Functional Nutrients for Epilepsy
Abstract
1. Introduction
2. General Overview of Epilepsy
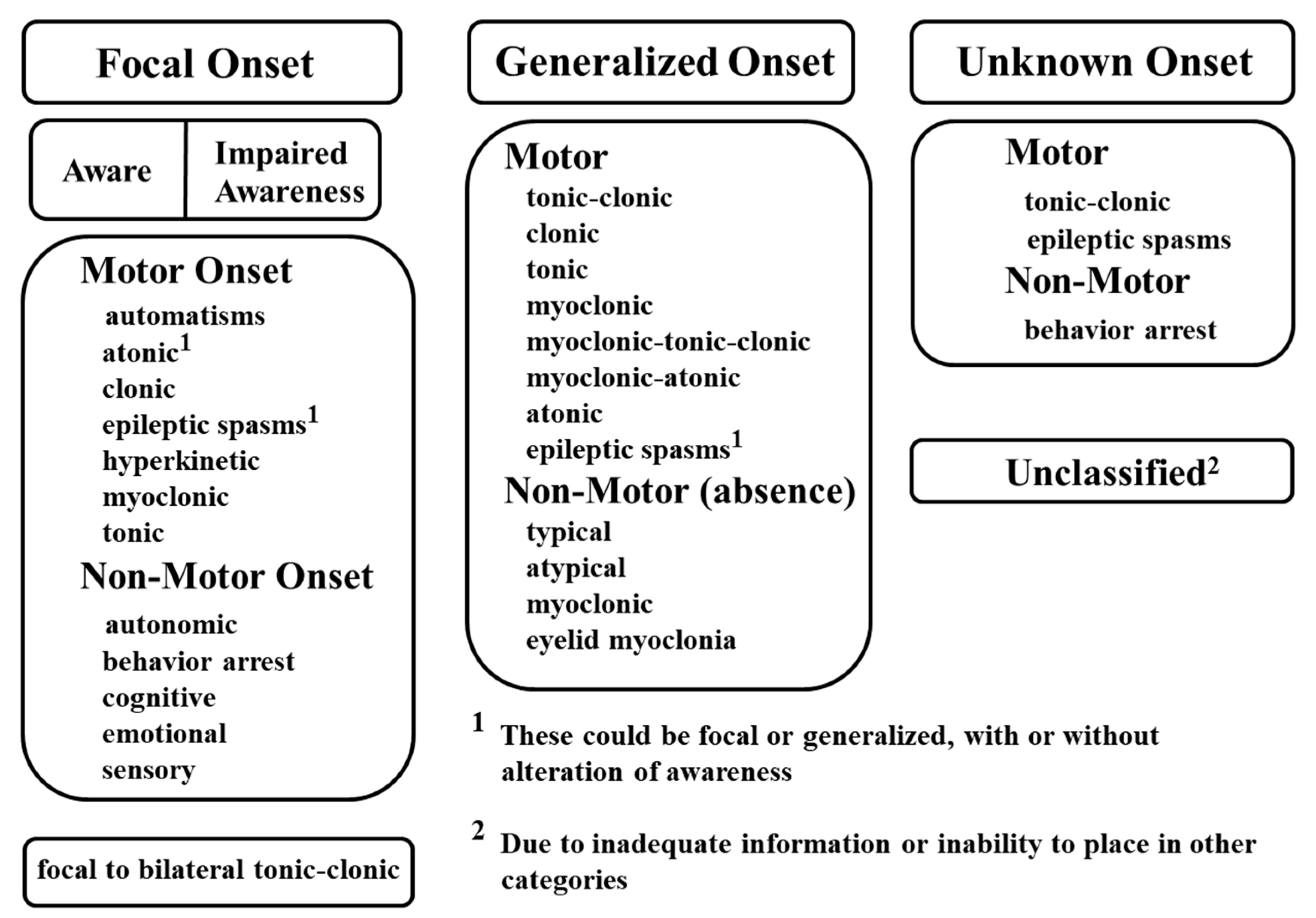
2.1. Classification of Seizures
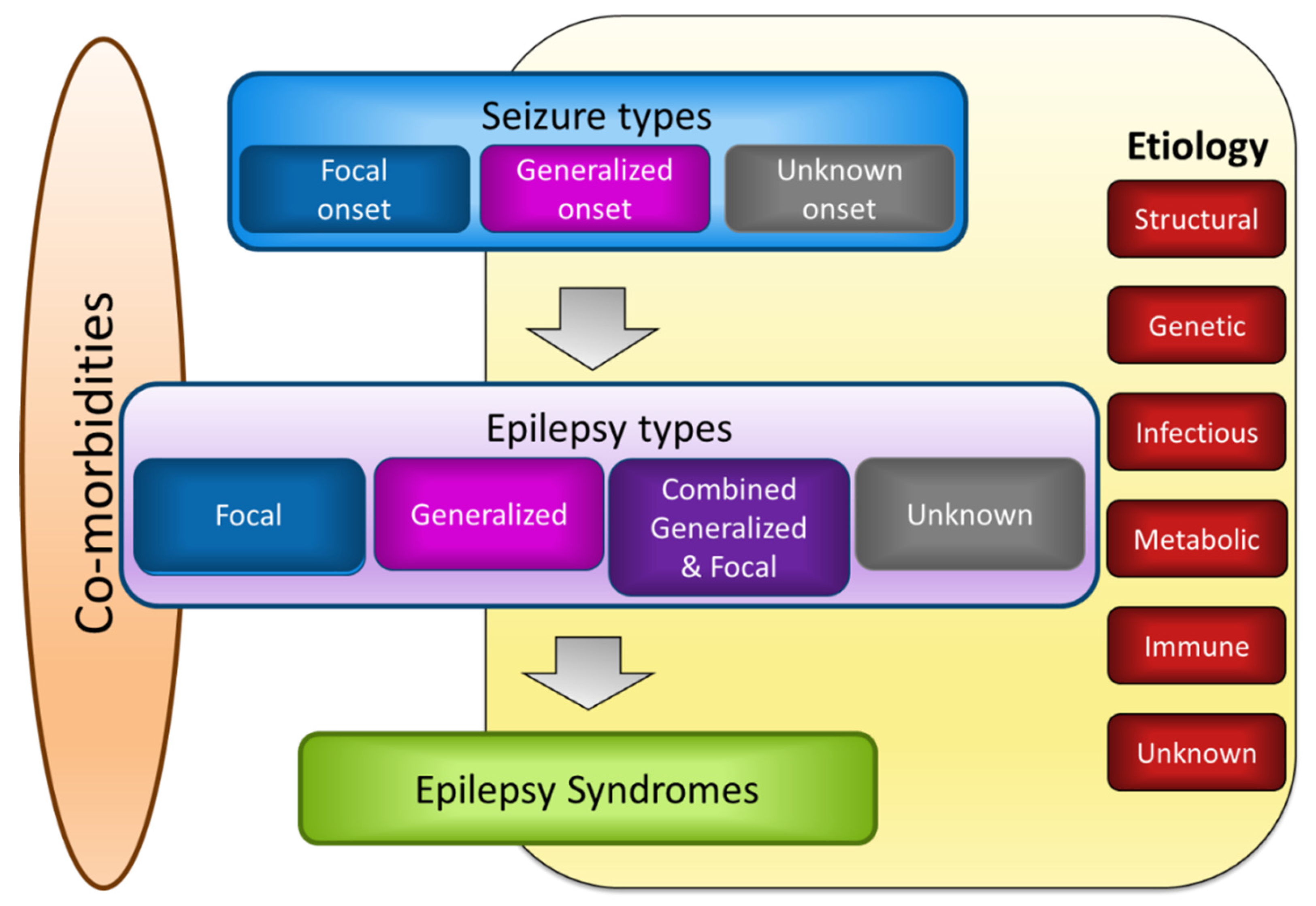
2.2. Classification of Epilepsy
3. Molecular Mechanisms of Epilepsy
3.1. Ion Channel and Neurotransmitter Dynamics in Epilepsy
3.2. Oxidative Stress in Epilepsy
3.3. Inflammation in Epilepsy
4. Functional Nutrients Beneficial in Epilepsy
4.1. Omega-3 Polyunsaturated Fatty Acids
4.2. Vitamin D3 (Cholecalciferol)
4.3. Vitamin E
4.4. Vitamin B6
4.5. Nutrients Potentially Beneficial for Refractory Epilepsy
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Epilepsy Fact sheets. Available online: https://www.who.int/news-room/fact-sheets/detail/epilepsy (accessed on 7 February 2019).
- Fisher, R.S.; Acevedo, C.; Arzimanoglou, A.; Bogacz, A.; Cross, J.H.; Elger, C.E.; Engel, J., Jr.; Forsgren, L.; French, J.A.; Glynn, M.; et al. ILAE official report: A practical clinical definition of epilepsy. Epilepsia 2014, 55, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, I.E.; Berkovic, S.; Capovilla, G.; Connolly, M.B.; French, J.; Guilhoto, L.; Hirsch, E.; Jain, S.; Mathern, G.W.; Moshe, S.L.; et al. ILAE classification of the epilepsies: Position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Schlanger, S.; Shinitzky, M.; Yam, D. Diet enriched with omega-3 fatty acids alleviates convulsion symptoms in epilepsy patients. Epilepsia 2002, 43, 103–104. [Google Scholar] [CrossRef] [PubMed]
- Yuen, A.W.; Sander, J.W.; Fluegel, D.; Patsalos, P.N.; Bell, G.S.; Johnson, T.; Koepp, M.J. Omega-3 fatty acid supplementation in patients with chronic epilepsy: A randomized trial. Epilepsy Behav. 2005, 7, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Al Khayat, H.A.; Awadalla, M.M.; Al Wakad, A.; Marwook, Z.A. Polyunsaturated fatty acids in children with idiopathic intractable epilepsy: Serum levels and therapeutic response. J. Pediatr. Neurol. 2010, 8, 175–185. [Google Scholar] [CrossRef]
- DeGiorgio, C.M.; Miller, P.R.; Harper, R.; Gornbein, J.; Schrader, L.; Soss, J.; Meymandi, S. Fish oil (n-3 fatty acids) in drug resistant epilepsy: A randomised placebo-controlled crossover study. J. Neurol. Neurosurg. Psychiatry 2015, 86, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Reda, D.M.; Abd-El-Fatah, N.K.; Omar Tel, S.; Darwish, O.A. Fish Oil Intake and Seizure Control in Children with Medically Resistant Epilepsy. N. Am. J. Med. Sci. 2015, 7, 317–321. [Google Scholar] [CrossRef]
- Omrani, S.; Taheri, M.; Omrani, M.D.; Arsang-Jang, S.; Ghafouri-Fard, S. The effect of omega-3 fatty acids on clinical and paraclinical features of intractable epileptic patients: A triple blind randomized clinical trial. Clin. Transl. Med. 2019, 8, 3. [Google Scholar] [CrossRef]
- DeGiorgio, C.M.; Miller, P.; Meymandi, S.; Gornbein, J.A. n-3 fatty acids (fish oil) for epilepsy, cardiac risk factors, and risk of SUDEP: Clues from a pilot, double-blind, exploratory study. Epilepsy Behav. 2008, 13, 681–684. [Google Scholar] [CrossRef]
- Christiansen, C.; Rodbro, P.; Sjo, O. “Anticonvulsant action” of vitamin D in epileptic patients? A controlled pilot study. Br. Med. J. 1974, 2, 258–259. [Google Scholar] [CrossRef]
- Hollo, A.; Clemens, Z.; Kamondi, A.; Lakatos, P.; Szucs, A. Correction of vitamin D deficiency improves seizure control in epilepsy: A pilot study. Epilepsy Behav. 2012, 24, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Tombini, M.; Palermo, A.; Assenza, G.; Pellegrino, G.; Benvenga, A.; Campana, C.; Naciu, A.M.; Assenza, F.; Lazzaro, V.D. Calcium metabolism serum markers in adult patients with epilepsy and the effect of vitamin D supplementation on seizure control. Seizure 2018, 58, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Ogunmekan, A.O.; Hwang, P.A. A randomized, double-blind, placebo-controlled, clinical trial of D-alpha-tocopheryl acetate (vitamin E), as add-on therapy, for epilepsy in children. Epilepsia 1989, 30, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Hom, A.C.; Weaver, R.C.; Aldersen, J.J. Efficacy of D-alpha-tocopheryl acetate as adjuctive antiepileptic agent in patients with refractory epilepsy and profound developmental disability: A prospective, randomized double-blind, placebo-controlled trial. Epilepsia 1991, 32, 62. [Google Scholar]
- Mehvari, J.; Motlagh, F.G.; Najafi, M.; Ghazvini, M.R.A.; Naeiru, A.A.; Zare, M. Effects of vitamin E on seizure frequency, electroencephalogram findings, and oxidative stress status of refractory epileptic patients. Adv. Biomed. Res. 2016, 5, 36. [Google Scholar]
- Hagberg, B.; Hamfelt, A.; Hansson, O. Epileptic Children with Disturbed Trytophan Metabolism Treated with Vitamin B6. Lancet 1964, 1, 145. [Google Scholar] [CrossRef]
- Hansson, O.; Hagberg, B. Effect of pyridoxine treatment in children with epilepsy. Acta Soc. Med. Ups. 1968, 73, 35–43. [Google Scholar]
- Jiao, F.Y.; Gao, D.Y.; Takuma, Y.; Wu, S.; Liu, Z.Y.; Zhang, X.K.; Lieu, N.S.; Ge, Z.L.; Chui, W.; Li, H.R.; et al. Randomized, controlled trial of high-dose intravenous pyridoxine in the treatment of recurrent seizures in children. Pediatr. Neurol. 1997, 17, 54–57. [Google Scholar] [CrossRef]
- Wang, H.S.; Kuo, M.F. Vitamin B6 related epilepsy during childhood. Chang Gung Med. J. 2007, 30, 396–401. [Google Scholar]
- Winesett, S.P.; Bessone, S.K.; Kossoff, E.H. The ketogenic diet in pharmacoresistant childhood epilepsy. Expert Rev. Neurother. 2015, 15, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, Y.; Wang, Y.; Tang, H.; Zhang, F.; Zhang, Y.; Zhao, Y. Ketogenic diet for treatment of intractable epilepsy in adults: A meta-analysis of observational studies. Epilepsia Open 2018, 3, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; Cross, J.H.; French, J.A.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshe, S.L.; Peltola, J.; Roulet Perez, E.; et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.T.; Berkovic, S.F.; Brodie, M.J.; Buchhalter, J.; Cross, J.H.; van Emde Boas, W.; Engel, J.; French, J.; Glauser, T.A.; Mathern, G.W.; et al. Revised terminology and concepts for organization of seizures and epilepsies: Report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 2010, 51, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Ran, X.; Li, J.; Shao, Q.; Chen, H.; Lin, Z.; Sun, Z.S.; Wu, J. EpilepsyGene: A genetic resource for genes and mutations related to epilepsy. Nucleic Acids Res. 2015, 43, D893–D899. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Xiu, X.; Song, Z. The molecular biology of genetic-based epilepsies. Mol. Neurobiol. 2014, 49, 352–367. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Yan, L.M.; Su, T.; He, N.; Lin, Z.J.; Wang, J.; Shi, Y.W.; Yi, Y.H.; Liao, W.P. Ion Channel Genes and Epilepsy: Functional Alteration, Pathogenic Potential, and Mechanism of Epilepsy. Neurosci. Bull. 2017, 33, 455–477. [Google Scholar] [CrossRef]
- Scharfman, H.E. The neurobiology of epilepsy. Curr. Neurol. Neurosci. Rep. 2007, 7, 348–354. [Google Scholar] [CrossRef]
- El Achkar, C.M.; Olson, H.E.; Poduri, A.; Pearl, P.L. The genetics of the epilepsies. Curr. Neurol. Neurosci. Rep. 2015, 15, 39. [Google Scholar] [CrossRef]
- Wang, J.; Lin, Z.J.; Liu, L.; Xu, H.Q.; Shi, Y.W.; Yi, Y.H.; He, N.; Liao, W.P. Epilepsy-associated genes. Seizure 2017, 44, 11–20. [Google Scholar] [CrossRef]
- Wolfart, J.; Laker, D. Homeostasis or channelopathy? Acquired cell type-specific ion channel changes in temporal lobe epilepsy and their antiepileptic potential. Front. Physiol. 2015, 6, 168. [Google Scholar] [CrossRef]
- Ogiwara, I.; Ito, K.; Sawaishi, Y.; Osaka, H.; Mazaki, E.; Inoue, I.; Montal, M.; Hashikawa, T.; Shike, T.; Fujiwara, T.; et al. De novo mutations of voltage-gated sodium channel alphaII gene SCN2A in intractable epilepsies. Neurology 2009, 73, 1046–1053. [Google Scholar] [CrossRef] [PubMed]
- Estacion, M.; Gasser, A.; Dib-Hajj, S.D.; Waxman, S.G. A sodium channel mutation linked to epilepsy increases ramp and persistent current of Nav1.3 and induces hyperexcitability in hippocampal neurons. Exp. Neurol. 2010, 224, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Veeramah, K.R.; O’Brien, J.E.; Meisler, M.H.; Cheng, X.; Dib-Hajj, S.D.; Waxman, S.G.; Talwar, D.; Girirajan, S.; Eichler, E.E.; Restifo, L.L.; et al. De novo pathogenic SCN8A mutation identified by whole-genome sequencing of a family quartet affected by infantile epileptic encephalopathy and SUDEP. Am. J. Hum. Genet. 2012, 90, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Audenaert, D.; Claes, L.; Ceulemans, B.; Lofgren, A.; Van Broeckhoven, C.; De Jonghe, P. A deletion in SCN1B is associated with febrile seizures and early-onset absence epilepsy. Neurology 2003, 61, 854–856. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.H.; Mantegazza, M.; Westenbroek, R.E.; Robbins, C.A.; Kalume, F.; Burton, K.A.; Spain, W.J.; McKnight, G.S.; Scheuer, T.; Catterall, W.A. Reduced sodium current in GABAergic interneurons in a mouse model of severe myoclonic epilepsy in infancy. Nat. Neurosci. 2006, 9, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.S.; Dutt, K.; Papale, L.A.; Dube, C.M.; Dutton, S.B.; de Haan, G.; Shankar, A.; Tufik, S.; Meisler, M.H.; Baram, T.Z.; et al. Altered function of the SCN1A voltage-gated sodium channel leads to gamma-aminobutyric acid-ergic (GABAergic) interneuron abnormalities. J. Biol. Chem. 2010, 285, 9823–9834. [Google Scholar] [CrossRef]
- Papale, L.A.; Beyer, B.; Jones, J.M.; Sharkey, L.M.; Tufik, S.; Epstein, M.; Letts, V.A.; Meisler, M.H.; Frankel, W.N.; Escayg, A. Heterozygous mutations of the voltage-gated sodium channel SCN8A are associated with spike-wave discharges and absence epilepsy in mice. Hum. Mol. Genet. 2009, 18, 1633–1641. [Google Scholar] [CrossRef]
- Singh, N.A.; Pappas, C.; Dahle, E.J.; Claes, L.R.F.; Pruess, T.H.; De Jonghe, P.; Thompson, J.; Dixon, M.; Gurnett, C.; Peiffer, A.; et al. A Role of SCN9A in Human Epilepsies, As a Cause of Febrile Seizures and As a Potential Modifier of Dravet Syndrome. PLoS Genet. 2009, 5, e1000649. [Google Scholar] [CrossRef]
- Chen, C.; Westenbroek, R.E.; Xu, X.; Edwards, C.A.; Sorenson, D.R.; Chen, Y.; McEwen, D.P.; O’Malley, H.A.; Bharucha, V.; Meadows, L.S.; et al. Mice lacking sodium channel beta1 subunits display defects in neuronal excitability, sodium channel expression, and nodal architecture. J. Neurosci. 2004, 24, 4030–4042. [Google Scholar] [CrossRef]
- Manolis, T.A.; Manolis, A.A.; Melita, H.; Manolis, A.S. Sudden unexpected death in epilepsy: The neuro-cardio-respiratory connection. Seizure 2019, 64, 65–73. [Google Scholar] [CrossRef]
- Kalume, F.; Westenbroek, R.E.; Cheah, C.S.; Yu, F.H.; Oakley, J.C.; Scheuer, T.; Catterall, W.A. Sudden unexpected death in a mouse model of Dravet syndrome. J. Clin. Investig. 2013, 123, 1798–1808. [Google Scholar] [CrossRef] [PubMed]
- Le Gal, F.; Korff, C.M.; Monso-Hinard, C.; Mund, M.T.; Morris, M.; Malafosse, A.; Schmitt-Mechelke, T. A case of SUDEP in a patient with Dravet syndrome with SCN1A mutation. Epilepsia 2010, 51, 1915–1918. [Google Scholar] [CrossRef] [PubMed]
- Delogu, A.B.; Spinelli, A.; Battaglia, D.; Dravet, C.; De Nisco, A.; Saracino, A.; Romagnoli, C.; Lanza, G.A.; Crea, F. Electrical and autonomic cardiac function in patients with Dravet syndrome. Epilepsia 2011, 52, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Wagnon, J.L.; Korn, M.J.; Parent, R.; Tarpey, T.A.; Jones, J.M.; Hammer, M.F.; Murphy, G.G.; Parent, J.M.; Meisler, M.H. Convulsive seizures and SUDEP in a mouse model of SCN8A epileptic encephalopathy. Hum. Mol. Genet. 2015, 24, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.A.; Westenskow, P.; Charlier, C.; Pappas, C.; Leslie, J.; Dillon, J.; Anderson, V.E.; Sanguinetti, M.C.; Leppert, M.F.; Consortium, B.P. KCNQ2 and KCNQ3 potassium channel genes in benign familial neonatal convulsions: Expansion of the functional and mutation spectrum. Brain 2003, 126, 2726–2737. [Google Scholar] [CrossRef]
- Peters, H.C.; Hu, H.; Pongs, O.; Storm, J.F.; Isbrandt, D. Conditional transgenic suppression of M channels in mouse brain reveals functions in neuronal excitability, resonance and behavior. Nat. Neurosci. 2005, 8, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Reid, C.A.; Berkovic, S.F.; Petrou, S. Mechanisms of human inherited epilepsies. Prog. Neurobiol. 2009, 87, 41–57. [Google Scholar] [CrossRef]
- Heron, S.E.; Phillips, H.A.; Mulley, J.C.; Mazarib, A.; Neufeld, M.Y.; Berkovic, S.F.; Scheffer, I.E. Genetic variation of CACNA1H in idiopathic generalized epilepsy. Ann. Neurol. 2004, 55, 595–596. [Google Scholar] [CrossRef]
- Becker, A.J.; Pitsch, J.; Sochivko, D.; Opitz, T.; Staniek, M.; Chen, C.C.; Campbell, K.P.; Schoch, S.; Yaari, Y.; Beck, H. Transcriptional upregulation of Cav3.2 mediates epileptogenesis in the pilocarpine model of epilepsy. J. Neurosci. 2008, 28, 13341–13353. [Google Scholar] [CrossRef]
- Su, H.; Sochivko, D.; Becker, A.; Chen, J.; Jiang, Y.; Yaari, Y.; Beck, H. Upregulation of a T-type Ca2+ channel causes a long-lasting modification of neuronal firing mode after status epilepticus. J. Neurosci. 2002, 22, 3645–3655. [Google Scholar] [CrossRef]
- Sanabria, E.R.; Su, H.; Yaari, Y. Initiation of network bursts by Ca2+-dependent intrinsic bursting in the rat pilocarpine model of temporal lobe epilepsy. J. Physiol. 2001, 532, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, B. Physiological changes during prolonged seizures and epileptic brain damage. Neuropadiatrie 1978, 9, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Bernard, C.; Cossart, R.; Hirsch, J.C.; Esclapez, M.; Ben-Ari, Y. What is GABAergic inhibition? How is it modified in epilepsy? Epilepsia 2000, 41, S90–S95. [Google Scholar] [CrossRef] [PubMed]
- Irier, H.A.; Shaw, R.; Lau, A.; Feng, Y.; Dingledine, R. Translational regulation of GluR2 mRNAs in rat hippocampus by alternative 3’ untranslated regions. J. Neurochem. 2009, 109, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Rogawski, M.A. AMPA receptors as a molecular target in epilepsy therapy. Acta Neurol. Scand. 2013, 127, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Sperk, G.; Drexel, M.; Pirker, S. Neuronal plasticity in animal models and the epileptic human hippocampus. Epilepsia 2009, 50, 29–31. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Pearson-Smith, J.N.; Patel, M. Metabolic Dysfunction and Oxidative Stress in Epilepsy. Int. J. Mol. Sci. 2017, 18, 2365. [Google Scholar] [CrossRef]
- Shin, E.J.; Jeong, J.H.; Chung, Y.H.; Kim, W.K.; Ko, K.H.; Bach, J.H.; Hong, J.S.; Yoneda, Y.; Kim, H.C. Role of oxidative stress in epileptic seizures. Neurochem. Int. 2011, 59, 122–137. [Google Scholar] [CrossRef]
- Stadtman, E.R. Protein oxidation in aging and age-related diseases. Ann. N. Y. Acad. Sci. 2001, 928, 22–38. [Google Scholar] [CrossRef]
- Wong-Ekkabut, J.; Xu, Z.; Triampo, W.; Tang, I.M.; Tieleman, D.P.; Monticelli, L. Effect of lipid peroxidation on the properties of lipid bilayers: A molecular dynamics study. Biophys. J. 2007, 93, 4225–4236. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, G.; Moraes, C.T.; Shanske, S.; Oh, S.J.; DiMauro, S. A new mtDNA mutation in the tRNA(Lys) gene associated with myoclonic epilepsy and ragged-red fibers (MERRF). Am. J. Hum. Genet. 1992, 51, 1213–1217. [Google Scholar] [PubMed]
- Ruhoy, I.S.; Saneto, R.P. The genetics of Leigh syndrome and its implications for clinical practice and risk management. Appl. Clin. Genet. 2014, 7, 221–234. [Google Scholar] [PubMed]
- Wu, S.B.; Ma, Y.S.; Wu, Y.T.; Chen, Y.C.; Wei, Y.H. Mitochondrial DNA mutation-elicited oxidative stress, oxidative damage, and altered gene expression in cultured cells of patients with MERRF syndrome. Mol. Neurobiol. 2010, 41, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Wojtala, A.; Karkucinska-Wieckowska, A.; Sardao, V.A.; Szczepanowska, J.; Kowalski, P.; Pronicki, M.; Duszynski, J.; Wieckowski, M.R. Modulation of mitochondrial dysfunction-related oxidative stress in fibroblasts of patients with Leigh syndrome by inhibition of prooxidative p66Shc pathway. Mitochondrion 2017, 37, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Brini, M.; Pinton, P.; King, M.P.; Davidson, M.; Schon, E.A.; Rizzuto, R. A calcium signaling defect in the pathogenesis of a mitochondrial DNA inherited oxidative phosphorylation deficiency. Nat. Med. 1999, 5, 951–954. [Google Scholar] [CrossRef]
- Kudin, A.P.; Baron, G.; Zsurka, G.; Hampel, K.G.; Elger, C.E.; Grote, A.; Weber, Y.; Lerche, H.; Thiele, H.; Nurnberg, P.; et al. Homozygous mutation in TXNRD1 is associated with genetic generalized epilepsy. Free Radic. Biol. Med. 2017, 106, 270–277. [Google Scholar] [CrossRef]
- Roma-Mateo, C.; Aguado, C.; Garcia-Gimenez, J.L.; Knecht, E.; Sanz, P.; Pallardo, F.V. Oxidative stress, a new hallmark in the pathophysiology of Lafora progressive myoclonus epilepsy. Free Radic. Biol. Med. 2015, 88, 30–41. [Google Scholar] [CrossRef]
- Roma-Mateo, C.; Aguado, C.; Garcia-Gimenez, J.L.; Ibanez-Cabellos, J.S.; Seco-Cervera, M.; Pallardo, F.V.; Knecht, E.; Sanz, P. Increased oxidative stress and impaired antioxidant response in Lafora disease. Mol. Neurobiol. 2015, 51, 932–946. [Google Scholar] [CrossRef]
- Kovac, S.; Dinkova Kostova, A.T.; Herrmann, A.M.; Melzer, N.; Meuth, S.G.; Gorji, A. Metabolic and Homeostatic Changes in Seizures and Acquired Epilepsy-Mitochondria, Calcium Dynamics and Reactive Oxygen Species. Int. J. Mol. Sci. 2017, 18, 1935. [Google Scholar] [CrossRef]
- Kunz, W.S.; Kudin, A.P.; Vielhaber, S.; Blumcke, I.; Zuschratter, W.; Schramm, J.; Beck, H.; Elger, C.E. Mitochondrial complex I deficiency in the epileptic focus of patients with temporal lobe epilepsy. Ann. Neurol. 2000, 48, 766–773. [Google Scholar] [CrossRef]
- Lopez, J.; Gonzalez, M.E.; Lorigados, L.; Morales, L.; Riveron, G.; Bauza, J.Y. Oxidative stress markers in surgically treated patients with refractory epilepsy. Clin. Biochem. 2007, 40, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Pecorelli, A.; Natrella, F.; Belmonte, G.; Miracco, C.; Cervellati, F.; Ciccoli, L.; Mariottini, A.; Rocchi, R.; Vatti, G.; Bua, A.; et al. NADPH oxidase activation and 4-hydroxy-2-nonenal/aquaporin-4 adducts as possible new players in oxidative neuronal damage presents in drug-resistant epilepsy. Biochim. Biophys. Acta 2015, 1852, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Rumia, J.; Marmol, F.; Sanchez, J.; Gimenez-Crouseilles, J.; Carreno, M.; Bargallo, N.; Boget, T.; Pintor, L.; Setoain, X.; Donaire, A.; et al. Oxidative stress markers in the neocortex of drug-resistant epilepsy patients submitted to epilepsy surgery. Epilepsy Res. 2013, 107, 75–81. [Google Scholar] [CrossRef]
- Ristic, A.J.; Savic, D.; Sokic, D.; Bogdanovic Pristov, J.; Nestorov, J.; Bascarevic, V.; Raicevic, S.; Savic, S.; Spasojevic, I. Hippocampal antioxidative system in mesial temporal lobe epilepsy. Epilepsia 2015, 56, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Yuzbasioglu, A.; Karatas, H.; Gursoy-Ozdemir, Y.; Saygi, S.; Akalan, N.; Soylemezoglu, F.; Dalkara, T.; Kocaefe, Y.C.; Ozguc, M. Changes in the expression of selenoproteins in mesial temporal lobe epilepsy patients. Cell. Mol. Neurobiol. 2009, 29, 1223–1231. [Google Scholar] [CrossRef]
- Liang, L.P.; Ho, Y.S.; Patel, M. Mitochondrial superoxide production in kainate-induced hippocampal damage. Neuroscience 2000, 101, 563–570. [Google Scholar] [CrossRef]
- Patel, M.; Liang, L.P.; Roberts, L.J., 2nd. Enhanced hippocampal F2-isoprostane formation following kainate-induced seizures. J. Neurochem. 2001, 79, 1065–1069. [Google Scholar] [CrossRef]
- Furukawa, A.; Kawamoto, Y.; Chiba, Y.; Takei, S.; Hasegawa-Ishii, S.; Kawamura, N.; Yoshikawa, K.; Hosokawa, M.; Oikawa, S.; Kato, M.; et al. Proteomic identification of hippocampal proteins vulnerable to oxidative stress in excitotoxin-induced acute neuronal injury. Neurobiol. Dis. 2011, 43, 706–714. [Google Scholar] [CrossRef]
- Ryan, K.; Backos, D.S.; Reigan, P.; Patel, M. Post-translational oxidative modification and inactivation of mitochondrial complex I in epileptogenesis. J. Neurosci. 2012, 32, 11250–11258. [Google Scholar] [CrossRef]
- Jarrett, S.G.; Liang, L.P.; Hellier, J.L.; Staley, K.J.; Patel, M. Mitochondrial DNA damage and impaired base excision repair during epileptogenesis. Neurobiol. Dis. 2008, 30, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Dal-Pizzol, F.; Klamt, F.; Vianna, M.M.; Schroder, N.; Quevedo, J.; Benfato, M.S.; Moreira, J.C.; Walz, R. Lipid peroxidation in hippocampus early and late after status epilepticus induced by pilocarpine or kainic acid in Wistar rats. Neurosci. Lett. 2000, 291, 179–182. [Google Scholar] [CrossRef]
- Liang, L.P.; Patel, M. Mitochondrial oxidative stress and increased seizure susceptibility in Sod2(-/+) mice. Free Radic. Biol. Med. 2004, 36, 542–554. [Google Scholar] [CrossRef] [PubMed]
- Melov, S.; Coskun, P.; Patel, M.; Tuinstra, R.; Cottrell, B.; Jun, A.S.; Zastawny, T.H.; Dizdaroglu, M.; Goodman, S.I.; Huang, T.T.; et al. Mitochondrial disease in superoxide dismutase 2 mutant mice. Proc. Natl. Acad. Sci. USA 1999, 96, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Virta, M.; Hurme, M.; Helminen, M. Increased frequency of interleukin-1beta (-511) allele 2 in febrile seizures. Pediatr. Neurol. 2002, 26, 192–195. [Google Scholar] [CrossRef]
- Al Morshedy, S.; Elsaadany, H.F.; Ibrahim, H.E.; Sherif, A.M.; Farghaly, M.A.; Allah, M.A.; Abouzeid, H.; Elashkar, S.S.; Hamed, M.E.; Fathy, M.M.; et al. Interleukin-1beta and interleukin-1receptor antagonist polymorphisms in Egyptian children with febrile seizures: A case-control study. Medicine (Baltimore) 2017, 96, e6370. [Google Scholar] [CrossRef]
- Devinsky, O.; Vezzani, A.; Najjar, S.; De Lanerolle, N.C.; Rogawski, M.A. Glia and epilepsy: Excitability and inflammation. Trends Neurosci. 2013, 36, 174–184. [Google Scholar] [CrossRef]
- Webster, K.M.; Sun, M.; Crack, P.; O’Brien, T.J.; Shultz, S.R.; Semple, B.D. Inflammation in epileptogenesis after traumatic brain injury. J. Neuroinflamm. 2017, 14, 10. [Google Scholar] [CrossRef]
- Van Vliet, E.A.; Aronica, E.; Vezzani, A.; Ravizza, T. Review: Neuroinflammatory pathways as treatment targets and biomarker candidates in epilepsy: Emerging evidence from preclinical and clinical studies. Neuropathol. Appl. Neurobiol. 2018, 44, 91–111. [Google Scholar] [CrossRef]
- Marchi, N.; Fan, Q.; Ghosh, C.; Fazio, V.; Bertolini, F.; Betto, G.; Batra, A.; Carlton, E.; Najm, I.; Granata, T.; et al. Antagonism of peripheral inflammation reduces the severity of status epilepticus. Neurobiol. Dis. 2009, 33, 171–181. [Google Scholar] [CrossRef]
- Zhao, H.; Zhu, C.; Huang, D. Microglial activation: An important process in the onset of epilepsy. Am. J. Transl. Res. 2018, 10, 2877–2889. [Google Scholar] [PubMed]
- Paudel, Y.N.; Semple, B.D.; Jones, N.C.; Othman, I.; Shaikh, M.F. High mobility group box 1 (HMGB1) as a novel frontier in epileptogenesis: From pathogenesis to therapeutic approaches. J. Neurochem. 2019, 149. [Google Scholar] [CrossRef] [PubMed]
- Maroso, M.; Balosso, S.; Ravizza, T.; Liu, J.; Aronica, E.; Iyer, A.M.; Rossetti, C.; Molteni, M.; Casalgrandi, M.; Manfredi, A.A.; et al. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat. Med. 2010, 16, 413–491. [Google Scholar] [CrossRef] [PubMed]
- De Sarro, G.; Russo, E.; Ferreri, G.; Giuseppe, B.; Flocco, M.A.; Di Paola, E.D.; De Sarro, A. Seizure susceptibility to various convulsant stimuli of knockout interleukin-6 mice. Pharmacol. Biochem. Behav. 2004, 77, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Peltola, J.; Hurme, M.; Miettinen, A.; Keranen, T. Elevated levels of interleukin-6 may occur in cerebrospinal fluid from patients with recent epileptic seizures. Epilepsy Res. 1998, 31, 129–133. [Google Scholar] [CrossRef]
- Riazi, K.; Galic, M.A.; Kuzmiski, J.B.; Ho, W.; Sharkey, K.A.; Pittman, Q.J. Microglial activation and TNF alpha production mediate altered CNS excitability following peripheral inflammation. Proc. Natl. Acad. Sci. USA 2008, 105, 17151–17156. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, M.S.; Blake, B.L.; McCown, T.J. Opposing actions of hippocampus TNF alpha receptors on limbic seizure susceptibility. Exp. Neurol. 2013, 247, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Balosso, S.; Ravizza, T.; Perego, C.; Peschon, J.; Campbell, I.L.; De Simoni, M.G.; Vezzani, A. Tumor necrosis factor-alpha inhibits seizures in mice via p75 receptors. Ann. Neurol. 2005, 57, 804–812. [Google Scholar] [CrossRef] [PubMed]
- Taha, A.Y.; Burnham, W.M.; Auvin, S. Polyunsaturated fatty acids and epilepsy. Epilepsia 2010, 51, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Pourmasoumi, M.; Vosoughi, N.; Derakhshandeh-Rishehri, S.M.; Assarroudi, M.; Heidari-Beni, M. Association of Omega-3 Fatty Acid and Epileptic Seizure in Epileptic Patients: A Systematic Review. Int. J. Prev. Med. 2018, 9, 36. [Google Scholar] [PubMed]
- Lund, E.K.; Harvey, L.J.; Ladha, S.; Clark, D.C.; Johnson, I.T. Effects of dietary fish oil supplementation on the phospholipid composition and fluidity of cell membranes from human volunteers. Ann. Nutr. Metab. 1999, 43, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Voskuyl, R.A.; Vreugdenhil, M.; Kang, J.X.; Leaf, A. Anticonvulsant effect of polyunsaturated fatty acids in rats, using the cortical stimulation model. Eur. J. Pharmacol. 1998, 341, 145–152. [Google Scholar] [CrossRef]
- Taha, A.Y.; Zahid, T.; Epps, T.; Trepanier, M.O.; Burnham, W.M.; Bazinet, R.P.; Zhang, L. Selective reduction of excitatory hippocampal sharp waves by docosahexaenoic acid and its methyl ester analog ex-vivo. Brain Res. 2013, 1537, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C.; Yaqoob, P. Omega-3 (n-3) fatty acids, cardiovascular disease and stability of atherosclerotic plaques. Cell. Mol. Biol. 2010, 56, 28–37. [Google Scholar] [PubMed]
- Puri, B.K.; Koepp, M.J.; Holmes, J.; Hamilton, G.; Yuen, A.W. A 31-phosphorus neurospectroscopy study of omega-3 long-chain polyunsaturated fatty acid intervention with eicosapentaenoic acid and docosahexaenoic acid in patients with chronic refractory epilepsy. Prostaglandins Leukot. Essent. Fatty Acids 2007, 77, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, M.; Hjelte, L.; Nilsson, S.; Amark, P. Plasma phospholipid fatty acids are influenced by a ketogenic diet enriched with n-3 fatty acids in children with epilepsy. Epilepsy Res. 2007, 73, 199–207. [Google Scholar] [CrossRef]
- Bromfield, E.; Dworetzky, B.; Hurwitz, S.; Eluri, Z.; Lane, L.; Replansky, S.; Mostofsky, D. A randomized trial of polyunsaturated fatty acids for refractory epilepsy. Epilepsy Behav. 2008, 12, 187–190. [Google Scholar] [CrossRef]
- Yuen, A.W.; Flugel, D.; Poepel, A.; Bell, G.S.; Peacock, J.L.; Sander, J.W. Non-randomized open trial of eicosapentaenoic acid (EPA), an omega-3 fatty acid, in ten people with chronic epilepsy. Epilepsy Behav. 2012, 23, 370–372. [Google Scholar] [CrossRef]
- Scorza, F.A.; Cysneiros, R.M.; Arida, R.M.; Terra, V.C.; Machado, H.R.; Rabello, G.M.M.; Albuquerque, M.; Cavalheiro, E.A. Fish consumption, contaminants and sudden unexpected death in epilepsy: Many more benefits than risks. Braz. J. Biol. 2010, 70, 665–670. [Google Scholar] [CrossRef]
- Saxena, A.; Jones, L.; Shankar, R.; McLean, B.; Newman, C.G.J.; Hamandi, K. Sudden unexpected death in epilepsy in children: A focused review of incidence and risk factors. J. Neurol. Neurosurg. Psychiatry 2018, 89, 1064–1070. [Google Scholar] [CrossRef]
- Tomson, T.; Walczak, T.; Sillanpaa, M.; Sander, J.W. Sudden unexpected death in epilepsy: A review of incidence and risk factors. Epilepsia 2005, 46, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Scorza, F.A.; Cysneiros, R.M.; Arida, R.M.; Terra-Bustamante, V.C.; de Albuquerque, M.; Cavalheiro, E.A. The other side of the coin: Beneficiary effect of omega-3 fatty acids in sudden unexpected death in epilepsy. Epilepsy Behav. 2008, 13, 279–283. [Google Scholar] [CrossRef] [PubMed]
- Yuen, A.W.C.; Sander, J.W. Is omega-3 fatty acid deficiency a factor contributing to refractory seizures and SUDEP? A hypothesis. Seizure 2004, 13, 104–107. [Google Scholar] [CrossRef]
- Scorza, C.A.; Cavalheiro, E.A.; Calderazzo, L.; de Almeida, A.C.G.; Scorza, F.A. Chew on this: Sardines are still a healthy choice against SUDEP. Epilepsy Behav. 2014, 41, 21–22. [Google Scholar] [CrossRef] [PubMed]
- Cysneiros, R.M.; Arida, R.M.; Terra, V.C.; Sonoda, E.Y.; Cavalheiro, E.A.; Scorza, F.A. To sushi or not to sushi: Can people with epilepsy have sushi from time to time? Epilepsy Behav. 2009, 16, 565–566. [Google Scholar] [CrossRef] [PubMed]
- Scorza, F.A.; Scorza, C.A.; Cavalheiro, E.A. Omega-3 fatty acids and SUDEP prevention. Lancet Neurol. 2016, 15, 1303. [Google Scholar] [CrossRef][Green Version]
- Taha, A.Y.; Trepanier, M.O.; Ciobanu, F.A.; Taha, N.M.; Ahmed, M.; Zeng, Q.; Cheuk, W.I.; Ip, B.; Filo, E.; Scott, B.W.; et al. A minimum of 3 months of dietary fish oil supplementation is required to raise amygdaloid afterdischarge seizure thresholds in rats—Implications for treating complex partial seizures. Epilepsy Behav. 2013, 27, 49–58. [Google Scholar] [CrossRef]
- Trepanier, M.O.; Taha, A.Y.; Mantha, R.L.; Ciobanu, F.A.; Zeng, Q.H.; Tchkhartichvili, G.M.; Domenichiello, A.F.; Bazinet, R.P.; Burnham, W.M. Increases in seizure latencies induced by subcutaneous docosahexaenoic acid are lost at higher doses. Epilepsy Res. 2012, 99, 225–232. [Google Scholar] [CrossRef]
- Flores-Mancilla, L.E.; Hernandez-Gonzalez, M.; Guevara, M.A.; Benavides-Haro, D.E.; Martinez-Arteaga, P. Long-term fish oil supplementation attenuates seizure activity in the amygdala induced by 3-mercaptopropionic acid in adult male rats. Epilepsy Behav. 2014, 33, 126–134. [Google Scholar] [CrossRef]
- Bandero, C.R.; Salvadori, M.G.; Gomes, A.T.; Dal Ri, N.M.; Furian, A.F.; Oliveira, M.S.; Rambo, L.M.; Scorza, F.A.; Cysneiros, R.M.; Emanuelli, T.; et al. Fish oil attenuates methylmalonate-induced seizures. Epilepsy Res. 2013, 105, 69–76. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.A.; Al-Qahtani, J.M.; El-Safty, S.A. Omega-3 polyunsaturated fatty acids in large doses attenuate seizures, cognitive impairment, and hippocampal oxidative DNA damage in young kindled rats. Neurosci. Lett. 2015, 584, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Trepanier, M.O.; Lim, J.; Lai, T.K.; Cho, H.J.; Domenichiello, A.F.; Chen, C.T.; Taha, A.Y.; Bazinet, R.P.; Burnham, W.M. Intraperitoneal administration of docosahexaenoic acid for 14days increases serum unesterified DHA and seizure latency in the maximal pentylenetetrazol model. Epilepsy Behav. 2014, 33, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, Y.; Itoh, K.; Tanaka, M.; Tsuji, M.; Kawamoto, T.; Kawato, S.; Vogel, C.F.A.; Yamazaki, T. Potentiation of 17 beta-estradiol synthesis in the brain and elongation of seizure latency through dietary supplementation with docosahexaenoic acid. Sci. Rep. 2017, 7, 6268. [Google Scholar] [CrossRef] [PubMed]
- Vreugdenhil, M.; Bruehl, C.; Voskuyl, R.A.; Kang, J.X.; Leaf, A.; Wadman, W.J. Polyunsaturated fatty acids modulate sodium and calcium currents in CA1 neurons. Proc. Natl. Acad. Sci. USA 1996, 93, 12559–12563. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.P.; Kim, H.I.; Shin, Y.K.; Lee, C.S.; Park, M.; Song, J.H. Effects of free fatty acids on sodium currents in rat dorsal root ganglion neurons. Brain Res. 2004, 1008, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Liin, S.I.; Karlsson, U.; Bentzen, B.H.; Schmitt, N.; Elinder, F. Polyunsaturated fatty acids are potent openers of human M-channels expressed in Xenopus laevis oocytes. Acta Physiol. 2016, 218, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Hamano, H.; Nabekura, J.; Nishikawa, M.; Ogawa, T. Docosahexaenoic acid reduces GABA response in substantia nigra neuron of rat. J. Neurophysiol. 1996, 75, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Nabekura, J.; Noguchi, K.; Witt, M.R.; Nielsen, M.; Akaike, N. Functional modulation of human recombinant gamma-aminobutyric acid type A receptor by docosahexaenoic acid. J. Biol. Chem. 1998, 273, 11056–11061. [Google Scholar] [CrossRef]
- Sogaard, R.; Werge, T.M.; Bertelsen, C.; Lundbye, C.; Madsen, K.L.; Nielsen, C.H.; Lundbaek, J.A. GABA(A) receptor function is regulated by lipid bilayer elasticity. Biochemistry 2006, 45, 13118–13129. [Google Scholar] [CrossRef]
- Ferrari, D.; Cysneiros, R.M.; Scorza, C.A.; Arida, R.M.; Cavalheiro, E.A.; de Almeida, A.C.; Scorza, F.A. Neuroprotective activity of omega-3 fatty acids against epilepsy-induced hippocampal damage: Quantification with immunohistochemical for calcium-binding proteins. Epilepsy Behav. 2008, 13, 36–42. [Google Scholar] [CrossRef]
- Cysneiros, R.M.; Ferrari, D.; Arida, R.M.; Terra, V.C.; de Almeida, A.C.; Cavalheiro, E.A.; Scorza, F.A. Qualitative analysis of hippocampal plastic changes in rats with epilepsy supplemented with oral omega-3 fatty acids. Epilepsy Behav. 2010, 17, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.H.; Chang, C.D.; Chen, P.H.; Su, J.R.; Chen, C.C.; Chaung, H.C. Docosahexaenoic acid and phosphatidylserine supplementations improve antioxidant activities and cognitive functions of the developing brain on pentylenetetrazol-induced seizure model. Brain Res. 2012, 1451, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Nejm, M.B.; Haidar, A.A.; Marques, M.J.; Hirata, A.E.; Nogueira, F.N.; Cavalheiro, E.A.; Scorza, F.A.; Cysneiros, R.M. Fish oil provides protection against the oxidative stress in pilocarpine model of epilepsy. Metab. Brain Dis. 2015, 30, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, T.L.; Vieira de Sousa, P.V.; de Almeida, S.S.; Nejm, M.B.; Vieira de Brito, J.M.; Cysneiros, R.M.; de Brito, M.V.; Salu, B.R.; Oliva, M.L.; Scorza, F.A.; et al. High serum levels of proinflammatory markers during epileptogenesis. Can omega-3 fatty acid administration reduce this process? Epilepsy Behav. 2015, 51, 300–305. [Google Scholar] [CrossRef] [PubMed]
- NIH Office of Dietary Supplements. Vitamin D. Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/#en11 (accessed on 9 November 2018).
- Pendo, K.; DeGiorgio, C.M. Vitamin D3 for the Treatment of Epilepsy: Basic Mechanisms, Animal Models, and Clinical Trials. Front. Neurol. 2016, 7, 218. [Google Scholar] [CrossRef] [PubMed]
- Eyles, D.W.; Burne, T.H.J.; McGrath, J.J. Vitamin D, effects on brain development, adult brain function and the links between low levels of vitamin D and neuropsychiatric disease. Front. Neuroendocrinol. 2013, 34, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Teagarden, D.L.; Meador, K.J.; Loring, D.W. Low vitamin D levels are common in patients with epilepsy. Epilepsy Res. 2014, 108, 1352–1356. [Google Scholar] [CrossRef] [PubMed]
- Offermann, G.; Pinto, V.; Kruse, R. Antiepileptic drugs and vitamin D supplementation. Epilepsia 1979, 20, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, J.R.; Mridula, K.R.; Rathnakishore, C.; Balaraju, B.; Bandaru, V.S. Association of 25-Hydroxyvitamin D Deficiency in Pediatric Epileptic Patients. Iran. J. Child Neurol. 2017, 11, 48–56. [Google Scholar]
- Garcia, V.C.; Martini, L.A. Vitamin D and cardiovascular disease. Nutrients 2010, 2, 426–437. [Google Scholar] [CrossRef]
- Scorza, F.A.; de Albuquerque, M.; Arida, R.M.; Terra, V.C.; Machado, H.R.; Cavalheiro, E.A. Benefits of sunlight: Vitamin D deficiency might increase the risk of sudden unexpected death in epilepsy. Med. Hypotheses 2010, 74, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Mann, M.C.; Exner, D.V.; Hemmelgarn, B.R.; Hanley, D.A.; Turin, T.C.; MacRae, J.M.; Wheeler, D.C.; Sola, D.Y.; Ramesh, S.; Ahmed, S.B. The VITAH Trial-Vitamin D Supplementation and Cardiac Autonomic Tone in Patients with End-Stage Kidney Disease on Hemodialysis: A Blinded, Randomized Controlled Trial. Nutrients 2016, 8, 608. [Google Scholar] [CrossRef] [PubMed]
- Mann, M.C.; Hemmelgarn, B.R.; Exner, D.V.; Hanley, D.A.; Turin, T.C.; Wheeler, D.C.; Sola, D.Y.; Ellis, L.; Ahmed, S.B. Vitamin D supplementation is associated with stabilization of cardiac autonomic tone in IgA nephropathy. Hypertension 2015, 66, 4–6. [Google Scholar] [CrossRef] [PubMed]
- Mann, M.C.; Exner, D.V.; Hemmelgarn, B.R.; Hanley, D.A.; Turin, T.C.; MacRae, J.M.; Ahmed, S.B. The VITAH trial VITamin D supplementation and cardiac Autonomic tone in Hemodialysis: A blinded, randomized controlled trial. BMC Nephrol. 2014, 15, 129. [Google Scholar] [CrossRef] [PubMed]
- Mann, M.C.; Exner, D.V.; Hemmelgarn, B.R.; Turin, T.C.; Sola, D.Y.; Ellis, L.; Ahmed, S.B. Vitamin D supplementation is associated with improved modulation of cardiac autonomic tone in healthy humans. Int. J. Cardiol. 2014, 172, 506–508. [Google Scholar] [CrossRef] [PubMed]
- Novak, J.L.; Miller, P.R.; Markovic, D.; Meymandi, S.K.; DeGiorgio, C.M. Risk Assessment for Sudden Death in Epilepsy: The SUDEP-7 Inventory. Front. Neurol. 2015, 6, 252. [Google Scholar] [CrossRef]
- DeGiorgio, C.M.; Miller, P.; Meymandi, S.; Chin, A.; Epps, J.; Gordon, S.; Gornbein, J.; Harper, R.M. RMSSD, a measure of vagus-mediated heart rate variability, is associated with risk factors for SUDEP: The SUDEP-7 Inventory. Epilepsy Behav. 2010, 19, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Siegel, A.; Malkowitz, L.; Moskovits, M.J.; Christakos, S. Administration of 1,25-dihydroxyvitamin D3 results in the elevation of hippocampal seizure threshold levels in rats. Brain Res. 1984, 298, 125–129. [Google Scholar] [CrossRef]
- Abdel-Wahab, A.F.; Afify, M.A.; Mahfouz, A.M.; Shahzad, N.; Bamagous, G.A.; Al Ghamdi, S.S. Vitamin D enhances antiepileptic and cognitive effects of lamotrigine in pentylenetetrazole-kindled rats. Brain Res. 2017, 1673, 78–85. [Google Scholar] [CrossRef]
- Kalueff, A.V.; Minasyan, A.; Tuohimaa, P. Anticonvulsant effects of 1,25-dihydroxyvitamin D in chemically induced seizures in mice. Brain Res. Bull. 2005, 67, 156–160. [Google Scholar] [CrossRef]
- Borowicz, K.K.; Morawska, M.; Furmanek-Karwowska, K.; Luszczki, J.J.; Czuczwar, S.J. Cholecalciferol enhances the anticonvulsant effect of conventional antiepileptic drugs in the mouse model of maximal electroshock. Eur. J. Pharmacol. 2007, 573, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Kalueff, A.V.; Minasyan, A.; Keisala, T.; Kuuslahti, M.; Miettinen, S.; Tuohimaa, P. Increased severity of chemically induced seizures in mice with partially deleted Vitamin D receptor gene. Neurosci. Lett. 2006, 394, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Brewer, L.D.; Thibault, V.; Chen, K.C.; Langub, M.C.; Landfield, P.W.; Porter, N.M. Vitamin D hormone confers neuroprotection in parallel with downregulation of L-type calcium channel expression in hippocampal neurons. J. Neurosci. 2001, 21, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, L.; Goulart, P.B.; Goncalves, R.; Pierozan, P.; Winkelmann-Duarte, E.C.; Woehl, V.M.; Pessoa-Pureur, R.; Silva, F.R.M.B.; Zamoner, A. 1 alpha,25-Dihydroxyvitamin D-3 mechanism of action: Modulation of L-type calcium channels leading to calcium uptake and intermediate filament phosphorylation in cerebral cortex of young rats. BBA Mol. Cell Res. 2012, 1823, 1708–1719. [Google Scholar]
- Dursun, E.; Gezen-Ak, D.; Yilmazer, S. The Influence of Vitamin D Treatment on the Inducible Nitric Oxide Synthase (INOS) Expression in Primary Hippocampal Neurons. Noro. Psikiyatr. Ars. 2014, 51, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre d’Hellencourt, C.; Montero-Menei, C.N.; Bernard, R.; Couez, D. Vitamin D3 inhibits proinflammatory cytokines and nitric oxide production by the EOC13 microglial cell line. J. Neurosci. Res. 2003, 71, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Bartels, L.E.; Jorgensen, S.P.; Agnholt, J.; Kelsen, J.; Hvas, C.L.; Dahlerup, J.F. 1,25-dihydroxyvitamin D3 and dexamethasone increase interleukin-10 production in CD4+ T cells from patients with Crohn’s disease. Int. Immunopharmacol. 2007, 7, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Chemistry, Nutrition, and Health-Promoting Properties of Hericium erinaceus (Lion’s Mane) Mushroom Fruiting Bodies and Mycelia and Their Bioactive Compounds. J. Agric. Food Chem. 2015, 63, 7108–7123. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.J.; Kim, J.E.; Jeong, K.H.; Lim, S.C.; Kim, S.Y.; Cho, K.O. The Neuroprotective Effect of Hericium erinaceus Extracts in Mouse Hippocampus after Pilocarpine-Induced Status Epilepticus. Int. J. Mol. Sci. 2019, 20. [Google Scholar] [CrossRef] [PubMed]
- NIH Office of Dietary Supplements. Vitamin E. Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional/ (accessed on 17 August 2018).
- Lloret, A.; Esteve, D.; Monllor, P.; Cervera-Ferri, A.; Lloret, A. The Effectiveness of Vitamin E Treatment in Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 879. [Google Scholar] [CrossRef]
- Sozen, E.; Demirel, T.; Ozer, N.K. Vitamin E: Regulatory role in the cardiovascular system. IUBMB Life 2019, 71, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Uchida, D.; Takaki, A.; Adachi, T.; Okada, H. Beneficial and Paradoxical Roles of Anti-Oxidative Nutritional Support for Non-Alcoholic Fatty Liver Disease. Nutrients 2018, 10, 977. [Google Scholar] [CrossRef]
- Raju, G.B.; Behari, M.; Prasad, K.; Ahuja, G.K. Randomized, double-blind, placebo-controlled, clinical trial of D-alpha-tocopherol (vitamin E) as add-on therapy in uncontrolled epilepsy. Epilepsia 1994, 35, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Ayyildiz, M.; Yildirim, M.; Agar, E. The effects of vitamin E on penicillin-induced epileptiform activity in rats. Exp. Brain Res. 2006, 174, 109–113. [Google Scholar] [CrossRef]
- Ambrogini, P.; Albertini, M.C.; Betti, M.; Galati, C.; Lattanzi, D.; Savelli, D.; Di Palma, M.; Saccomanno, S.; Bartolini, D.; Torquato, P.; et al. Neurobiological Correlates of Alpha-Tocopherol Antiepileptogenic Effects and MicroRNA Expression Modulation in a Rat Model of Kainate-Induced Seizures. Mol. Neurobiol. 2018, 55, 7822–7838. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.L.; Burnham, W.M.; Bishai, A.; Hwang, P.A. The anticonvulsant effects of vitamin E: A further evaluation. Can. J. Neurol. Sci. 1992, 19, 201–203. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.L.; Burnham, W.M.; Hwang, P.A. An evaluation of the anticonvulsant effects of vitamin E. Epilepsy Res. 1990, 6, 12–17. [Google Scholar] [CrossRef]
- Ribeiro, M.C.; de Avila, D.S.; Schneider, C.Y.; Hermes, F.S.; Furian, A.F.; Oliveira, M.S.; Rubin, M.A.; Lehmann, M.; Krieglstein, J.; Mello, C.F. alpha-Tocopherol protects against pentylenetetrazol- and methylmalonate-induced convulsions. Epilepsy Res. 2005, 66, 185–194. [Google Scholar] [CrossRef]
- Ambrogini, P.; Minelli, A.; Galati, C.; Betti, M.; Lattanzi, D.; Ciffolilli, S.; Piroddi, M.; Galli, F.; Cuppini, R. Post-seizure alpha-tocopherol treatment decreases neuroinflammation and neuronal degeneration induced by status epilepticus in rat hippocampus. Mol. Neurobiol. 2014, 50, 246–256. [Google Scholar] [CrossRef]
- Zakharova, I.O.; Sokolova, T.V.; Vlasova, Y.A.; Bayunova, L.V.; Rychkova, M.P.; Avrova, N.F. alpha-Tocopherol at Nanomolar Concentration Protects Cortical Neurons against Oxidative Stress. Int. J. Mol. Sci. 2017, 18, 216. [Google Scholar] [CrossRef]
- Ferri, P.; Cecchini, T.; Ambrogini, P.; Betti, M.; Cuppini, R.; Del Grande, P.; Ciaroni, S. alpha-Tocopherol affects neuronal plasticity in adult rat dentate gyrus: The possible role of PKCdelta. J. Neurobiol. 2006, 66, 793–810. [Google Scholar] [CrossRef] [PubMed]
- Brodie, C.; Bogi, K.; Acs, P.; Lorenzo, P.S.; Baskin, L.; Blumberg, P.M. Protein kinase C delta (PKCdelta) inhibits the expression of glutamine synthetase in glial cells via the PKCdelta regulatory domain and its tyrosine phosphorylation. J. Biol. Chem. 1998, 273, 30713–30718. [Google Scholar] [CrossRef] [PubMed]
- Osakada, F.; Hashino, A.; Kume, T.; Katsuki, H.; Kaneko, S.; Akaike, A. Neuroprotective effects of alpha-tocopherol on oxidative stress in rat striatal cultures. Eur. J. Pharmacol. 2003, 465, 15–22. [Google Scholar] [CrossRef]
- Betti, M.; Minelli, A.; Ambrogini, P.; Ciuffoli, S.; Viola, V.; Galli, F.; Canonico, B.; Lattanzi, D.; Colombo, E.; Sestili, P.; et al. Dietary supplementation with alpha-tocopherol reduces neuroinflammation and neuronal degeneration in the rat brain after kainic acid-induced status epilepticus. Free Radic. Res. 2011, 45, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Zaja-Milatovic, S.; Gupta, R.C.; Aschner, M.; Montine, T.J.; Milatovic, D. Pharmacologic suppression of oxidative damage and dendritic degeneration following kainic acid-induced excitotoxicity in mouse cerebrum. Neurotoxicology 2008, 29, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Tome, A.R.; Feng, D.; Freitas, R.M. The effects of alpha-tocopherol on hippocampal oxidative stress prior to in pilocarpine-induced seizures. Neurochem. Res. 2010, 35, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Simeone, K.A.; Matthews, S.A.; Samson, K.K.; Simeone, T.A. Targeting deficiencies in mitochondrial respiratory complex I and functional uncoupling exerts anti-seizure effects in a genetic model of temporal lobe epilepsy and in a model of acute temporal lobe seizures. Exp. Neurol. 2014, 251, 84–90. [Google Scholar] [CrossRef]
- NIH Office of Dietary Supplements. Vitamin B6. Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/VitaminB6-HealthProfessional/ (accessed on 17 September 2018).
- Subar, A.F.; Krebs-Smith, S.M.; Cook, A.; Kahle, L.L. Dietary sources of nutrients among US adults, 1989 to 1991. J. Am. Diet. Assoc. 1998, 98, 537–547. [Google Scholar] [CrossRef]
- Bowling, F.G. Pyridoxine supply in human development. Semin. Cell Dev. Biol. 2011, 22, 611–618. [Google Scholar] [CrossRef]
- Di Salvo, M.L.; Contestabile, R.; Safo, M.K. Vitamin B(6) salvage enzymes: Mechanism, structure and regulation. Biochim. Biophys. Acta 2011, 1814, 1597–1608. [Google Scholar] [CrossRef]
- Spector, R.; Greenwald, L.L. Transport and metabolism of vitamin B6 in rabbit brain and choroid plexus. J. Biol. Chem. 1978, 253, 2373–2379. [Google Scholar] [PubMed]
- Fox, J.T.; Tullidge, G.M. Pyridoxine (Vitamin B6) in epilepsy; a clinical trial. Lancet 1946, 2, 345. [Google Scholar] [CrossRef]
- Livingston, S.; Jeng, M.H.; Petersen, D.C. Ineffectiveness of pyridoxine (Vitamin B6) in the treatment of epilepsy. Pediatrics 1955, 16, 250–251. [Google Scholar] [PubMed]
- Heeley, A.F.; Piesowicz, A.T.; McCubbing, D.G. The biochemical and clinical effect of pyridoxine in children with brain disorders. Clin. Sci. 1968, 35, 381–389. [Google Scholar] [PubMed]
- Plecko, B.; Stockler, S. Vitamin B6 dependent seizures. Can. J. Neurol. Sci. 2009, 36, 73–77. [Google Scholar]
- Mills, P.B.; Surtees, R.A.; Champion, M.P.; Beesley, C.E.; Dalton, N.; Scambler, P.J.; Heales, S.J.; Briddon, A.; Scheimberg, I.; Hoffmann, G.F.; et al. Neonatal epileptic encephalopathy caused by mutations in the PNPO gene encoding pyridox(am)ine 5’-phosphate oxidase. Hum. Mol. Genet. 2005, 14, 1077–1086. [Google Scholar] [CrossRef] [PubMed]
- Mills, P.B.; Camuzeaux, S.S.M.; Footitt, E.J.; Mills, K.A.; Gissen, P.; Fisher, L.; Das, K.B.; Varadkar, S.M.; Zuberi, S.; McWilliam, R.; et al. Epilepsy due to PNPO mutations: Genotype, environment and treatment affect presentation and outcome. Brain 2014, 137, 1350–1360. [Google Scholar] [CrossRef]
- Moore, C.A.; Ward, J.C.; Rivas, M.L.; Magill, H.L.; Whyte, M.P. Infantile hypophosphatasia: Autosomal recessive transmission to two related sibships. Am. J. Med. Genet. 1990, 36, 15–22. [Google Scholar] [CrossRef]
- Waymire, K.G.; Mahuren, J.D.; Jaje, J.M.; Guilarte, T.R.; Coburn, S.P.; MacGregor, G.R. Mice lacking tissue non-specific alkaline phosphatase die from seizures due to defective metabolism of vitamin B-6. Nat. Genet. 1995, 11, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Van Karnebeek, C.D.; Tiebout, S.A.; Niermeijer, J.; Poll-The, B.T.; Ghani, A.; Coughlin, C.R., 2nd; Van Hove, J.L.; Richter, J.W.; Christen, H.J.; Gallagher, R.; et al. Pyridoxine-Dependent Epilepsy: An Expanding Clinical Spectrum. Pediatr. Neurol. 2016, 59, 6–12. [Google Scholar] [CrossRef]
- Pena, I.A.; Marques, L.A.; Laranjeira, A.B.; Yunes, J.A.; Eberlin, M.N.; MacKenzie, A.; Arruda, P. Mouse lysine catabolism to aminoadipate occurs primarily through the saccharopine pathway; implications for pyridoxine dependent epilepsy (PDE). Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Jagadeesh, S.; Suresh, B.; Murugan, V.; Suresh, S.; Salomans, G.S.; Struys, E.A.; Jacobs, C. Pyridoxine-dependent epilepsy owing to antiquitin deficiency—Mutation in the ALDH7A1 gene. Paediatr. Int. Child Health 2013, 33, 113–115. [Google Scholar] [CrossRef] [PubMed]
- Pena, I.A.; Roussel, Y.; Daniel, K.; Mongeon, K.; Johnstone, D.; Weinschutz Mendes, H.; Bosma, M.; Saxena, V.; Lepage, N.; Chakraborty, P.; et al. Pyridoxine-Dependent Epilepsy in Zebrafish Caused by Aldh7a1 Deficiency. Genetics 2017, 207, 1501–1518. [Google Scholar] [CrossRef] [PubMed]
- NIH Office of Dietary Supplements. Vitamin C. Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/ (accessed on 18 September 2018).
- Tome Ada, R.; Ferreira, P.M.; Freitas, R.M. Inhibitory action of antioxidants (ascorbic acid or alpha-tocopherol) on seizures and brain damage induced by pilocarpine in rats. Arq. Neuropsiquiatr. 2010, 68, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Harrison, F.E.; May, J.M. Vitamin C function in the brain: Vital role of the ascorbate transporter SVCT2. Free Radic. Biol. Med. 2009, 46, 719–730. [Google Scholar] [CrossRef] [PubMed]
- Santos, I.M.; Tome Ada, R.; Saldanha, G.B.; Ferreira, P.M.; Militao, G.C.; Freitas, R.M. Oxidative stress in the hippocampus during experimental seizures can be ameliorated with the antioxidant ascorbic acid. Oxid. Med. Cell. Longev. 2009, 2, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Ramirez, M.; Razo-Juarez, L.I.; Sauer-Ramirez, J.L.; Gonzalez-Trujano, M.E.; Salgado-Ceballos, H.; Orozco-Suarez, S. Anticonvulsive effect of vitamin C on pentylenetetrazol-induced seizures in immature rats. Pharmacol. Biochem. Behav. 2010, 97, 267–272. [Google Scholar] [CrossRef]
- Schneider Oliveira, M.; Flavia Furian, A.; Freire Royes, L.F.; Rechia Fighera, M.; de Carvalho Myskiw, J.; Gindri Fiorenza, N.; Mello, C.F. Ascorbate modulates pentylenetetrazol-induced convulsions biphasically. Neuroscience 2004, 128, 721–728. [Google Scholar] [CrossRef]
- Ayyildiz, M.; Coskun, S.; Yildirim, M.; Agar, E. The effects of ascorbic acid on penicillin-induced epileptiform activity in rats. Epilepsia 2007, 48, 1388–1395. [Google Scholar] [CrossRef]
- Das, A.; Sarwar, M.S.; Hossain, M.S.; Karmakar, P.; Islam, M.S.; Hussain, M.E.; Banik, S. Elevated Serum Lipid Peroxidation and Reduced Vitamin C and Trace Element Concentrations Are Correlated With Epilepsy. Clin. EEG Neurosci. 2019, 50, 63–72. [Google Scholar] [CrossRef]
- Desagher, S.; Glowinski, J.; Premont, J. Pyruvate protects neurons against hydrogen peroxide-induced toxicity. J. Neurosci. 1997, 17, 9060–9067. [Google Scholar] [CrossRef] [PubMed]
- Kao, K.K.; Fink, M.P. The biochemical basis for the anti-inflammatory and cytoprotective actions of ethyl pyruvate and related compounds. Biochem. Pharmacol. 2010, 80, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Popova, I.; Malkov, A.; Ivanov, A.I.; Samokhina, E.; Buldakova, S.; Gubkina, O.; Osypov, A.; Muhammadiev, R.S.; Zilberter, T.; Molchanov, M.; et al. Metabolic correction by pyruvate halts acquired epilepsy in multiple rodent models. Neurobiol. Dis. 2017, 106, 244–254. [Google Scholar] [CrossRef]
- Brodie, M.J. Antiepileptic drug therapy the story so far. Seizure 2010, 19, 650–655. [Google Scholar] [CrossRef] [PubMed]
- Botchway, B.O.A.; Moore, M.K.; Akinleye, F.O.; Iyer, I.C.; Fang, M. Nutrition: Review on the Possible Treatment for Alzheimer’s Disease. J. Alzheimers Dis. 2018, 61, 867–883. [Google Scholar] [CrossRef] [PubMed]
- Larsson, S.C. Dietary Approaches for Stroke Prevention. Stroke 2017, 48, 2905–2911. [Google Scholar] [CrossRef] [PubMed]
- Hang, L.; Basil, A.H.; Lim, K.L. Nutraceuticals in Parkinson’s Disease. Neuromolecular Med. 2016, 18, 306–321. [Google Scholar] [CrossRef]
- Szwajgier, D.; Borowiec, K.; Pustelniak, K. The Neuroprotective Effects of Phenolic Acids: Molecular Mechanism of Action. Nutrients 2017, 9, 477. [Google Scholar] [CrossRef]
- Castro, O.W.; Upadhya, D.; Kodali, M.; Shetty, A.K. Resveratrol for Easing Status Epilepticus Induced Brain Injury, Inflammation, Epileptogenesis, and Cognitive and Memory Dysfunction-Are We There Yet? Front. Neurol. 2017, 8, 603. [Google Scholar] [CrossRef]
- Ojemann, L.M.; Nelson, W.L.; Shin, D.S.; Rowe, A.O.; Buchanan, R.A. Tian ma, an ancient Chinese herb, offers new options for the treatment of epilepsy and other conditions. Epilepsy Behav. 2006, 8, 376–383. [Google Scholar] [CrossRef]
- Kulkarni, S.K.; Dhir, A. Withania somnifera: An Indian ginseng. Prog. Neuropsychopharmacol. Biol. Psychiatry 2008, 32, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Vishnoi, S.; Raisuddin, S.; Parvez, S. Glutamate Excitotoxicity and Oxidative Stress in Epilepsy: Modulatory Role of Melatonin. J. Environ. Pathol. Toxicol. Oncol. 2016, 35, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Dalton, K.; Dalton, M.J. Characteristics of pyridoxine overdose neuropathy syndrome. Acta Neurol. Scand. 1987, 76, 8–11. [Google Scholar] [CrossRef] [PubMed]
| Author | Number of Patients | Age (years) | Study Type | Intervention | Duration | Any Other Intervention | Final Result | |
|---|---|---|---|---|---|---|---|---|
| Experimental (Exp) | Control (Con) | |||||||
| Schlanger et al., 2002 [4] | n = 5 | Range: 12–26 | Observational | 5 g DHA, EPA, ALA | - | 24 weeks | Anticonvulsive drugs | Decreased seizures after intervention |
| Yuen et al., 2005 [5] | n = 57 (Exp: 13, Con: 22) | Range: 19–65 | Randomized, double-blind, placebo-controlled trial | 1000 mg fish oil (171 mg EPA, 112 mg DHA, <100 IU Vit A, <40 IU Vit D) | Placebo (70% palm olein, 15% rapeseed oil, 15% sunflower oil) | 12 weeks | Antiepileptic drugs | First 6 weeks: five had 50% reduction in seizures Second 6 weeks: no difference |
| Puri et al., 2007 [106] | n = 7 (Exp: 3, Con: 4) | Mean ± SD: 50.7 ± 13.6 (Exp), 40.5 ± 12.0 (Con) | Randomized, double-blind, placebo-controlled trial | 1000 mg fish oil (171 mg EPA, 112 mg DHA, <100 IU Vit A, <40 IU Vit D) | Placebo (70% palm olein, 15% rapeseed oil, 15% sunflower oil) | 12 weeks | - | No significant correlations between seizures and changes in spectroscopic resonances |
| Dahlin et al., 2007 [107] | n = 25 | Range: 1.5–18.1 | Prospective cohort | 1–2 g fish oil with meal, four times/day | - | 48 weeks | Antiepileptic drugs | No effect on seizures |
| Bromfield et al., 2008 [108] | n = 21, (Exp: 12, Con: 9) | Range: 25–55 (Exp), 22–62 (Con) | Randomized, double-blind, placebo-controlled trial | 2.2 mg EPA and DHA (3:2 ratio) 1.1 g/day (1 week) → 1.1 g, two times/day (3 weeks) | Mineral oil | 4 weeks | Antiepileptic drugs | No effect on seizures |
| DeGiorgio et al., 2008 [10] | n = 11 | Range: 18–65 | Randomized, double-blind, two periods crossover clinical trial, 6 weeks washout period | 1200 mg fish oil/day (216 mg EPA, 144 mg DHA) | Soybean oil 8 capsules/day | 30 weeks | Antiepileptic drugs | No effect on seizures Restoration of heart rate variability |
| Al Khayat et al., 2010 [6] | n = 20 | Range: 3–10 | Observational | 1000 mg PUFA (700 mg DHA and 300 mg EPA) | 6 months | Antiepileptic drugs | Decreased seizure frequency, seizure duration and seizure severity after intervention | |
| Yuen et al., 2012 [109] | n = 10 | Range: 23–75 | Observational | 500 mg EPA with 10 mg mixed tocopherols/capsule, 2 capsules/day | - | 12 weeks | Antiepileptic drugs | No effect on seizures Reduced seizure severity in one person |
| DeGiorgio et al., 2015 [7] | n = 24 | Range: 18–56 | Randomized, double-blind, three periods crossover clinical trial, twice 6 weeks washout | Fish oil capsule (216 mg EPA, 144 mg DHA (360 mg fatty acids/capsules)) Low-dose group: 1080 mg/day High-dose group: 2160 mg/day | 3 corn oil capsules/twice day | 42 weeks | Antiepileptic drugs | Decreased seizures in low-dose fish oil |
| Reda et al., 2015 [8] | n = 70 | Range: 4–12 Mean ± SD: 6.9 ± 2.5 (Exp), 6.6 ± 2.4 (Con) | Randomized, single-blind trial | 1200 mg fish oil (240 mg DHA, 360 mg EPA, Vit E) | Corn oil | 12 weeks | Antiepileptic drugs | Elevated the seizure threshold in fish oil supplementation group |
| Omrani et al., 2019 [9] | n = 50 | Range: 18–55 | Randomized, triple-blind, placebo-controlled trial | Omega-3 fatty acids capsules (120 mg DHA, 180 mg EPA plus Vit E) | Placebo capsule | 16 weeks (two times/ day) | Antiepileptic drugs in refractory epilepsy | Decreased seizure frequency and duration in omega-3 fatty acids supplementation group |
| Author | Number of Patients | Age (Years) | Study Type | Subgroup | Duration | Any Other Intervention | Final Results | |
|---|---|---|---|---|---|---|---|---|
| PWE | Control (Con) | |||||||
| Christiansen et al., 1974 [11] | n = 23 | Range: 6–27 | Observational | A: N = 9, 4000 IU, 16,000 IU/day Vit D3 B: N = 14, 8000 IU/day Vit D3 | 84 days | Antiepileptic drugs | Reduction in the number of seizures after intervention | |
| Hollo et al., 2012 [12] | n = 13 | Range: 19–60 | Observational | Vit D3 | 90 days | - | 40% decrease in seizures after intervention | |
| Tombini et al., 2018 [13] | n = 202 (PWE: 160, Con: 42) | Mean ± SD: 50.6 ± 19.3 | Case-sectional cohort | Cholecalciferol 100,000 IU/week (Vit D deficiency) Cholecalciferol 100,000 IU/2 weeks (Vit D insufficiency) | Cholecalciferol 100,000 IU/week (Vit D deficiency) Cholecalciferol 100,000 IU/2 weeks (Vit D insufficiency) | 3 months | Antiepileptic drugs | PWE showed low Vit D No effect on drug-resistant seizures |
| Author | Number of Patients | Age (years) | Study Type | Intervention | Duration | Any Other Intervention | Final Results | |
|---|---|---|---|---|---|---|---|---|
| Experimental (Exp) | Control (Con) | |||||||
| Ogunmekan et al., 1989 [14] | n = 24 (Exp: 12, Con: 12) | Range: 6–17 | Randomized, double-blind, placebo-controlled clinical trial | Vit E | Placebo | 3 months | Antiepileptic drugs | >60% reduction in seizure frequency |
| Hom et al., 1991 [15] | n = 52 (Exp: 27, Con: 25) | - | Randomized, double-blind, placebo-controlled trial | Vit E | Placebo | 3 months | Antiepileptic drugs | Reduction in seizure frequency: 30% of all patients Reduction in seizure frequency: 58% of medically stable patients |
| Raju et al., 1994 [166] | n = 43 | - | Randomized, double-blind, two periods crossover trial | Vit E and then placebo | Placebo and then Vit E | 6 months (for two treatments) | Antiepileptic drugs | No significant change in seizure frequency |
| Mehvari et al., 2016 [16] | n = 65 (Exp: 32, Con: 33) | Mean ± SD: 28.8 ± 5.3 (Exp), 28.6 ± 8.8 (Con) | Randomized, double-blind, placebo-controlled trial | 400 IU/day Vit E | Placebo | 6 months | Antiepileptic drugs | Reduction in seizure frequency Improved EEG findings |
| Author | Number of Patients | Age (years) | Study Type | Intervention | Duration | Any Other Intervention | Final Result | |
|---|---|---|---|---|---|---|---|---|
| Experimental (Exp) | Control (Con) | |||||||
| Fox and Tullidge, 1946 [186] | n = 8 | Range: 14–15 | Observational | Four patients: 100 mg pyridoxine/day for 3 weeks and then 100 mg pyridoxine/day for 4 weeks Two patients: 20 mg/day for 4 weeks and then 100 mg pyridoxine/day for 4 weeks Two patients: only 20 mg/day for 8 weeks | - | 7–8 weeks | - | No effects |
| Livingston et al., 1955 [187] | n = 31 | Range: 0.5–14 | Observational | 20 mg (two times/day); pyridoxine dosage was increased to 100 mg/day | - | At least 1 mo | Antiepileptic drugs | Pyridoxine failed to control seizures Larger doses of pyridoxine increased the number of seizures |
| Hagberg et al., 1964 [17] | n = 3 | Range: 1–3 | Observational | One patient: 60 mg Vit B6 Two patients: 160 mg Vit B6 | - | NR | Antiepileptic drugs | Improved EEG findings, normal development, decreased mental changes, reduced dosage of anticonvulsive drugs |
| Hansson and Hagberg, 1968 [18] | n = 56 | Children | Observational | 160–300 mg pyridoxine | At least 6 weeks | Antiepileptic drugs | Five patients showed significant clinical improvement; side effects for only one patient | |
| Heeley et al., 1968 [188] | n = 70 | Range: 0.2–11 | Observational | 30 mg/day pyridoxine | - | 1–4 weeks | Antiepileptic drugs | No clinical improvement |
| Jiao et al., 1997 [19] | n = 90, (Exp: 40, Con: 50) | Range: 0.1–12 | Randomized, controlled trial | 30 or 50 mg/day pyridoxine | No treatments | NR | Antiepileptic drugs | Recurrent seizures were resolved |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-E.; Cho, K.-O. Functional Nutrients for Epilepsy. Nutrients 2019, 11, 1309. https://doi.org/10.3390/nu11061309
Kim J-E, Cho K-O. Functional Nutrients for Epilepsy. Nutrients. 2019; 11(6):1309. https://doi.org/10.3390/nu11061309
Chicago/Turabian StyleKim, Ji-Eun, and Kyung-Ok Cho. 2019. "Functional Nutrients for Epilepsy" Nutrients 11, no. 6: 1309. https://doi.org/10.3390/nu11061309
APA StyleKim, J.-E., & Cho, K.-O. (2019). Functional Nutrients for Epilepsy. Nutrients, 11(6), 1309. https://doi.org/10.3390/nu11061309





