Influence of Diets with Varying Essential/Nonessential Amino Acid Ratios on Mouse Lifespan
Abstract
1. Introduction
2. Materials and methods
2.1. Animals
2.2. Diet Composition
- (1)
- (2)
- EAA-30% diet (EAA-poor diet) and thus NEAA-rich diet (70% of NEAA).
- (3)
- NEAA-100% diet contained exclusively NEAA as the source of nitrogen (thus 0% of EAA).
- (4)
- Casein-Prot diet contained only whole casein protein of highest quality.
- (5)
- Casein-AA (casein-like) diet contained free AAs equivalent to the composition of casein (EAA/NEAA = 49/51).
- (6)
- A commercial standard rodent laboratory diet (StD) (Mucedola srl, Milan, Italy) was also used.
2.3. Data and Sample Collections
2.4. Blood and Urine Analysis
2.5. Statistical Analysis
3. Results
3.1. Parameters Evaluation after 2 Months
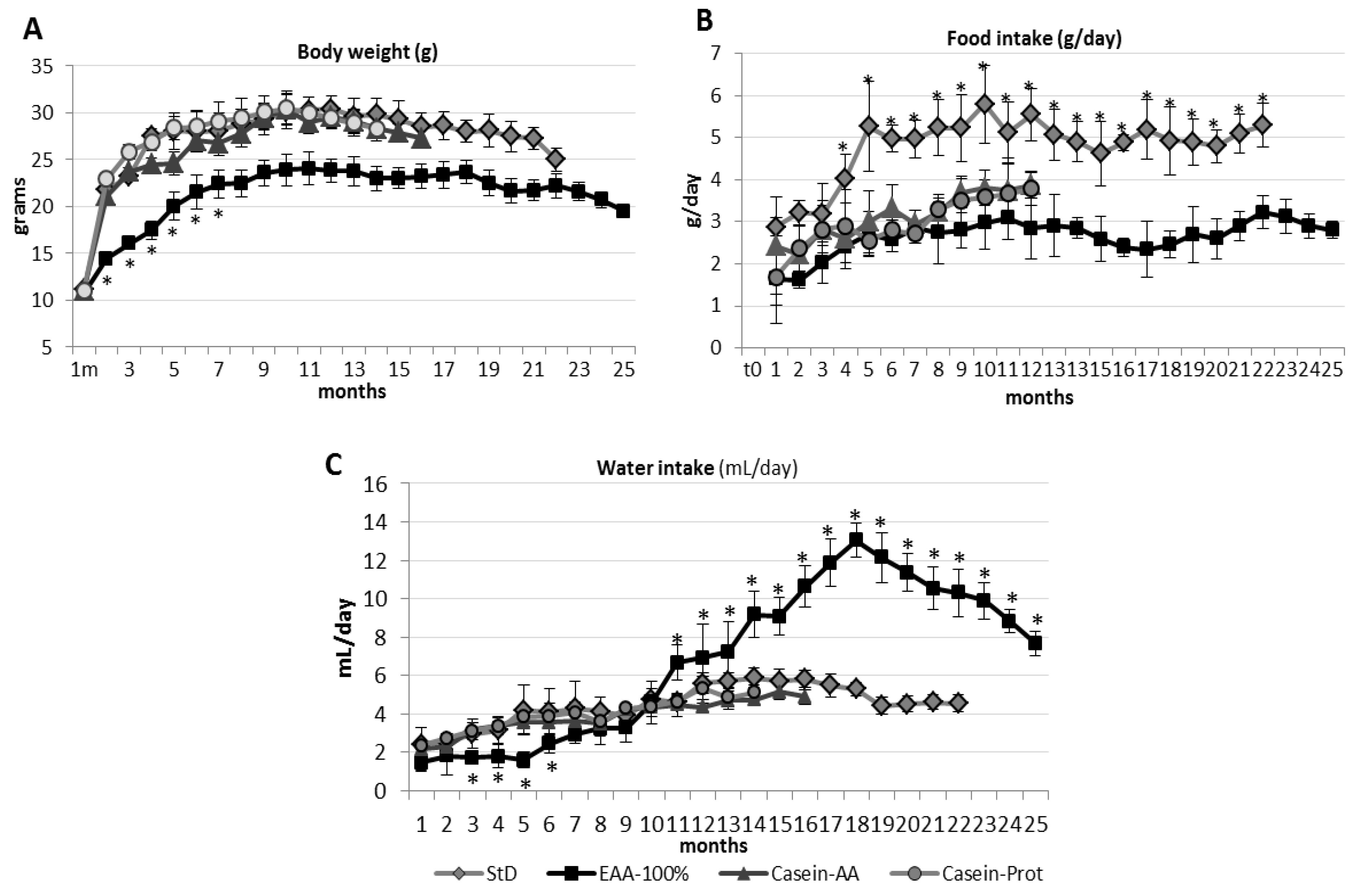
3.1.1. Body Weight (BW) and Length (BL)
3.1.2. Food and Water Consumption
3.1.3. Organ Weights
3.1.4. Blood and Urinary Parameters
3.2. Parameter Evaluation after 12 Months.
3.2.1. Body Weight and Length
3.2.2. Food and Water Consumption
3.2.3. Organ Weights
3.2.4. Blood and Urinary Parameters
3.3. Parameter Evaluation after 18 Months.
3.3.1. Body Weight and Length.
3.3.2. Food and Water Consumption
3.3.3. Organ Weights.
3.3.4. Blood and Urinary Parameters
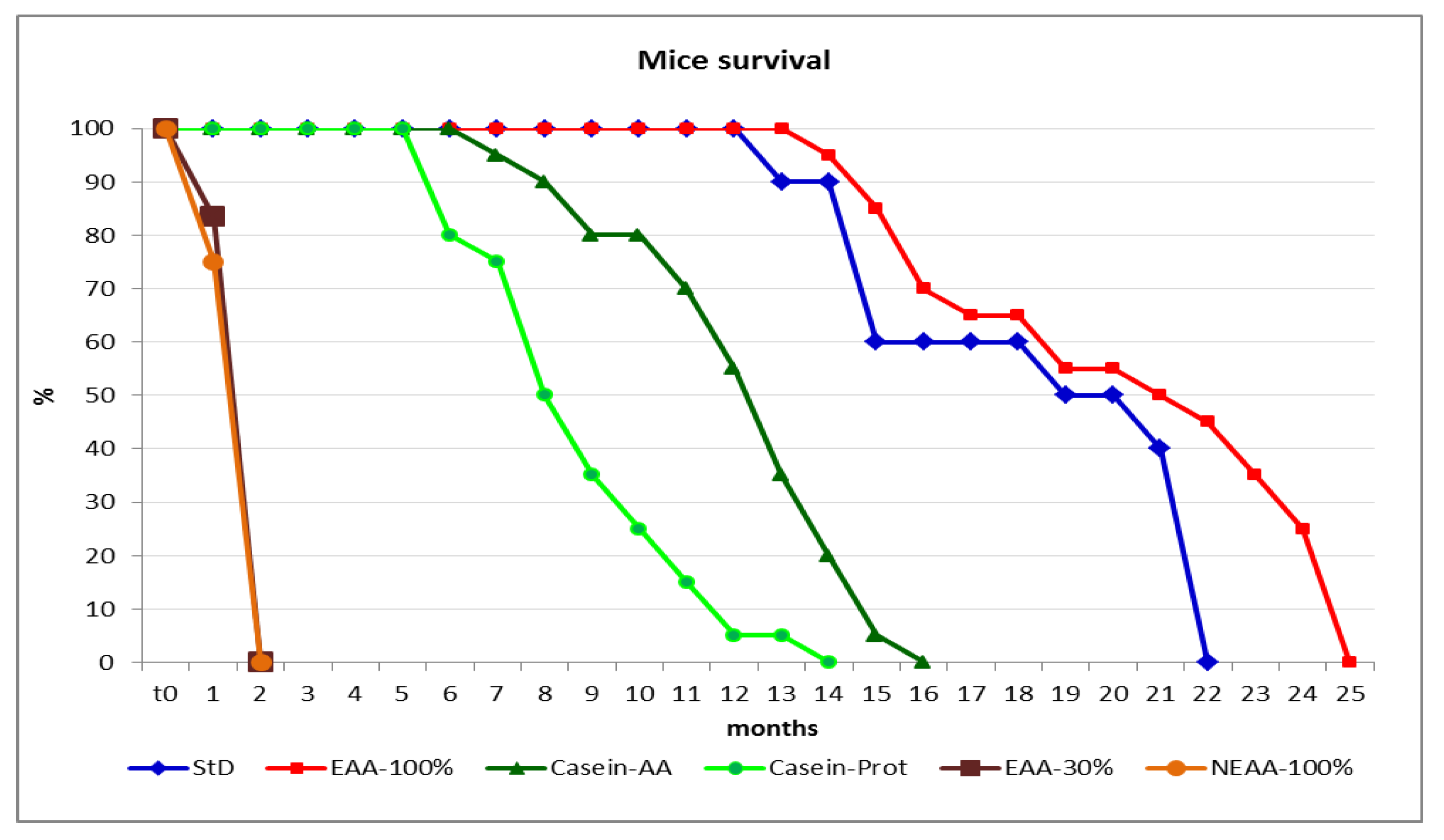
3.4. Parameter Evaluation after 22 Months
4. Discussion
4.1. Clinical Implications
4.2. Study limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Compliance with Ethical Standards
Abbreviations
References
- Pasini, E.; Corsetti, G.; Aquilani, R.; Romano, C.; Picca, A.; Calvani, R.; Dioguardi, F.S. Protein-amino acid metabolism disarrangements: The hidden enemy of chronic age-related conditions. Nutrients 2018, 10, 391. [Google Scholar] [CrossRef] [PubMed]
- Guigoz, Y. The mini nutritional assessment review of the literature: What does it tell us? J. Nutr. Health Aging 2006, 10, 466–485. [Google Scholar] [PubMed]
- Wu, G. Amino acids: Metabolism, functions, and nutrition. Amino Acids 2009, 37, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Yin, Y.; Wu, G. Dietary essentiality of “nutritionally non-essential amino acids” for animals and humans. Exp. Biol. Med. (Maywood) 2015, 240, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Borman, A.; Wood, T.R.; Balck, H.C.; Anderson, E.G.; Oesterling, M.J.; Womack, M.; Rose, W.C. The role of arginine in growth with some observations on the effects of argininic acid. J. Biol. Chem. 1946, 166, 585–594. [Google Scholar] [PubMed]
- Reeds, P.J. Dispensable and indispensable amino acids for humans. J. Nutr. 2000, 130, 1835S–1840S. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Yin, Y.; Liu, G.; Yu, X.; Li, Y.; Yang, G.; Li, T.; Wu, G. Effect of dietary arginine supplementation on reproductive performance of mice with porcine circovirus type 2 infection. Amino Acids 2012, 42, 2089–2094. [Google Scholar] [CrossRef]
- Rose, W.C.; Oesterling, M.J.; Womack, M.J. Comparative growth on diets containing ten and 19 amino acids, with further observations upon the role of glutamic and aspartic acids. Biol. Chem. 1948, 176, 753–762. [Google Scholar]
- Wu, G.; Wu, Z.; Dai, Z.; Yang, Y.; Wang, W.; Liu, C.; Wang, B.; Wang, J.; Yin, Y. Dietary requirements of “nutritionally non-essential amino acids” by animals and humans. Amino Acids 2013, 44, 1107–1113. [Google Scholar] [CrossRef]
- Civitarese, A.E.; Carling, S.; Heilbronn, L.K.; Hulver, M.H.; Ukropcova, B.; Deutsch, W.A.; Smith, S.R.; Ravussin, E.; CALERIE Pennington Team. Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007, 4, e76. [Google Scholar] [CrossRef]
- D’Antona, G.; Ragni, M.; Cardile, A.; Tedesco, L.; Dossena, M.; Bruttini, F.; Caliaro, F.; Corsetti, G.; Bottinelli, R.; Carruba, M.O.; et al. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 2010, 12, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, G.; Pasini, E.; D’Antona, G.; Nisoli, E.; Flati, V.; Assanelli, D.; Dioguardi, F.S.; Bianchi, R. Morphometric changes induced by amino acid supplementation in skeletal and cardiac muscles of old mice. Am. J. Cardiol. 2008, 101, S26–S34. [Google Scholar] [CrossRef] [PubMed]
- Flati, V.; Pasini, E.; D’Antona, G.; Speca, S.; Toniato, E.; Martinotti, S. Intracellular mechanisms of metabolism regulation: The role of signaling via the mammalian target of rapamycin pathway and other routes. Am. J. Cardiol. 2008, 101, 16E–21E. [Google Scholar] [CrossRef] [PubMed]
- Stacchiotti, A.; Corsetti, G.; Lavazza, A.; Rezzani, R. Microscopic features of mitochondria rejuvenation by amino acids. In Current Microscopy Contributions to Advances in Science and Technology; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2012; pp. 286–294. [Google Scholar]
- Corsetti, G.; Stacchiotti, A.; Tedesco, L.; D’Antona, G.; Pasini, E.; Dioguardi, F.S.; Nisoli, E.; Rezzani, R. Essential amino acid supplementation decreases liver damage induced by chronic ethanol consumption in rats. Int. J. Immunopathol. Pharmacol. 2011, 24, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, L.; Corsetti, G.; Ruocco, C.; Ragni, M.; Rossi, F.; Carruba, M.O.; Valerio, A.; Nisoli, E. A specific amino acid formula prevents alcoholic liver disease in rodents. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G566–G582. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, G.; D’Antona, G.; Ruocco, C.; Stacchiotti, A.; Romano, C.; Tedesco, L.; Dioguardi, F.S.; Rezzani, R.; Nisoli, E. Dietary supplementation with essential amino acids boots the beneficial effects of rosuvastatin on mouse kidney. Amino Acids 2014, 6, 2189–2203. [Google Scholar] [CrossRef][Green Version]
- Corsetti, G.; Romano, C.; Pasini, E.; Marzetti, E.; Calvani, R.; Picca, A.; Flati, V.; Dioguardi, F.S. Diet enrichment with a specific essential free amino acid mixture improves healing of undressed wounds in aged rats. Exp. Geront. 2017, 96, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Bonfili, L.; Cecarini, V.; Cuccioloni, M.; Angeletti, M.; Flati, V.; Corsetti, G.; Pasini, E.; Dioguardi, F.S.; Eleuteri, A.M. Essential amino acid mixtures drive cancer cells to apoptosis through proteasome inhibition and autophagy activation. FEBS J. 2017, 284, 1726–1737. [Google Scholar] [CrossRef]
- Dioguardi, F.S. Clinical use of amino acids as dietary supplement: Pros and cons. J. Cachexia Sarcopenia Muscle 2011, 2, 75–80. [Google Scholar] [CrossRef]
- Wanders, D.; Forney, L.A.; Stone, K.P.; Burk, D.H.; Pierse, A.; Gettys, T.W. FGF21 mediates the thermogenic and insulin-sensitizing effects of dietary methionine restriction but not its effects on hepatic lipid metabolism. Diabetes 2017, 66, 858–867. [Google Scholar] [CrossRef]
- Cummings, N.E.M.; Williams, E.M.; Kasza, I. Restoration of metabolic health by decreased consumption of branched-chain amino acids. J. Physiol. 2018, 596, 623–645. [Google Scholar] [CrossRef] [PubMed]
- Wanders, D.; Stone, K.P.; Dille, K.; Simon, J.; Pierse, A.; Gettys, T.W. Metabolic responses to dietary leucine restriction involve remodeling of adipose tissue and enhanced hepatic insulin signaling. Biofactors 2015, 41, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Simpson, S.J.; Le Couteur, D.G.; Raubenheimer, D.; Solon-Biet, S.M.; Cooney, G.J.; Cogger, V.C.; Fontana, L. Dietary protein, aging and nutritional geometry. Ageing Res. Rev. 2017, 39, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Le Couteur, D.G.; Solon-Biet, S.; Wahl, D.; Cogger, V.C.; Willcox, B.J.; Willcox, D.C.; Raubenheimer, D.; Simpson, S.J. New Horizons: Dietary protein, ageing and the Okinawan ratio. Age Ageing 2016, 45, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Le Couteur, D.G.; Solon-Biet, S.; Cogger, V.C.; Mitchell, S.J.; Senior, A.; de Cabo, R.; Raubenheimer, D.; Simpson, S.J. The impact of low-protein high-carbohydrate diets on aging and lifespan. Cell Mol. Life Sci. 2016, 73, 1237–1252. [Google Scholar] [CrossRef]
- Corsetti, G.; Pasini, E.; Romano, C.; Calvani, R.; Picca, A.; Marzetti, E.; Flati, V.; Dioguardi, F.S. Body weight loss and tissue wasting in late middle-aged mice on slightly imbalanced essential/non-essential amino acids diet. Front. Med. (Lausanne) 2018, 17, 136. [Google Scholar] [CrossRef] [PubMed]
- Pasini, E.; Aquilani, R.; Dioguardi, F.S.; D’Antona, G.; Gheorghiade, M.; Taegtmeyer, H. Hypercatabolic syndrome: Molecular basis and effects of nutritional supplements with amino acids. Am. J. Cardiol. 2008, 101, 11E–15E. [Google Scholar] [CrossRef]
- Reeves, P.G.; Forrest, H.; Nielsen, F.H.; Fahey, G.C., Jr. AIN-93 Purified diets for laboratory rodents: Final report of the american institute of nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Blouet, C.; Mariotti, F.; Azzout-Marniche, D.; Bos, C.; Mathé, V.; Tomé, D.; Huneau, J.F. The reduced energy intake of rats fed a high-protein low-carbohydrate diet explains the lower fat deposition, but macronutrient substitution accounts for the improved glycemic control. J. Nutr. 2006, 136, 1849–1854. [Google Scholar] [CrossRef] [PubMed]
- French, T. Acute phase proteins. In The Clinical Chemistry of Laboratory Animals; Loeb, W.F., Quimby, F.W., Eds.; Pergamon Press: Oxford, UK, 1989; Volume 1, pp. 201–235. [Google Scholar]
- Ouellet, G.; Malhotra, R.; Penne, E.L.; Usvya, L.; Levin, N.W.; Kotanko, P. Neutrophil-lymphocyte ratio as a novel predictor of survival in chronic hemodialysis patients. Clin. Nephrol. 2016, 85, 191–198. [Google Scholar] [CrossRef]
- Prats-Puig, A.; Gispert-Saüch, M.; Díaz-Roldán, F.; Carreras-Badosa, G.; Osiniri, I.; Planella-Colomer, M.; Mayol, L.; de Zegher, F.; Ibánez, L.; Bassols, J.; et al. Neutrophil-to-lymphocyte ratio: An inflammation marker related to cardiovascular risk in children. Thromb. Haemost. 2015, 114. [Google Scholar] [CrossRef]
- Evan’s Awesome A/B Tools. Available online: http://www.evanmiller.org/ab-testing/survival-curves.html (accessed on 8 May 2019).
- Volpi, E.; Kobayashi, H.; Sheffield-Moore, M.; Mittendorfer, B.; Wolfe, R.R. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am. J. Clin. Nutr. 2003, 78, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Mitrache, C.; Passweg, J.R.; Libura, J.; Petrikkos, L.; Seiler, W.O.; Gratwohl, A.; Stähelin, H.B.; Tichelli, A. Anemia: An indicator for malnutrition in the elderly. Ann. Hematol. 2001, 80, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Ou, S.M.; Chen, Y.T.; Hung, S.C.; Shih, C.J.; Lin, C.H.; Chiang, C.K.; Tarng, D.C. Taiwan Geriatric Kidney Disease (TGKD) Research Group: Association of estimated glomerular filtration rate with all-cause and cardiovascular mortality: The role of malnutrition-inflammation-cachexia syndrome. J. Cachexia Sarcopenia Muscle 2016, 7, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Shih, A.W.; McFarlane, A.; Verhovsek, M. Haptoglobin testing in hemolysis: Measurement and interpretation. Am. J. Hematol. 2014, 89, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.C.; Hyen Baek, J.; Hassell, K.; Nuss, R.; Eigenberger, P.; Lisk, C.; Loomis, Z.; Maltzahn, J.; Stenmark, K.R.; Nozik-Grayck, E.; et al. Hemoglobin-induced lung vascular oxidation, inflammation, and remodeling contribute to the progression of hypoxic pulmonary hypertension and is attenuated in rats with repeated-dose haptoglobin administration. Free Radic. Biol. Med. 2015, 82, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Romano, C.; Corsetti, G.; Pasini, E.; Flati, V.; Dioguardi, F.S. Dietary modifications of nitrogen intake decreases inflammation and promotes rejuvenation of spleen in aged mice. J. Food Nutr. Res. 2018, 6, 419–432. [Google Scholar] [CrossRef][Green Version]
- Potier, M.; Darcela, N.; Tome, D. Protein, amino acids and the control of food intake. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 54–58. [Google Scholar] [CrossRef]
- Dubos, R.; Schaedler, R.W.; Costello, R. Lasting biological effects of early environmental influences. 1. Conditioning of adult size by prenatal and postnatal nutrition. J. Exp. Med. 1968, 127, 783–799. [Google Scholar] [CrossRef]
- Goettsch, M. Comparative protein requirement of the rat and mouse for growth, reproduction and lactation using casein diets. J. Nutr. 1960, 70, 307–312. [Google Scholar] [CrossRef]
- Miner-Williams, W.; Deglaire, A.; Benamouzig, R.; Fuller, M.F.; Tomé, D.; Moughan, P.J. Endogenous proteins in terminal ileal digesta of adult subjects fed a casein-based diet. Am. J. Clin. Nutr. 2012, 96, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Jin, U.-H.; Lee, S.-O.; Sridharan, G.; Lee, K.; Davidson, L.A.; Jayaraman, A.; Chapkin, R.S.; Alaniz, R.; Safe, S. Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol. Pharmacol. 2014, 85, 777–788. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Wu, L.; Xu, Z.; Li, T.; Yao, K.; Cui, Z.; Yin, Y.; Wu, G. Low-protein diets affect ileal amino acid digestibility and gene expression of digestive enzymes in growing and finishing pigs. Amino Acids 2015, 48, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Rondanelli, M.; Aquilani, R.; Verri, M.; Boschi, F.; Pasini, E.; Perna, S.; Faliva, A.; Condino, A.M. Plasma kinetics of essential amino acids following their ingestion as free formula or as dietary protein components. Aging Clin. Exp. Res. 2017, 29, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Jäger, R.; Kerksick, C.M.; Campbell, B.I.; Cribb, P.J.; Wells, S.D.; Skwiat, T.M.; Purpura, M.; Ziegenfuss, T.N.; Ferrando, A.A.; Arent, S.M.; et al. International Society of Sports Nutrition Position Stand: Protein and exercise. J. Int. Soc. Sports Nutr. 2017, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Ables, G.P.; Johnson, J.E. Pleiotropic responses to methionine restriction. Exp. Gerontol. 2017, 94, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Levitt, D.G.; Levitt, M.D. Human serum albumin homeostasis: A new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int. J. Gen. Med. 2016, 9, 229–255. [Google Scholar] [CrossRef] [PubMed]
- Gowda, S.; Desai, P.B.; Kulkarni, S.S.; Hull, V.V.; Math, A.A.; Vernekar, S.N. Markers of renal function tests. N. Am. J. Med. Sci. 2010, 2, 170–173. [Google Scholar]
- Miller, R.A.; Chrisp, C.; Atchley, W. Differential longevity in mouse stocks selected for early life growth trajectory. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, B455–B461. [Google Scholar] [CrossRef]
- He, Q.; Morris, B.J.; Grove, J.S.; Petrovitch, H.; Ross, W.; Masaki, K.H.; Rodriguez, B.; Chen, R.; Donlon, T.A.; Willcox, D.C.; et al. Shorter men live longer: Association of height with longevity and FOXO3 genotype in american men of japanese ancestry. PLoS ONE 2014, 9, e94385. [Google Scholar] [CrossRef] [PubMed]
- Aquilani, R.; Zuccarelli, G.C.; Condino, A.M.; Catani, M.; Rutili, C.; Del Vecchio, C.; Pisano, P.; Verri, M.; Iadarola, P.; Viglio, S.; et al. Despite inflammation, supplemented essential amino acids may improve circulating levels of albumin and hemoglobin in patients after hip fractures. Nutrients 2017, 9, 637. [Google Scholar] [CrossRef] [PubMed]
- Boselli, M.; Aquilani, R.; Maestri, R.; Achilli, M.P.; Arrigoni, N.; Pasini, E.; Condino, A.M.; Boschi, F.; Dossena, M.; Buonocore, D.; et al. Inflammation and rehabilitation outcomes in patients with nontraumatic intracranial hemorrhage. NeuroRehabilitation 2018, 42, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Meng, Q.; Wang, C.; Li, H.; Huang, Z.; Chen, S.; Xiao, F.; Guo, F. Leucine deprivation decreases fat mass by stimulation of lipolysis in white adipose tissue and upregulation of uncoupling protein 1 (UCP1) in brown adipose tissue. Diabetes 2010, 59, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Partridge, L.; Longo, V.D. Extending healthy life span--from yeast to humans. Science 2010, 328, 321–326. [Google Scholar] [CrossRef]

| Nutrients | EAA-100% | Casein-AA | Casein-Prot | StD | EAA-30% | NEAA-100% |
|---|---|---|---|---|---|---|
| KCal/Kg | 3995 | 3995 | 3995 | 3952 | 3995 | 3995 |
| Carbohydrates % | 61.76 | 61.76 | 61.76 | 54.61 | 61.76 | 61.76 |
| Lipids % | 6.12 | 6.12 | 6.12 | 7.5 | 6.12 | 6.12 |
| Nitrogen % | 20 * | 20 * | 20 ^ | 21.8 ° | 20 * | 20 * |
| Proteins: % of total nitrogen content | 0 | 0 | 100 | 95.93 | 0 | 0 |
| Free AAs: % of total nitrogen content | 100 | 100 | 0 | 4.07 | 100 | 100 |
| EAA/NEAA (% in grams) | 100/0 | 49/51 | - | - | 30/70 | 0/100 |
| Free AAs composition (%) | ||||||
| L-Leucine (bcaa) | 31.25 | 9.5 | -- | -- | 9.4 | -- |
| L-Isoleucine (bcaa) | 15.62 | 6 | -- | -- | 4.7 | -- |
| L-Valine (bcaa) | 15.62 | 6.5 | -- | -- | 4.7 | -- |
| L-Lysine | 16.25 | 7 | -- | 0.97 | 6.24 | -- |
| L-Threonine | 8.75 | 4 | -- | -- | 2.7 | -- |
| L-Hystidine | 3.75 | 2.8 | -- | -- | 1.1 | -- |
| L-Phenylalanine | 2.5 | 5 | -- | -- | 0.8 | -- |
| L-Cysteine | -- | 0.8 | -- | -- | -- | -- |
| L-Cystine | 3.75 | -- | -- | 0.39 | 1.1 | -- |
| L-Methionine | 1.25 | 2.5 | -- | 0.45 | 0.4 | -- |
| L-Tyrosine | 0.75 | 5 | -- | -- | 2.6 | 1.0 |
| L-Triptophan | 0.5 | 1.3 | -- | 0.28 | 0.01 | -- |
| L-Alanine | -- | 3.2 | -- | -- | 24.0 | 35.0 |
| L-Glycine | -- | 2.4 | -- | 0.88 | 10.39 | 15.0 |
| L-Arginine | -- | 3.4 | -- | 1.1 | 13.5 | 14.0 |
| L-Proline | -- | 9.5 | -- | -- | 8.2 | 12.0 |
| L-Glutamine | -- | 9.5 | -- | -- | 3.0 | 12.0 |
| L-Serine | -- | 5.1 | -- | -- | 4.1 | 6.0 |
| L-Glutamic Acid | -- | 9.5 | -- | -- | 2.5 | 2.0 |
| L-Asparagine | -- | 3.5 | -- | -- | 0.79 | 2.0 |
| L-Aspartic Acid | -- | 3.5 | -- | -- | 1.1 | 1.0 |
| NEAA-100% | EAA-30% | EAA-100% | StD | Casein-Prot | Casein-AA | |||
|---|---|---|---|---|---|---|---|---|
| 2 months | F | p | ||||||
| Body W. (g) | 7.09 ± 0.41 *^ | 7.31 ± 0.52 *^ | 14.93 ± 0.62 * | 22.35 ± 1.9 | 22.02 ± 1.2 | 21.84 ± 0.9 | 238.26 | 0.000 |
| Body L. (cm) | 6.61 ± 0.1 *^ | 6.63 ± 0.12 *^ | 9.15 ± 0.13 * | 9.66 ± 0.1 | 9.62 ± 0.2 | 9.47 ± 0.17 | 1024.88 | 0.000 |
| Heart (g) | 0.08 ± 0.015* | 0.08 ± 0.01* | 0.08 ± 0.03 * | 0.13 ± 0.006 | - | - | 9.75 | 0.000 |
| Kidneys (g) | 0.12 ± 0.008*^ | 0.12 ± 0.009*^ | 0.24 ± 0.028 * | 0.40 ± 0.05 | - | - | 105.71 | 0.000 |
| Liver (g) | 0.32 ± 0.049*^ | 0.34 ± 0.61*^ | 0.75 ± 0.066 * | 1.10 ± 0.17 | - | - | 70.95 | 0.000 |
| Spleen (g) | 0.02 ± 0.004 * | 0.02 ± 0.003 * | 0.05 ± 0.008 * | 0.11 ± 0.032 | - | - | 32.35 | 0.000 |
| rpWAT (g) | 0 * | 0 * | 0.02 ± 0.002 * | 0.11 ± 0.02 | - | - | 123.97 | 0.000 |
| BAT (g) | 0.02 ± 0.01*^ | 0.02 ± 0.009*^ | 0.09 ± 0.01 * | 0.13 ± 0.015 | - | - | 112.59 | 0.000 |
| Triceps (g) | 0.07 ± 0.01*^ | 0.06 ± 0.002*^ | 0.10 ± 0.011 * | 0.19 ± 0.01 | - | - | 215.38 | 0.000 |
| 12 months | F | p | ||||||
| Body W. (g) | - | - | 24.51 ± 1.9 * | 31.78 ± 1.69 | 30.39 ± 2.79 ^ | 29.56 ± 1.0 ^ | 12.95 | 0.000 |
| Body L. (cm) | - | - | 9.82 ± 0.12 | 10.0 ± 0.13 | 10.01 ± 0.14 | 9.87 ± 0.13 | 2.65 | 0.085 |
| Heart (g) | - | - | 0.15 ± 0.02 *° | 0.23 ± 0.03 | 0.18 ± 0.02 | 0.22 ± 0.05 | 6.51 | 0.000 |
| Kidneys (g) | - | - | 0.46 ± 0.06 *°§ | 0.8 ± 0.1 | 0.7 ± 0.05 | 0.69 ± 0.06 | 21.0 | 0.000 |
| Liver (g) | - | - | 1.36 ± 0.13 *° | 1.79 ± 0.2 | 1.56 ± 0.2 | 1.7 ± 0.2 | 5.13 | 0.011 |
| Spleen (g) | - | - | 0.12 ± 0.03 | 0.15 ± 0.06 | 0.18 ± 0.02 | 0.15 ± 0.04 | 1.85 | 0.179 |
| rpWAT (g) | - | - | 0.07 ± 0.02 *§ | 0.17 ± 0.01 | 0.16 ± 0.08 | 0.09 ± 0.03 * | 6.39 | 0.005 |
| BAT (g) | - | - | 0.13 ± 0.02 § | 0.2 ± 0.04 | 0.23 ± 0.06 | 0.17 ± 0.03 | 5.62 | 0.008 |
| Triceps (g) | - | - | 0.18 ± 0.01 *°§ | 0.26 ± 0.02 | 0.31 ± 0.02 * | 0.34 ± 0.03 * | 54.35 | 0.000 |
| 18 months | t | p | ||||||
| Body W. (g) | - | - | 23.67 ± 1.15 * | 28.31 ± 1.04 | - | - | 6.692 | 0.000 |
| Body L. (cm) | - | - | 9.79 ± 0.11 | 9.95 ± 0.13 | - | - | 2.101 | 0.069 |
| Heart (g) | - | - | 0.15 ± 0.01 * | 0.2 ± 0.01 | - | - | 7.906 | 0.000 |
| Kidneys (g) | - | - | 0.47 ± 0.04 * | 0.68 ± 0.07 | - | - | 5.824 | 0.000 |
| Liver (g) | - | - | 1.18 ± 0.09 * | 1.6 ± 0.15 | - | - | 5.369 | 0.000 |
| Spleen (g) | - | - | 0.08 ± 0.03 * | 0.13 ± 0.04 | - | - | 2.236 | 0.000 |
| rpWAT (g) | - | - | 0.07 ± 0.03 * | 0.11 ± 0.02 | - | - | 2.481 | 0.038 |
| BAT (g) | - | - | 0.12 ± 0.01 * | 0.17 ± 0.01 | - | - | 7.906 | 0.000 |
| Triceps (g) | - | - | 0.15 ± 0.01 * | 0.21 ± 0.01 | - | - | 9.487 | 0.000 |
| 22 months | t | p | ||||||
| Body W. (g) | - | - | 22.2 ± 1.56 * | 25.01 ± 1.2 | - | - | 4.515 | 0.000 |
| Body L. (cm) | - | - | 9.75 ± 0.12 | 9.81 ± 0.14 | - | - | 0.794 | 0.444 |
| NEAA-100% | EAA-30% | EAA-100% | StD | Casein-Prot | Casein-AA | |||
|---|---|---|---|---|---|---|---|---|
| Blood 2 months | F | p | ||||||
| Glucose (mg/dL) | 108.3 ± 6.7 *^ | 111.7 ± 7.9 * | 122.04 ± 8.1 | 127.3 ± 9.6 | - | - | 8.47 | 0.000 |
| Erythrocytes (M/μL) | 7.75 ± 1.93 | 8.01 ± 1.32 | 9.04 ± 0.58 | 9.13 ± 0.29 | - | - | 1.69 | 0.210 |
| Hemoglobin (g/dL) | 10.23 ± 2.37 *^ | 11.46 ± 2.15 *^ | 14.18 ± 1.16 | 14.8 ± 0.46 | - | - | 8.04 | 0.002 |
| NLR | 1.88 ± 1.02 *^ | 1.64 ± 0.93 *^ | 0.68 ± 0.26 | 0.67 ± 0.25 | - | - | 3.95 | 0.028 |
| Albumin (g/L) | 22.58 ± 1.68 *^ | 24.75 ± 1.56 * | 26.8 ± 1.83 | 28.66 ± 2.57 | - | - | 9.03 | 0.000 |
| Creatinine (μmol/L) | 25.92 ± 4.54 | 24.95 ± 3.9 | 22.8 ± 1.75 | 23.82 ± 1.89 | - | - | 0.86 | 0.480 |
| Haptoglobin (mg/mL) | 0.02 ± 0.01 *^ | 0.04 ± 0.01 *^ | 0.13 ± 0.02 * | 0.18 ± 0.02 | - | - | 113.83 | 0.000 |
| Urine 3 months | ||||||||
| Albumin (g/L) | 0.9 ± 0.4 *^ | 0.8 ± 0.6 *^ | 2.2 ± 0.4 | 1.8 ± 0.3 | - | - | 12.19 | 0.000 |
| Creatinine (μmol/L) | 5502 ± 443 *^ | 5653 ± 520 *^ | 3956 ± 824 | 4066 ± 1027 | - | - | 7.49 | 0.002 |
| Blood 12 months | F | p | ||||||
| Glucose (mg/dL) | - | - | 119.3 ± 11.4 | 124.3 ± 19.4 | 127.25 ± 14 | 113.25 ± 4.3 | 1.04 | 0.40 |
| Erythrocytes (M/μL) | - | - | 9.65 ± 0.62 | 9.93 ± 0.37 | 9.28 ± 0.33 | 9.8 ± 0.28 | 2.23 | 0.124 |
| Hemoglobin (g/dL) | - | - | 13.35 ± 1.02 | 14.4 ± 1.5 | 14.32 ± 0.3 | 14.4 ± 0.2 | 1.54 | 0.243 |
| NLR | - | - | 0.71 ± 0.1 | 0.72 ± 0.12 | 0.65 ± 0.07 | 0.67 ± 0.14 | 0.45 | 0.723 |
| Albumin (g/L) | - | - | 29.7 ± 1.7 | 30.88 ± 2.1 | 46.2 ± 8.03 *^ | 31.6 ± 6.6 ^ | 11.09 | 0.000 |
| Creatinine (μmol/L) | - | - | 46.9 ± 2.3 | 42.9 ± 5.7 | 72.33 ± 12.7 *^ | 57.0 ± 12.4 | 9.75 | 0.000 |
| Haptoglobin (mg/mL) | - | - | 0.10 ± 0.04 * | 0.15 ± 0.07 | 4.53 ± 0.15 *^ | 4.97 ± 0.06 *^ | 3968 | 0.000 |
| Urine 12 months | ||||||||
| Albumin (g/L) | - | - | 2.13 ± 0.23 | 1.94 ± 0.21 | 1.3 ± 0.19 *^ | 1.02 ± 0.35 *^ | 21.46 | 0.000 |
| Creatinine (μmol/L) | - | - | 3581 ± 526 | 3832 ± 364 | 2934 ± 355 *^ | 2595 ± 222.7 *^ | 11.13 | 0.000 |
| Blood 18 months | t | p | ||||||
| Glucose (mg/dL) | - | - | 127.2 ± 8.3 | 133.4 ± 12.6 | - | - | 0.919 | 0.385 |
| Erythrocytes (M/μL) | - | - | 9.73 ± 0.25 | 9.97 ± 1.06 | - | - | 0.493 | 0.635 |
| Hemoglobin (g/dL) | - | - | 14.23 ± 0.15 | 14.87 ± 1.4 | - | - | 1.016 | 0.339 |
| NLR | - | - | 0.95 ± 0.2 * | 1.44 ± 0.08 | - | - | 5.087 | 0.000 |
| Albumin (g/L) | - | - | 29.1 ± 0.7 * | 27.4 ± 0.78 | - | - | 2.332 | 0.04 |
| Creatinine (μmol/L) | - | - | 38.3 ± 2.69 | 37.8 ± 1.45 | - | - | 0.366 | 0.724 |
| Haptoglobin (mg/mL) | - | - | 0.33 ± 0.15 * | 0.56 ± 0.16 | - | - | 2.268 | 0.05 |
| Urine 18 months | ||||||||
| Albumin (g/L) | - | - | 1.58 ± 0.99 * | 3.49 ± 0.87 | - | - | 3.241 | 0.01 |
| Creatinine (μmol/L) | - | - | 2954.9 ± 927 | 3421.7 ± 670 | - | - | 0.913 | 0.388 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, C.; Corsetti, G.; Flati, V.; Pasini, E.; Picca, A.; Calvani, R.; Marzetti, E.; Dioguardi, F.S. Influence of Diets with Varying Essential/Nonessential Amino Acid Ratios on Mouse Lifespan. Nutrients 2019, 11, 1367. https://doi.org/10.3390/nu11061367
Romano C, Corsetti G, Flati V, Pasini E, Picca A, Calvani R, Marzetti E, Dioguardi FS. Influence of Diets with Varying Essential/Nonessential Amino Acid Ratios on Mouse Lifespan. Nutrients. 2019; 11(6):1367. https://doi.org/10.3390/nu11061367
Chicago/Turabian StyleRomano, Claudia, Giovanni Corsetti, Vincenzo Flati, Evasio Pasini, Anna Picca, Riccardo Calvani, Emanuele Marzetti, and Francesco Saverio Dioguardi. 2019. "Influence of Diets with Varying Essential/Nonessential Amino Acid Ratios on Mouse Lifespan" Nutrients 11, no. 6: 1367. https://doi.org/10.3390/nu11061367
APA StyleRomano, C., Corsetti, G., Flati, V., Pasini, E., Picca, A., Calvani, R., Marzetti, E., & Dioguardi, F. S. (2019). Influence of Diets with Varying Essential/Nonessential Amino Acid Ratios on Mouse Lifespan. Nutrients, 11(6), 1367. https://doi.org/10.3390/nu11061367











