Preparation and Identification of Novel Antihypertensive Peptides from the In Vitro Gastrointestinal Digestion of Marine Cobia Skin Hydrolysates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hydrolysates of Cobia Skin
2.3. Biochemical Analysis
2.4. Determination of Peptides
2.5. Measurement of Free Amino Acid Content
2.6. ACE Inhibition Assay
2.7. In Vitro Gastrointestinal Digestion
2.8. Gel Filtration
2.9. Isolation of Bioactive Peptides with ACE-Inhibitory Activity
2.10. Identification of Antihypertensive Peptide Sequences
2.11. Blood Pressure Measurement
2.12. Statistical Data Treatment
3. Results and Discussion
3.1. IC50 and Soluble Protein, Peptide, and Free Amino Acid Contents
3.2. Antihypertensive Effect of Cobia Skin Protein Hydrolysate
3.3. In Vitro Digestive Stability and ACE inhibition of the Cobia Skin Hydrolysates
3.4. Separation and Assessment of Potent ACE-Inhibitory Peptides
3.5. Amino Acid Sequences and ACE-Inhibitory Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PN-5 | hydrolysate from Protease N hydrolysis for 5 h |
| PX-5 | hydrolysate from Protamex hydrolysis for 5 h |
| ACE | angiotensin I-converting enzyme |
| MW | molecular weight |
| SBP | systolic blood pressure |
| DBP | diastolic blood pressure |
| SHRs | spontaneously hypertensive rats |
| BW | body weight |
| RP-HPLC | reverse-phase high-performance liquid chromatography |
| IER | inhibitory efficiency ratio |
| HHL | hippuryl- L-histidyl-L-leucine |
| MWCO | molecular weight cut-off |
| HA | hippuric acid |
References
- Lopez, A.D.; Murray, C.C. The global burden of disease, 1990–2020. Nat. Med. 1998, 4, 1241–1243. [Google Scholar] [CrossRef] [PubMed]
- Yee, S.; Amidon, G.L. Oral absorption of angiotensin-converting enzyme inhibitors and peptide prodrugs. In Peptide-Based Drug Design; Taylor, M.D., Amidon, G.L., Eds.; American Chemistry Society, NW: Washington, DC, USA, 1995; pp. 137–147. [Google Scholar]
- Alderman, C.P. Adverse effects of the angiotensin-converting enzyme inhibitors. Ann. Pharmacother. 1996, 30, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Al Shohaib, S.; Raweily, E. Acute tubular necrosis due to captopril. Am. J. Nephrol. 2000, 20, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Vercruysse, L.; Camp, J.V.; Smagghe, G. ACE inhibitory peptides derived from enzymatic hydrolysates of animal muscle protein: A review. J. Agric. Food Chem. 2005, 53, 8106–8115. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.W.; Tsai, J.S.; Sun, P.B. Purification of Angiotensin I-converting enzyme inhibitory peptides and antihypertensive effect of milk produced by protease-facilitated lactic fermentation. Int. Dairy J. 2007, 17, 641–647. [Google Scholar] [CrossRef]
- Chen, G.W.; Tsa, J.S.; Sun, P.B. Cardiovascular effects of whey from prozyme 6-facilitated lactic acid bacteria fermentation of milk. J. Food Biochem. 2007, 31, 639–655. [Google Scholar] [CrossRef]
- Tsai, J.S.; Chen, T.J.; Pan, B.S.; Gong, S.D.; Chung, M.Y. Antihypertensive effect of bioactive peptides produced by protease-facilitated lactic acid fermentation of milk. Food Chem. 2008, 106, 552–558. [Google Scholar] [CrossRef]
- Suetsuna, K. Identification of antihypertensive peptides from peptic digest of the short-necked clam Tapes philippinarum and the pearl oyster Pinctada fucata martensii. Fisheries Sci. 2002, 68, 233–235. [Google Scholar] [CrossRef]
- Tsai, J.S.; Chen, J.L.; Pan, B.S. ACE-inhibitory peptides identified from the muscle protein hydrolysate of hard clam (Meretrix lusoria). Process Biochem. 2008, 43, 743–747. [Google Scholar] [CrossRef]
- Terashima, M.; Baba, T.; Ikenmoto, N.; Katayama, M.; Morimoto, T.; Matsumura, S. Novel angiotensin-converting enzyme (ACE) inhibitory peptides derived from boneless chicken leg meat. J. Agric. Food Chem. 2010, 58, 7432–7436. [Google Scholar] [CrossRef]
- Jahandideh, F.; Majumder, K.; Chakrabarti, S.; Morton, J.S.; Panahi, S.; Kaufman, S.; Davidge, S.T.; Wu, J. Beneficial effects of simulated gastro-intestinal digests of fried egg and its fractions on blood pressure, plasma lipids and oxidative stress in spontaneously hypertensive rats. PLoS ONE 2014, 9, e115006. [Google Scholar] [CrossRef] [PubMed]
- Jahandideh, F.; Chakrabarti, S.; Majumder, K.; Li, Q.; Panahi, S.; Morton, J.S.; Davidge, S.T.; Wu, J. Egg white protein hydrolysate reduces blood pressure, improves vascular relaxation and modifies aortic angiotensin II receptors expression in spontaneously hypertensive rats. J. Funct. Foods 2016, 27, 667–673. [Google Scholar] [CrossRef] [Green Version]
- Majumder, K.; Wu, J. Purification and characterisation of angiotensin I converting enzyme (ACE) inhibitory peptides derived from enzymatic hydrolysate of ovotransferrin. Food Chem. 2011, 126, 1614–1619. [Google Scholar] [CrossRef] [PubMed]
- Majumder, K.; Chakrabarti, S.; Morton, J.S.; Panahi, S.; Kaufman, S.; Davidge, S.T.; Wu, J. Egg-derived tri-peptide IRW exerts antihypertensive effects in spontaneously hypertensive rats. PLoS ONE 2013, 8, e82829. [Google Scholar] [CrossRef] [PubMed]
- Majumder, K.; Chakrabarti, S.; Morton, J.S.; Panahi, S.; Kaufman, S.; Davidge, S.T.; Wu, J. Egg-derived ACE-inhibitory peptides IQW and LKP reduce blood pressure in spontaneously hypertensive rats. J. Funct. Foods 2015, 13, 50–60. [Google Scholar] [CrossRef]
- Jang, J.H.; Jeong, S.C.; Kim, J.H.; Lee, Y.H.; Ju, Y.C.; Lee, J.S. Characterisation of a new antihypertensiveangiotensin I-converting enzyme inhibitory peptide from Pleurotus cornucopiae. Food Chem. 2011, 127, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Suetsuna, K.; Chen, J.R. Identification of antihypertensive peptides from peptic digest of two microalgae, Chlorella vulgaris and Spirulina platensis. Mar. Biotechnol. 2001, 3, 305–309. [Google Scholar] [CrossRef]
- Xie, J.; Chen, X.; Wu, J.; Zhang, Y.; Zhou, Y.; Zhang, L.; Tang, Y.J.; Wei, D. Antihypertensive effects, molecular docking study, and isothermal titration calorimetry assay of angiotensin I-converting enzyme inhibitory peptides from Chlorella vulgaris. J. Agric. Food Chem. 2018, 66, 1359–1368. [Google Scholar] [CrossRef]
- Lin, Y.H.; Chen, G.W.; Yeh, C.H.; Song, H.; Tsai, J.S. Purification and identification of angiotensin I-converting enzyme inhibitory peptides and the antihypertensive effect of Chlorella sorokiniana protein hydrolysates. Nutrients 2018, 10, 1397. [Google Scholar] [CrossRef]
- Wijesekara, I.; Qian, Z.J.; Ryu, B.; Ngo, D.H.; Kim, S.K. Purification and identification of antihypertensive peptides from seaweed pipefish (Syngnathus schlegeli) muscle protein hydrolysate. Food Res. Int. 2011, 44, 703–707. [Google Scholar] [CrossRef]
- Lin, H.C.; Alashi, A.M.; Aluko, R.E.; Sun, P.B.; Chang, Y.W. Antihypertensive properties of tilapia (Oreochromis spp.) frame and skin enzymatic protein hydrolysates. Food Nutr. Res. 2018, 61, 1391666. [Google Scholar] [CrossRef] [PubMed]
- Fahmi, A.; Morimura, S.; Guo, H.C.; Shigematsu, T.; Kida, K.; Uemura, Y. Production of angiotensin I converting enzyme inhibitory peptides from sea bream scales. Process Biochem. 2004, 39, 1195–1200. [Google Scholar] [CrossRef]
- Byun, H.G.; Kim, S.K. Purification and characterization of angiotensin I converting enzyme (ACE) inhibitory peptides from Alaska pollack (Theragra chalcogramma) skin. Process Biochem. 2001, 36, 1155–1162. [Google Scholar] [CrossRef]
- Lin, L.; Shun, L.; Bafang, L. Angiotensin-I-converting enzyme (ACE)-inhibitory and antihypertensive properties of squid skin gelatin hydrolysates. Food Chem. 2012, 131, 225–230. [Google Scholar] [CrossRef]
- Alemán, A.; Gómez-Guillén, M.C.; Montero, G. Identification of ace-inhibitory peptides from squid skin collagen after in vitro gastrointestinal digestion. Food Res. Int. 2013, 51, 790–795. [Google Scholar] [CrossRef]
- Ngo, D.H.; Vo, T.S.; Ryu, B.M.; Kim, S.K. Angiotensin-I-converting enzyme (ACE) inhibitory peptides from Pacific cod skin gelatin using ultrafiltration membranes. Process Biochem. 2016, 51, 1622–1628. [Google Scholar] [CrossRef]
- Yang, P.; Jiang, Y.; Hong, P.; Cao, W. Angiotensin I converting enzyme inhibitory activity and antihypertensive effect in spontaneously hypertensive rats of cobia (Rachycentron canadum) head papain hydrolysate. Food Sci. Technol. Int. 2012, 19, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Ditty, J.G.; Shaw, R.F. Larval development, distribution, and ecology of cobia, Rachycentron canadum, (Family: Rachycentridae) in the northern Gulf of Mexico. Fish Bull 1992, 90, 668–677. [Google Scholar]
- Liao, I.C.; Huang, S.T.; Tsai, W.S.; Hsueh, M.C.; Chang, L.C.; Leaño, E. Cobia culture in Taiwan: Currents status and problems. Aquaculture 2004, 237, 155–165. [Google Scholar] [CrossRef]
- Shih, Y.C. Marine environmental management and development strategy for marine aquaculture in Taiwan: Cobia case study. Ad. Oceanogr. Mar. Biol. 2018, 1, AOMB.MS.ID.000504. [Google Scholar]
- Liu, S.C.; Li, D.T.; Hong, P.Z.; Zhang, C.H.; Yang, P.; Ji, H.W. Evaluation of protein nutritional value in different tissues of cobia. Food Sci. Technol. 2009, 35, 158–160. (In Chinese) [Google Scholar]
- Kristinsson, H.G.; Rasco, B.A. Biochemical and functional properties of Atlantic salmon (Salmo salar) muscle hydrolyzed with various alkaline proteases. J. Agric. Food Chem. 2000, 48, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Shahidi, F.; Han, X.Q.; Synowiecki, J. Production and characteristics of protein hydrolysates from capelin (Mallotus villosus). Food Chem. 1995, 53, 285–293. [Google Scholar] [CrossRef]
- Je, J.Y.; Qian, Z.J.; Lee, S.H.; Byun, H.G.; Kim, S.K. Purification and antioxidant properties of bigeye tuna (Thunnus obesus) dark muscle peptide on free radicalmediated oxidative systems. J. Med. Food 2008, 11, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.J.; Je, J.Y.; Kim, S.K. Antihypertensive effect of angiotensin I converting enzyme-inhibitory peptide from hydrolysates of bigeye tuna dark muscle, Thunnus obesus. J. Agric. Food Chem. 2007, 55, 8398–8403. [Google Scholar] [CrossRef] [PubMed]
- Je, J.Y.; Qian, Z.J.; Byun, H.G.; Kim, S.K. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process Biochem. 2007, 42, 840–846. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, Y.T.; Byun, H.G.; Nam, K.S.; Joo, D.S.; Shahidi, F. Isolation and characterization of antioxidative peptides from gelatin hydrolysate of Allaska Pollack skin. J. Agric. Food Chem. 2001, 49, 1984–1989. [Google Scholar] [CrossRef]
- Lee, S.H.; Qian, Z.J.; Kim, S.K. A novel angiotensin I converting enzyme inhibitory peptides from tuna frame protein hydrolysate and its antihypertensive effect in spontaneously hypertensive rats. Food Chem. 2010, 118, 96–102. [Google Scholar] [CrossRef]
- Lowry, O.H.; Resebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Cooper, T.G. Spectrophotometry. In The Tools of Biochemistry, 1st ed.; Wiley-Interscience: Hoboken, NJ, USA, 1977; pp. 53–55. ISBN 0471171166. [Google Scholar]
- Church, F.C.; Swaisgood, H.E.; Porter, H.D.; Catignani, G.L. Spectrophotometric assay using o-phthaldialdehyde for determination of proteolysis in milk and isolated milk proteins. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Doi, E.; Shibata, D.; Matoba, T. Modified colorimetric ninhydrin methods for peptidase assay. Anal. Biochem. 1981, 118, 173–184. [Google Scholar] [CrossRef]
- Cushman, D.W.; Cheung, H.S. Spectrophotometric assay and properties of the angiotensin-converting enzyme of rabbit lung. Biochem. Parmacol. 1971, 20, 1637–1648. [Google Scholar] [CrossRef]
- Wu, J.; Ding, X. Characterization of inhibition and stability of soy-protein-derived angiotensin I-converting enzyme inhibitory peptides. Food Res. Int. 2002, 35, 367–375. [Google Scholar] [CrossRef]
- Lin, Y.H.; Tsai, J.S.; Chen, G.W. Purification and identification of hypocholesterolemic peptides from freshwater clam hydrolysate with in vitro gastrointestinal digestion. J. Food Biochem. 2017, 41, e12385. [Google Scholar] [CrossRef]
- UniProt Database. Available online: http://www.uniprot.org (accessed on 1 January 2019).
- Pairwise Sequence Alignment Software. Available online: https://www.ebi.ac.uk/Tools/psa/ (accessed on 1 January 2019).
- SAS Institute Inc. SAS® 9.4 TS1M5 User’s Guide; SAS Institute Press: Cary, NC, USA, 2019; p. 35. [Google Scholar]
- Tsai, J.S.; Lin, T.C.; Chen, J.L.; Pan, B.S. The inhibitory effects of freshwater clam (Corbicula fluminea, Muller) muscle protein hydrolysates on angiotensin I converting enzyme. Process Biochem. 2006, 41, 2276–2281. [Google Scholar] [CrossRef]
- Kuba, M.; Shinjo, S.; Yasuda, M. Antihypertensive and hypocholesterolem effects of tofuyo in spontaneously hypertensive rats. J. Health Sci. 2004, 50, 670–673. [Google Scholar] [CrossRef]
- Kaiser, S.; Martin, M.; Lunow, D.; Rudolph, S.; Mertten, S.; Möckel, U.; Deußen, A.; Henle, T. Tryptophan-containing dipeptides are bioavailable and inhibit plasma human angiotensin-converting enzyme in vivo. Int. Dairy J. 2016, 52, 107–114. [Google Scholar] [CrossRef]
- Ruiz, J.Á.G.; Ramos, M.; Recio, J. Angiotensin converting enzyme inhibitory activity of peptides isolated from Manchego cheese. Stability under simulated gastrointestinal digestion. Int. Dairy J. 2004, 14, 1075–1080. [Google Scholar] [CrossRef]
- Gu, R.Z.; Chen, Y.L.; Liu, W.Y.; Yi, W.X.; Cai, M.Y. Angiotensin I-converting enzyme inhibitory activity of low-molecular-weight peptides from Atlantic salmon (Salmo salar L.) skin. Food Res. Int. 2011, 44, 1536–1540. [Google Scholar] [CrossRef]
- Lee, J.K.; Jeon, J.K.; Byun, H.G. Antihypertensive effect of novel angiotensin I converting enzyme inhibitory peptide from chum salmon (Oncorhynchus keta) skin in spontaneously hypertensive rats. J. Funct. Foods 2014, 7, 381–389. [Google Scholar] [CrossRef]
- Hernández-Ledesma, B.; Contreras, M.M.; Recio, I. Antihypertensive peptides: Production, bioavailability, and incorporation into foods. Adv. Coll. Interface Sci. 2011, 165, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, S.; Kobayashi, Y.; Kita, E.; Imamura, Y.; Toyama, S. Antihypertensive effects of tryptic hydrolysate of casein on normotensive and hypertensive volunteers. J. Jpn. Soc. Nutr. Food Sci. 1992, 45, 513–517. [Google Scholar] [CrossRef]
- Kuba, M.; Tanaka, K.; Tawata, S.; Takeda, Y.; Yasuda, M. Angiotensin I-converting enzyme inhibitory peptides isolated from tofuyo fermented soybean food. Biosci. Biotechnol. Biochem. 2003, 67, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Hosokawa, M.; Miyashita, K.; Takahashi, K. Inhibition properties of dipeptides from salmon muscle hydrolysate on angiotensin I-converting enzyme. Int. J. Food Sci. Technol. 2006, 41, 383–386. [Google Scholar] [CrossRef]
- Panyayai, T.; Sangsawad, P.; Pacharawongsakda, E.; Sawatdichaikul, O.; Tongsima, S.; Choowongkomon, K. The potential peptides against angiotensin-I converting enzyme through a virtual tripeptide-constructing library. Comput. Biol. Chem. 2018, 77, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Henda, Y.B.; Labidi, A.; Arnaudin, I.; Bridiau, N.; Delatouche, R.; Maugard, T.; Piot, J.M.; Sannier, F.; Thiéry, V.; Bordenave-Juchereau, S. Measuring angiotensin-I converting enzyme inhibitory activity by microl plate assays: Comparison using marine cryptides and tentative threshold determinations with captopril and losartan. J. Agric. Food Chem. 2013, 61, 10685–10690. [Google Scholar] [CrossRef]
- Balgir, P.P.; Sharma, M. Biopharmaceutical potential of ACE-inhibitory peptides. J. Proteom. Bioinform. 2017, 10, 171–177. [Google Scholar] [CrossRef]
- Daskaya-Dikmen, C.; Yucetepe, A.; Karbancioglu-Guler, F.; Daskaya, H.; Ozcelik, B. Angiotensin-I-converting enzyme (ACE)-inhibitory peptides from plants. Nutrients 2017, 9, 316. [Google Scholar] [CrossRef]
- Mora, L.; Gallego, M.; Toldrá, F. ACEI-inhibitory peptides naturally generated in meat and meat products and their health relevance. Nutrients 2018, 10, 1259. [Google Scholar] [CrossRef]
- He, R.; Ma, H.; Zhao, W.; Qu, W.; Zhao, J.; Luo, L.; Zhu, W. Modeling the QSAR of ACE-inhibitory peptides with ANN and its applied illustration. Int. J. Pept. 2012. [Google Scholar] [CrossRef]
- Wu, J.; Aluko, R.E.; Nakai, S. Structural requirements of angiotensin I-converting enzyme inhibitory peptides: Quantitative structure−activity relationship study of di- and tripeptides. J. Agric. Food Chem. 2006, 54, 732–738. [Google Scholar] [CrossRef] [PubMed]
- Li, G.H.; Le, G.W.; Shi, Y.H.; Shrestha, S. Angiotensin-I-converting enzyme inhibitory peptides derived from food proteins and their physiological and pharmacological effects. Nutr. Res. 2004, 24, 469–486. [Google Scholar] [CrossRef]
- Cheung, H.S.; Wang, F.L.; Ondetti, M.A.; Sabo, E.F.; Cushman, D.W. Binding of peptide substrates and inhibitors of angiotensin-converting enzyme. J. Biol. Chem. 1980, 255, 401–407. [Google Scholar] [PubMed]
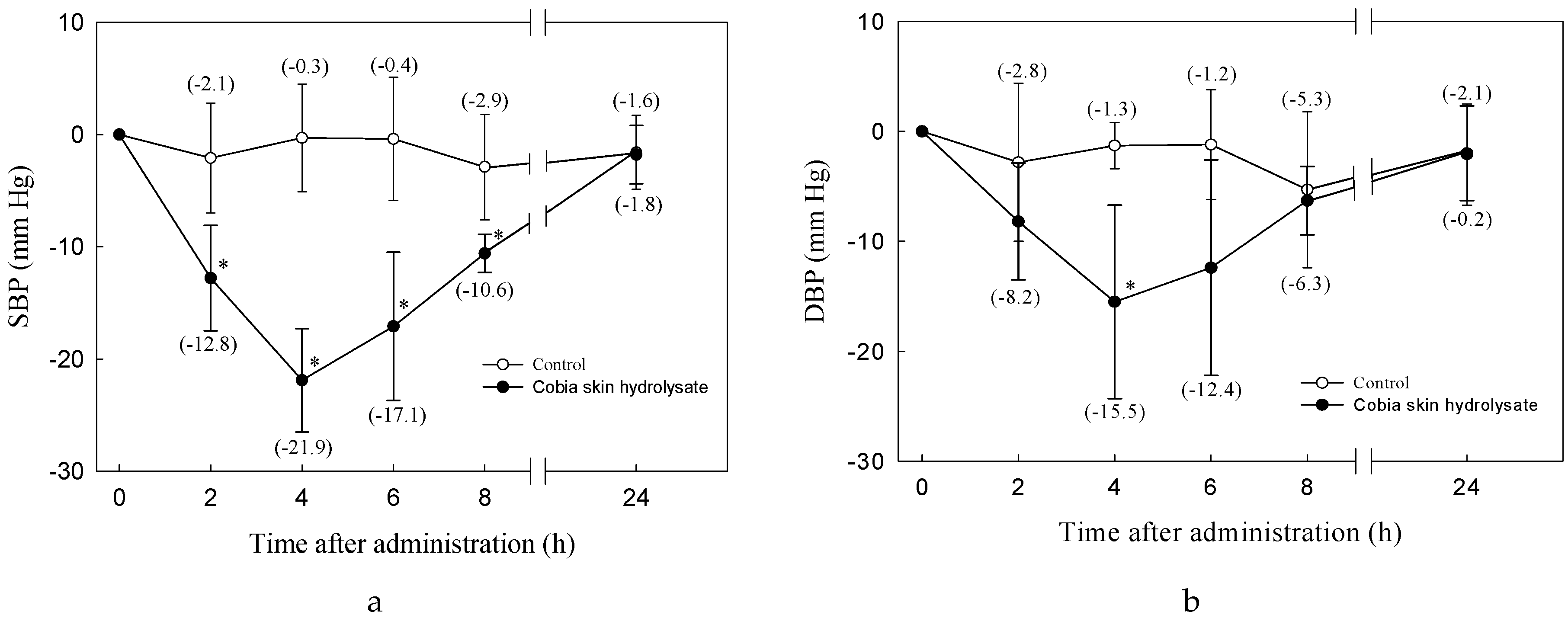
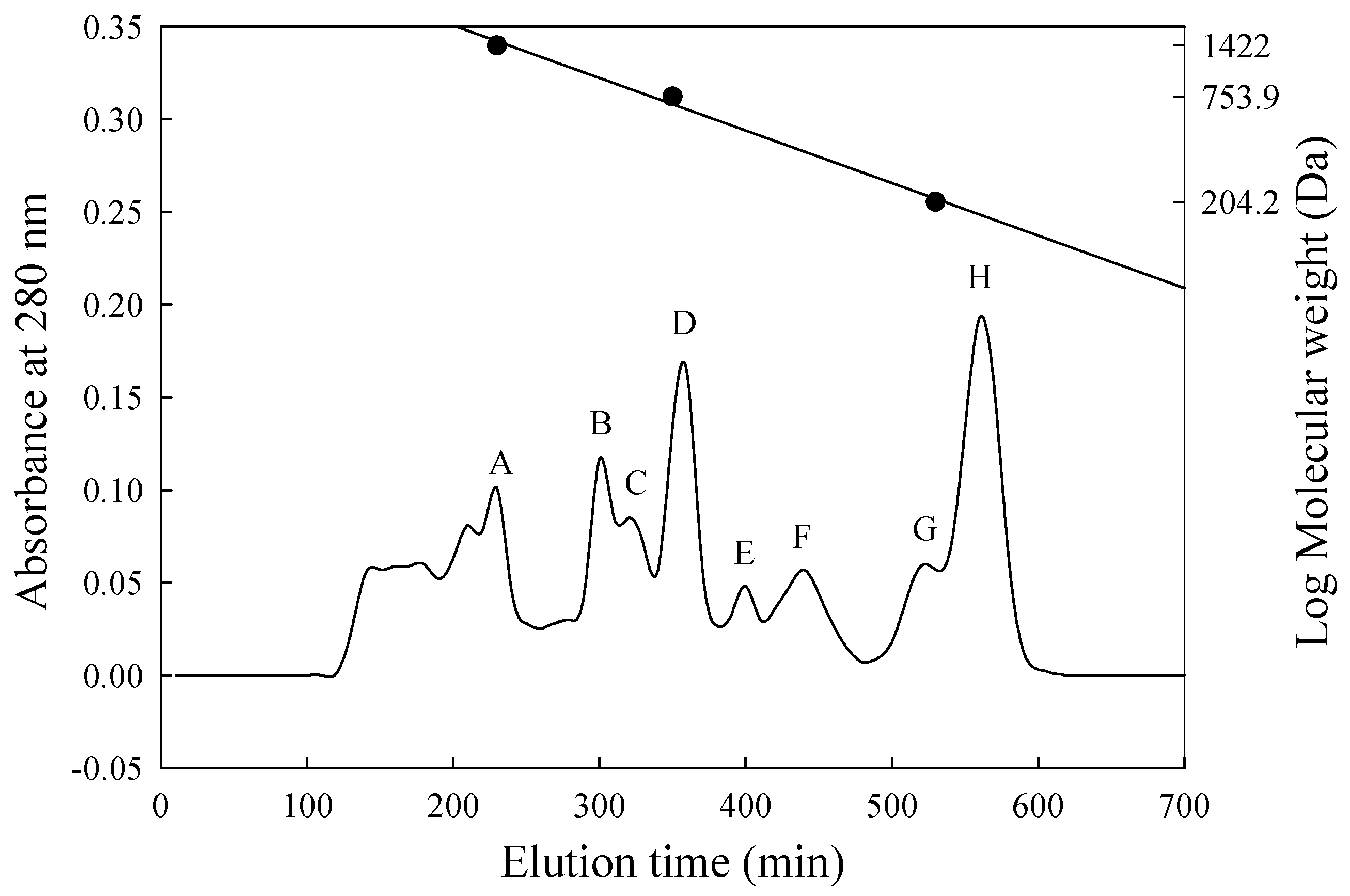
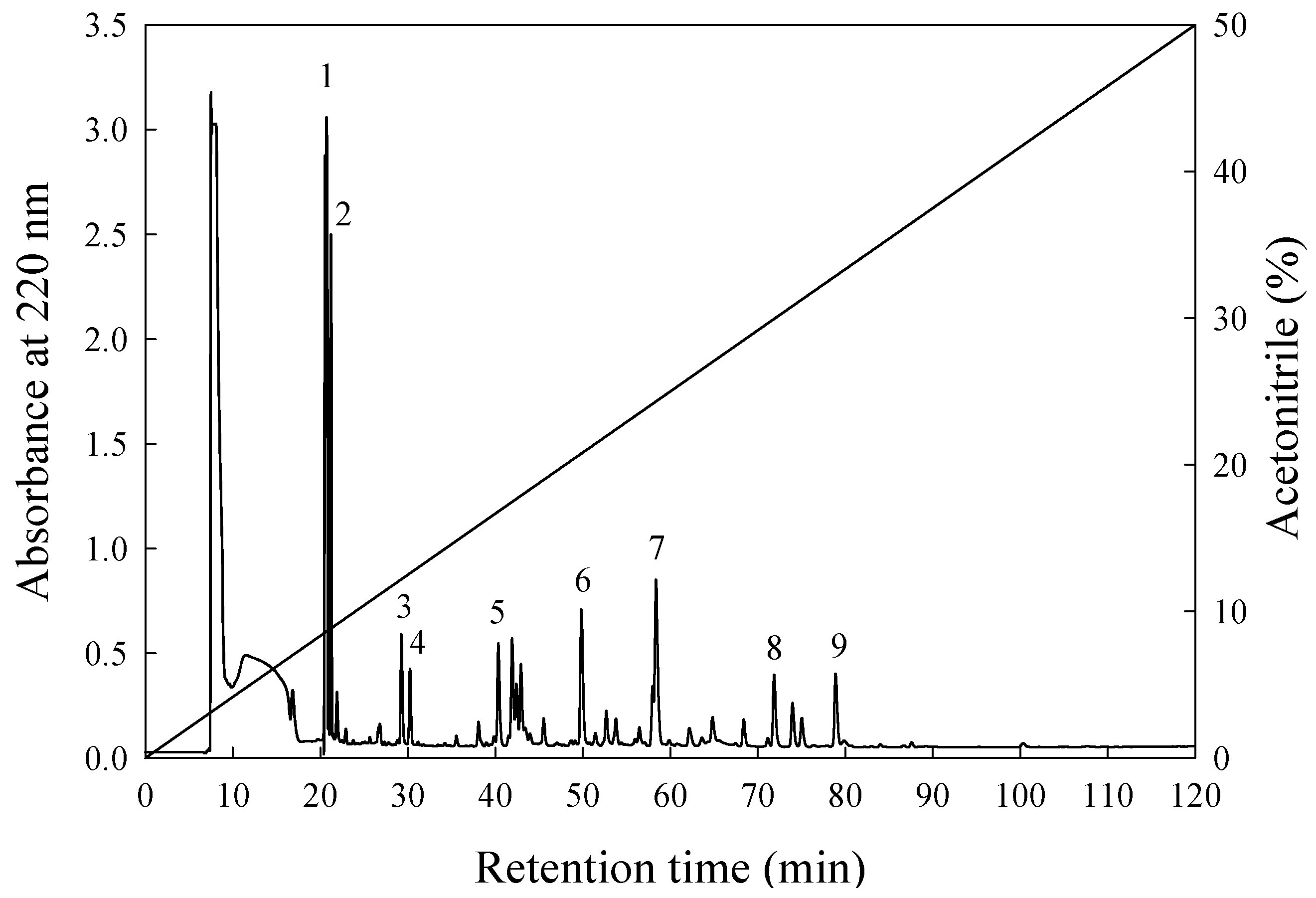
| Sample | Soluble Protein | Peptide Content | Free Amino Acid (mg/g) | IC50 1 (mg/mL) |
|---|---|---|---|---|
| (mg/g) | (mg/g) | |||
| PX-5 2 | 612.0 ± 9.0 b | 270.0 ± 7.0 a | 75.5 ± 0.2 a | 0.221 ± 0.005 a |
| PN-5 3 | 531.0 ± 7.0 a | 400.0 ± 9.0 b | 97.3 ± 0.8 b | 0.291 ± 0.005 b |
| Sample | Protease | IC50 (mg/mL) |
|---|---|---|
| PX-5 | Control | 0.221 ± 0.005 b |
| PX-5P 1 | 0.217 ± 0.005 b | |
| PX-5G 2 | 0.304 ± 0.005 a | |
| PN-5 | Control | 0.291 ± 0.005 c |
| PN-5P 1 | 0.308 ± 0.003 b | |
| PN-5G 2 | 0.384 ± 0.002 a |
| Fraction | Molecular Weight (Da) | Inhibition (%) | Peptide Content (mg/mL) | IER 1 (%/mg/mL) |
|---|---|---|---|---|
| A | 1670–1370 | 72.3 | 0.706 | 102.4 |
| B | 1130–870 | 56.5 | 1.190 | 47.5 |
| C | 870–760 | 71.8 | 0.820 | 87.6 |
| D | 760–630 | 57.2 | 0.397 | 144.1 |
| E | 630–450 | 65.2 | 0.042 | 1552.4 |
| F | 450–350 | 34.0 | 0.028 | 1214.3 |
| G | 270–220 | 22.2 | 0.083 | 267.5 |
| H | 210–160 | 22.2 | 0.022 | 1009.1 |
| Bioactive Substances | Inhibition (%) | Peptide Concentration (mg/mL) | IER1 (%/mg/mL) |
|---|---|---|---|
| E1 | 2.7 | 0.009 | 300.0 |
| E2 | 6.5 | ─2 | ─2 |
| E3 | 55.6 | 0.073 | 761.6 |
| E4 | 15.3 | 0.109 | 140.4 |
| E5 | 7.9 | 0.024 | 329.2 |
| E6 | 41.9 | 0.048 | 872.9 |
| E7 | 81.1 | 0.058 | 1398.3 |
| E8 | 93.4 | 0.028 | 3335.7 |
| E9 | 42.3 | 0.014 | 3021.4 |
| Peak | Sequence | IC50 (μM) | IC50 (mg/mL) |
|---|---|---|---|
| E6 | Trp-Ala-Ala | 118.50 ± 2.80 | 0.04110 |
| E7 | Ala-Trp-Trp | 9.40 ± 0.60 | 0.00434 |
| E8 | Ile-Trp-Trp | 0.51 ± 0.10 | 0.00026 |
| E9 | Trp-Leu | 26.80 ± 0.90 | 0.00851 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-H.; Chen, C.-A.; Tsai, J.-S.; Chen, G.-W. Preparation and Identification of Novel Antihypertensive Peptides from the In Vitro Gastrointestinal Digestion of Marine Cobia Skin Hydrolysates. Nutrients 2019, 11, 1351. https://doi.org/10.3390/nu11061351
Lin Y-H, Chen C-A, Tsai J-S, Chen G-W. Preparation and Identification of Novel Antihypertensive Peptides from the In Vitro Gastrointestinal Digestion of Marine Cobia Skin Hydrolysates. Nutrients. 2019; 11(6):1351. https://doi.org/10.3390/nu11061351
Chicago/Turabian StyleLin, Yu-Hsin, Chun-An Chen, Jenn-Shou Tsai, and Guan-Wen Chen. 2019. "Preparation and Identification of Novel Antihypertensive Peptides from the In Vitro Gastrointestinal Digestion of Marine Cobia Skin Hydrolysates" Nutrients 11, no. 6: 1351. https://doi.org/10.3390/nu11061351
APA StyleLin, Y.-H., Chen, C.-A., Tsai, J.-S., & Chen, G.-W. (2019). Preparation and Identification of Novel Antihypertensive Peptides from the In Vitro Gastrointestinal Digestion of Marine Cobia Skin Hydrolysates. Nutrients, 11(6), 1351. https://doi.org/10.3390/nu11061351





