Modulation of Adenosine Receptors and Antioxidative Effect of Beer Extracts in in Vitro Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Cell Viability Assay
2.4. Beer Extracts Preparation
2.5. Total RNA Isolation and Preparation of cDNA
2.6. Quantitative Real Time RT-PCR Analysis
2.7. Radioligand Binding Assays
2.7.1. Adenosine A1 Receptor in C6 and SH-SY5Y Cells
2.7.2. Adenosine A2A Receptor in SH-SY5Y Cells
2.8. Protein Determination
2.9. Statistical and Data Analysis
3. Results
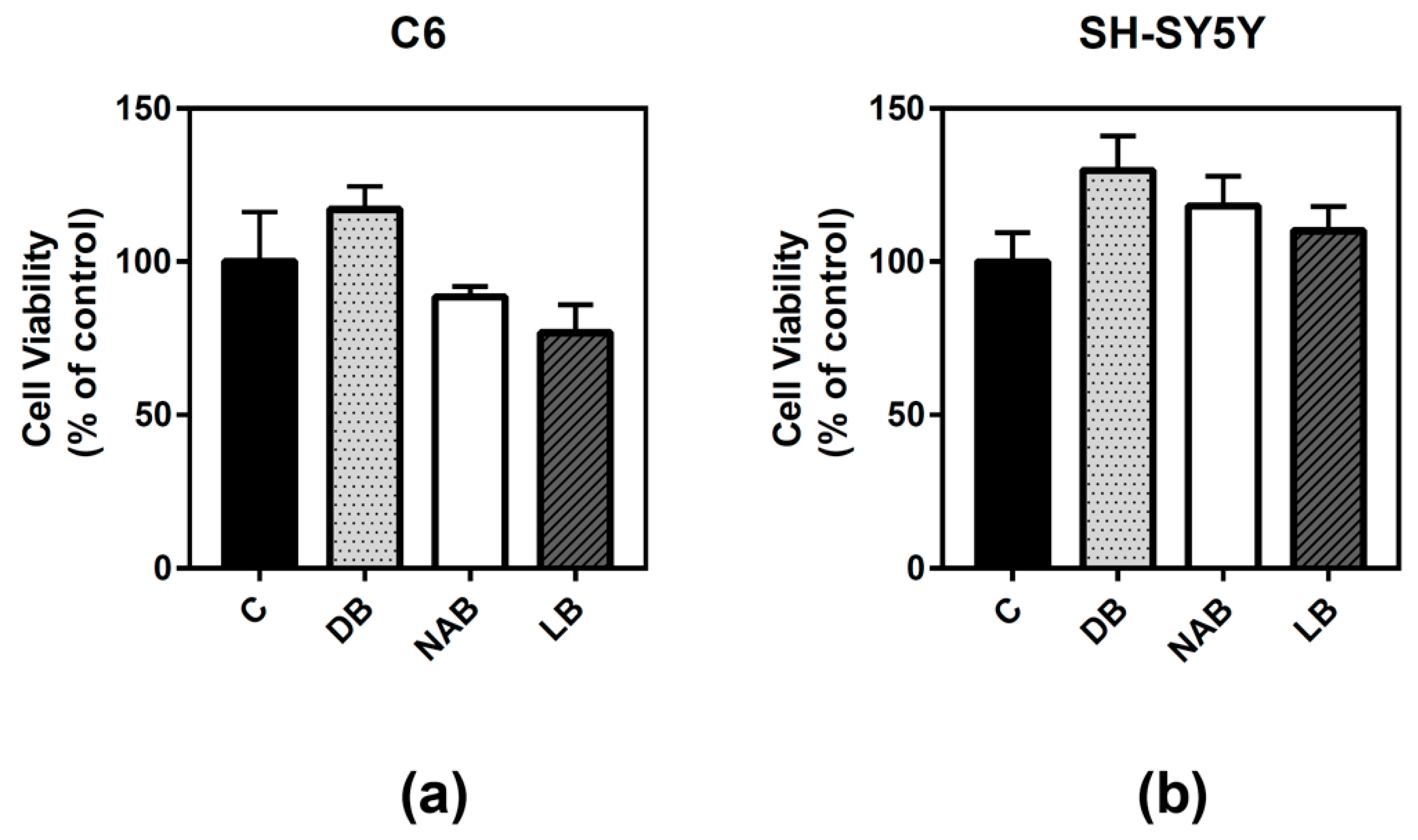
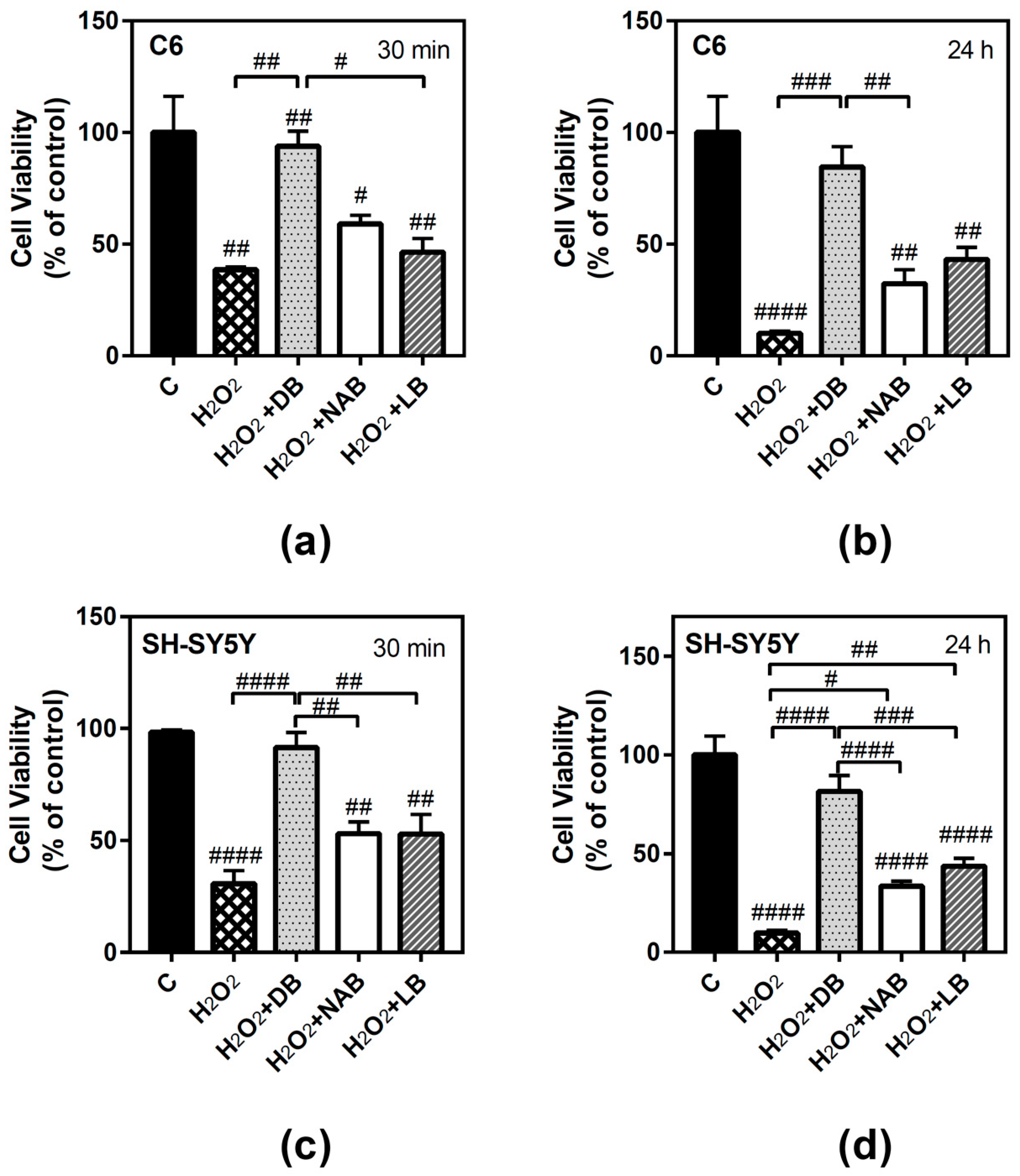
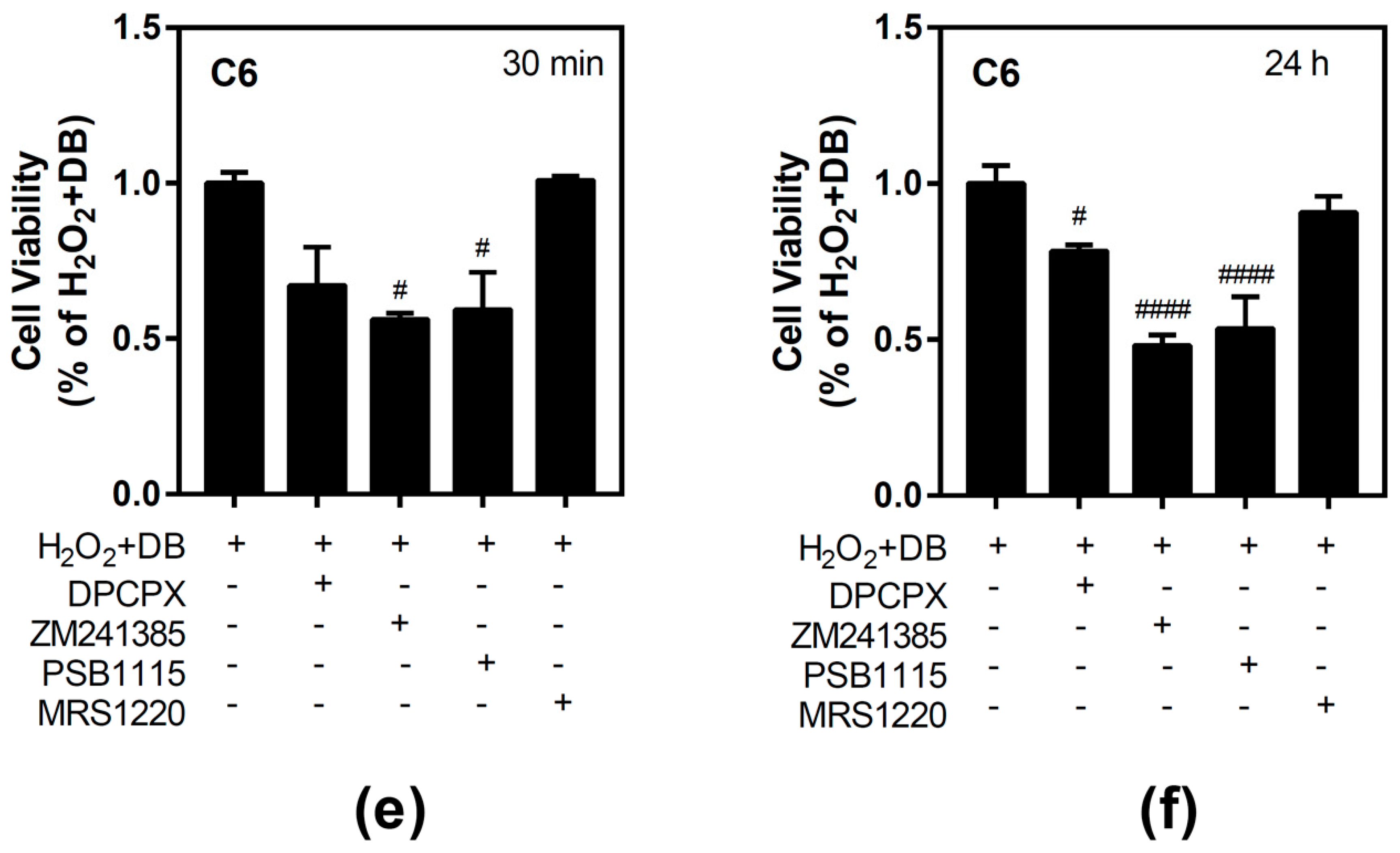
3.1. Effect of Beer Extracts on C6 and SH-SY5Y Cell Viability
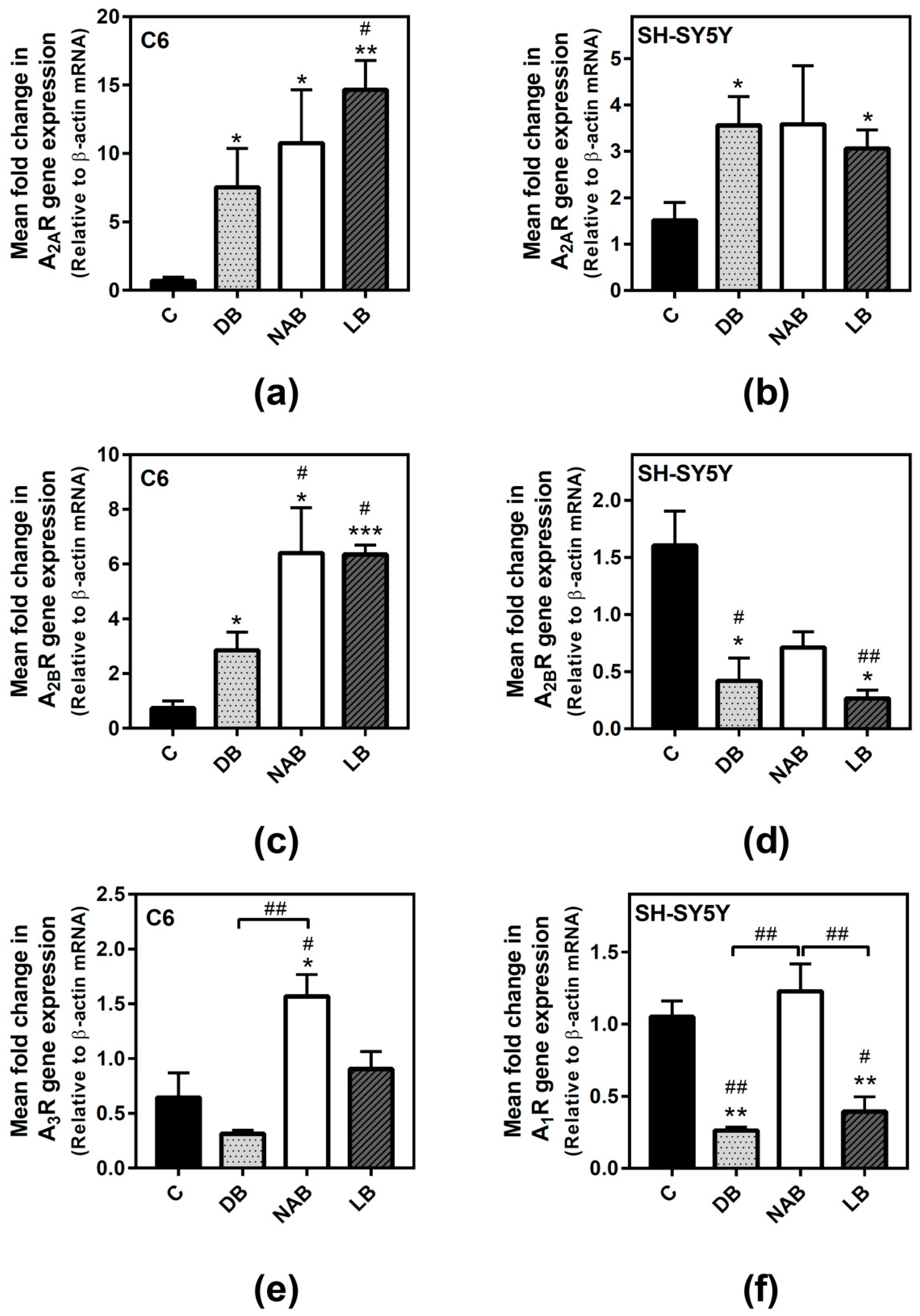
3.2. Effect of Beer Extracts on Adenosine Receptor Gene Expression in C6 and SH-SY5Y Cells
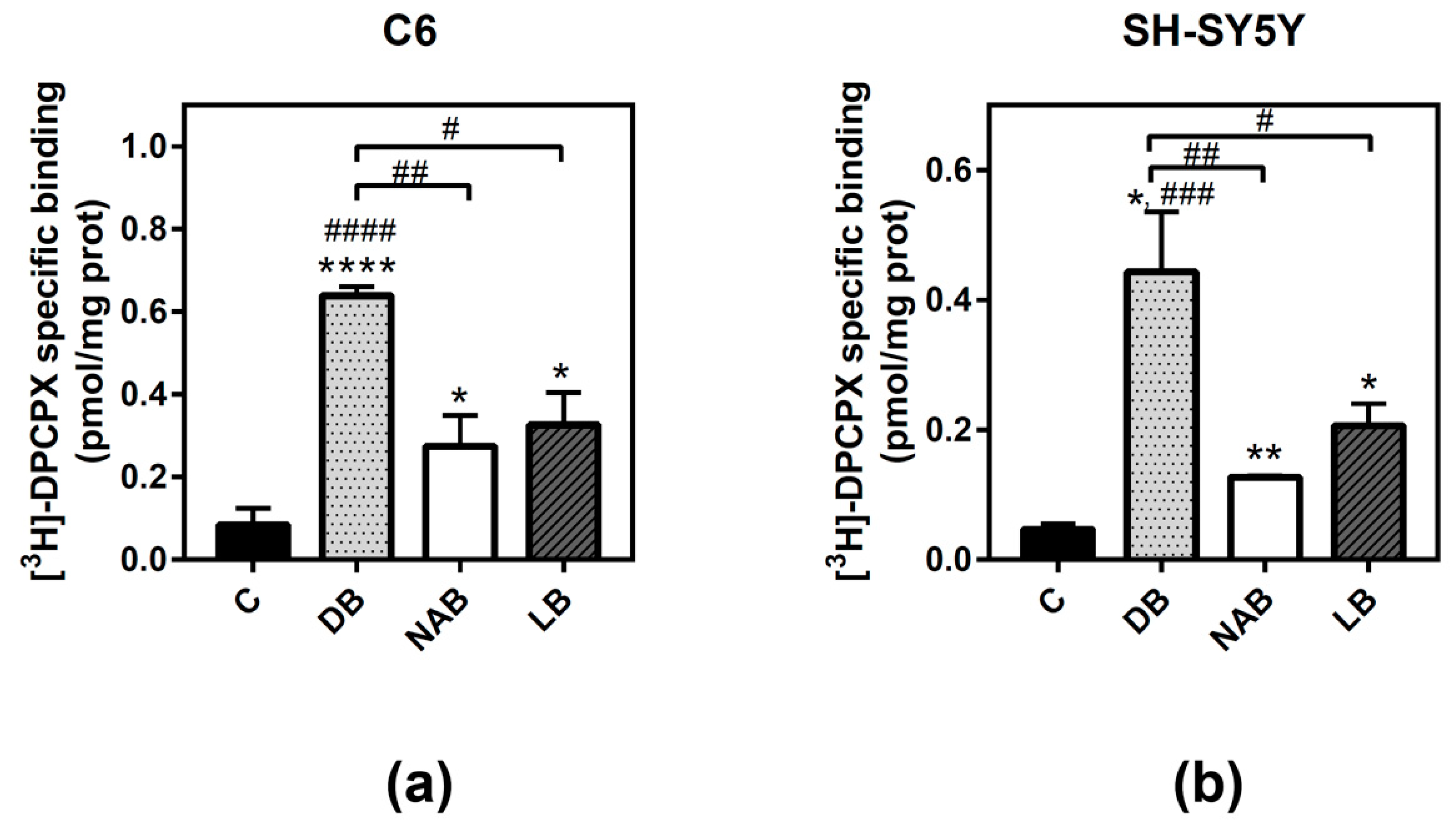
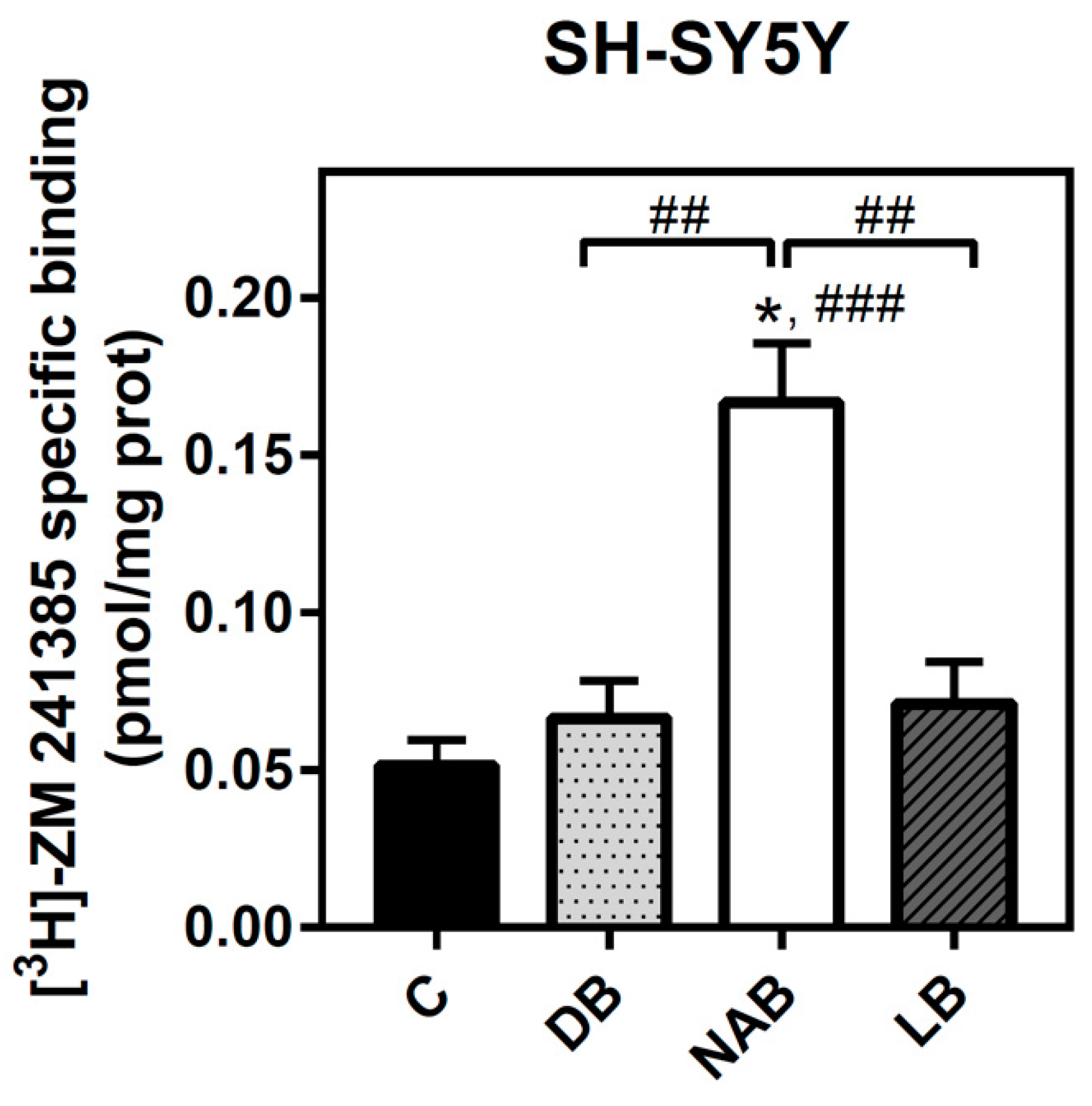
3.3. Effect of Beer Extracts on Adenosine A1 and A2A Receptors Levels
3.4. Antioxidant Ability of Beer Extracts in C6 and SH-SY5Y Cells
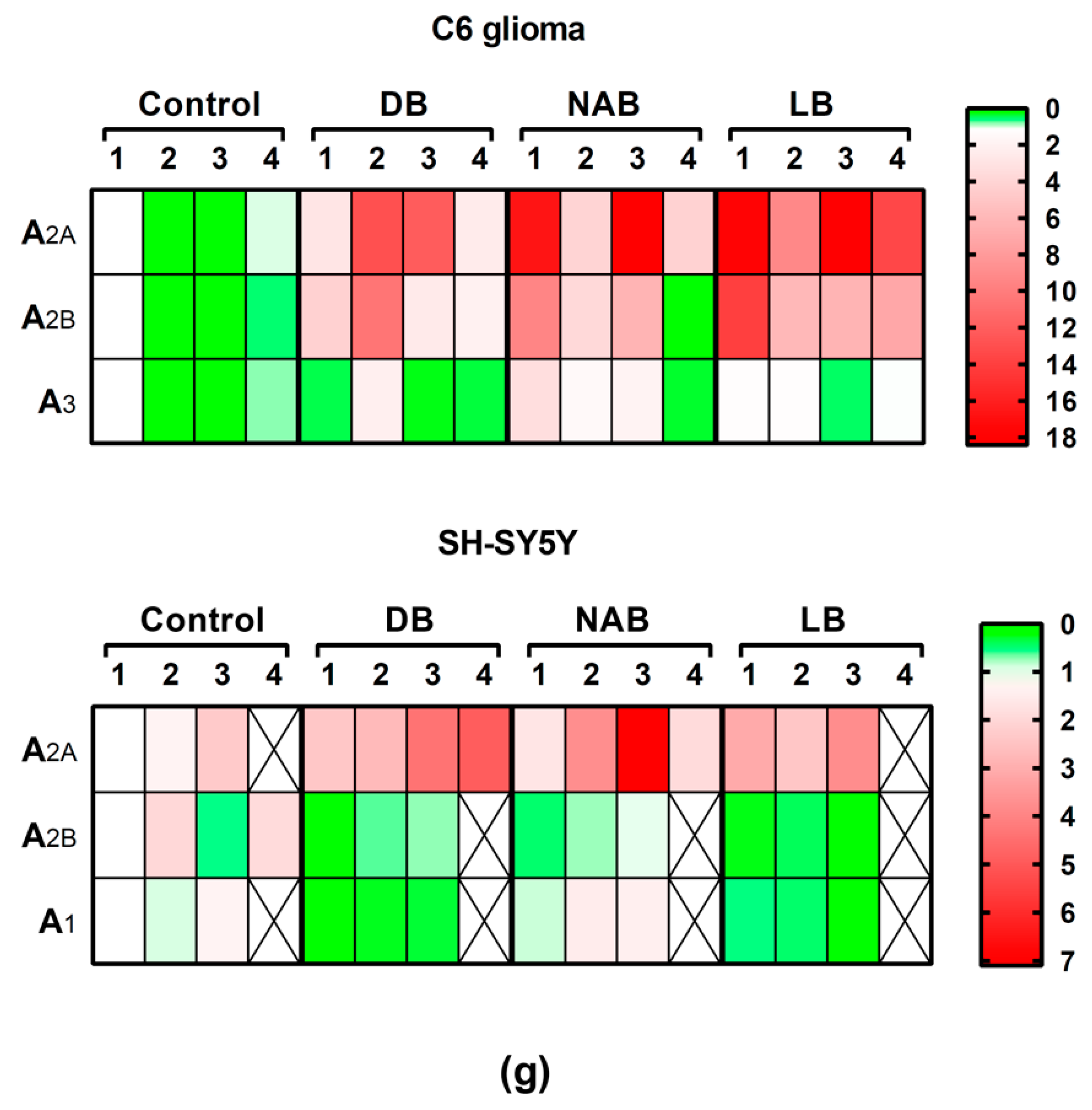
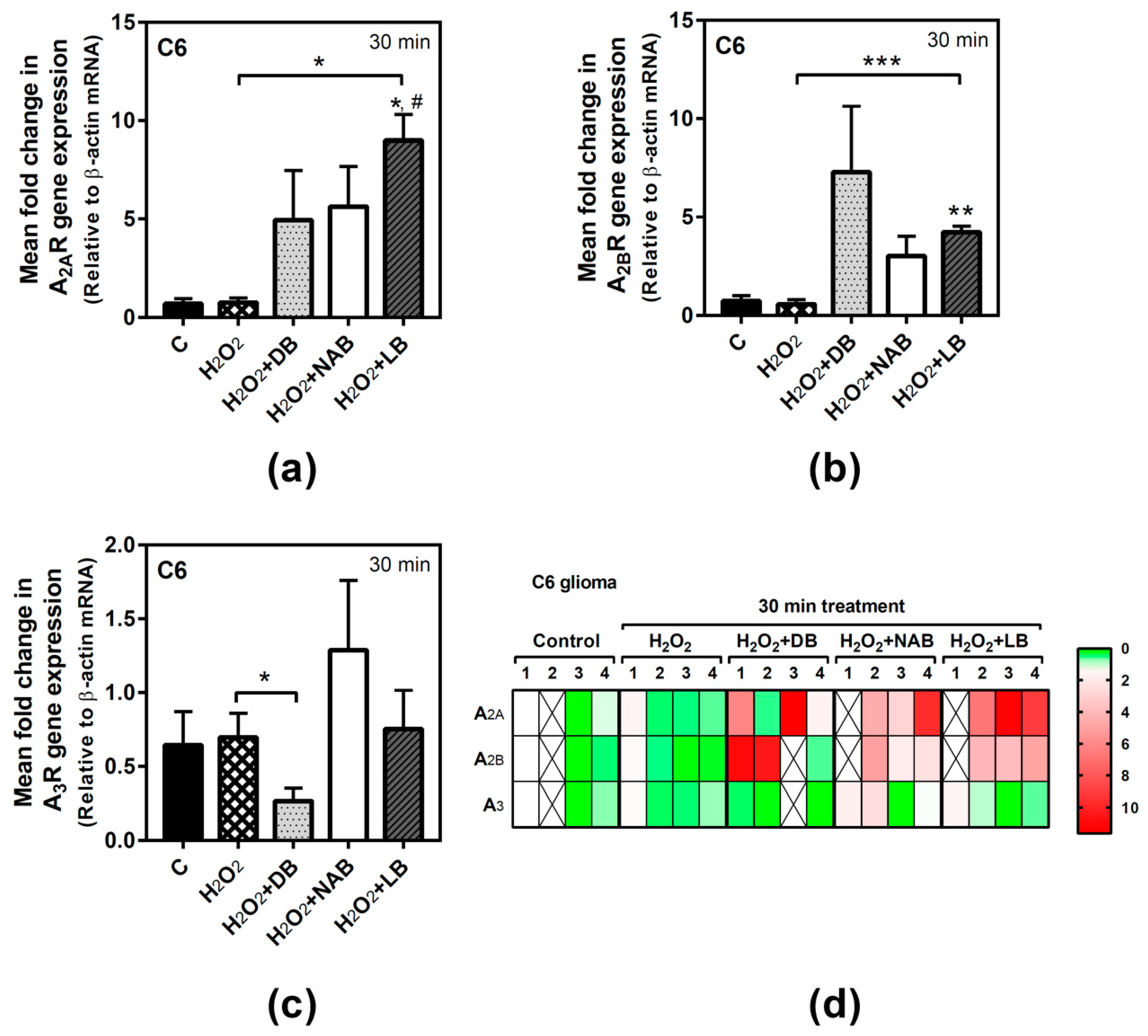
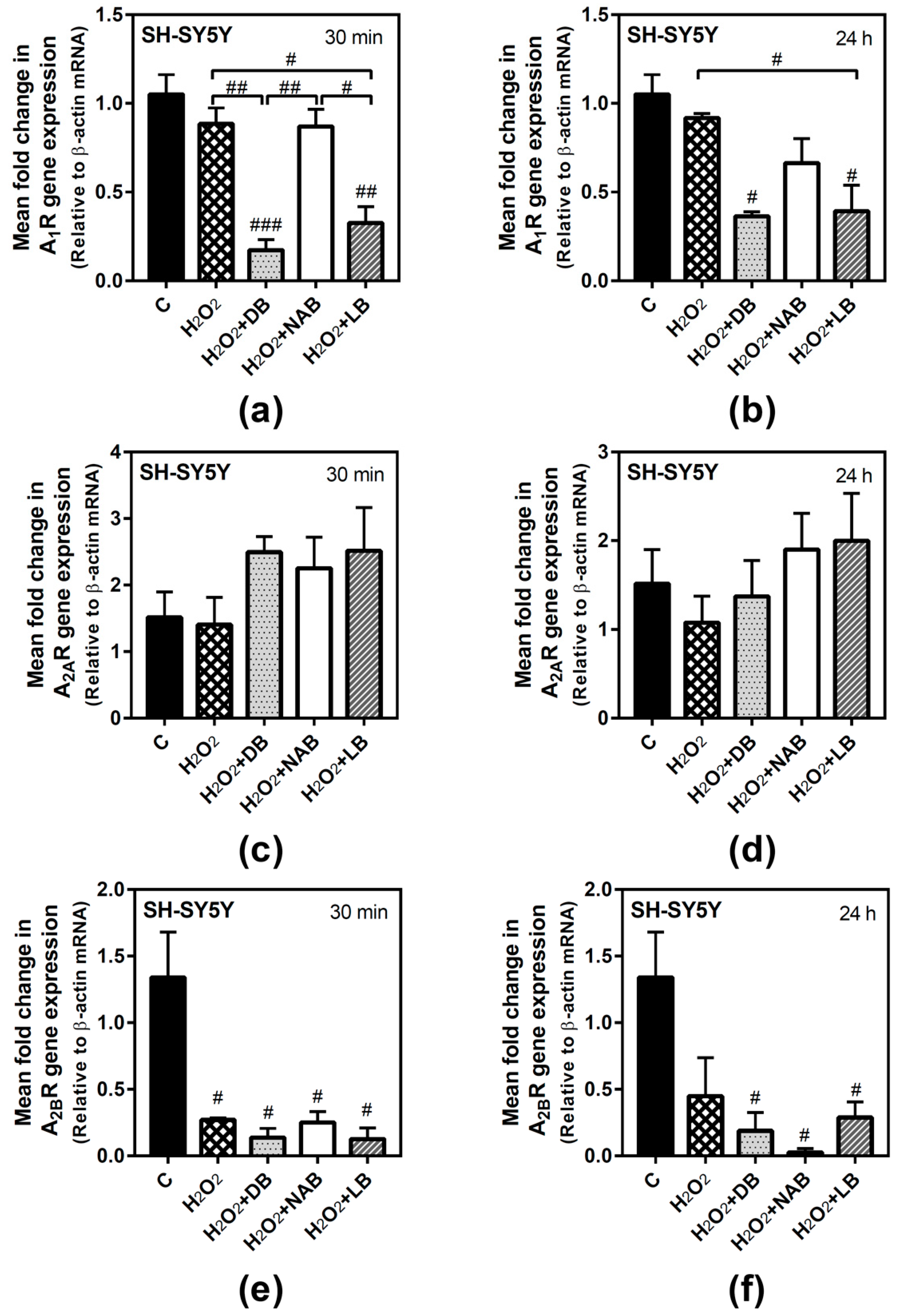
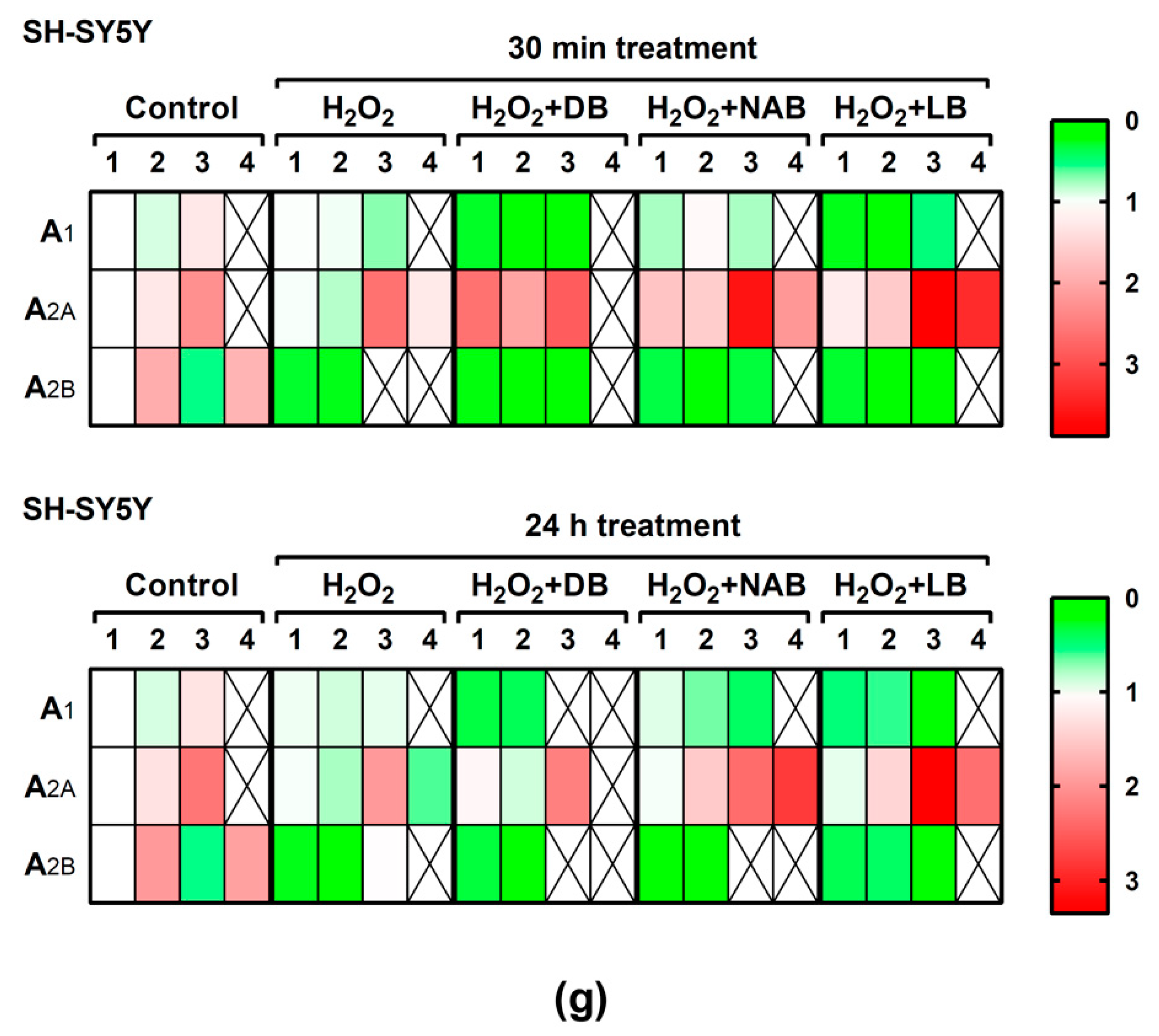
3.5. Effect of Beer Extracts and Oxidative Stress on Adenosine Receptors Gene Expression in C6 and SH-SY5Y Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gustafson, D.R.; Clare Morris, M.; Scarmeas, N.; Shah, R.C.; Sijben, J.; Yaffe, K.; Zhu, X. New Perspectives on Alzheimer’s Disease and Nutrition. J. Alzheimer’s Dis. 2015, 46, 1111–1127. [Google Scholar] [CrossRef]
- Hernando-Requejo, V. Nutrición y deterioro cognitivo. Nutrición Hospitalaria 2016, 33, 49–52. [Google Scholar] [CrossRef]
- Airoldi, C.; Ferla, B.L.; D’Orazio, G.; Ciaramelli, C.; Palmioli, A. Flavonoids in the Treatment of Alzheimer’s and Other Neurodegenerative Diseases. Curr. Med. Chem. 2018, 25, 3228–3246. [Google Scholar] [CrossRef]
- Barberger-Gateau, P.; Jutand, M.A.; Letenneur, L.; Larrieu, S.; Tavernier, B.; Berr, C. Correlates of regular fish consumption in French elderly community dwellers: Data from the Three-City study. Eur. J. Clin. Nutr. 2005, 59, 817–825. [Google Scholar] [CrossRef]
- Piazzon, A.; Forte, M.; Nardini, M. Characterization of Phenolics Content and Antioxidant Activity of Different Beer Types. J. Agric. Food Chem. 2010, 58, 10677–10683. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Zhang, B.; Ge, C.; Peng, S.; Fang, J. Xanthohumol, a Polyphenol Chalcone Present in Hops, Activating Nrf2 Enzymes to Confer Protection against Oxidative Damage in PC12 Cells. J. Agric. Food Chem. 2015, 63, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Gobinath, T.; Salomy, A.; Nisha, M.; Kandasamy, M.; Essa, M.M.; Jayachandran, K.S.; Anusuyadevi, M. Biophysical interaction of resveratrol with sirtuin pathway: Significance in Alzheimer’s disease. Front. Neurosci. 2018, 1, 1380–1390. [Google Scholar]
- Neves, M.F.; Trombin, V.G.; Lopes, F.F.; Kalaki, R.; Milan, P. World consumption of beverages. In The Orange Juice Business; Wageningen Academic Publishers: Wageningen, The Netherlands, 2011. [Google Scholar]
- Tafulo, P.A.R.; Queirós, R.B.; Delerue-Matos, C.M.; Sales, M.G.F. Control and comparison of the antioxidant capacity of beers. Food Res. Int. 2010, 43, 1702–1709. [Google Scholar] [CrossRef]
- Buiatti, S. Beer Composition: An Overview. Beer Health Dis. Prev. 2009, 213–225. [Google Scholar]
- Bamforth, C.W. Nutritional aspects of beer—A review. Nutr. Res. 2002, 22, 227–237. [Google Scholar] [CrossRef]
- de Gaetano, G.; Costanzo, S.; Di Castelnuovo, A.; Badimon, L.; Bejko, D.; Alkerwi, A.; Chiva-Blanch, G.; Estruch, R.; La Vecchia, C.; Panico, S.; et al. Effects of moderate beer consumption on health and disease: A consensus document. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 443–467. [Google Scholar] [CrossRef] [PubMed]
- Pai, T.V.; Sawant, S.Y.; Ghatak, A.A.; Chaturvedi, P.A.; Gupte, A.M.; Desai, N.S. Characterization of Indian beers: Chemical composition and antioxidant potential. J. Food Sci. Technol. 2015, 52, 1414–1423. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.; Raza, S.S.; Javed, H.; Ahmad, A.; Khan, A.; Islam, F.; Safhi, M.M.; Islam, F. Rutin Protects Dopaminergic Neurons from Oxidative Stress in an Animal Model of Parkinson’s Disease. Neurotox. Res. 2012, 22, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Guo, X.; Park, Y.; Wang, J.; Huang, X.; Hollenbeck, A.; Blair, A.; Chen, H. Alcohol Consumption, Types of Alcohol, and Parkinson’s Disease. PLoS ONE 2013, 8, e66452. [Google Scholar] [CrossRef]
- Gonzalez-Muñoz, M.J.; Meseguer, I.; Sanchez-Reus, M.I.; Schultz, A.; Olivero, R.; Benedí, J.; Sánchez-Muniz, F.J. Beer consumption reduces cerebral oxidation caused by aluminum toxicity by normalizing gene expression of tumor necrotic factor alpha and several antioxidant enzymes. Food Chem. Toxicol. 2008, 46, 1111–1118. [Google Scholar] [CrossRef]
- Kok, E.H.; Karppinen, T.T.; Luoto, T.; Alafuzoff, I.; Karhunen, P.J. Beer Drinking Associates with Lower Burden of Amyloid Beta Aggregation in the Brain: Helsinki Sudden Death Series. Alcohol. Clin. Exp. Res. 2016, 40, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Ano, Y.; Dohata, A.; Taniguchi, Y.; Hoshi, A.; Uchida, K.; Takashima, A.; Nakayama, H. Iso-α-acids, Bitter Components of Beer, Prevent Inflammation and Cognitive Decline Induced in a Mouse Model of Alzheimer’s Disease. J. Biol. Chem. 2017, 292, 3720–3728. [Google Scholar] [CrossRef]
- Stranahan, A.M.; Mattson, M.P. Recruiting Adaptive Cellular Stress Responses for Successful Brain Aging. Nat. Rev. Neurosci. 2012, 13, 209–216. [Google Scholar] [CrossRef]
- Pohanka, M. Alzheimer’s disease and oxidative stress: A review. Curr. Med. Chem. 2014, 21, 356–364. [Google Scholar] [CrossRef]
- Jha, A.B.; Panchal, S.S.; Shah, A. Ellagic acid: Insights into its neuroprotective and cognitive enhancement effects in sporadic Alzheimer’s disease. Pharmacol. Biochem. Behav. 2018, 175, 33–46. [Google Scholar] [CrossRef]
- Brose, R.D.; Lehrmann, E.; Zhang, Y.; Reeves, R.H.; Smith, K.D.; Mattson, M.P. Hydroxyurea attenuates oxidative, metabolic, and excitotoxic stress in rat hippocampal neurons and improves spatial memory in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2018, 72, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xia, N.; Förstermann, U. Cardiovascular effects and molecular targets of resveratrol. Nitric Oxide 2012, 26, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Melgar, A.; Albasanz, J.L.; Guixa-Gonzalez, R.; Saleh, N.; Selent, J.; Martin, M. The antioxidant resveratrol acts as a non-selective adenosine receptor agonist. Free Radic. Biol. Med. 2019, 135, 261–273. [Google Scholar] [CrossRef]
- Ribeiro, J.A.; Sebastião, A.M.; de Mendonça, A. Adenosine receptors in the nervous system: Pathophysiological implications. Prog. Neurobiol. 2002, 68, 377–392. [Google Scholar] [CrossRef]
- Rahman, A. The Role of Adenosine in Alzheimer’s Disease. Curr. Neuropharmacol. 2009, 7, 207–216. [Google Scholar] [CrossRef]
- Albasanz, J.L.; Perez, S.; Barrachina, M.; Ferrer, I.; Martín, M. Up-regulation of Adenosine Receptors in the Frontal Cortex in Alzheimer’s Disease. Brain Pathol. 2008, 18, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Kolahdouzan, M.; Hamadeh, M.J. The neuroprotective effects of caffeine in neurodegenerative diseases. CNS Neurosci. Ther. 2017, 23, 272–290. [Google Scholar] [CrossRef]
- Nehlig, A. Effects of coffee/caffeine on brain health and disease: What should I tell my patients? Pract. Neurol. 2016, 16, 89–95. [Google Scholar] [CrossRef]
- Borea, P.A.; Gessi, S.; Merighi, S.; Varani, K. Adenosine as a Multi-Signalling Guardian Angel in Human Diseases: When, Where and How Does it Exert its Protective Effects? Trends Pharmacol. Sci. 2016, 37, 419–434. [Google Scholar] [CrossRef]
- Wei, C.J.; Li, W.; Chen, J.F. Normal and abnormal functions of adenosine receptors in the central nervous system revealed by genetic knockout studies. Biochim. Biophys. Acta 2011, 1808, 1358–1379. [Google Scholar] [CrossRef]
- Martire, A.; Lambertucci, C.; Pepponi, R.; Ferrante, A.; Benati, N.; Buccioni, M.; Dal Ben, D.; Marucci, G.; Klotz, K.N.; Volpini, R.; et al. Neuroprotective potential of adenosine A1 receptor partial agonists in experimental models of cerebral ischemia. J. Neurochem. 2019, 149, 211–230. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, S.H.; Hueng, D.Y.; Syu, J.P.; Liao, C.C.; Wu, Y.C. Cordycepin induces apoptosis of C6 glioma cells through the adenosine 2A receptor-p53-caspase-7-PARP pathway. Chem. Biol. Interact. 2014, 216, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Castillo, C.A.; Leon, D.; Ruiz, M.A.; Albasanz, J.L.; Martin, M. Modulation of adenosine A1 and A2A receptors in C6 glioma cells during hypoxia: Involvement of endogenous adenosine. J. Neurochem. 2008, 105, 2315–2329. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, A.; Bavaresco, L.; Wink, M.R.; Jacques-Silva, M.C.; Delgado-Canedo, A.; Lenz, G.; Battastini, A.M. Indomethacin stimulates activity and expression of ecto-5’-nucleotidase/CD73 in glioma cell lines. Eur. J. Pharmacol. 2007, 569, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Appel, E.; Kazimirsky, G.; Ashkenazi, E.; Kim, S.G.; Jacobson, K.A.; Brodie, C. Roles of BCL-2 and caspase 3 in the adenosine A3 receptor-induced apoptosis. J. Mol. Neurosci. 2001, 17, 285–292. [Google Scholar] [CrossRef]
- Castillo, C.A.; León, D.A.; Ballesteros-Yáñez, I.; Albasanz, J.L.; Martín, M. Glutamate differently modulates excitatory and inhibitory adenosine receptors in neuronal and glial cells. Neurochem. Int. 2010, 57, 33–42. [Google Scholar] [CrossRef]
- Giunta, S.; Andriolo, V.; Castorina, A. Dual blockade of the A1 and A2A adenosine receptor prevents amyloid beta toxicity in neuroblastoma cells exposed to aluminum chloride. Int. J. Biochem. Cell Biol. 2014, 54, 122–136. [Google Scholar] [CrossRef]
- Giust, D.; Da Ros, T.; Martín, M.; Albasanz, J.L. [60]Fullerene derivative modulates adenosine and metabotropic glutamate receptors gene expression: A possible protective effect against hypoxia. J. Nanobiotechnol. 2014, 12, 27. [Google Scholar] [CrossRef]
- Falsini, M.; Catarzi, D.; Varano, F.; Dal Ben, D.; Marucci, G.; Buccioni, M.; Volpini, R.; Di Cesare Mannelli, L.; Ghelardini, C.; Colotta, V. Novel 8-amino-1,2,4-triazolo[4,3-a]pyrazin-3-one derivatives as potent human adenosine A1 and A2A receptor antagonists. Evaluation of their protective effect against beta-amyloid-induced neurotoxicity in SH-SY5Y cells. Bioorg. Chem. 2019, 87, 380–394. [Google Scholar] [CrossRef]
- Ferreira, D.G.; Batalha, V.L.; Vicente Miranda, H.; Coelho, J.E.; Gomes, R.; Goncalves, F.Q.; Real, J.I.; Rino, J.; Albino-Teixeira, A.; Cunha, R.A.; et al. Adenosine A2A Receptors Modulate alpha-Synuclein Aggregation and Toxicity. Cereb. Cortex 2017, 27, 718–730. [Google Scholar] [CrossRef]
- Razali, N.; Agarwal, R.; Agarwal, P.; Froemming, G.R.A.; Tripathy, M.; Ismail, N.M. IOP lowering effect of topical trans-resveratrol involves adenosine receptors and TGF-beta2 signaling pathways. Eur. J. Pharmacol. 2018, 838, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Melgar, A.; Albasanz, J.L.; Palomera-Avalos, V.; Pallas, M.; Martin, M. Resveratrol Modulates and Reverses the Age-Related Effect on Adenosine-Mediated Signalling in SAMP8 Mice. Mol. Neurobiol. 2019, 56, 2881–2895. [Google Scholar] [CrossRef] [PubMed]
- Castillo, C.A.; Albasanz, J.L.; Fernández, M.; Martín, M. Endogenous Expression of Adenosine A1, A2 and A3 Receptors in Rat C6 Glioma Cells. Neurochem. Res. 2007, 32, 1056–1070. [Google Scholar] [CrossRef] [PubMed]
- Buira, S.P.; Albasanz, J.L.; Dentesano, G.; Moreno, J.; Martín, M.; Ferrer, I.; Barrachina, M. DNA methylation regulates adenosine A2A receptor cell surface expression levels. J. Neurochem. 2010, 112, 1273–1285. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [PubMed]
- Huang, W.-J.; Zhang, X.; Chen, W.-W. Association between alcohol and Alzheimer’s disease. Exp. Ther. Med. 2016, 12, 1247–1250. [Google Scholar] [CrossRef] [PubMed]
- Solfrizzi, V.; D’Introno, A.; Colacicco, A.M.; Capurso, C.; Parigi, A.D.; Baldassarre, G.; Scapicchio, P.; Scafato, E.; Amodio, M.; Capurso, A.; et al. Alcohol consumption, mild cognitive impairment, and progression to dementia. Neurology 2007, 68, 1790–1799. [Google Scholar] [CrossRef]
- Solfrizzi, V.; Panza, F.; Frisardi, V.; Seripa, D.; Logroscino, G.; Imbimbo, B.P.; Pilotto, A. Diet and Alzheimer’s disease risk factors or prevention: The current evidence. Expert Rev. Neurother. 2011, 11, 677–708. [Google Scholar] [CrossRef]
- Bhat, A.H.; Dar, K.B.; Anees, S.; Zargar, M.A.; Masood, A.; Sofi, M.A.; Ganie, S.A. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 2015, 74, 101–110. [Google Scholar] [CrossRef]
- Formella, I.; Svahn, A.J.; Radford, R.A.W.; Don, E.K.; Cole, N.J.; Hogan, A.; Lee, A.; Chung, R.S.; Morsch, M. Real-time visualization of oxidative stress-mediated neurodegeneration of individual spinal motor neurons in vivo. Redox Biol. 2018, 19, 226–234. [Google Scholar] [CrossRef]
- Perry, G.; Cash, A.D.; Smith, M.A. Alzheimer Disease and Oxidative Stress. J. BioMed. Biotechnol. 2002, 2, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Ilomaki, J.; Jokanovic, N.; Tan, E.C.; Lonnroos, E. Alcohol Consumption, Dementia and Cognitive Decline: An Overview of Systematic Reviews. Curr. Clin. Pharmacol. 2015, 10, 204–2012. [Google Scholar] [CrossRef] [PubMed]
- Ruitenberg, A.; Swieten, v.J.C.; Witteman, J.C.M.; Mehta, K.M.; Duijn, v.C.M.; Hofman, A.; Breteler, M.M.B. Alcohol consumption and risk of dementia: The Rotterdam Study. Lancet 2002, 359, 281–286. [Google Scholar] [CrossRef]
- Heymann, D.; Stern, Y.; Cosentino, S.; Tatarina-Nulman, O.; Dorrejo, J.N.; Gu, Y. The association between alcohol use and the progression of Alzheimer’s disease. Curr. Alzheimer Res. 2016, 13, 1356–1362. [Google Scholar] [CrossRef] [PubMed]
- Langballe, E.M.; Ask, H.; Holmen, J.; Stordal, E.; Saltvedt, I.; Selbæk, G.; Fikseaunet, A.; Bergh, S.; Nafstad, P.; Tambs, K. Alcohol consumption and risk of dementia up to 27 years later in a large, population-based sample: The HUNT study, Norway. Eur. J. Epidemiol. 2015, 30, 1049–1056. [Google Scholar] [CrossRef] [PubMed]
- Luchsinger, J.A.; Tang, M.-X.; Siddiqui, M.; Shea, S.; Mayeux, R. Alcohol intake and risk of dementia. J. Am. Geriatr. Soc. 2004, 52, 540–546. [Google Scholar] [CrossRef]
- Neafsey, E.J.; Collins, M.A. Moderate alcohol consumption and cognitive risk. Neuropsychiat. Dis. Treat 2011, 7, 465–484. [Google Scholar] [CrossRef]
- Xu, W.; Wang, H.; Wan, Y.; Tan, C.; Li, J.; Tan, L.; Yu, J.-T. Alcohol consumption and dementia risk: A dose–response meta-analysis of prospective studies. Eur. J. Epidemiol. 2017, 32, 31–42. [Google Scholar] [CrossRef]
- Arranz, S.; Chiva-Blanch, G.; Valderas-Martínez, P.; Medina-Remón, A.; Lamuela-Raventós, R.M.; Estruch, R. Wine, Beer, Alcohol and Polyphenols on Cardiovascular Disease and Cancer. Nutrients 2012, 4, 759–781. [Google Scholar] [CrossRef]
- Peña, A.; Meseguer, I.; González-Muñoz, M.J. Influence of moderate beer consumption on aluminium toxico-kynetics: Acute study. Nutricion Hospitalaria 2007, 22, 371–376. [Google Scholar]
- Collins, M.A.; Neafsey, E.J.; Mukamal, K.J.; Gray, M.O.; Parks, D.A.; Das, D.K.; Korthuis, R.J. Alcohol in Moderation, Cardioprotection and Neuroprotection: Epidemiological Considerations and Mechanistic Studies. Alcohol Clin. Exp. Res. 2009, 33, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Fogarasi, A.-L.; Kun, S.; Tankó, G.; Stefanovits-Bányai, É.; Hegyesné-Vecseri, B. A comparative assessment of antioxidant properties, total phenolic content of einkorn, wheat, barley and their malts. Food Chem. 2015, 167, 1–6. [Google Scholar] [CrossRef]
- Koren, D.; Orbán, C.; Galló, N.; Kun, S.; Vecseri-Hegyes, B.; Kun-Farkas, G. Folic acid content and antioxidant activity of different types of beers available in Hungarian retail. J. Food Sci. Technol. 2017, 54, 1158–1167. [Google Scholar] [CrossRef] [PubMed]
- Spreng, S.; Hofmann, T. Activity-Guided Identification of in Vitro Antioxidants in Beer. J. Agric. Food Chem. 2018, 66, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Sivanantham, B.; Krishnan, U.; Rajendiran, V. Amelioration of oxidative stress in differentiated neuronal cells by rutin regulated by a concentration switch. Biomed. Pharmacother. 2018, 108, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Rekha, K.R.; Inmozhi Sivakamasundari, R. Geraniol Protects Against the Protein and Oxidative Stress Induced by Rotenone in an In Vitro Model of Parkinson’s Disease. Neurochem. Res. 2018. [Google Scholar] [CrossRef]
- Beking, K.; Vieira, A. Flavonoid intake and disability-adjusted life years due to Alzheimer’s and related dementias: A population-based study involving twenty-three developed countries. Public Health Nutr. 2010, 13, 1403–1409. [Google Scholar] [CrossRef]
- Kim, Y.A.; Lim, S.-Y.; Rhee, S.-H.; Park, K.Y.; Kim, C.-H.; Choi, B.T.; Lee, S.J.; Park, Y.-M.; Choi, Y.H. Resveratrol inhibits inducible nitric oxide synthase and cyclooxygenase-2 expression in β-amyloid-treated C6 glioma cells. Int. J. Mol. Med. 2006, 17, 1069–1075. [Google Scholar] [CrossRef]
- Luo, L.; Bai, R.; Zhao, Y.; Li, J.; Wei, Z.; Wang, F.; Sun, B. Protective Effect of Grape Seed Procyanidins against H2 O2 -Induced Oxidative Stress in PC-12 Neuroblastoma Cells: Structure-Activity Relationships. J. Food Sci. 2018. [Google Scholar] [CrossRef]
- Ristivojević, P.M.; Morlock, G.E. Effect-directed classification of biological, biochemical and chemical profiles of 50 German beers. Food Chem. 2018, 260, 344–353. [Google Scholar] [CrossRef]
- Česlová, L.; Holčapek, M.; Fidler, M.; Drštičková, J.; Lísa, M. Characterization of prenylflavonoids and hop bitter acids in various classes of Czech beers and hop extracts using high-performance liquid chromato...PubMed—NCBI. J. Chromatogr. A 2009, 1216, 7249–7257. [Google Scholar] [CrossRef] [PubMed]
- Almeida, C.; Duarte, I.F.; Barros, A.; Rodrigues, J.; Spraul, M.; Gil, A.M. Composition of Beer by 1H NMR Spectroscopy: Effects of Brewing Site and Date of Production. J. Agric. Food Chem. 2006, 54, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Cunha, R.A. Neuroprotection by adenosine in the brain: From A1 receptor activation to A2A receptor blockade. Purinergic Signal. 2005, 1, 111–134. [Google Scholar] [CrossRef] [PubMed]
- Stone, T.W.; Ceruti, S.; Abbracchio, M.P. Adenosine Receptors and Neurological Disease: Neuroprotection and Neurodegeneration. Handb. Exp. Pharmacol. 2009, 193, 535–587. [Google Scholar]
- Albasanz, J.L.; Rodríguez, A.; Ferrer, I.; Martín, M. Up-regulation of adenosine A1 receptors in frontal cortex from Pick’s disease cases. Eur. J. Neurosci. 2007, 26, 3501–3508. [Google Scholar] [CrossRef]
- Villar-Menéndez, I.; Porta, S.; Buira, S.P.; Pereira-Veiga, T.; Díaz-Sánchez, S.; Albasanz, J.L.; Ferrer, I.; Martín, M.; Barrachina, M. Increased striatal adenosine A2A receptor levels is an early event in Parkinson’s disease-related pathology and it is potentially regulated by miR-34b. Neurobiol. Dis. 2014, 69, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Woods, L.T.; Ajit, D.; Camden, J.M.; Erb, L.; Weisman, G.A. Purinergic receptors as potential therapeutic targets in Alzheimer’s disease. Neuropharmacology 2016, 104, 169–179. [Google Scholar] [CrossRef]
- Albasanz, J.L.; León, D.; Ruíz, M.A.; Fernández, M.; Martín, M. Adenosine A1 receptor agonist treatment up-regulates rat brain metabotropic glutamate receptors. Biochim. Biophys. Acta Mol. Cell Res. 2002, 1593, 69–75. [Google Scholar] [CrossRef][Green Version]
- Reichelt, M.E.; Shanu, A.; Willems, L.; Witting, P.K.; Ellis, N.A.; Blackburn, M.R.; Headrick, J.P. Endogenous adenosine selectively modulates oxidant stress via the A1 receptor in ischemic hearts. Antioxid. Redox Signal. 2009, 11, 2641–2650. [Google Scholar] [CrossRef]
- Zhou, X.; Teng, B.; Tilley, S.; Mustafa, S.J. A1 adenosine receptor negatively modulates coronary reactive hyperemia via counteracting A2A-mediated H2O2 production and KATP opening in isolated mouse hearts. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1668–H1679. [Google Scholar] [CrossRef]
- Narayan, P.; Mentzer, R.M.; Lasley, R.D. Adenosine A1 receptor activation reduces reactive oxygen species and attenuates stunning in ventricular myocytes. J. Mol. Cell. Cardiol. 2001, 33, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Mendes, F.B.; Bergamin, L.S.; Dos Santos Stuepp, C.; Braganhol, E.; Terroso, T.; Pohlmann, A.R.; Guterres, S.S.; Battastini, A.M. Alpha-bisabolol Promotes Glioma Cell Death by Modulating the Adenosinergic System. Anticancer Res. 2017, 37, 1819–1823. [Google Scholar] [CrossRef] [PubMed]
- Bobermin, L.D.; Roppa, R.H.A.; Quincozes-Santos, A. Adenosine receptors as a new target for resveratrol-mediated glioprotection. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 634–647. [Google Scholar] [CrossRef]
- Chen, S.-Q.; Wang, Z.-S.; Ma, Y.-X.; Zhang, W.; Lu, J.-L.; Liang, Y.-R.; Zheng, X.-Q.; Chen, S.-Q.; Wang, Z.-S.; Ma, Y.-X.; et al. Neuroprotective Effects and Mechanisms of Tea Bioactive Components in Neurodegenerative Diseases. Molecules 2018, 23, 512. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alonso-Andrés, P.; Martín, M.; Albasanz, J.L. Modulation of Adenosine Receptors and Antioxidative Effect of Beer Extracts in in Vitro Models. Nutrients 2019, 11, 1258. https://doi.org/10.3390/nu11061258
Alonso-Andrés P, Martín M, Albasanz JL. Modulation of Adenosine Receptors and Antioxidative Effect of Beer Extracts in in Vitro Models. Nutrients. 2019; 11(6):1258. https://doi.org/10.3390/nu11061258
Chicago/Turabian StyleAlonso-Andrés, Patricia, Mairena Martín, and José Luis Albasanz. 2019. "Modulation of Adenosine Receptors and Antioxidative Effect of Beer Extracts in in Vitro Models" Nutrients 11, no. 6: 1258. https://doi.org/10.3390/nu11061258
APA StyleAlonso-Andrés, P., Martín, M., & Albasanz, J. L. (2019). Modulation of Adenosine Receptors and Antioxidative Effect of Beer Extracts in in Vitro Models. Nutrients, 11(6), 1258. https://doi.org/10.3390/nu11061258