Effect of Postoperative Oral Intake on Prognosis for Esophageal Cancer
Abstract
1. Introduction
2. Participants and Methods
2.1. Participants
2.2. Perioperative Nutritional Management
2.3. Classification According to Postoperative Oral Intake
2.4. Survey Items
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characteristics, Preoperative Characteristics, and Nutrient Intake between the Study Groups (POI Group vs. Control Group)
3.2. Nutrient Intake and Nutritional Status during the Follow-Up Period between the Study Groups (POI Group vs. Control Group)
3.3. Long-Term Outcomes after Esophagectomy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kawasaki, T.; Sata, T. Perioperative innate immunity. J. UOEH 2011, 33, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, H.; Hansen, H.S.; Cederqvist, C.; Løber, J. The prognostic significance of weight loss and its integration in stage-grouping of oesophageal cancer. Acta Chir. Scand. 1982, 148, 363–366. [Google Scholar] [PubMed]
- Onodera, T.; Goseki, N.; Kosaki, G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. J. Jpn. Surg. Soc. 1984, 85, 1001–1005. [Google Scholar]
- O’Gorman, P.; McMillan, D.C.; McArdle, C.S. Longitudinal study of weight, appetite, performance status, and inflammation in advanced gastrointestinal cancer. Nutr. Cancer 1999, 35, 127–129. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.Z.; Shen, Q.; Zhang, H.B. Expression and significance of immunosuppressive acidic protein (IAP) in human esophageal squamous cell carcinoma. Zhonghua Zhong Liu Za Zhi 1994, 16, 125–127. [Google Scholar]
- Hartl, W.H.; Jauch, K.-W. Metabolic self-destruction in critically ill patients: Origins, mechanisms and therapeutic principles. Nutrition 2014, 30, 261–267. [Google Scholar] [CrossRef] [PubMed]
- van Niekerk, G.; Davis, T.; Engelbrecht, A.-M. Hyperglycaemia in critically ill patients: The immune system’s sweet tooth. Crit. Care 2017, 21, 202. [Google Scholar] [CrossRef]
- Mecott, G.A.; Al-Mousawi, A.M.; Gauglitz, G.G.; Herndon, D.N.; Jeschke, M.G. The role of hyperglycemia in burned patients: Evidence-based studies. Shock 2010, 33, 5–13. [Google Scholar] [CrossRef]
- Kight, C.E. Nutrition considerations in esophagectomy patients. Nutr. Clin. Pr. 2008, 23, 521–528. [Google Scholar] [CrossRef] [PubMed]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient. J. Parenter. Enter. Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef]
- Singer, P.; Berger, M.M.; Van den Berghe, G.; Biolo, G.; Calder, P.; Forbes, A.; Griffiths, R.; Kreyman, G.; Leverve, X.; Pichard, C.; et al. ESPEN guidelines on parenteral nutrition: Intensive care. Clin. Nutr. 2009, 28, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Casaer, M.P.; Mesotten, D.; Hermans, G.; Wouters, P.J.; Schetz, M.; Meyfroidt, G.; Van Cromphaut, S.; Ingels, C.; Meersseman, P.; Muller, J.; et al. Early versus late parenteral nutrition in critically ill adults. N. Engl. J. Med. 2011, 6365, 506–517. [Google Scholar] [CrossRef] [PubMed]
- Doig, G.S.; Simpson, F.; Sweetman, E.A.; Finfer, S.R.; Cooper, D.J.; Heighes, P.T.; Davies, A.R.; O’Leary, M.; Solano, T.; Peake, S.; et al. Early parenteral nutrition in critically ill patients with short-term relative contraindications to early enteral nutrition. JAMA 2013, 309, 2130. [Google Scholar] [CrossRef] [PubMed]
- Harvey, S.E.; Parrott, F.; Harrison, D.A.; Bear, D.E.; Segaran, E.; Beale, R.; Bellingan, G.; Leonard, R.; Mythen, M.G.; Rowan, K.M. Trial of the route of early nutritional support in critically ill adults. N. Engl. J. Med. 2014, 371, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- Reintam Blaser, A.; Starkopf, J.; Alhazzani, W.; Berger, M.M.; Casaer, M.P.; Deane, A.M.; Fruhwald, S.; Hiesmayr, M.; Ichai, C.; Jakob, S.M.; et al. Early enteral nutrition in critically ill patients: ESICM clinical practice guidelines. Intensive Care Med. 2017, 43, 380–398. [Google Scholar] [CrossRef] [PubMed]
- Lassen, K. Consensus review of optimal perioperative care in colorectal surgery. Arch. Surg. 2009, 144, 961. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Y.; Wang, Z.; Sun, H.; Zhang, R. A video demonstration of the Li’s anastomosis-the key part of the “non-tube no fasting” fast track program for resectable esophageal carcinoma. J. Thorac. Dis. 2015, 7, 1264–1268. [Google Scholar] [PubMed]
- Zhu, Z.; Li, Y.; Zheng, Y.; Sun, H.; Liu, X.; Zhang, R.; Wang, Z.; Liu, S.; Chen, X.; Hua, X.; et al. Chewing 50 times per bite could help to resume oral feeding on the first postoperative day following minimally invasive oesophagectomy. Eur. J. Cardio-Thorac. Surg. 2018, 53, 325–330. [Google Scholar] [CrossRef]
- Smeets, P.A.M.; Erkner, A.; de Graaf, C. Cephalic phase responses and appetite. Nutr. Rev. 2010, 68, 643–655. [Google Scholar] [CrossRef]
- Teff, K. Nutritional implications of the cephalic-phase reflexes: Endocrine responses. Appetite 2000, 34, 206–213. [Google Scholar] [CrossRef]
- Nederkoorn, C.; Smulders, F.T.Y.; Jansen, A. Cephalic phase responses, craving and food intake in normal subjects. Appetite 2000, 35, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Habu, D.; Lee, S.; Kishida, S.; Osugi, H. Enteral diet enriched with ω-3 fatty acid improves oxygenation after thoracic esophagectomy for cancer: A randomized controlled trial. World J. Surg. 2017, 41, 1584–1594. [Google Scholar] [CrossRef]
- Osugi, H.; Takemura, M.; Lee, S.; Nishikawa, T.; Fukuhara, K.; Iwasaki, H.; Higashino, M. Thoracoscopic esophagectomy for intrathoracic esophageal cancer. Ann. Thorac. Cardiovasc. Surg. 2005, 11, 221–227. [Google Scholar] [PubMed]
- Sobin, L.H.; Gospodarowicz, M.K.; Wittekind, C. (Eds.) TNM Classification of Malignant Tumours, 7th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2011; ISBN 978-1-444-35896-4. [Google Scholar]
- Martin, L.; Lagergren, J.; Jia, C.; Lindblad, M.; Rouvelas, I.; Viklund, P. The influence of needle catheter jejunostomy on weight development after oesophageal cancer surgery in a population-based study. Eur. J. Surg. Oncol. 2007, 33, 713–717. [Google Scholar] [CrossRef] [PubMed]
- Sato, M. Nutritional assessment of gastric cancer patients: Estimation of risk of postoperative complication from preoperative nutritional states. J. Jpn. Surg. Soc. 1982, 83, p66–p77. [Google Scholar]
- Ignacio De Ulíbarri, J.; González-Madroño, A.; De Villar, N.G.P.; González, P.; González, B.; Mancha, A.; Rodríguez, F.; Fernández, G. CONUT: A tool for controlling nutritional status. First validation in a hospital population. Nutr. Hosp. 2005, 20, 38–45. [Google Scholar] [PubMed]
- Demirtas, H.; Hedeker, D. An imputation strategy for incomplete longitudinal ordinal data. Stat. Med. 2008, 27, 4086–4093. [Google Scholar] [CrossRef]
- Rubin, D.B. Multiple Imputation for Nonresponse in Surveys; Wiley Series in Probability and Statistics; Rubin, D.B., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1987; ISBN 9780470316696. [Google Scholar]
- Okada, G.; Momoki, C.; Matsumoto, Y.; Nakamura, Y.; Hayashi, F.; Yasui, Y.; Habu, D.; Nadai, J.; Matsuda, Y.; Kishida, S.; et al. The effects of preoperative weight loss on nutritional status and prognosis for esophageal cancer. Jacobs J. Gastroenterol. Hepatol. 2016, 3, 1–10. [Google Scholar]
- Okada, G.; Matsumoto, Y.; Hayashi, F.; Kato, K.; Momoki, C.; Yasui, Y.; Habu, D.; Edagawa, E.; Matsuda, Y.; Kishida, S.; et al. Nutritional changes and factors contributing to postoperative weight recovery after esophagectomy. Esophagus 2017, 14, 343–350. [Google Scholar] [CrossRef]
- Marik, P.E.; Zaloga, G.P. Early enteral nutrition in acutely ill patients: A systematic review. Crit. Care Med. 2001, 29, 2264–2270. [Google Scholar] [CrossRef]
- Parikh, H.G.; Miller, A.; Chapman, M.; Moran, J.L.; Peake, S.L. Calorie delivery and clinical outcomes in the critically ill: A systematic review and meta-analysis. Crit Care Resusc. 2016, 18, 17–24. [Google Scholar] [PubMed]
- Rice, T.W.; Wheeler, A.P.; Thompson, B.T.; Steingrub, J.; Hite, R.D.; Moss, M.; Morris, A.; Dong, N.; Rock, P. Initial trophic vs. full enteral feeding in patients with acute lung injury: The EDEN randomized trial. JAMA J. Am. Med. Assoc. 2012, 307, 795–803. [Google Scholar]
- Arabi, Y.M.; Aldawood, A.S.; Haddad, S.H.; Al-Dorzi, H.M.; Tamim, H.M.; Jones, G.; Mehta, S.; McIntyre, L.; Solaiman, O.; Sakkijha, M.H.; et al. Permissive underfeeding or standard enteral feeding in critically ill adults. N. Engl. J. Med. 2015, 372, 2398–2408. [Google Scholar] [CrossRef] [PubMed]
- Needham, D.M.; Dinglas, V.D.; Bienvenu, O.J.; Colantuoni, E.; Wozniak, A.W.; Rice, T.W.; Hopkins, R.O. One year outcomes in patients with acute lung injury randomised to initial trophic or full enteral feeding: Prospective follow-up of EDEN randomised trial. BMJ 2013, 346, f1532. [Google Scholar] [CrossRef] [PubMed]
- Berkelmans, G.H.K.; Fransen, L.; Weijs, T.J.; Lubbers, M.; Nieuwenhuijzen, G.A.P.; Ruurda, J.P.; Kouwenhoven, E.A.; van Det, M.J.; Rosman, C.; van Hillegersberg, R.; et al. The long-term effects of early oral feeding following minimal invasive esophagectomy. Dis. Esophagus 2018, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Laughton, W.B.; Powley, T.L. Localization of efferent function in the dorsal motor nucleus of the vagus. Am. J. Physiol. 1987, 252, R13–R25. [Google Scholar] [CrossRef] [PubMed]
- Christensen, C.M.; Navazesh, M. Anticipatory salivary flow to the sight of different foods. Appetite 1984, 5, 307–315. [Google Scholar] [CrossRef]
- Cummings, D.E.; Overduin, J. Gastrointestinal regulation of food intake. J. Clin. Investig. 2007, 117, 13–23. [Google Scholar] [CrossRef]
- Love, J.A.; Yi, E.; Smith, T.G. Autonomic pathways regulating pancreatic exocrine secretion. Auton. Neurosci. 2007, 133, 19–34. [Google Scholar] [CrossRef]
- Teff, K.L.; Mattes, R.D.; Engelman, K. Cephalic phase insulin release in normal weight males: Verification and reliability. Am. J. Physiol. 1991, 261, E430–E436. [Google Scholar] [CrossRef]
- McGregor, I.S.; Lee, A.M. Changes in respiratory quotient elicited in rats by a conditioned stimulus predicting food. Physiol. Behav. 1998, 63, 227–232. [Google Scholar] [CrossRef]
- LeBlanc, J. Nutritional implications of cephalic phase thermogenic responses. Appetite 2000, 34, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Boelens, P.G.; Heesakkers, F.F.B.M.; Luyer, M.D.P.; van Barneveld, K.W.Y.; de Hingh, I.H.J.T.; Nieuwenhuijzen, G.A.P.; Roos, A.N.; Rutten, H.J.T. Reduction of postoperative ileus by early enteral nutrition in patients undergoing major rectal surgery. Ann. Surg. 2014, 259, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Van den Heijkant, T.C.; Costes, L.M.M.; van der Lee, D.G.C.; Aerts, B.; Osinga-de Jong, M.; Rutten, H.R.M.; Hulsewé, K.W.E.; de Jonge, W.J.; Buurman, W.A.; Luyer, M.D.P. Randomized clinical trial of the effect of gum chewing on postoperative ileus and inflammation in colorectal surgery. Br. J. Surg. 2015, 102, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, T.; De Haan, J.J.; Luyer, M.D.P.; Verbaeys, I.; Hadfoune, M.; Dejong, C.H.C.; Buurman, W.A.; Greve, J.W.M. Cholecystokinin/cholecystokinin-1 receptor-mediated peripheral activation of the afferent vagus by enteral nutrients attenuates inflammation in rats. Ann. Surg. 2010, 252, 376–382. [Google Scholar] [CrossRef] [PubMed]
- Lassen, K.; Kjæve, J.; Fetveit, T.; Tranø, G.; Sigurdsson, H.K.; Horn, A.; Revhaug, A. Allowing normal food at will after major upper gastrointestinal surgery does not increase morbidity: A randomized multicenter trial. Ann. Surg. 2008, 247, 721–729. [Google Scholar] [CrossRef]
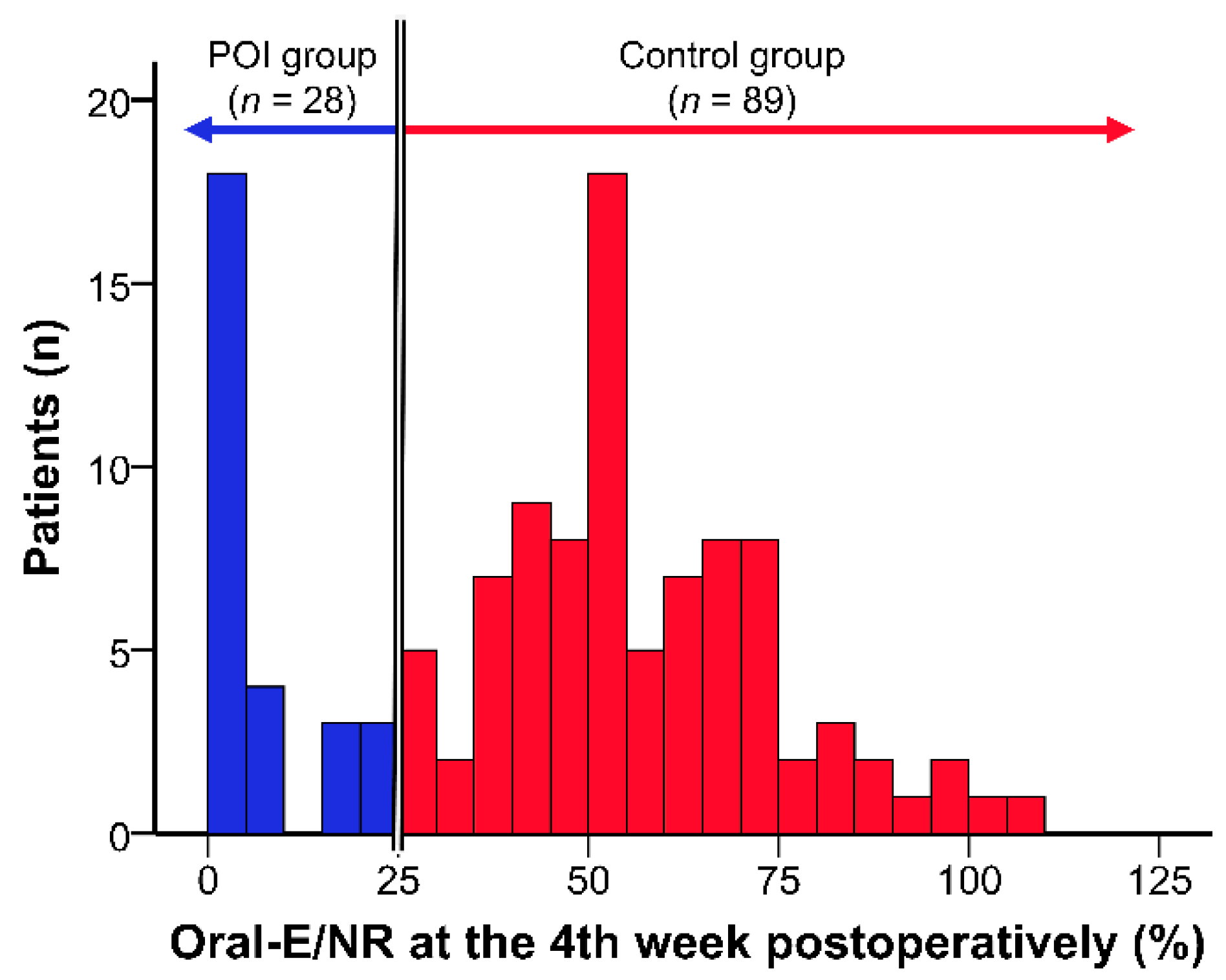
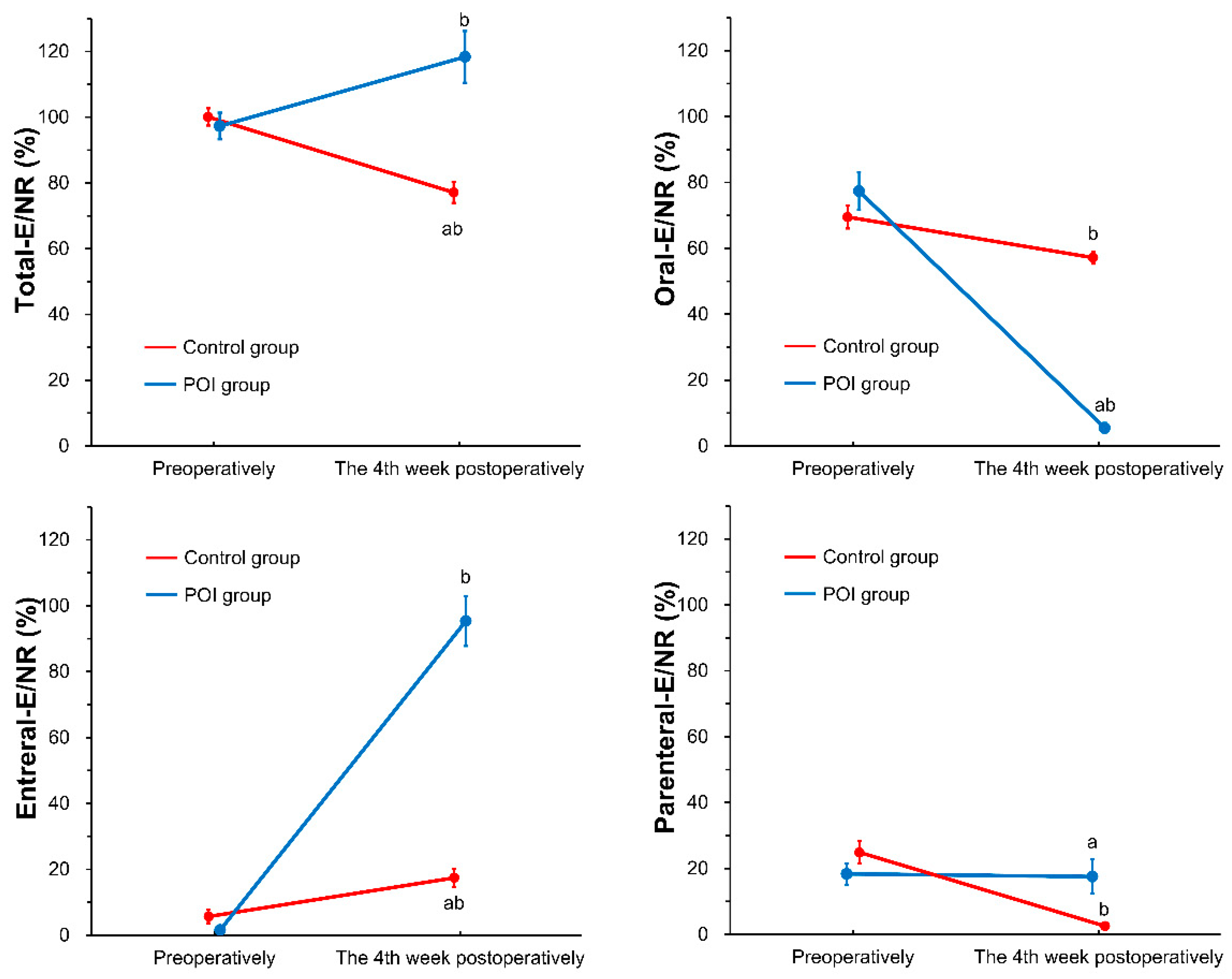
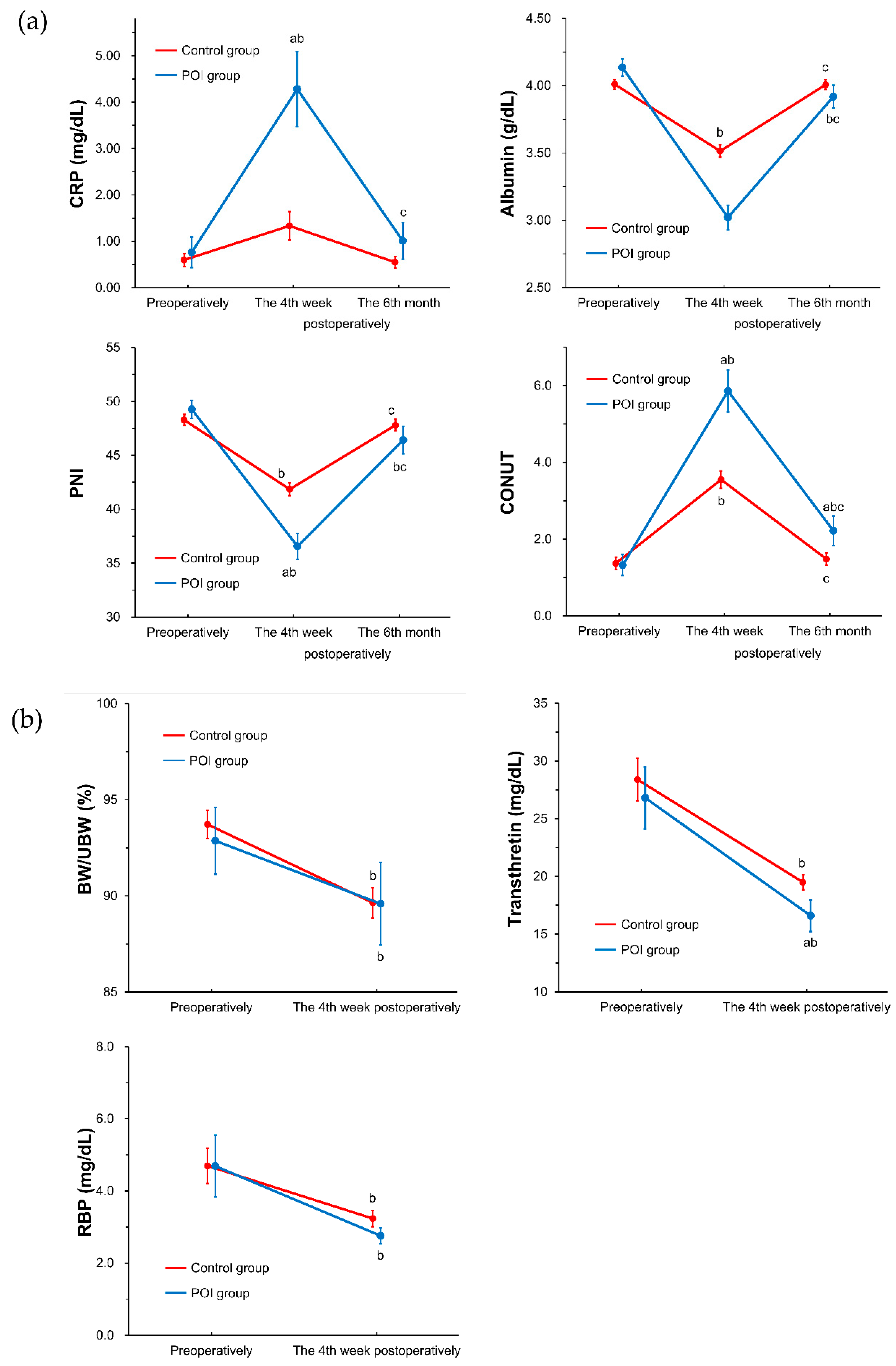

| Total (n = 117) | Control Group (n = 89) | POI Group (n = 28) | p-Value (Control vs. POI) | |
|---|---|---|---|---|
| Gender (Men/Women) | 88/29 | 68/21 | 20/8 | 0.595 |
| Age (years) | 64.0 ± 7.9 | 63.7 ± 7.5 | 65.0 ± 9.2 | 0.435 |
| Height (cm) | 163.4 ± 7.8 | 164.0 ± 7.5 | 161.5 ± 8.6 | 0.137 |
| Weight (kg) | 56.0 ± 8.9 | 56.0 ± 9.4 | 56.0 ± 7.5 | 1.000 |
| BMI (kg/m2) | 20.9 ± 2.7 | 20.8 ± 2.7 | 21.5 ± 2.7 | 0.203 |
| UBW (kg) | 60.2 ± 10.0 | 59.9 ± 9.5 | 61.1 ± 11.3 | 0.567 |
| Preoperative morbidities | ||||
| Mastication disorder | 10 (8.5%) | 7 (7.9%) | 3 (10.7%) | 0.638 |
| Dysphagia | 10 (8.5%) | 9 (10.1%) | 1 (3.6%) | 0.280 |
| Obstruction | 42 (35.9%) | 33 (37.1%) | 9 (32.1%) | 0.635 |
| Glucose intolerance | 30 (20.4) | 23 (25.8%) | 7 (25.0%) | 0.929 |
| Hypertension | 32 (27.4%) | 24 (27.0%) | 8 (28.6%) | 0.868 |
| Dyslipidemia | 8 (6.8%) | 5 (5.6%) | 3 (10.7%) | 0.351 |
| Tumor stage (0/IA/IB/IIA/IIB/IIIA/IIIB/IV) | 13/24/9/12/17/24/0/18 | 9/17/6/11/13/21/0/12 | 4/7/3/1/4/3/0/6 | 0.502 |
| Operative procedure (VATS/Open esophagectomy) | 76/41 | 56/33 | 20/8 | 0.411 |
| Esophageal reconstruction (Gastric conduit/Jejunal graft) | 110/7 | 84/5 | 26/2 | 0.767 |
| Postoperative complications | 77 (65.8%) | 51 (57.3%) | 26 (92.9%) | <0.001 |
| Postoperative morbidity | 61 (52.1%) | 39 (43.8%) | 22 (78.6%) | 0.001 |
| Postoperative meal start (POD) | 13.9 ± 13.6 | 10.1 ± 3.9 | 26.1 ± 23.7 | 0.002 |
| Postoperative meal interruption | 61 (52.1%) | 39 (43.8%) | 22 (78.6%) | 0.001 |
| Neoadjuvant therapy | 60 (51.3%) | 48 (53.9%) | 12 (42.9%) | 0.306 |
| Postoperative hospital stay (days) | 36.3 ± 26.3 | 26.7 ± 8.3 | 66.9 ± 38.4 | <0.001 |
| Postoperative chemotherapy within 1 year postoperatively * | 55 (47.0%) | 43 (48.3%) | 11 (39.3%) | 0.403 |
| Recurrence within 1 year postoperatively * | 37 (31.6%) | 26 (29.2%) | 10 (35.7%) | 0.516 |
| Postoperative mean survival time (years) | 4.5 ± 2.3 | 4.7 ± 2.2 | 3.5 ± 2.4 | 0.016 |
| Total (n = 117) | Control Group (n = 89) | POI Group (n = 28) | p-Value (Control vs. POI) | |
|---|---|---|---|---|
| Preoperatively | ||||
| Energy intake in the hospital (kcal/day) | 1567 ± 323 | 1582 ± 324 | 1521 ± 319 | 0.390 |
| Via oral route (kcal/day) | 1136 ± 505 | 1115 ± 520 | 1204 ± 458 | 0.422 |
| Via enteral route (kcal/day) | 70 ± 262 | 86 ± 295 | 21 ± 81 | 0.065 |
| Via parenteral route (kcal/day) | 361 ± 429 | 381 ± 462 | 297 ± 302 | 0.369 |
| Protein intake in the hospital (g/day) | 61.1 ± 13.8 | 61.4 ± 14.2 | 60.3 ± 12.8 | 0.717 |
| Per preoperative weight (g/kg) | 1.12 ± 0.31 | 1.12 ± 0.33 | 1.09 ± 0.26 | 0.656 |
| Via oral route (g/day) | 46.1 ± 21.0 | 45.2 ± 21.9 | 48.9 ± 18.1 | 0.415 |
| Via enteral route (g/day) | 3.0 ± 11.6 | 3.7 ± 13.2 | 0.8 ± 2.9 | 0.056 |
| Via parenteral route (g/day) | 12.1 ± 13.8 | 12.5 ± 14.6 | 10.6 ± 10.9 | 0.524 |
| The 4th week postoperatively | ||||
| Nutrient requirement (kcal/day) | 1599 ± 192 | 1605 ± 200 | 1578 ± 192 | 0.514 |
| Energy intake in the hospital (kcal/day) | 1366 ± 564 | 1211 ± 413 | 1856 ± 693 | <0.001 |
| Via oral route (kcal/day) | 716 ± 434 | 913 ± 279 | 88 ± 156 | <0.001 |
| Via enteral route (kcal/day) | 557 ± 698 | 260 ± 373 | 1501 ± 654 | <0.001 |
| Via parenteral route (kcal/day) | 92 ± 240 | 38 ± 103 | 268 ± 414 | <0.001 |
| Protein intake in the hospital (g/day) | 57.6 ± 26.7 | 51.3 ± 19.5 | 77.5 ± 32.4 | <0.001 |
| Per postoperative weight (g/kg) | 1.10 ± 0.50 | 0.99 ± 0.43 | 1.45 ± 0.57 | <0.001 |
| Via oral route (g/day) | 30.8 ± 20.6 | 39.4 ± 15.4 | 3.5 ± 5.8 | <0.001 |
| Via enteral route (g/day) | 24.4 ± 31.0 | 11.4 ± 16.8 | 65.6 ± 30.1 | <0.001 |
| Via parenteral route (g/day) | 2.7 ± 8.1 | 0.9 ± 3.5 | 8.4 ± 14.1 | 0.010 |
| Factors | Objective Variables | Control | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|---|---|
| Crude HR (95% CI) | p-Value | Adjusted HR (95% CI) | p-Value | |||
| Gender | Male | Female | 1.85 (0.77–4.45) | 0.167 | ||
| Age | ≥65 | <65 | 1.22 (0.64–2.32) | 0.548 | ||
| Tumor stage | III–IV | 0–II | 3.51 (1.82–6.74) | <0.001 | 3.72 (1.92–7.20) | <0.001 |
| Operation procedure | Open esophagectomy | VATS | 2.23 (1.17–4.26) | 0.015 | ||
| Esophageal reconstruction | Jejunal graft | Gastric conduit | 0.91 (0.22–3.77) | 0.905 | ||
| Neoadjuvant therapy | Present | Absent | 1.96 (1.01–3.82) | 0.048 | 2.33 (1.16–4.70) | 0.018 |
| Preoperative morbidities | ||||||
| Mastication disorder | Present | Absent | 2.25 (0.87–5.78) | 0.093 | ||
| Dysphagia | Present | Absent | 1.83 (0.65–5.18) | 0.253 | ||
| Obstruction | Present | Absent | 1.42 (0.74–2.75) | 0.292 | ||
| Postoperative complications | Present (77) | Absent (40) | 0.80 (0.41–1.54) | 0.498 | ||
| Postoperative morbidity | Present (61) | Absent (56) | 1.32 (0.69–2.53) | 0.410 | ||
| Postoperative meal interruption | Present (61) | Absent (56) | 1.07 (0.56–2.03) | 0.847 | ||
| Preoperative weight loss | ≥5% weight loss | <5% weight loss | 1.70 (0.88–3.28) | 0.112 | ||
| Energy intake at preoperatively | <80% oral-E/total-E | ≥80% oral-E/total-E | 2.14 (1.12–4.07) | 0.021 | ||
| Energy intake at the 4th week postoperatively | <25% oral-E/NR (POI group) | ≥25% oral-E/NR (Control group) | 1.99 (1.00–3.98) | 0.051 | 2.70 (1.30–5.61) | 0.008 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okada, G.; Momoki, C.; Habu, D.; Kambara, C.; Fujii, T.; Matsuda, Y.; Lee, S.; Osugi, H. Effect of Postoperative Oral Intake on Prognosis for Esophageal Cancer. Nutrients 2019, 11, 1338. https://doi.org/10.3390/nu11061338
Okada G, Momoki C, Habu D, Kambara C, Fujii T, Matsuda Y, Lee S, Osugi H. Effect of Postoperative Oral Intake on Prognosis for Esophageal Cancer. Nutrients. 2019; 11(6):1338. https://doi.org/10.3390/nu11061338
Chicago/Turabian StyleOkada, Genya, Chika Momoki, Daiki Habu, Chisako Kambara, Tamotsu Fujii, Yasunori Matsuda, Shigeru Lee, and Harushi Osugi. 2019. "Effect of Postoperative Oral Intake on Prognosis for Esophageal Cancer" Nutrients 11, no. 6: 1338. https://doi.org/10.3390/nu11061338
APA StyleOkada, G., Momoki, C., Habu, D., Kambara, C., Fujii, T., Matsuda, Y., Lee, S., & Osugi, H. (2019). Effect of Postoperative Oral Intake on Prognosis for Esophageal Cancer. Nutrients, 11(6), 1338. https://doi.org/10.3390/nu11061338





