti-inflammatory drugs (NSAIDs) and IBD [94]. In a cohort study, investigators found that the regular use of NSAIDs may increase CD activity [95]. However, a systematic review did not find a reliable association between the use of NSAIDs and risk of CD and UC exacerbation [96]. NSAIDs are capable of disrupting the intestinal epithelial barrier, and this may predispose the individual to the invasion of pathogens and may affect the intestinal homeostasis. The conflicting results regarding the association between NSAIDs and IBD may reflect different methodologies but also the participation of other uncontrolled environmental factors generating unanticipated and confounding effects.
Smoking is a well-established modifying factor in IBD, with opposing effects in CD and UC [97], but the mechanism by which it regulates intestinal inflammation is still unclear. The composition of cigarettes is heterogeneous and complex with several chemical elements that may affect different targets in the intestinal cells and modulate the microbiota [98,99]. Other evidence suggests that chemicals released from cigarette smoking can interfere with functions related to the immune response, such as the production of cytokines [100], and significantly impact the microbiota [101].
The functionality of vitamin D, an important regulator of mucosal immunity, is critically related to the exposure to sun with natural UV light [102]. Therefore, low sunlight exposure has been proposed to be a risk factor for the development of IBD, especially CD [103,104]. This is in line with the incidence and prevalence rates of IBD being greater in the Northern Hemisphere, where UV light is markedly lower [105]. However, the proposed association between IBD, particularly CD, and the northern latitudes should be interpreted not only in terms of geography, but in a wider context, where several other variables interact in a complex and dynamic concert, with largely unpredictable outcomes.
6. Epidemiological Associations between Diet and IBD
Although a consistent correlation between environmental factors and the pathogenesis of several disorders is usually difficult to prove due to the intervening effect of potentially confounding variables and the uncertain dynamics of exposures, associations between IBD and diet have been reported. The realization that the increase in the worldwide incidence of IBD and other immune-mediated diseases began in more developed and industrialized countries, particularly in North America and Northern Europe, has led investigators to hypothesize the global spread of a “Westernized” lifestyle [106,107,108].
The observation that the risk of developing IBD increases when people move from a low-risk to high-risk area appears to further support the role of environmental factors in the detriment of genetic predisposition, and reinforces the utility of studies examining immigration. For example, distinct investigations showed that immigrants to Israel had a higher prevalence of IBD than populations born in Israel [107,109]. Another population-based study demonstrated that in Sweden, a high incidence region, second-generation but not first-generation immigrants developed IBD at rates comparable to the native Swedish population. These results appear to underscore the importance of early-life exposures in determining disease initiation [110].
Epidemiological studies investigating the South Asian population indicate that the incidence and prevalence of adult IBD are significantly lower than in North America or the U.K. [111,112,113,114]. However, higher incidences of IBD in patients from South Asia have been reported in adult individuals who subsequently migrated to the U.K. [115], and in children migrating to British Columbia, Canada, at rates higher than in other ethnicities, including children of Western European descent [116]. In another similar study analyzing a migrant group to investigate the role of environmental factors in the development of IBD, Middle Eastern migrants to Australia had a significantly higher risk compared with the Caucasian population. The results, including the analysis of additional exposures such as smoking, antibiotic usage, and hygiene markers, suggested that migrants might be more sensitive to environmental challenges influencing the gut microbiome [117]. This thought is further supported by the results of a population-based study in the Asia-Pacific area in which the investigators suggested that early childhood factors and markers of altered intestinal microbiota may modulate the risk of IBD later in life [118].
Another study analyzing an Asian population combined a series of Chinese patients with IBD with the sequences from RISK and PRISM cohorts of IBD patients from the United States for a meta-analysis comparing gut microbiome profiles across ethnic groups. The investigators found that the gut dysbiosis observed in IBD is similar among Chinese and Western populations [119]. This may lead to the question of whether the gut microbiota provides universal biomarkers in IBD regardless of ethnicity. Results from another study suggest the existence of diverse gut microbial taxa with differential patterns of abundance common to various immune-mediated inflammatory diseases (IMIDs), including IBD [120]. These findings appear to corroborate the possible use of microbial taxa patterns as biomarkers for the detection and diagnosis of IBD, but also support the idea that the gut microbiota may constitute a common component of the etiology of IMIDs.
Several observational studies have attempted to characterize dietary patterns that could contribute to a higher risk of IBD development. These studies pointed to an increased risk of IBD among people consuming larger proportions of meat and fats, and a lower risk among people with diets rich in fiber, fruits, and vegetables [121,122]. However, as in other complex diseases, it may be difficult to interpret the role of individual risk factors, and an analysis of dietary patterns should consider exposures to groups of foods [5], distinct food components, and cultural habits, including specificities in food preparation.
7. Diet and Epigenetic Modifications in IBD
The epigenome, an intersection between the environment and the genome, has been implicated in the regulation of gene expression and cellular functions, and plays an essential role in the delineation of phenotypes and their preservation [21]. Novel information has contributed to an improved understanding of the role of epigenetic modifications in defining the molecular basis of IBD [123,124]. Regarding IBD, the first epigenetic modifications associated with disease pathogenesis were based on DNA methylation studies [125].
Currently, among the environmental factors, dietary factors were found to be powerful stimuli, being associated with peculiar patterns of gene expression and epigenetic signatures [126]. For instance, the diet supplies substrates for DNA methylation and can regulate the enzymatic activity required in the one-carbon cycle. Consequently, elements such as folate, choline, and water-soluble B vitamins have been implicated in methylation patterns [123,127], which are essential elements for the synthesis and repair of DNA that modulate gene expression [128]. Experimental evidence obtained also supports the idea that dietary nutraceuticals possess potential as epigenetic modulators. For example, data from studies involving DNA methylation and chromatin repair indicate that polyphenols can regulate gene expression [129]. Polyphenols are secondary plant metabolites, common components of vegetables, fruits, green tea, and red wine, and have been recognized for their antioxidant properties. In addition, studies have shown beneficial effects of polyphenols through the modulation of NF-κB expression, and chromatin remodeling via the modulation of histone deacetylases and DNA methyltransferase activities, reversing altered gene expression [130]. In experimental IBD, resveratrol, a natural phenolic compound, was shown to decrease inflammatory cytokines and profibrotic factors, supporting a potential therapeutic benefit [131].
Curcumin, another natural product commonly used in cooking, has been investigated in experimental models of IBD, demonstrating beneficial effects mediated by the suppression of proinflammatory mediators [132]. In a randomized, multicenter, double-blind, placebo-controlled trial, curcumin has shown beneficial results in maintenance therapy for patients with UC [133]. Although the exact mechanisms by which curcumin may exert a wide range of biological actions have not been established, effects have been attributed to the regulation of histone acetylation/deacetylation and the expression of various microRNAs [134].
Several nutrients have been shown to modulate immune responses, potentially counteracting inflammatory processes [135], in actions also mediated through epigenetic regulation [123,136]. Processed foods, deficient in micronutrients including selenium and folate, have been implicated in the progression of diseases, such as colorectal cancer and possibly IBD [21,137,138,139]. In an experimental model, a selenium-deficient diet was shown to result in markedly hypomethylated colon DNA [140]. Selenium supplementation was able to prevent tissue damage by modulating the expression of key genes responsible for inflammation in experimental IBD [141]. Although low levels of selenium have been detected in the serum of patients with IBD [142], studies in human IBD involving potential effects of selenium supplementation are still limited [21].
To investigate the mechanisms underlying the potential beneficial effects of the Mediterranean diet, an intervention with two different diets, one rich in nuts and the other one in extra-virgin olive oil, was conducted. The evaluation of the methylation status of peripheral white blood cells genes showed that both diets influenced methylation, characteristically of genes such as CPT1B and GNAS involved in intermediate metabolism, inflammation, and the signal transduction process. Therefore, the investigators concluded that the expected beneficial effects of the Mediterranean diet, supplemented with nuts or extra-virgin olive oil, could be mediated, at least in part, through epigenetic modifications [16,143,144].
In addition to the direct effects of dietary constituents on epigenetic modifications in human intestinal tissues, the resident microbiota has been shown to alter host histone acetylation and methylation. In this regard, particularly short-chain fatty acids such as acetate, butyrate, and propionate, mostly produced by the microbial fermentation of fiber, appear to play a critical role in the epigenetic control of the inflammatory response. In a diet poor in fiber, the inhibition of SCFA production was correlated with disturbed chromatin effects, which were restored following supplementation with SCFA [145]. In human IBD, SCFA-producing bacteria (Roseburia) and butyrate-producing bacteria (Faecalibacterium) are reduced [146]. However, the application of butyrate-producing bacteria as nutraceuticals in humans has been questioned and needs further investigation [123,147]. Figure 1 summarizes the potential interactions between diet and the microbiota and other environmental factors, resulting in epigenetic modifications, exemplifying the effects of different diets with their respective major characteristics.
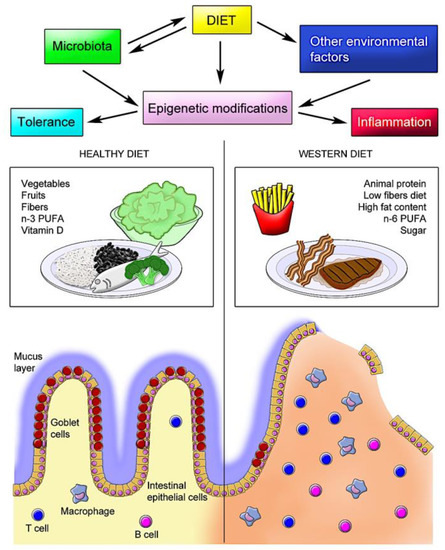
Figure 1.
Schematic model of diet–host interactions in the intestine. The interaction between dietary elements and the intestinal mucosa is highly complex and, in normal conditions, results in a tolerogenic response. However, in genetically predisposed individuals, the interplay of specific dietary constituents with the resident microbiota and other environmental factors induces epigenetic modifications that affect the immune response, further compromising the epithelial barrier and defense mechanisms, leading to chronic inflammation, as observed in inflammatory bowel disease (IBD).
8. Clinical Effects of Dietary Factors
Clinical experience shows that patients usually associate their symptoms or disease relapses with the ingestion of certain foods and, as a result, they often change their diet empirically, with variable and, at most, temporary effects. In practice, decisions on dietary modifications usually do not follow professional nutritional advice and may have detrimental consequences [17,148,149,150]. In a prospective study investigating the dietary influence in the course of UC, researchers observed that a large ingestion of meat, more so of red and processed meat, in addition to ethanol, protein, sulfur, and sulfate, enhanced the chances of triggering a flare [151,152]. In the case of patients with CD, a diet rich in total fat, saturated fat, monounsaturated fatty acids, and a higher ratio of omega-6:omega-3 polyunsaturated fatty acids (PUFAs) was related to disease relapses [153,154]. Of note, the oral administration of iron sulfate or heme in dietary iron, which is present in meat, was shown to increase the severity of chemically-induced colitis in rodents [155,156]. However, although a substantial number of iron-deficient patients tolerate oral iron poorly, only a small percentage of patients with IBD have disease relapses attributed to oral iron [157]. Anemia is a common complication of IBD, mostly multifactorial, but frequently related to iron deficiency due to loss from bleeding and decreased iron absorption or ingestion [158]. Although many physicians have concerns regarding the management of iron deficiency in IBD, current data suggest that oral iron therapy should be preferred in mild cases or during clinical remission, unless patients are intolerant or have an inadequate response [159]. The aggravation of experimental colitis following dietary iron has been associated with a marked reduction in the fecal abundance of Firmicutes and Bacteroidetes, and an increase in Proteobacteria [160]. Hence, poor outcomes following oral iron replacement in patients with IBD could be related to exacerbation of the intestinal dysbiosis.
9. Dietary Interventions in IBD
Simple dietary interventions usually improve gastrointestinal symptoms in patients with IBD, including a reduction in the ingestion of dairy products and also fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPS) [161]. More recent data have demonstrated the possible roles of specific dietary therapy in IBD. For example, dietary therapies, such as exclusive enteral nutrition (EEN), have been successfully used to induce remission in early or new-onset CD [162]. Theoretically, EEN involves the isolated use of medical formulas in a liquid form without exposure to other foods, usually for six to eight weeks [5]. This strategy is apparently more feasible in the pediatric population, in contrast to adults. Investigations have demonstrated that EEN may induce remission in more than 60% of children with CD followed by a significant reduction in inflammatory markers, including the erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and fecal calprotectin [5,163,164,165]. Grover et al. conducted a prospective open label study to assess mucosal healing before and after eight weeks of EEN, and concluded that the therapy is effective for inducing early clinical, biochemical, mucosal, and transmural remission, and was associated with improved outcomes at one year [166].
Given practical concerns and clinical limitations, other research groups have employed a different strategy based on the combination of partial enteral nutrition (PEN) with a specific exclusion diet granting access to whole foods. The diet was evaluated in a retrospective cohort of children and young adults with mild-to-moderate CD, and remission was achieved in 70% of patients. Clinical improvement was followed by the reduction in ESR and CRP and was associated with mucosal healing [167]. Afterward, this diet—low in animal fat, rich in complex carbohydrates, and with a moderate exposure to soluble fiber—was evaluated for the induction of remission in children and adults failing biologics. More than 60% of the patients achieved clinical remission, with a marked decrease in inflammatory markers [168].
Other important theoretical dietary elements, fiber, and prebiotics supplements have been evaluated as interventions in CD. However, a systematic review of randomized controlled trials found no evidence for the use of fiber or prebiotics to induce or maintain remission in CD [169]. No evidence for the restriction of fiber in the absence of obstructive symptoms or stricturing disease has been established for CD. In another study, a semi-vegetarian diet with considerable amounts of dietary fiber was shown to induce a high remission rate with no negative effects [170]. The same group investigated the effect of infliximab combined with a plant-based diet as first-line therapy for patients with CD naïve to biologics and found that the combined therapy can induce remission in most cases [171].
Although the most consistent studies regarding dietary interventions in IBD usually refer to CD, previous systematic evaluations performed by independent groups have supported the potential beneficial effects of diet in both CD and UC according to patients’ perceptions [149,172]. In an observational prospective cohort study, patients with UC in clinical remission were followed for one year to investigate the effect of diet on disease relapse. The investigators identified an increased risk of UC relapses associated with the consumption of meat (particularly red and processed meat), eggs, and alcoholic beverages, which have been attributed to the high dietary content of sulfur [151]. In addition to red meat and eggs, milk and dairy products contain high concentrations of cysteine, which can be used by sulfate-reducing bacteria (SRB) to produce hydrogen sulfide (H2S) [173,174]. H2S, in turn, has been shown to induce proinflammatory effects in the colon and intestinal epithelial cells [175,176,177]. Some studies have demonstrated the increased presence of SRB and H2S production in the colon of patients with UC [178,179,180]. However, despite all evidence suggesting a link between sulfur or sulfate-containing foods and UC, current data do not allow a causal association. While more controlled studies regarding SRB and H2S in UC are awaited, patients should be aware of the potential detrimental effects of high-sulfur-containing dietary products.
The nutritional theories of IBD pathogenesis involving carbohydrates, for example, have been based on the increasing evidence of the beneficial effects of the Specific Carbohydrate Diet in the treatment of both CD and UC, resulting in improvement in clinical parameters and inflammatory biomarkers [181,182,183]. The hypothesis that carbohydrate variation increases the predisposition to IBD has been supported by the evidence of resultant immune dysfunction, mucosal barrier defects, and dysbiosis, which are represented by the Westernized dietary features [1,184,185]. Carbohydrate monotony could be beneficial in IBD [186]. This has been corroborated by a successful report using the Paleo diet for a small cohort of patients with IBD [187]. Paleo is also an exclusion diet, supposedly representing habits of the Paleolithic era, consisting of a non-cereal, plant-based diet, including non-domesticated meats, and a low intake of carbohydrates, which are taken from a monotonous source. The rational for the use of carbohydrate monotony derives from the usually lower prevalence of IBD in rural or less developed communities consuming locally-produced seasonal products [16], in contrast to the modern Westernized diet, for which the digestive tract would theoretically not have had time to adapt [188].
In terms of dietary supplements, vitamin D appears to have a role in IBD. Vitamin D3 (animal sources and skin exposure to ultraviolet light) and vitamin D2 (plant sources) are first hydroxylated in the liver, and then in the kidney and extrarenal tissues, including intestinal macrophages, into the active form 1,25-dihydroxyvitamin D. The active vitamin D binds to its specific receptor in different tissues, including immune cells, modulating gene expression. Vitamin D deficiency has been frequently seen in patients with IBD; therefore, it has been regarded as a protective factor against disease development [189,190]. Particularly in patients with CD, vitamin D deficiency has been consistently associated with disease activity [79,191,192]. Vitamin D3 supplementation for patients with CD has been suggested to alleviate clinical manifestations [193]. In another clinical investigation, a randomized double-blind placebo-controlled study, dietary supplementation with 1200 IU vitamin D3 daily for 12 months significantly increased the vitamin D levels, but only modestly reduced the risk of disease relapse [194].
Currently, the exact mechanisms by which vitamin D may reduce the severity of CD are still unclear. In a study using peripheral blood mononuclear cells, investigators found that vitamin D was able to reduce the expression of pro-inflammatory M1 cytokines but did not induce the anti-inflammatory M2 phenotype [195]. In other experimental studies, vitamin D deficiency, or even its impaired signaling, were shown to worsen different models of experimental colitis through multiple effects, including epithelial barrier disruption [196,197], reduced mucosal immunity [198], and an association with gut dysbiosis [199,200].
10. Future Directions
Among the environmental changes associated with the progressive and global expansion of IBD, diet has emerged as an essential regulatory element of the gut microbiome, which in turn has been strongly associated with the pathogenesis of several complex chronic inflammatory and autoimmune disorders. In addition to the involvement in dysbiosis, dietary components have been shown to interfere in intestinal homeostasis, modulating the barrier function and innate immunity mechanisms. Therefore, the accumulated scientific evidence appears to position diet as a central element both in disease pathogenesis as a critical risk factor and also as a potential opportunity for therapeutic interventions. Currently, several clinical trials are being conducted in different institutions and, hopefully, novel approaches to dietary therapy will be available in the near future.
Considering the progressive burden due to IBD anticipated in the next decades, dietary interventions appear particularly interesting, presumably due to the expected irrelevant side effects, lower costs, and potential application in the prevention of disease initiation or relapses. For the moment, however, the simplest approach includes the avoidance of foods that patients self-identify as worsening their symptoms, in addition to high-fat, high-carbohydrate meals and processed foods. Notably, dietary measures should be considered within the wider context of the patient’s routine, not only in terms of food quality, but also changes in lifestyle, including dietary habits. For this purpose, while the results of large prospective controlled studies are awaited to provide more specific dietary guidance to patients, it is important to reinforce the role of multidisciplinary teams, including nutritionists, for offering better attention to and individualized support for patients with IBD.
Author Contributions
F.C. participated in the investigation and writing—original draft preparation; H.S.P.d.S. participated in the conceptualization, investigation, data curation, writing—review and editing, supervision, and funding acquisition.
Funding
This research was funded by Brazilian Research Council (CNPq), grant number 302401/2016-4, and FAPERJ (Fundação Carlos Chagas Filho de Amparo a Pesquisa do Estado do Rio de Janeiro), grant number E26/202.781/2017. The APC was funded by Fundação CAPES/PROEX, grant number 8882.331795/2010-01.
Acknowledgments
This research was funded by Brazilian Research Council (CNPq), grant number 302401/2016-4, and FAPERJ (Fundação Carlos Chagas Filho de Amparo a Pesquisa do Estado do Rio de Janeiro), grant number E26/202.781/2017. The APC was funded by Fundação CAPES/PROEX, grant number 8882.331795/2010-01. We thank Claudio Bernardazzi for his assistance with the figure.
Conflicts of Interest
The authors declare no conflict of interest.
References
- de Souza, H.S.; Fiocchi, C. Immunopathogenesis of ibd: Current state of the art. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 13–27. [Google Scholar] [CrossRef]
- Siebert, U.; Wurm, J.; Gothe, R.M.; Arvandi, M.; Vavricka, S.R.; von Kanel, R.; Begre, S.; Sulz, M.C.; Meyenberger, C.; Sagmeister, M. Swiss IBDCSG: Predictors of temporary and permanent work disability in patients with inflammatory bowel disease: Results of the swiss inflammatory bowel disease cohort study. Inflamm. Bowel Dis. 2013, 19, 847–855. [Google Scholar] [CrossRef]
- Knights, D.; Lassen, K.G.; Xavier, R.J. Advances in inflammatory bowel disease pathogenesis: Linking host genetics and the microbiome. Gut 2013, 62, 1505–1510. [Google Scholar] [CrossRef]
- de Souza, H.S.P.; Fiocchi, C.; Iliopoulos, D. The ibd interactome: An integrated view of aetiology, pathogenesis and therapy. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 739–749. [Google Scholar] [CrossRef]
- Levine, A.; Sigall Boneh, R.; Wine, E. Evolving role of diet in the pathogenesis and treatment of inflammatory bowel diseases. Gut 2018, 67, 1726–1738. [Google Scholar] [CrossRef]
- Uniken Venema, W.T.; Voskuil, M.D.; Dijkstra, G.; Weersma, R.K.; Festen, E.A. The genetic background of inflammatory bowel disease: From correlation to causality. J. Pathol. 2017, 241, 146–158. [Google Scholar] [CrossRef]
- Rook, G.A. Hygiene hypothesis and autoimmune diseases. Clin. Rev. Allergy Immunol. 2012, 42, 5–15. [Google Scholar] [CrossRef]
- Park, S.J.; Kim, W.H.; Cheon, J.H. Clinical characteristics and treatment of inflammatory bowel disease: A comparison of eastern and western perspectives. World J. Gastroenterol. 2014, 20, 11525–11537. [Google Scholar] [CrossRef]
- Ng, S.C.; Tang, W.; Ching, J.Y.; Wong, M.; Chow, C.M.; Hui, A.J.; Wong, T.C.; Leung, V.K.; Tsang, S.W.; Yu, H.H.; et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn’s and colitis epidemiology study. Gastroenterology 2013, 145, 158–165. [Google Scholar] [CrossRef]
- Kaplan, G.G. The global burden of ibd: From 2015 to 2025. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 720–727. [Google Scholar] [CrossRef]
- Bernstein, C.N. Review article: Changes in the epidemiology of inflammatory bowel disease-clues for aetiology. Aliment. Pharmacol. Ther. 2017, 46, 911–919. [Google Scholar] [CrossRef]
- Marion-Letellier, R.; Savoye, G.; Ghosh, S. Ibd: In food we trust. J. Crohn’s Colitis 2016, 10, 1351–1361. [Google Scholar] [CrossRef]
- Duffy, L.C.; Raiten, D.J.; Hubbard, V.S.; Starke-Reed, P. Progress and challenges in developing metabolic footprints from diet in human gut microbial cometabolism. J. Nutr. 2015, 145, 1123S–1130S. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Ley, R.E.; Hamady, M.; Lozupone, C.; Turnbaugh, P.J.; Ramey, R.R.; Bircher, J.S.; Schlegel, M.L.; Tucker, T.A.; Schrenzel, M.D.; Knight, R.; et al. Evolution of mammals and their gut microbes. Science 2008, 320, 1647–1651. [Google Scholar] [CrossRef]
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696. [Google Scholar] [CrossRef]
- Lewis, J.D.; Abreu, M.T. Diet as a trigger or therapy for inflammatory bowel diseases. Gastroenterology 2017, 152, 398–414.e6. [Google Scholar] [CrossRef]
- Calder, P.C. Polyunsaturated fatty acids and inflammation. Biochem. Soc. Trans. 2005, 33, 423–427. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef]
- Barcenilla, A.; Pryde, S.E.; Martin, J.C.; Duncan, S.H.; Stewart, C.S.; Henderson, C.; Flint, H.J. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl. Environ. Microbiol. 2000, 66, 1654–1661. [Google Scholar] [CrossRef]
- Rapozo, D.C.; Bernardazzi, C.; de Souza, H.S. Diet and microbiota in inflammatory bowel disease: The gut in disharmony. World J. Gastroenterol. 2017, 23, 2124–2140. [Google Scholar] [CrossRef]
- Man, S.M.; Kaakoush, N.O.; Mitchell, H.M. The role of bacteria and pattern-recognition receptors in Crohn’s disease. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 152–168. [Google Scholar] [CrossRef]
- Takahashi, K.; Nishida, A.; Fujimoto, T.; Fujii, M.; Shioya, M.; Imaeda, H.; Inatomi, O.; Bamba, S.; Sugimoto, M.; Andoh, A. Reduced abundance of butyrate-producing bacteria species in the fecal microbial community in Crohn’s disease. Digestion 2016, 93, 59–65. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, X.; Li, L. Human gut microbiome: The second genome of human body. Protein Cell 2010, 1, 718–725. [Google Scholar] [CrossRef]
- Harris, R.A.; Shah, R.; Hollister, E.B.; Tronstad, R.R.; Hovdenak, N.; Szigeti, R.; Versalovic, J.; Kellermayer, R. Colonic mucosal epigenome and microbiome development in children and adolescents. J. Immunol. Res. 2016, 2016, 9170162. [Google Scholar] [CrossRef]
- Guslandi, M. Role of probiotics in Crohn’s disease and in pouchitis. J. Clin. Gastroenterol. 2015, 49 (Suppl. 1), S46–S49. [Google Scholar] [CrossRef]
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.A.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563. [Google Scholar] [CrossRef]
- Sokol, H. Probiotics and antibiotics in ibd. Dig. Dis. 2014, 32 (Suppl. 1), 10–17. [Google Scholar] [CrossRef]
- Ishikawa, H.; Akedo, I.; Umesaki, Y.; Tanaka, R.; Imaoka, A.; Otani, T. Randomized controlled trial of the effect of bifidobacteria-fermented milk on ulcerative colitis. J. Am. Coll. Nutr. 2003, 22, 56–63. [Google Scholar] [CrossRef]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vazquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef]
- Forsyth, C.B.; Shaikh, M.; Bishehsari, F.; Swanson, G.; Voigt, R.M.; Dodiya, H.; Wilkinson, P.; Samelco, B.; Song, S.; Keshavarzian, A. Alcohol feeding in mice promotes colonic hyperpermeability and changes in colonic organoid stem cell fate. Alcohol. Clin. Exp. Res. 2017, 41, 2100–2113. [Google Scholar] [CrossRef]
- Cani, P.D.; Bibiloni, R.; Knauf, C.; Waget, A.; Neyrinck, A.M.; Delzenne, N.M.; Burcelin, R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008, 57, 1470–1481. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Cox, A.J.; West, N.P.; Cripps, A.W. Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 2015, 3, 207–215. [Google Scholar] [CrossRef]
- Frank, D.N.; Robertson, C.E.; Hamm, C.M.; Kpadeh, Z.; Zhang, T.; Chen, H.; Zhu, W.; Sartor, R.B.; Boedeker, E.C.; Harpaz, N.; et al. Disease phenotype and genotype are associated with shifts in intestinal-associated microbiota in inflammatory bowel diseases. Inflamm. Bowel Dis. 2011, 17, 179–184. [Google Scholar] [CrossRef]
- Maeda, Y.; Kurakawa, T.; Umemoto, E.; Motooka, D.; Ito, Y.; Gotoh, K.; Hirota, K.; Matsushita, M.; Furuta, Y.; Narazaki, M.; et al. Dysbiosis contributes to arthritis development via activation of autoreactive t cells in the intestine. Arthr. Rheumatol. 2016, 68, 2646–2661. [Google Scholar] [CrossRef]
- Ott, S.J.; Musfeldt, M.; Wenderoth, D.F.; Hampe, J.; Brant, O.; Folsch, U.R.; Timmis, K.N.; Schreiber, S. Reduction in diversity of the colonic mucosa associated bacterial microflora in patients with active inflammatory bowel disease. Gut 2004, 53, 685–693. [Google Scholar] [CrossRef]
- Wang, H.; Shi, P.; Zuo, L.; Dong, J.; Zhao, J.; Liu, Q.; Zhu, W. Dietary non-digestible polysaccharides ameliorate intestinal epithelial barrier dysfunction in il-10 knockout mice. J. Crohn’s Colitis 2016, 10, 1076–1086. [Google Scholar] [CrossRef]
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory t cells. Nature 2013, 504, 446–450. [Google Scholar] [CrossRef]
- Desai, M.S.; Seekatz, A.M.; Koropatkin, N.M.; Kamada, N.; Hickey, C.A.; Wolter, M.; Pudlo, N.A.; Kitamoto, S.; Terrapon, N.; Muller, A.; et al. A dietary fiber-deprived gut microbiota degrades the colonic mucus barrier and enhances pathogen susceptibility. Cell 2016, 167, 1339–1353.e21. [Google Scholar] [CrossRef]
- Agus, A.; Denizot, J.; Thevenot, J.; Martinez-Medina, M.; Massier, S.; Sauvanet, P.; Bernalier-Donadille, A.; Denis, S.; Hofman, P.; Bonnet, R.; et al. Western diet induces a shift in microbiota composition enhancing susceptibility to adherent-invasive E. coli infection and intestinal inflammation. Sci. Rep. 2016, 6, 19032. [Google Scholar] [CrossRef]
- Ma, X.; Torbenson, M.; Hamad, A.R.; Soloski, M.J.; Li, Z. High-fat diet modulates non-cd1d-restricted natural killer t cells and regulatory t cells in mouse colon and exacerbates experimental colitis. Clin. Exp. Immunol. 2008, 151, 130–138. [Google Scholar] [CrossRef]
- Kim, K.A.; Gu, W.; Lee, I.A.; Joh, E.H.; Kim, D.H. High fat diet-induced gut microbiota exacerbates inflammation and obesity in mice via the tlr4 signaling pathway. PLoS ONE 2012, 7, e47713. [Google Scholar] [CrossRef]
- Liu, Z.; Brooks, R.S.; Ciappio, E.D.; Kim, S.J.; Crott, J.W.; Bennett, G.; Greenberg, A.S.; Mason, J.B. Diet-induced obesity elevates colonic tnf-alpha in mice and is accompanied by an activation of wnt signaling: A mechanism for obesity-associated colorectal cancer. J. Nutr. Biochem. 2012, 23, 1207–1213. [Google Scholar] [CrossRef]
- Progatzky, F.; Sangha, N.J.; Yoshida, N.; McBrien, M.; Cheung, J.; Shia, A.; Scott, J.; Marchesi, J.R.; Lamb, J.R.; Bugeon, L.; et al. Dietary cholesterol directly induces acute inflammasome-dependent intestinal inflammation. Nat. Commun. 2014, 5, 5864. [Google Scholar] [CrossRef]
- Joossens, M.; Huys, G.; Cnockaert, M.; De Preter, V.; Verbeke, K.; Rutgeerts, P.; Vandamme, P.; Vermeire, S. Dysbiosis of the faecal microbiota in patients with Crohn’s disease and their unaffected relatives. Gut 2011, 60, 631–637. [Google Scholar] [CrossRef]
- Varela, E.; Manichanh, C.; Gallart, M.; Torrejon, A.; Borruel, N.; Casellas, F.; Guarner, F.; Antolin, M. Colonisation by Faecalibacterium prausnitzii and maintenance of clinical remission in patients with ulcerative colitis. Aliment. Pharmacol. Ther. 2013, 38, 151–161. [Google Scholar] [CrossRef]
- Wright, E.K.; Kamm, M.A.; Teo, S.M.; Inouye, M.; Wagner, J.; Kirkwood, C.D. Recent advances in characterizing the gastrointestinal microbiome in Crohn’s disease: A systematic review. Inflamm. Bowel Dis. 2015, 21, 1219–1228. [Google Scholar]
- Sokol, H.; Leducq, V.; Aschard, H.; Pham, H.P.; Jegou, S.; Landman, C.; Cohen, D.; Liguori, G.; Bourrier, A.; Nion-Larmurier, I.; et al. Fungal microbiota dysbiosis in ibd. Gut 2017, 66, 1039–1048. [Google Scholar] [CrossRef]
- Pascal, V.; Pozuelo, M.; Borruel, N.; Casellas, F.; Campos, D.; Santiago, A.; Martinez, X.; Varela, E.; Sarrabayrouse, G.; Machiels, K.; et al. A microbial signature for Crohn’s disease. Gut 2017, 66, 813–822. [Google Scholar] [CrossRef]
- Powell, N.; MacDonald, T.T. Recent advances in gut immunology. Parasite Immunol. 2017, 39. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Oshima, K.; Suda, W.; Nagano, Y.; Nishikawa, H.; Fukuda, S.; Saito, T.; Narushima, S.; Hase, K.; et al. Treg induction by a rationally selected mixture of clostridia strains from the human microbiota. Nature 2013, 500, 232–236. [Google Scholar] [CrossRef]
- Sefik, E.; Geva-Zatorsky, N.; Oh, S.; Konnikova, L.; Zemmour, D.; McGuire, A.M.; Burzyn, D.; Ortiz-Lopez, A.; Lobera, M.; Yang, J.; et al. Mucosal immunology. Individual intestinal symbionts induce a distinct population of rorgamma(+) regulatory t cells. Science 2015, 349, 993–997. [Google Scholar] [CrossRef]
- Atarashi, K.; Tanoue, T.; Ando, M.; Kamada, N.; Nagano, Y.; Narushima, S.; Suda, W.; Imaoka, A.; Setoyama, H.; Nagamori, T.; et al. Th17 cell induction by adhesion of microbes to intestinal epithelial cells. Cell 2015, 163, 367–380. [Google Scholar] [CrossRef]
- Tan, T.G.; Sefik, E.; Geva-Zatorsky, N.; Kua, L.; Naskar, D.; Teng, F.; Pasman, L.; Ortiz-Lopez, A.; Jupp, R.; Wu, H.J.; et al. Identifying species of symbiont bacteria from the human gut that, alone, can induce intestinal th17 cells in mice. Proc. Natl. Acad. Sci. USA 2016, 113, E8141–E8150. [Google Scholar] [CrossRef]
- Viladomiu, M.; Kivolowitz, C.; Abdulhamid, A.; Dogan, B.; Victorio, D.; Castellanos, J.G.; Woo, V.; Teng, F.; Tran, N.L.; Sczesnak, A.; et al. Iga-coated E. coli enriched in Crohn’s disease spondyloarthritis promote th17-dependent inflammation. Sci. Transl. Med. 2017, 9, eaaf9655. [Google Scholar] [CrossRef]
- Ivanov, I.I.; Atarashi, K.; Manel, N.; Brodie, E.L.; Shima, T.; Karaoz, U.; Wei, D.; Goldfarb, K.C.; Santee, C.A.; Lynch, S.V.; et al. Induction of intestinal th17 cells by segmented filamentous bacteria. Cell 2009, 139, 485–498. [Google Scholar] [CrossRef]
- Statovci, D.; Aguilera, M.; MacSharry, J.; Melgar, S. The impact of western diet and nutrients on the microbiota and immune response at mucosal interfaces. Front. Immunol. 2017, 8, 838. [Google Scholar] [CrossRef]
- Martinez-Medina, M.; Denizot, J.; Dreux, N.; Robin, F.; Billard, E.; Bonnet, R.; Darfeuille-Michaud, A.; Barnich, N. Western diet induces dysbiosis with increased e coli in ceabac10 mice, alters host barrier function favouring AIEC colonisation. Gut 2014, 63, 116–124. [Google Scholar] [CrossRef]
- Tilg, H.; Moschen, A.R. Food, immunity, and the microbiome. Gastroenterology 2015, 148, 1107–1119. [Google Scholar] [CrossRef]
- Devkota, S.; Wang, Y.; Musch, M.W.; Leone, V.; Fehlner-Peach, H.; Nadimpalli, A.; Antonopoulos, D.A.; Jabri, B.; Chang, E.B. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in il10-/- mice. Nature 2012, 487, 104–108. [Google Scholar] [CrossRef]
- Silveira, A.L.M.; Ferreira, A.V.M.; de Oliveira, M.C.; Rachid, M.A.; da Cunha Sousa, L.F.; Dos Santos Martins, F.; Gomes-Santos, A.C.; Vieira, A.T.; Teixeira, M.M. Preventive rather than therapeutic treatment with high fiber diet attenuates clinical and inflammatory markers of acute and chronic dss-induced colitis in mice. Eur. J. Nutr. 2017, 56, 179–191. [Google Scholar] [CrossRef]
- Nickerson, K.P.; McDonald, C. Crohn’s disease-associated adherent-invasive escherichia coli adhesion is enhanced by exposure to the ubiquitous dietary polysaccharide maltodextrin. PLoS ONE 2012, 7, e52132. [Google Scholar] [CrossRef]
- Nickerson, K.P.; Chanin, R.; McDonald, C. Deregulation of intestinal anti-microbial defense by the dietary additive, maltodextrin. Gut Microbes 2015, 6, 78–83. [Google Scholar] [CrossRef]
- Hold, G.L.; Smith, M.; Grange, C.; Watt, E.R.; El-Omar, E.M.; Mukhopadhya, I. Role of the gut microbiota in inflammatory bowel disease pathogenesis: What have we learnt in the past 10 years? World J. Gastroenterol. 2014, 20, 1192–1210. [Google Scholar] [CrossRef]
- Colombel, J.F. Decade in review-ibd: Ibd-genes, bacteria and new therapeutic strategies. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 652–654. [Google Scholar] [CrossRef]
- Zhernakova, A.; Kurilshikov, A.; Bonder, M.J.; Tigchelaar, E.F.; Schirmer, M.; Vatanen, T.; Mujagic, Z.; Vila, A.V.; Falony, G.; Vieira-Silva, S.; et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016, 352, 565–569. [Google Scholar] [CrossRef]
- Figliuolo, V.R.; Dos Santos, L.M.; Abalo, A.; Nanini, H.; Santos, A.; Brittes, N.M.; Bernardazzi, C.; de Souza, H.S.P.; Vieira, L.Q.; Coutinho-Silva, R.; et al. Sulfate-reducing bacteria stimulate gut immune responses and contribute to inflammation in experimental colitis. Life Sci. 2017, 189, 29–38. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Birchenough, G.M.H.; Stahlman, M.; Arike, L.; Johansson, M.E.V.; Hansson, G.C.; Backhed, F. Bifidobacteria or fiber protects against diet-induced microbiota-mediated colonic mucus deterioration. Cell Host Microbe 2018, 23, 27–40.e7. [Google Scholar] [CrossRef]
- James, S.L.; Christophersen, C.T.; Bird, A.R.; Conlon, M.A.; Rosella, O.; Gibson, P.R.; Muir, J.G. Abnormal fibre usage in uc in remission. Gut 2015, 64, 562–570. [Google Scholar] [CrossRef]
- Vinolo, M.A.; Rodrigues, H.G.; Nachbar, R.T.; Curi, R. Regulation of inflammation by short chain fatty acids. Nutrients 2011, 3, 858–876. [Google Scholar] [CrossRef]
- Hartog, A.; Belle, F.N.; Bastiaans, J.; de Graaff, P.; Garssen, J.; Harthoorn, L.F.; Vos, A.P. A potential role for regulatory t-cells in the amelioration of dss induced colitis by dietary non-digestible polysaccharides. J. Nutr. Biochem. 2015, 26, 227–233. [Google Scholar] [CrossRef]
- Roberts, C.L.; Keita, A.V.; Duncan, S.H.; O’Kennedy, N.; Soderholm, J.D.; Rhodes, J.M.; Campbell, B.J. Translocation of Crohn’s disease escherichia coli across m-cells: Contrasting effects of soluble plant fibres and emulsifiers. Gut 2010, 59, 1331–1339. [Google Scholar] [CrossRef]
- Andersen, V.; Chan, S.; Luben, R.; Khaw, K.T.; Olsen, A.; Tjonneland, A.; Kaaks, R.; Grip, O.; Bergmann, M.M.; Boeing, H.; et al. Fibre intake and the development of inflammatory bowel disease: A european prospective multi-centre cohort study (epic-ibd). J. Crohn’s Colitis 2018, 12, 129–136. [Google Scholar] [CrossRef]
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96. [Google Scholar] [CrossRef]
- Chassaing, B.; Van de Wiele, T.; De Bodt, J.; Marzorati, M.; Gewirtz, A.T. Dietary emulsifiers directly alter human microbiota composition and gene expression ex vivo potentiating intestinal inflammation. Gut 2017, 66, 1414–1427. [Google Scholar] [CrossRef]
- Khalili, H.; Malik, S.; Ananthakrishnan, A.N.; Garber, J.J.; Higuchi, L.M.; Joshi, A.; Peloquin, J.; Richter, J.M.; Stewart, K.O.; Curhan, G.C.; et al. Identification and characterization of a novel association between dietary potassium and risk of Crohn’s disease and ulcerative colitis. Front. Immunol. 2016, 7, 554. [Google Scholar] [CrossRef]
- Mayneris-Perxachs, J.; Bolick, D.T.; Leng, J.; Medlock, G.L.; Kolling, G.L.; Papin, J.A.; Swann, J.R.; Guerrant, R.L. Protein- and zinc-deficient diets modulate the murine microbiome and metabolic phenotype. Am. J. Clin. Nutr. 2016, 104, 1253–1262. [Google Scholar] [CrossRef]
- Del Pinto, R.; Pietropaoli, D.; Chandar, A.K.; Ferri, C.; Cominelli, F. Association between inflammatory bowel disease and vitamin d deficiency: A systematic review and meta-analysis. Inflamm. Bowel Dis. 2015, 21, 2708–2717. [Google Scholar] [CrossRef]
- de Bruyn, J.R.; van Heeckeren, R.; Ponsioen, C.Y.; van den Brink, G.R.; Lowenberg, M.; Bredenoord, A.J.; Frijstein, G.; D’Haens, G.R. Vitamin d deficiency in Crohn’s disease and healthy controls: A prospective case-control study in the netherlands. J. Crohn’s Colitis 2014, 8, 1267–1273. [Google Scholar] [CrossRef]
- Maaser, C.; Langholz, E.; Gordon, H.; Burisch, J.; Ellul, P.; Ramirez, V.H.; Karakan, T.; Katsanos, K.H.; Krustins, E.; Levine, A.; et al. European Crohn’s and colitis organisation topical review on environmental factors in ibd. J. Crohn’s Colitis 2017, 11, 905–920. [Google Scholar] [CrossRef]
- Strachan, D.P. Hay fever, hygiene, and household size. BMJ 1989, 299, 1259–1260. [Google Scholar] [CrossRef]
- Yazdanbakhsh, M.; Matricardi, P.M. Parasites and the hygiene hypothesis: Regulating the immune system? Clin. Rev. Allergy Immunol. 2004, 26, 15–24. [Google Scholar] [CrossRef]
- Feillet, H.; Bach, J.F. Increased incidence of inflammatory bowel disease: The price of the decline of infectious burden? Curr. Opin. Gastroenterol. 2004, 20, 560–564. [Google Scholar] [CrossRef]
- Foster, A.; Jacobson, K. Changing incidence of inflammatory bowel disease: Environmental influences and lessons learnt from the south Asian population. Front. Pediatr. 2013, 1, 34. [Google Scholar] [CrossRef]
- Ponder, A.; Long, M.D. A clinical review of recent findings in the epidemiology of inflammatory bowel disease. Clin. Epidemiol. 2013, 5, 237–247. [Google Scholar]
- Kaplan, G.G.; Hubbard, J.; Korzenik, J.; Sands, B.E.; Panaccione, R.; Ghosh, S.; Wheeler, A.J.; Villeneuve, P.J. The inflammatory bowel diseases and ambient air pollution: A novel association. Am. J. Gastroenterol. 2010, 105, 2412–2419. [Google Scholar] [CrossRef]
- Ungaro, R.; Bernstein, C.N.; Gearry, R.; Hviid, A.; Kolho, K.L.; Kronman, M.P.; Shaw, S.; Van Kruiningen, H.; Colombel, J.F.; Atreja, A. Antibiotics associated with increased risk of new-onset Crohn’s disease but not ulcerative colitis: A meta-analysis. Am. J. Gastroenterol. 2014, 109, 1728–1738. [Google Scholar] [CrossRef]
- Aniwan, S.; Tremaine, W.J.; Raffals, L.E.; Kane, S.V.; Loftus, E.V., Jr. Antibiotic use and new-onset inflammatory bowel disease in Olmsted county, Minnesota: A population-based case-control study. J. Crohn’s Colitis 2018, 12, 137–144. [Google Scholar] [CrossRef]
- Shaw, S.Y.; Blanchard, J.F.; Bernstein, C.N. Association between the use of antibiotics in the first year of life and pediatric inflammatory bowel disease. Am. J. Gastroenterol. 2010, 105, 2687–2692. [Google Scholar] [CrossRef]
- Hviid, A.; Svanstrom, H.; Frisch, M. Antibiotic use and inflammatory bowel diseases in childhood. Gut 2011, 60, 49–54. [Google Scholar] [CrossRef]
- Hildebrand, H.; Malmborg, P.; Askling, J.; Ekbom, A.; Montgomery, S.M. Early-life exposures associated with antibiotic use and risk of subsequent Crohn’s disease. Scand. J. Gastroenterol. 2008, 43, 961–966. [Google Scholar] [CrossRef]
- Cornish, J.A.; Tan, E.; Simillis, C.; Clark, S.K.; Teare, J.; Tekkis, P.P. The risk of oral contraceptives in the etiology of inflammatory bowel disease: A meta-analysis. Am. J. Gastroenterol. 2008, 103, 2394–2400. [Google Scholar] [CrossRef]
- Felder, J.B.; Korelitz, B.I.; Rajapakse, R.; Schwarz, S.; Horatagis, A.P.; Gleim, G. Effects of nonsteroidal antiinflammatory drugs on inflammatory bowel disease: A case-control study. Am. J. Gastroenterol. 2000, 95, 1949–1954. [Google Scholar] [CrossRef]
- Long, M.D.; Kappelman, M.D.; Martin, C.F.; Chen, W.; Anton, K.; Sandler, R.S. Role of nonsteroidal anti-inflammatory drugs in exacerbations of inflammatory bowel disease. J. Clin. Gastroenterol. 2016, 50, 152–156. [Google Scholar] [CrossRef]
- Moninuola, O.O.; Milligan, W.; Lochhead, P.; Khalili, H. Systematic review with meta-analysis: Association between acetaminophen and nonsteroidal anti-inflammatory drugs (nsaids) and risk of Crohn’s disease and ulcerative colitis exacerbation. Aliment. Pharmacol. Ther. 2018, 47, 1428–1439. [Google Scholar] [CrossRef]
- Cosnes, J. Tobacco and ibd: Relevance in the understanding of disease mechanisms and clinical practice. Best Pract. Res. Clin. Gastroenterol. 2004, 18, 481–496. [Google Scholar] [CrossRef]
- Parkes, G.C.; Whelan, K.; Lindsay, J.O. Smoking in inflammatory bowel disease: Impact on disease course and insights into the aetiology of its effect. J. Crohn’s Colitis 2014, 8, 717–725. [Google Scholar] [CrossRef]
- Biedermann, L.; Fournier, N.; Misselwitz, B.; Frei, P.; Zeitz, J.; Manser, C.N.; Pittet, V.; Juillerat, P.; von Kanel, R.; Fried, M.; et al. High rates of smoking especially in female Crohn’s disease patients and low use of supportive measures to achieve smoking cessation—Data from the swiss ibd cohort study. J. Crohn’s Colitis 2015, 9, 819–829. [Google Scholar] [CrossRef]
- Sher, M.E.; Bank, S.; Greenberg, R.; Sardinha, T.C.; Weissman, S.; Bailey, B.; Gilliland, R.; Wexner, S.D. The influence of cigarette smoking on cytokine levels in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 1999, 5, 73–78. [Google Scholar] [CrossRef]
- Biedermann, L.; Brulisauer, K.; Zeitz, J.; Frei, P.; Scharl, M.; Vavricka, S.R.; Fried, M.; Loessner, M.J.; Rogler, G.; Schuppler, M. Smoking cessation alters intestinal microbiota: Insights from quantitative investigations on human fecal samples using fish. Inflamm. Bowel Dis. 2014, 20, 1496–1501. [Google Scholar] [CrossRef]
- Adorini, L.; Penna, G. Dendritic cell tolerogenicity: A key mechanism in immunomodulation by vitamin d receptor agonists. Hum. Immunol. 2009, 70, 345–352. [Google Scholar] [CrossRef]
- Jorgensen, S.P.; Hvas, C.L.; Agnholt, J.; Christensen, L.A.; Heickendorff, L.; Dahlerup, J.F. Active Crohn’s disease is associated with low vitamin d levels. J. Crohn’s Colitis 2013, 7, e407–e413. [Google Scholar] [CrossRef]
- Limketkai, B.N.; Bayless, T.M.; Brant, S.R.; Hutfless, S.M. Lower regional and temporal ultraviolet exposure is associated with increased rates and severity of inflammatory bowel disease hospitalisation. Aliment. Pharmacol. Ther. 2014, 40, 508–517. [Google Scholar] [CrossRef]
- Holmes, E.A.; Xiang, F.; Lucas, R.M. Variation in incidence of pediatric Crohn’s disease in relation to latitude and ambient ultraviolet radiation: A systematic review and analysis. Inflamm. Bowel Dis. 2015, 21, 809–817. [Google Scholar] [CrossRef]
- Magnus, P.; Jaakkola, J.J. Secular trend in the occurrence of asthma among children and young adults: Critical appraisal of repeated cross sectional surveys. BMJ 1997, 314, 1795–1799. [Google Scholar] [CrossRef]
- Lee, D.; Albenberg, L.; Compher, C.; Baldassano, R.; Piccoli, D.; Lewis, J.D.; Wu, G.D. Diet in the pathogenesis and treatment of inflammatory bowel diseases. Gastroenterology 2015, 148, 1087–1106. [Google Scholar] [CrossRef]
- Alcalde-Cabero, E.; Almazan-Isla, J.; Garcia-Merino, A.; de Sa, J.; de Pedro-Cuesta, J. Incidence of multiple sclerosis among european economic area populations, 1985–2009: The framework for monitoring. BMC Neurol. 2013, 13, 58. [Google Scholar] [CrossRef]
- Krawiec, J.; Odes, H.S.; Lasry, Y.; Krugliak, P.; Weitzman, S. Aspects of the epidemiology of Crohn’s disease in the Jewish population in Beer Sheva, Israel. Isr. J. Med. Sci. 1984, 20, 16–21. [Google Scholar]
- Li, X.; Sundquist, J.; Hemminki, K.; Sundquist, K. Risk of inflammatory bowel disease in first- and second-generation immigrants in Sweden: A nationwide follow-up study. Inflamm. Bowel Dis. 2011, 17, 1784–1791. [Google Scholar] [CrossRef]
- Sood, A.; Midha, V.; Sood, N.; Bhatia, A.S.; Avasthi, G. Incidence and prevalence of ulcerative colitis in Punjab, north India. Gut 2003, 52, 1587–1590. [Google Scholar] [CrossRef]
- Khosla, S.N.; Girdhar, N.K.; Lal, S.; Mishra, D.S. Epidemiology of ulcerative colitis in hospital and select general population of northern India. J. Assoc. Phys. India 1986, 34, 405–407. [Google Scholar]
- Loftus, C.G.; Loftus, E.V., Jr.; Harmsen, W.S.; Zinsmeister, A.R.; Tremaine, W.J.; Melton, L.J., 3rd; Sandborn, W.J. Update on the incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted county, Minnesota, 1940–2000. Inflamm. Bowel Dis. 2007, 13, 254–261. [Google Scholar] [CrossRef]
- Bernstein, C.N.; Wajda, A.; Svenson, L.W.; MacKenzie, A.; Koehoorn, M.; Jackson, M.; Fedorak, R.; Israel, D.; Blanchard, J.F. The epidemiology of inflammatory bowel disease in Canada: A population-based study. Am. J. Gastroenterol. 2006, 101, 1559–1568. [Google Scholar] [CrossRef]
- Fellows, I.W.; Freeman, J.G.; Holmes, G.K. Crohn’s disease in the city of derby, 1951–1985. Gut 1990, 31, 1262–1265. [Google Scholar] [CrossRef]
- Pinsk, V.; Lemberg, D.A.; Grewal, K.; Barker, C.C.; Schreiber, R.A.; Jacobson, K. Inflammatory bowel disease in the south Asian pediatric population of British Columbia. Am. J. Gastroenterol. 2007, 102, 1077–1083. [Google Scholar] [CrossRef]
- Ko, Y.; Butcher, R.; Leong, R.W. Epidemiological studies of migration and environmental risk factors in the inflammatory bowel diseases. World J. Gastroenterol. 2014, 20, 1238–1247. [Google Scholar] [CrossRef]
- Ng, S.C.; Tang, W.; Leong, R.W.; Chen, M.; Ko, Y.; Studd, C.; Niewiadomski, O.; Bell, S.; Kamm, M.A.; de Silva, H.J.; et al. Environmental risk factors in inflammatory bowel disease: A population-based case-control study in Asia-Pacific. Gut 2015, 64, 1063–1071. [Google Scholar] [CrossRef]
- Zhou, Y.; Xu, Z.Z.; He, Y.; Yang, Y.; Liu, L.; Lin, Q.; Nie, Y.; Li, M.; Zhi, F.; Liu, S.; et al. Gut microbiota offers universal biomarkers across ethnicity in inflammatory bowel disease diagnosis and infliximab response prediction. MSystems 2018, 3, e00188-17. [Google Scholar] [CrossRef]
- Forbes, J.D.; Chen, C.Y.; Knox, N.C.; Marrie, R.A.; El-Gabalawy, H.; de Kievit, T.; Alfa, M.; Bernstein, C.N.; Van Domselaar, G. A comparative study of the gut microbiota in immune-mediated inflammatory diseases-does a common dysbiosis exist? Microbiome 2018, 6, 221. [Google Scholar] [CrossRef]
- Hou, J.K.; Abraham, B.; El-Serag, H. Dietary intake and risk of developing inflammatory bowel disease: A systematic review of the literature. Am. J. Gastroenterol. 2011, 106, 563–573. [Google Scholar] [CrossRef]
- Ananthakrishnan, A.N. Epidemiology and risk factors for ibd. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef]
- Aleksandrova, K.; Romero-Mosquera, B.; Hernandez, V. Diet, gut microbiome and epigenetics: Emerging links with inflammatory bowel diseases and prospects for management and prevention. Nutrients 2017, 9, 962. [Google Scholar] [CrossRef]
- Dabritz, J.; Menheniott, T.R. Linking immunity, epigenetics, and cancer in inflammatory bowel disease. Inflamm. Bowel Dis. 2014, 20, 1638–1654. [Google Scholar] [CrossRef]
- Barnett, M.; Bermingham, E.; McNabb, W.; Bassett, S.; Armstrong, K.; Rounce, J.; Roy, N. Investigating micronutrients and epigenetic mechanisms in relation to inflammatory bowel disease. Mutat. Res. 2010, 690, 71–80. [Google Scholar] [CrossRef]
- Gallou-Kabani, C.; Vige, A.; Gross, M.S.; Junien, C. Nutri-epigenomics: Lifelong remodelling of our epigenomes by nutritional and metabolic factors and beyond. Clin. Chem. Lab. Med. 2007, 45, 321–327. [Google Scholar] [CrossRef]
- Anderson, O.S.; Sant, K.E.; Dolinoy, D.C. Nutrition and epigenetics: An interplay of dietary methyl donors, one-carbon metabolism and DNA methylation. J. Nutr. Biochem. 2012, 23, 853–859. [Google Scholar] [CrossRef]
- Lu, Q.; Qiu, X.; Hu, N.; Wen, H.; Su, Y.; Richardson, B.C. Epigenetics, disease, and therapeutic interventions. Ageing Res. Rev. 2006, 5, 449–467. [Google Scholar] [CrossRef]
- Remely, M.; Lovrecic, L.; de la Garza, A.L.; Migliore, L.; Peterlin, B.; Milagro, F.I.; Martinez, A.J.; Haslberger, A.G. Therapeutic perspectives of epigenetically active nutrients. Br. J. Pharmacol. 2015, 172, 2756–2768. [Google Scholar] [CrossRef]
- Russell, W.R.; Duncan, S.H.; Scobbie, L.; Duncan, G.; Cantlay, L.; Calder, A.G.; Anderson, S.E.; Flint, H.J. Major phenylpropanoid-derived metabolites in the human gut can arise from microbial fermentation of protein. Mol. Nutr. Food Res. 2013, 57, 523–535. [Google Scholar] [CrossRef]
- Rahal, K.; Schmiedlin-Ren, P.; Adler, J.; Dhanani, M.; Sultani, V.; Rittershaus, A.C.; Reingold, L.; Zhu, J.; McKenna, B.J.; Christman, G.M.; et al. Resveratrol has antiinflammatory and antifibrotic effects in the peptidoglycan-polysaccharide rat model of Crohn’s disease. Inflamm. Bowel Dis. 2012, 18, 613–623. [Google Scholar] [CrossRef]
- Hanai, H.; Sugimoto, K. Curcumin has bright prospects for the treatment of inflammatory bowel disease. Curr. Pharm. Des. 2009, 15, 2087–2094. [Google Scholar] [CrossRef]
- Hanai, H.; Iida, T.; Takeuchi, K.; Watanabe, F.; Maruyama, Y.; Andoh, A.; Tsujikawa, T.; Fujiyama, Y.; Mitsuyama, K.; Sata, M.; et al. Curcumin maintenance therapy for ulcerative colitis: Randomized, multicenter, double-blind, placebo-controlled trial. Clin. Gastroenterol. Hepatol. Off. Clin. Prac. J. Am. Gastroenterol. Assoc. 2006, 4, 1502–1506. [Google Scholar]
- Reuter, S.; Gupta, S.C.; Park, B.; Goel, A.; Aggarwal, B.B. Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr. 2011, 6, 93–108. [Google Scholar] [CrossRef]
- Burdge, G.C.; Hoile, S.P.; Lillycrop, K.A. Epigenetics: Are there implications for personalised nutrition? Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 442–447. [Google Scholar] [CrossRef]
- Beaudet, A.L. Epigenetics and complex human disease: Is there a role in ibd? J. Pediatr. Gastroenterol. Nutr. 2008, 46 (Suppl. 1), E2. [Google Scholar]
- Garfinkel, M.D.; Ruden, D.M. Chromatin effects in nutrition, cancer, and obesity. Nutrition 2004, 20, 56–62. [Google Scholar] [CrossRef]
- Wani, N.A.; Hamid, A.; Kaur, J. Folate status in various pathophysiological conditions. IUBMB life 2008, 60, 834–842. [Google Scholar] [CrossRef]
- McKay, J.A.; Williams, E.A.; Mathers, J.C. Gender-specific modulation of tumorigenesis by folic acid supply in the APC mouse during early neonatal life. Br. J. Nutr. 2008, 99, 550–558. [Google Scholar] [CrossRef]
- Davis, C.D.; Uthus, E.O.; Finley, J.W. Dietary selenium and arsenic affect DNA methylation in vitro in caco-2 cells and in vivo in rat liver and colon. J. Nutr. 2000, 130, 2903–2909. [Google Scholar] [CrossRef]
- Tirosh, O.; Levy, E.; Reifen, R. High selenium diet protects against tnbs-induced acute inflammation, mitochondrial dysfunction, and secondary necrosis in rat colon. Nutrition 2007, 23, 878–886. [Google Scholar] [CrossRef]
- Castro Aguilar-Tablada, T.; Navarro-Alarcon, M.; Quesada Granados, J.; Samaniego Sanchez, C.; Rufian-Henares, J.A.; Nogueras-Lopez, F. Ulcerative colitis and Crohn’s disease are associated with decreased serum selenium concentrations and increased cardiovascular risk. Nutrients 2016, 8, 780. [Google Scholar] [CrossRef]
- Arpon, A.; Milagro, F.I.; Razquin, C.; Corella, D.; Estruch, R.; Fito, M.; Marti, A.; Martinez-Gonzalez, M.A.; Ros, E.; Salas-Salvado, J.; et al. Impact of consuming extra-virgin olive oil or nuts within a mediterranean diet on DNA methylation in peripheral white blood cells within the predimed-navarra randomized controlled trial: A role for dietary lipids. Nutrients 2017, 10, 15. [Google Scholar] [CrossRef]
- Kau, A.L.; Ahern, P.P.; Griffin, N.W.; Goodman, A.L.; Gordon, J.I. Human nutrition, the gut microbiome and the immune system. Nature 2011, 474, 327–336. [Google Scholar] [CrossRef]
- Krautkramer, K.A.; Kreznar, J.H.; Romano, K.A.; Vivas, E.I.; Barrett-Wilt, G.A.; Rabaglia, M.E.; Keller, M.P.; Attie, A.D.; Rey, F.E.; Denu, J.M. Diet-microbiota interactions mediate global epigenetic programming in multiple host tissues. Mol. Cell 2016, 64, 982–992. [Google Scholar] [CrossRef]
- Kostic, A.D.; Xavier, R.J.; Gevers, D. The microbiome in inflammatory bowel disease: Current status and the future ahead. Gastroenterology 2014, 146, 1489–1499. [Google Scholar] [CrossRef]
- Eeckhaut, V.; Ducatelle, R.; Sas, B.; Vermeire, S.; Van Immerseel, F. Progress towards butyrate-producing pharmabiotics: Butyricicoccus pullicaecorum capsule and efficacy in tnbs models in comparison with therapeutics. Gut 2014, 63, 367. [Google Scholar] [CrossRef]
- Limdi, J.K.; Aggarwal, D.; McLaughlin, J.T. Dietary practices and beliefs in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 2016, 22, 164–170. [Google Scholar] [CrossRef]
- Zallot, C.; Quilliot, D.; Chevaux, J.B.; Peyrin-Biroulet, C.; Gueant-Rodriguez, R.M.; Freling, E.; Collet-Fenetrier, B.; Williet, N.; Ziegler, O.; Bigard, M.A.; et al. Dietary beliefs and behavior among inflammatory bowel disease patients. Inflamm. Bowel Dis. 2013, 19, 66–72. [Google Scholar] [CrossRef]
- Tanaka, M.; Kawakami, A.; Iwao, Y.; Fukushima, T.; Yamamoto-Mitani, N. Coping strategies for possible flare-ups and their perceived effectiveness in patients with inflammatory bowel disease. Gastroenterology nursing. Off. J. Soc. Gastroenterol. Nur. Assoc. 2016, 39, 42–47. [Google Scholar] [CrossRef]
- Jowett, S.L.; Seal, C.J.; Pearce, M.S.; Phillips, E.; Gregory, W.; Barton, J.R.; Welfare, M.R. Influence of dietary factors on the clinical course of ulcerative colitis: A prospective cohort study. Gut 2004, 53, 1479–1484. [Google Scholar] [CrossRef]
- Magee, E.A.; Edmond, L.M.; Tasker, S.M.; Kong, S.C.; Curno, R.; Cummings, J.H. Associations between diet and disease activity in ulcerative colitis patients using a novel method of data analysis. Nutr. J. 2005, 4, 7. [Google Scholar] [CrossRef]
- Guerreiro, C.S.; Ferreira, P.; Tavares, L.; Santos, P.M.; Neves, M.; Brito, M.; Cravo, M. Fatty acids, il6, and tnfalpha polymorphisms: An example of nutrigenetics in Crohn’s disease. Am. J. Gastroenterol. 2009, 104, 2241–2249. [Google Scholar] [CrossRef]
- Ferreira, P.; Cravo, M.; Guerreiro, C.S.; Tavares, L.; Santos, P.M.; Brito, M. Fat intake interacts with polymorphisms of caspase9, fasligand and ppargamma apoptotic genes in modulating Crohn’s disease activity. Clin. Nutr. 2010, 29, 819–823. [Google Scholar] [CrossRef]
- Reifen, R.; Matas, Z.; Zeidel, L.; Berkovitch, Z.; Bujanover, Y. Iron supplementation may aggravate inflammatory status of colitis in a rat model. Dig. Dis. Sci. 2000, 45, 394–397. [Google Scholar] [CrossRef]
- Carrier, J.; Aghdassi, E.; Cullen, J.; Allard, J.P. Iron supplementation increases disease activity and vitamin e ameliorates the effect in rats with dextran sulfate sodium-induced colitis. J. Nutr. 2002, 132, 3146–3150. [Google Scholar] [CrossRef]
- de Silva, A.D.; Tsironi, E.; Feakins, R.M.; Rampton, D.S. Efficacy and tolerability of oral iron therapy in inflammatory bowel disease: A prospective, comparative trial. Aliment. Pharmacol. Ther. 2005, 22, 1097–1105. [Google Scholar] [CrossRef]
- Goldberg, N.D. Iron deficiency anemia in patients with inflammatory bowel disease. Clin. Exp. Gastroenterol. 2013, 6, 61–70. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Ainsworth, M.; Coskun, M.; Weiss, G. Management of iron-deficiency anemia in inflammatory bowel disease: A systematic review. Medicine 2015, 94, e963. [Google Scholar] [CrossRef]
- Mahalhal, A.; Williams, J.M.; Johnson, S.; Ellaby, N.; Duckworth, C.A.; Burkitt, M.D.; Liu, X.; Hold, G.L.; Campbell, B.J.; Pritchard, D.M.; et al. Oral iron exacerbates colitis and influences the intestinal microbiome. PLoS ONE 2018, 13, e0202460. [Google Scholar] [CrossRef]
- Colombel, J.F.; Shin, A.; Gibson, P.R. AGA clinical practice update on functional gastrointestinal symptoms in patients with inflammatory bowel disease: Expert review. Clin. Gastroenterol. Hepatol. Off. Clin. Prac. J. Am. Gastroenterol. Assoc. 2019, 17, 380–390.e1. [Google Scholar]
- Ruemmele, F.M.; Veres, G.; Kolho, K.L.; Griffiths, A.; Levine, A.; Escher, J.C.; Amil Dias, J.; Barabino, A.; Braegger, C.P.; Bronsky, J.; et al. Consensus guidelines of ecco/espghan on the medical management of pediatric Crohn’s disease. J. Crohn’s Colitis 2014, 8, 1179–1207. [Google Scholar] [CrossRef]
- Lee, D.; Baldassano, R.N.; Otley, A.R.; Albenberg, L.; Griffiths, A.M.; Compher, C.; Chen, E.Z.; Li, H.; Gilroy, E.; Nessel, L.; et al. Comparative effectiveness of nutritional and biological therapy in North American children with active Crohn’s disease. Inflamm. Bowel Dis. 2015, 21, 1786–1793. [Google Scholar] [CrossRef]
- Connors, J.; Basseri, S.; Grant, A.; Giffin, N.; Mahdi, G.; Noble, A.; Rashid, M.; Otley, A.; Van Limbergen, J. Exclusive enteral nutrition therapy in paediatric Crohn’s disease results in long-term avoidance of corticosteroids: Results of a propensity-score matched cohort analysis. J. Crohn’s Colitis 2017, 11, 1063–1070. [Google Scholar] [CrossRef]
- Cohen-Dolev, N.; Sladek, M.; Hussey, S.; Turner, D.; Veres, G.; Koletzko, S.; Martin de Carpi, J.; Staiano, A.; Shaoul, R.; Lionetti, P.; et al. Differences in outcomes over time with exclusive enteral nutrition compared with steroids in children with mild to moderate Crohn’s disease: Results from the growth cd study. J. Crohn’s Colitis 2018, 12, 306–312. [Google Scholar] [CrossRef]
- Grover, Z.; Burgess, C.; Muir, R.; Reilly, C.; Lewindon, P.J. Early mucosal healing with exclusive enteral nutrition is associated with improved outcomes in newly diagnosed children with luminal Crohn’s disease. J. Crohn’s Colitis 2016, 10, 1159–1164. [Google Scholar] [CrossRef]
- Sigall-Boneh, R.; Pfeffer-Gik, T.; Segal, I.; Zangen, T.; Boaz, M.; Levine, A. Partial enteral nutrition with a Crohn’s disease exclusion diet is effective for induction of remission in children and young adults with Crohn’s disease. Inflamm. Bowel Dis. 2014, 20, 1353–1360. [Google Scholar] [CrossRef]
- Sigall Boneh, R.; Sarbagili Shabat, C.; Yanai, H.; Chermesh, I.; Ben Avraham, S.; Boaz, M.; Levine, A. Dietary therapy with the Crohn’s disease exclusion diet is a successful strategy for induction of remission in children and adults failing biological therapy. J. Crohn’s Colitis 2017, 11, 1205–1212. [Google Scholar] [CrossRef]
- Wedlake, L.; Slack, N.; Andreyev, H.J.; Whelan, K. Fiber in the treatment and maintenance of inflammatory bowel disease: A systematic review of randomized controlled trials. Inflamm. Bowel Dis. 2014, 20, 576–586. [Google Scholar] [CrossRef]
- Chiba, M.; Tsuji, T.; Nakane, K.; Komatsu, M. High amount of dietary fiber not harmful but favorable for Crohn disease. Perm. J. 2015, 19, 58–61. [Google Scholar] [CrossRef]
- Chiba, M.; Tsuji, T.; Nakane, K.; Tsuda, S.; Ishii, H.; Ohno, H.; Watanabe, K.; Ito, M.; Komatsu, M.; Sugawara, T. Induction with infliximab and a plant-based diet as first-line (ipf) therapy for Crohn disease: A single-group trial. Perm. J. 2017, 21, 17-009. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.B.; Lee, D.; Long, M.D.; Kappelman, M.D.; Martin, C.F.; Sandler, R.S.; Lewis, J.D. Dietary patterns and self-reported associations of diet with symptoms of inflammatory bowel disease. Dig. Dis. Sci. 2013, 58, 1322–1328. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.; Ellis, C.J.; Furne, J.K.; Springfield, J.; Levitt, M.D. Fecal hydrogen sulfide production in ulcerative colitis. Am. J. Gastroenterol. 1998, 93, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Magee, E.A.; Richardson, C.J.; Hughes, R.; Cummings, J.H. Contribution of dietary protein to sulfide production in the large intestine: An in vitro and a controlled feeding study in humans. Am. J. Clin. Nutr. 2000, 72, 1488–1494. [Google Scholar] [CrossRef] [PubMed]
- Medani, M.; Collins, D.; Docherty, N.G.; Baird, A.W.; O’Connell, P.R.; Winter, D.C. Emerging role of hydrogen sulfide in colonic physiology and pathophysiology. Inflamm. Bowel Dis. 2011, 17, 1620–1625. [Google Scholar] [CrossRef] [PubMed]
- Attene-Ramos, M.S.; Nava, G.M.; Muellner, M.G.; Wagner, E.D.; Plewa, M.J.; Gaskins, H.R. DNA damage and toxicogenomic analyses of hydrogen sulfide in human intestinal epithelial FHS 74 int cells. Environ. Mol. Mutagen. 2010, 51, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Babidge, W.; Millard, S.; Roediger, W. Sulfides impair short chain fatty acid beta-oxidation at acyl-coa dehydrogenase level in colonocytes: Implications for ulcerative colitis. Mol. Cell. Biochem. 1998, 181, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, M.C.; Beatty, E.R.; Cummings, J.H. The contribution of sulphate reducing bacteria and 5-aminosalicylic acid to faecal sulphide in patients with ulcerative colitis. Gut 2000, 46, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Loubinoux, J.; Bronowicki, J.P.; Pereira, I.A.; Mougenel, J.L.; Faou, A.E. Sulfate-reducing bacteria in human feces and their association with inflammatory bowel diseases. FEMS Microbiol. Ecol. 2002, 40, 107–112. [Google Scholar] [CrossRef]
- Khalil, N.A.; Walton, G.E.; Gibson, G.R.; Tuohy, K.M.; Andrews, S.C. In vitro batch cultures of gut microbiota from healthy and ulcerative colitis (uc) subjects suggest that sulphate-reducing bacteria levels are raised in uc and by a protein-rich diet. Int. j. Food Sci. Nutr. 2014, 65, 79–88. [Google Scholar] [CrossRef]
- Suskind, D.L.; Wahbeh, G.; Cohen, S.A.; Damman, C.J.; Klein, J.; Braly, K.; Shaffer, M.; Lee, D. Patients perceive clinical benefit with the specific carbohydrate diet for inflammatory bowel disease. Dig. Dis. Sci. 2016, 61, 3255–3260. [Google Scholar] [CrossRef] [PubMed]
- Burgis, J.C.; Nguyen, K.; Park, K.T.; Cox, K. Response to strict and liberalized specific carbohydrate diet in pediatric Crohn’s disease. World J. Gastroenterol. 2016, 22, 2111–2117. [Google Scholar] [CrossRef] [PubMed]
- Obih, C.; Wahbeh, G.; Lee, D.; Braly, K.; Giefer, M.; Shaffer, M.L.; Nielson, H.; Suskind, D.L. Specific carbohydrate diet for pediatric inflammatory bowel disease in clinical practice within an academic ibd center. Nutrition 2016, 32, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Britto, S.; Kellermayer, R. Carbohydrate monotony as protection and treatment for inflammatory bowel disease. J. Crohn’s Colitis 2019. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, C. Tailoring treatment to the individual patient—Will inflammatory bowel disease medicine be personalized? Dig. Dis. 2015, 33 (Suppl. 1), 82–89. [Google Scholar] [CrossRef]
- Nagy-Szakal, D.; Mir, S.A.; Ross, M.C.; Tatevian, N.; Petrosino, J.F.; Kellermayer, R. Monotonous diets protect against acute colitis in mice: Epidemiologic and therapeutic implications. J. Pediatr. Gastroenterol. Nutr. 2013, 56, 544–550. [Google Scholar] [CrossRef]
- Konijeti, G.G.; Kim, N.; Lewis, J.D.; Groven, S.; Chandrasekaran, A.; Grandhe, S.; Diamant, C.; Singh, E.; Oliveira, G.; Wang, X.; et al. Efficacy of the autoimmune protocol diet for inflammatory bowel disease. Inflamm. Bowel Dis. 2017, 23, 2054–2060. [Google Scholar] [CrossRef]
- Knight-Sepulveda, K.; Kais, S.; Santaolalla, R.; Abreu, M.T. Diet and inflammatory bowel disease. Gastroenterol. Hepatol. 2015, 11, 511–520. [Google Scholar]
- van der Sloot, K.W.J.; Amini, M.; Peters, V.; Dijkstra, G.; Alizadeh, B.Z. Inflammatory bowel diseases: Review of known environmental protective and risk factors involved. Inflamm. Bowel Dis. 2017, 23, 1499–1509. [Google Scholar] [CrossRef]
- Schaffler, H.; Schmidt, M.; Huth, A.; Reiner, J.; Glass, A.; Lamprecht, G. Clinical factors are associated with vitamin d levels in ibd patients: A retrospective analysis. J. Dig. Dis. 2018, 19, 24–32. [Google Scholar] [CrossRef]
- Suibhne, T.N.; Cox, G.; Healy, M.; O’Morain, C.; O’Sullivan, M. Vitamin d deficiency in Crohn’s disease: Prevalence, risk factors and supplement use in an outpatient setting. J. Crohn’s Colitis 2012, 6, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Alrefai, D.; Jones, J.; El-Matary, W.; Whiting, S.J.; Aljebreen, A.; Mirhosseini, N.; Vatanparast, H. The association of vitamin d status with disease activity in a cohort of Crohn’s disease patients in Canada. Nutrients 2017, 9, 1112. [Google Scholar] [CrossRef] [PubMed]
- Narula, N.; Cooray, M.; Anglin, R.; Muqtadir, Z.; Narula, A.; Marshall, J.K. Impact of high-dose vitamin d3 supplementation in patients with Crohn’s disease in remission: A pilot randomized double-blind controlled study. Dig. Dis. Sci. 2017, 62, 448–455. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, S.P.; Agnholt, J.; Glerup, H.; Lyhne, S.; Villadsen, G.E.; Hvas, C.L.; Bartels, L.E.; Kelsen, J.; Christensen, L.A.; Dahlerup, J.F. Clinical trial: Vitamin d3 treatment in Crohn’s disease—A randomized double-blind placebo-controlled study. Aliment. Pharmacol. Ther. 2010, 32, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Dionne, S.; Duchatelier, C.F.; Seidman, E.G. The influence of vitamin d on m1 and m2 macrophages in patients with Crohn’s disease. Innate Immun. 2017, 23, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, Y.; Golan, M.A.; Annunziata, M.L.; Du, J.; Dougherty, U.; Kong, J.; Musch, M.; Huang, Y.; Pekow, J.; et al. Intestinal epithelial vitamin d receptor signaling inhibits experimental colitis. J. Clin. Investig. 2013, 123, 3983–3996. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Y.G.; Lu, R.; Xia, Y.; Zhou, D.; Petrof, E.O.; Claud, E.C.; Chen, D.; Chang, E.B.; Carmeliet, G.; et al. Intestinal epithelial vitamin d receptor deletion leads to defective autophagy in colitis. Gut 2015, 64, 1082–1094. [Google Scholar] [CrossRef]
- Ryz, N.R.; Lochner, A.; Bhullar, K.; Ma, C.; Huang, T.; Bhinder, G.; Bosman, E.; Wu, X.; Innis, S.M.; Jacobson, K.; et al. Dietary vitamin d3 deficiency alters intestinal mucosal defense and increases susceptibility to Citrobacter rodentium-induced colitis. Am. J. Ophysiol. Gastrointest. Liver physiol. 2015, 309, G730–G742. [Google Scholar] [CrossRef]
- Ooi, J.H.; Li, Y.; Rogers, C.J.; Cantorna, M.T. Vitamin d regulates the gut microbiome and protects mice from dextran sodium sulfate-induced colitis. J. Nutr. 2013, 143, 1679–1686. [Google Scholar] [CrossRef]
- Jin, D.; Wu, S.; Zhang, Y.G.; Lu, R.; Xia, Y.; Dong, H.; Sun, J. Lack of vitamin d receptor causes dysbiosis and changes the functions of the murine intestinal microbiome. Clin. Ther. 2015, 37, 996–1009.e7. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).