The Association of Breakfast Frequency and Cardiovascular Disease (CVD) Risk Factors among Adolescents in Malaysia
Abstract
1. Introduction
2. Methods
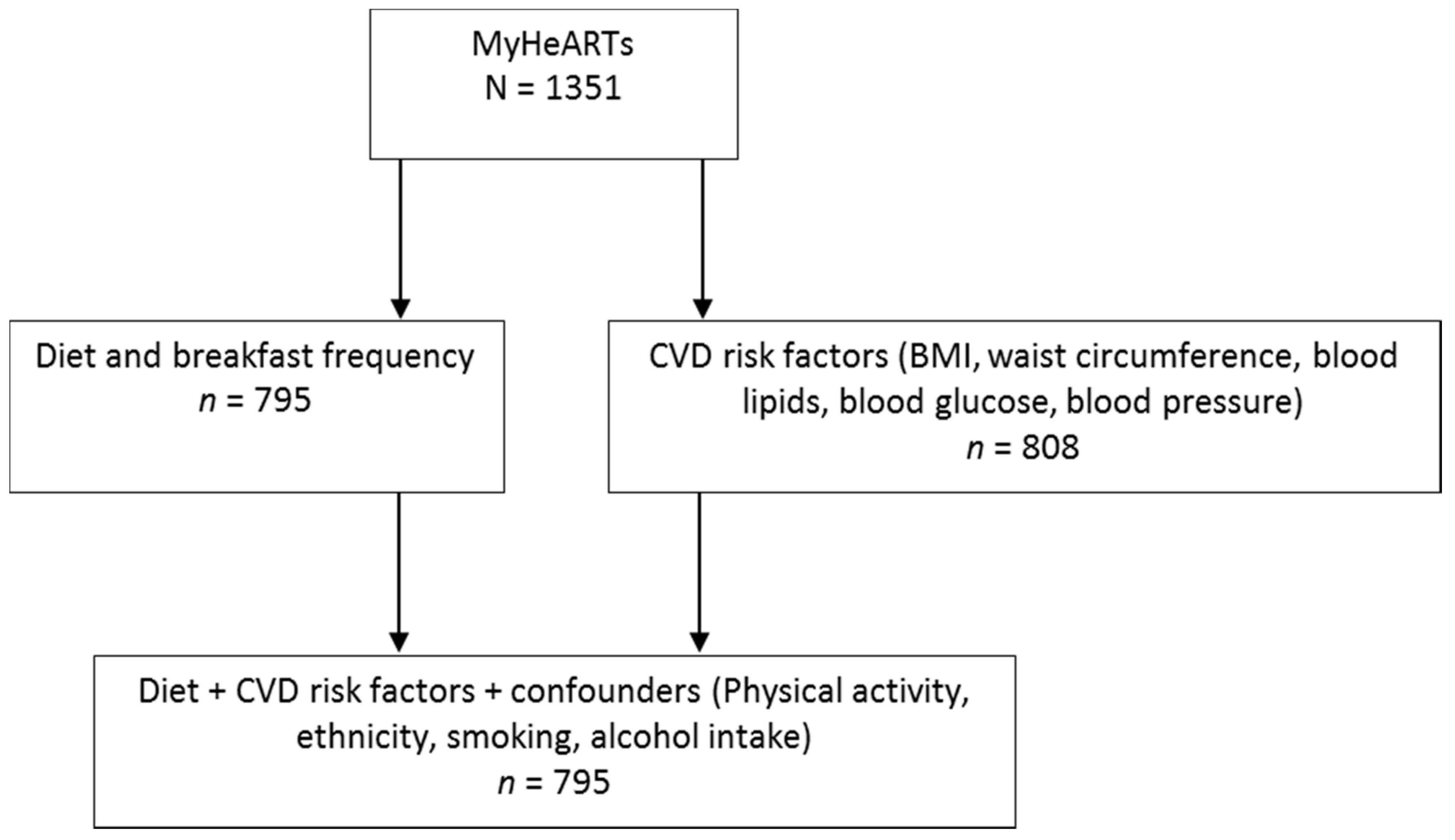
2.1. Study Overview and Population
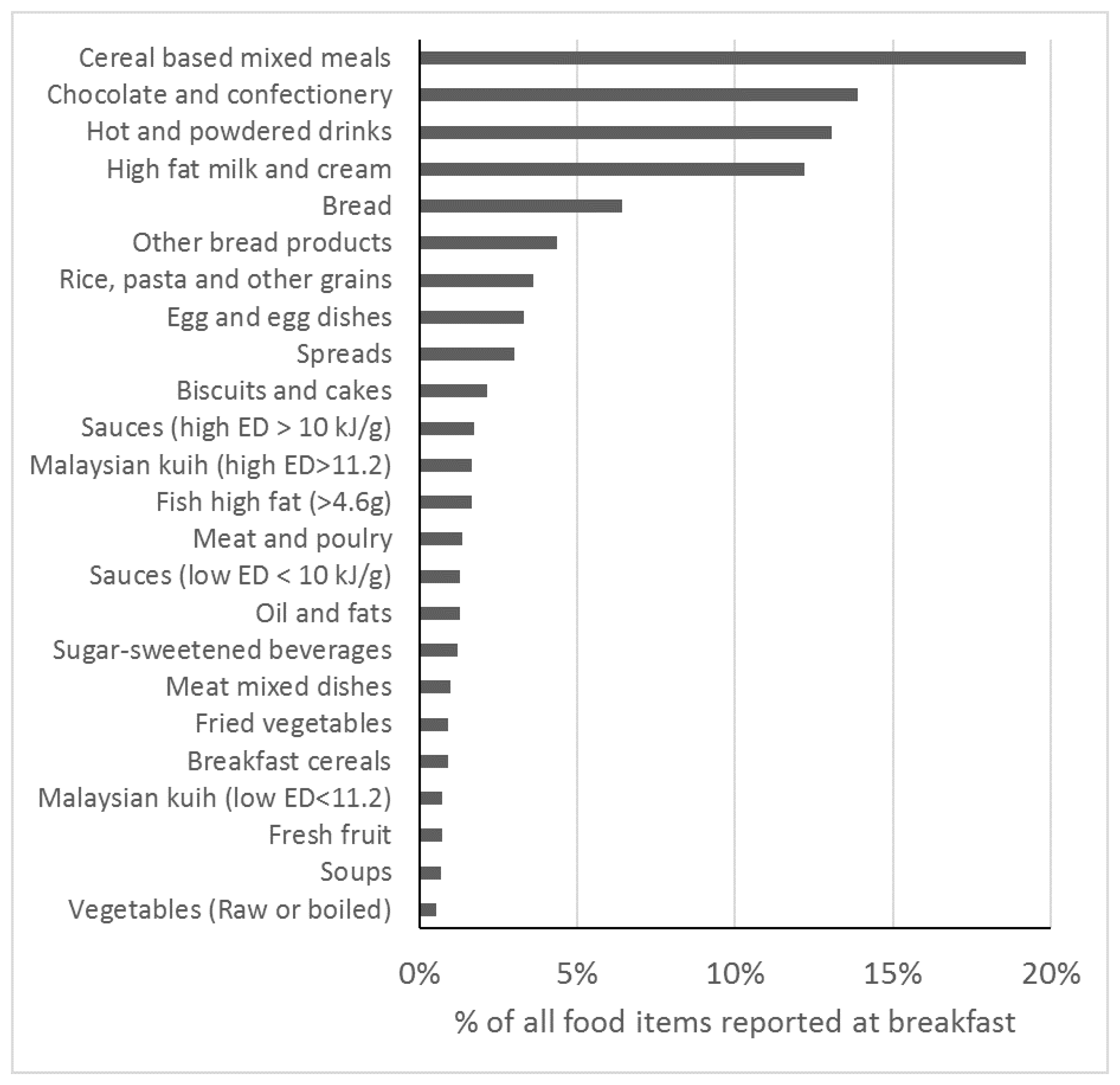
2.2. Dietary Assessment
2.3. Anthropometric and Clinical Measurements
2.4. Confounders
2.5. Statistical Analysis
3. Results
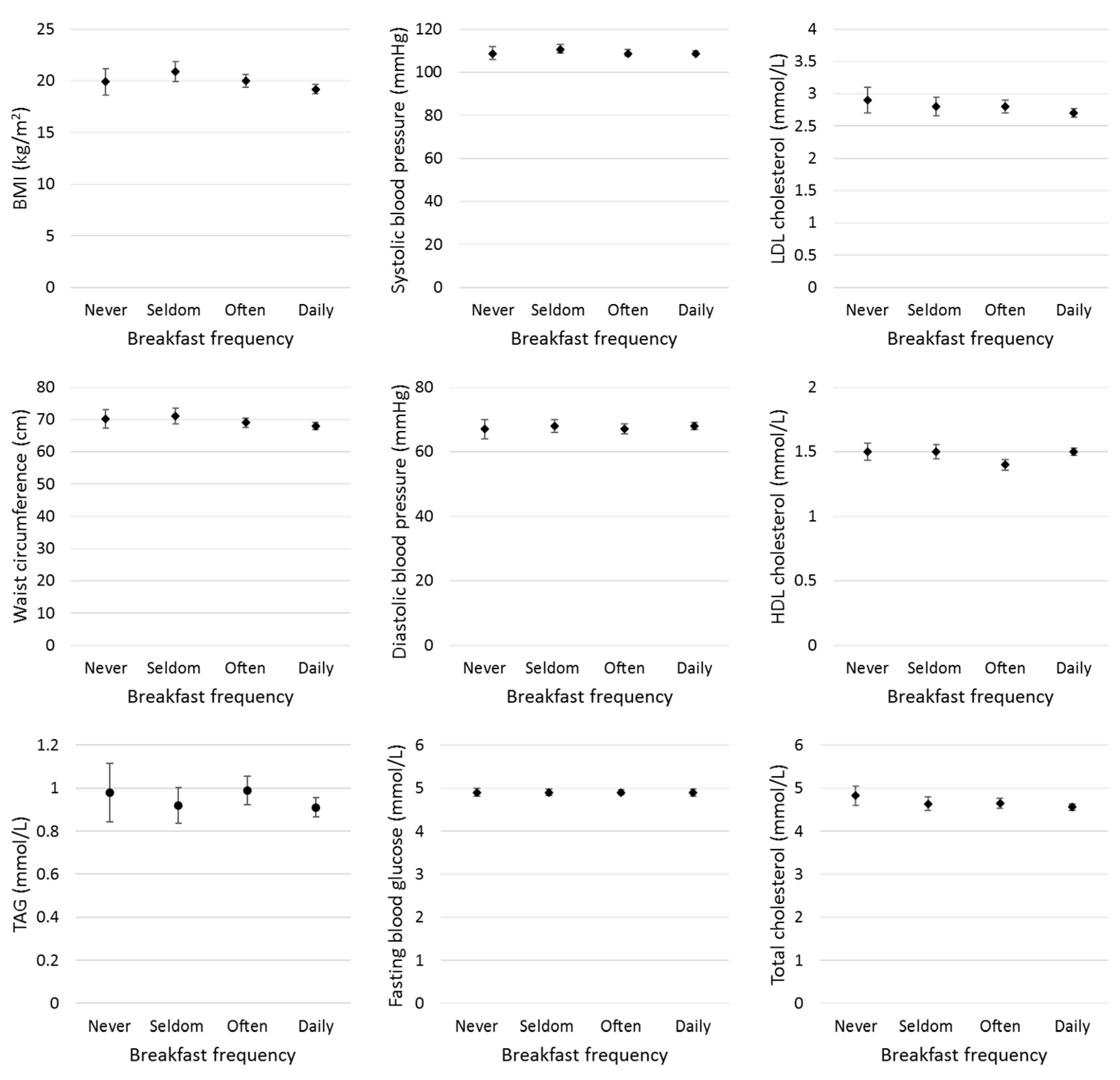
3.1. Mean of Cardiovascular Disease Risk Factors
3.2. Association of Breakfast Consumption and cardiovascular disease (CVD) Risk Factors
4. Discussion
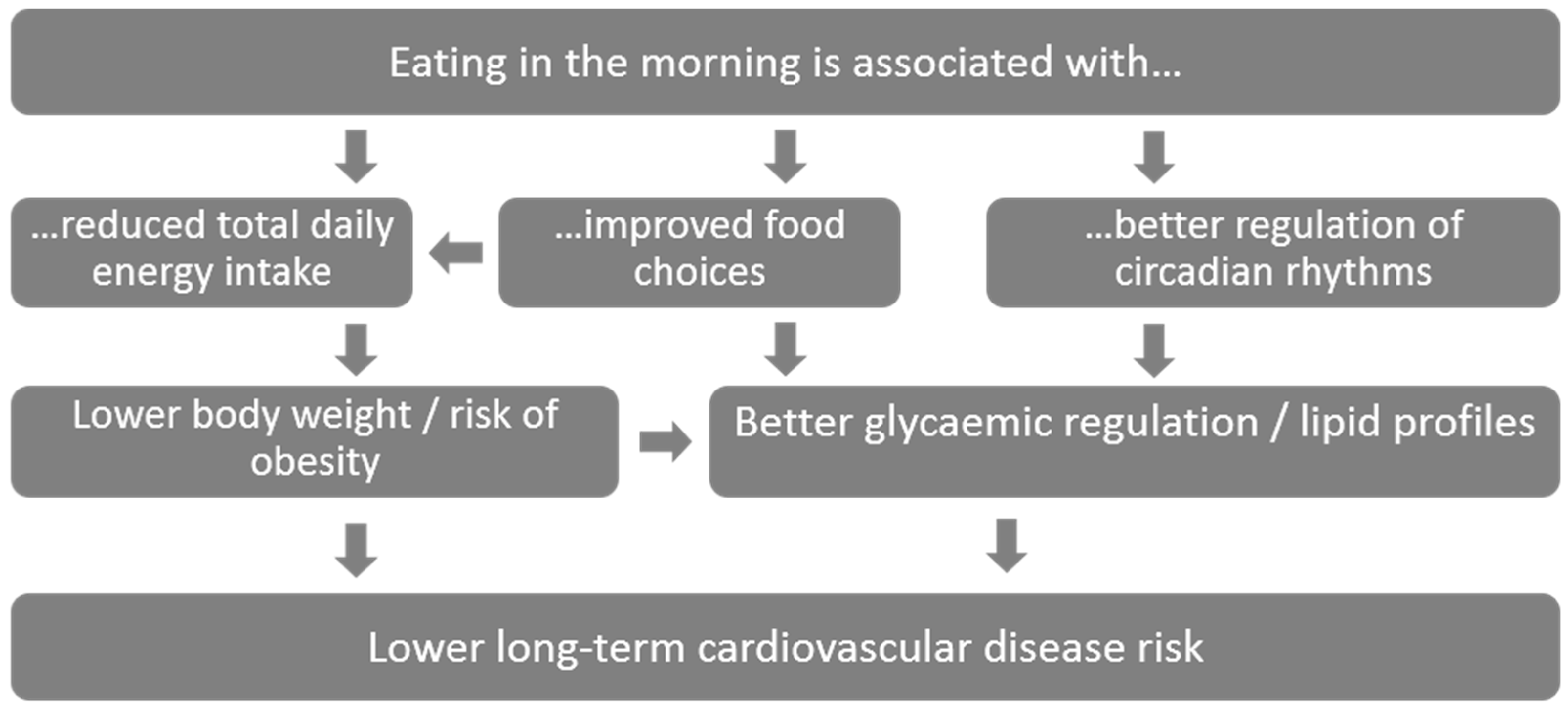
4.1. Breakfast Associations with Cardiovascular Disease Risk Factors
4.2. Comparisons of Breakfast Definitions
4.3. Breakfast Composition and Meal Timing
4.4. Strengths and Limitations
5. Conclusion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Financial Support
References
- Mendis, S.; Puska, P.; Norrving, B. Global Atlas on Cardiovascular Disease Prevention and Control; World Health Organization: Geneva, Switzerland.
- Noncommunicable Diseases Country Profiles 2014. Available online: https://www.who.int/nmh/publications/ncd-profiles-2014/en/ (accessed on 30 June 2017).
- Raitakari, O.T.; Juonala, M.; Kahonen, M.; Taittonen, L.; Laitinen, T.; Maki-Torkko, N.; Jarvisalo, M.J.; Uhari, M.; Jokinen, E.; Ronnemaa, T.; et al. Cardiovascular risk factors in childhood and carotid artery intima-media thickness in adulthood: the Cardiovascular Risk in Young Finns Study. JAMA 2003, 290, 2277–2283. [Google Scholar] [CrossRef]
- Ng, M.; Fleming, T.; Robinson, M.; Thomson, B.; Graetz, N.; Margono, C.; Mullany, E.C.; Biryukov, S.; Abbafati, C.; Abera, S.F.; et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 2013, 384, 766–781. [Google Scholar] [CrossRef]
- Brown, A.W.; Bohan Brown, M.M.; Allison, D.B. Belief beyond the evidence: using the proposed effect of breakfast on obesity to show 2 practices that distort scientific evidence. Am. J. Clin. Nutr. 2013. [Google Scholar] [CrossRef] [PubMed]
- Horikawa, C.; Kodama, S.; Yachi, Y.; Heianza, Y.; Hirasawa, R.; Ibe, Y.; Saito, K.; Shimano, H.; Yamada, N.; Sone, H. Skipping breakfast and prevalence of overweight and obesity in Asian and Pacific regions: A meta-analysis. Prev. Med. 2011, 53, 260–267. [Google Scholar] [CrossRef]
- Purslow, L.R.; Sandhu, M.S.; Forouhi, N.; Young, E.H.; Luben, R.N.; Welch, A.A.; Khaw, K.T.; Bingham, S.A.; Wareham, N.J. Energy Intake at Breakfast and Weight Change: Prospective Study of 6,764 Middle-aged Men and Women. Am. J. Epidemiol. 2008, 167, 188–192. [Google Scholar] [CrossRef]
- Mente, A.; de Koning, L.; Shannon, H.S.; Anand, S.S. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch. Intern. Med. 2009, 169, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Farshchi, H.R.; Taylor, M.A.; Macdonald, I.A. Deleterious effects of omitting breakfast on insulin sensitivity and fasting lipid profiles in healthy lean women. Am. J. Clin. Nutr. 2005, 81, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Clayton, D.J.; Stensel, D.J.; James, L.J. Effect of breakfast omission on subjective appetite, metabolism, acylated ghrelin and GLP-17-36 during rest and exercise. Nutrition 2016, 32, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Bo, S.; Fadda, M.; Castiglione, A.; Ciccone, G.; De Francesco, A.; Fedele, D.; Guggino, A.; Parasiliti Caprino, M.; Ferrara, S.; Vezio Boggio, M.; et al. Is the timing of caloric intake associated with variation in diet-induced thermogenesis and in the metabolic pattern? A randomized cross-over study. Int. J. Obes. (Lond) 2015, 39, 1689–1695. [Google Scholar] [CrossRef] [PubMed]
- Bandin, C.; Scheer, F.A.; Luque, A.J.; Avila-Gandia, V.; Zamora, S.; Madrid, J.A.; Gomez-Abellan, P.; Garaulet, M. Meal timing affects glucose tolerance, substrate oxidation and circadian-related variables: A randomized, crossover trial. Int. J. Obes. (Lond) 2015, 39, 828–833. [Google Scholar] [CrossRef]
- Betts, J.A.; Richardson, J.D.; Chowdhury, E.A.; Holman, G.D.; Tsintzas, K.; Thompson, D. The causal role of breakfast in energy balance and health: a randomized controlled trial in lean adults. Am. J. Clin. Nutr. 2014, 100, 539–547. [Google Scholar] [CrossRef]
- Chowdhury, E.A.; Richardson, J.D.; Holman, G.D.; Tsintzas, K.; Thompson, D.; Betts, J.A. The causal role of breakfast in energy balance and health: A randomized controlled trial in obese adults. Am. J. Clin. Nutr. 2016, 103, 747–756. [Google Scholar] [CrossRef]
- Dhurandhar, E.J.; Dawson, J.; Alcorn, A.; Larsen, L.H.; Thomas, E.A.; Cardel, M.; Bourland, A.C.; Astrup, A.; St-Onge, M.P.; Hill, J.O.; et al. The effectiveness of breakfast recommendations on weight loss: a randomized controlled trial. Am. J. Clin. Nutr. 2014, 100, 507–513. [Google Scholar] [CrossRef]
- Amiel, S.A.; Sherwin, R.S.; Simonson, D.C.; Lauritano, A.A.; Tamborlane, W.V. Impaired insulin action in puberty. A contributing factor to poor glycemic control in adolescents with diabetes. N. Engl. J. Med. 1986, 315, 215–219. [Google Scholar] [CrossRef]
- Hannon, T.S.; Janosky, J.; Arslanian, S.A. Longitudinal study of physiologic insulin resistance and metabolic changes of puberty. Pediatr. Res. 2006, 60, 759–763. [Google Scholar] [CrossRef]
- Crowley, S.J.; Acebo, C.; Carskadon, M.A. Sleep, circadian rhythms, and delayed phase in adolescence. Sleep Med. 2007, 8, 602–612. [Google Scholar] [CrossRef]
- Jakubowicz, D.; Wainstein, J.; Landau, Z.; Raz, I.; Ahren, B.; Chapnik, N.; Ganz, T.; Menaged, M.; Barnea, M.; Bar-Dayan, Y. Influences of Breakfast on Clock Gene Expression and Postprandial Glycemia in Healthy Individuals and Individuals With Diabetes: A Randomized Clinical Trial. Diabetes. Care 2017, 40, 1573–1579. [Google Scholar] [CrossRef]
- Zakrzewski, J.K.; Gillison, F.B.; Cumming, S.; Church, T.S.; Katzmarzyk, P.T.; Broyles, S.T.; Champagne, C.M.; Chaput, J.P.; Denstel, K.D.; Fogelholm, M.; et al. Associations between breakfast frequency and adiposity indicators in children from 12 countries. Int. J. Obes. Suppl. 2015, 5, S80–S88. [Google Scholar] [CrossRef] [PubMed]
- Blondin, S.A.; Anzman-Frasca, S.; Djang, H.C.; Economos, C.D. Breakfast consumption and adiposity among children and adolescents: An updated review of the literature. Pediatr. Obes. 2016. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.S.; Mohd Nasir, M.T. Eating Behaviors among Female Adolescents in Kuantan District, Pahang, Malaysia. Pak. J. Nutr. 2009, 8, 425–432. [Google Scholar] [CrossRef]
- Koo, H.-C.; Abdul Jalil, S.N.; Ruzita, A.T. Breakfast Eating Pattern and Ready-to-Eat Cereals Consumption among Schoolchildren in Kuala Lumpur. Malays. J. Med. Sci. 2015, 22, 32–39. [Google Scholar]
- Law, L.S.; Mohd-Nasir, M.T.; Hazizi, A.S. Factors associated with breakfast skipping among school going adolescents in Sarawak, Malaysia. Malays. J. Nutr. 2013, 19, 401–407. [Google Scholar]
- Ming, F.M.; Ying, G.C.; Kassim, S.Z.M. Eating patterns of school children and adolescent in Kuala Lumpur. Malays. J. Nutr. 2006, 12, 1–10. [Google Scholar]
- Nurul-Fadhilah, A.; Teo, P.S.; Huybrechts, I.; Foo, L.H. Infrequent breakfast consumption is associated with higher body adiposity and abdominal obesity in Malaysian school-aged adolescents. PLoS ONE 2013, 8, e59297. [Google Scholar] [CrossRef]
- Abdelaal, M.; le Roux, C.W.; Docherty, N.G. Morbidity and mortality associated with obesity. Ann. Transl. Med. 2017, 5, 161. [Google Scholar] [CrossRef] [PubMed]
- Donin, A.S.; Nightingale, C.M.; Owen, C.G.; Rudnicka, A.R.; Perkin, M.R.; Jebb, S.A.; Stephen, A.M.; Sattar, N.; Cook, D.G.; Whincup, P.H. Regular Breakfast Consumption and Type 2 Diabetes Risk Markers in 9- to 10-Year-Old Children in the Child Heart and Health Study in England (CHASE): A Cross-Sectional Analysis. PLoS Med. 2014, 11, e1001703. [Google Scholar] [CrossRef] [PubMed]
- Hallström, L.; Labayen, I.; Ruiz, J.R.; Patterson, E.; Vereecken, C.A.; Breidenassel, C.; Gottrand, F.; Huybrechts, I.; Manios, Y.; Mistura, L.; et al. Breakfast consumption and CVD risk factors in European adolescents: the HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) Study. Publi. Health Nutr. 2013, 1296–1305. [Google Scholar] [CrossRef]
- Marlatt, K.L.; Farbakhsh, K.; Dengel, D.R.; Lytle, L.A. Breakfast and fast food consumption are associated with selected biomarkers in adolescents. Prev. Med. Rep. 2016, 3, 49–52. [Google Scholar] [CrossRef]
- Jaaskelainen, A.; Schwab, U.; Kolehmainen, M.; Pirkola, J.; Jarvelin, M.R.; Laitinen, J. Associations of meal frequency and breakfast with obesity and metabolic syndrome traits in adolescents of Northern Finland Birth Cohort 1986. Nutr. Metab. Cardiovasc. Dis. 2013, 23, 1002–1009. [Google Scholar] [CrossRef]
- Ho, C.-Y.; Huang, Y.-C.; Lo, Y.-T.C.; Wahlqvist, M.L.; Lee, M.-S. Breakfast is associated with the metabolic syndrome and school performance among Taiwanese children. Res. Dev. Dis. 2015, 43, 179–188. [Google Scholar] [CrossRef]
- Yoshinaga, M.; Hatake, S.; Tachikawa, T.; Shinomiya, M.; Miyazaki, A.; Takahashi, H. Impact of Lifestyles of Adolescents and Their Parents on Cardiovascular Risk Factors in Adolescents. J. Atheroscler. Thromb. 2011, 18, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Ahadi, Z.; Qorbani, M.; Kelishadi, R.; Ardalan, G.; Motlagh, M.E.; Asayesh, H.; Zeynali, M.; Chinian, M.; Larijani, B.; Shafiee, G.; et al. Association between breakfast intake with anthropometric measurements, blood pressure and food consumption behaviors among Iranian children and adolescents: The CASPIAN-IV study. Publ. Health 2015, 129, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.J.; Gall, S.L.; McNaughton, S.A.; Blizzard, L.; Dwyer, T.; Venn, A.J. Skipping breakfast: longitudinal associations with cardiometabolic risk factors in the Childhood Determinants of Adult Health Study. Am. J. Clin. Nutr. 2010, 92, 1316–1325. [Google Scholar] [CrossRef]
- Wennberg, M.; Gustafsson, P.E.; Wennberg, P.; Hammarstrom, A. Poor breakfast habits in adolescence predict the metabolic syndrome in adulthood. Publ. Health Nutr. 2015, 18, 122–129. [Google Scholar] [CrossRef]
- Brion, M.J.; Lawlor, D.A.; Matijasevich, A.; Horta, B.; Anselmi, L.; Araujo, C.L.; Menezes, A.M.; Victora, C.G.; Smith, G.D. What are the causal effects of breastfeeding on IQ, obesity and blood pressure? Evidence from comparing high-income with middle-income cohorts. Int. J. Epidemiol. 2011, 40, 670–680. [Google Scholar] [CrossRef]
- Hazreen, M.A.; Su, T.T.; Jalaludin, M.Y.; Dahlui, M.; Chinna, K.; Ismail, M.; Murray, L.; Cantwell, M.; Al Sadat, N.; MyHe, A.R.T.S.G. An exploratory study on risk factors for chronic non-communicable diseases among adolescents in Malaysia: overview of the Malaysian Health and Adolescents Longitudinal Research Team study (The MyHeART study). BMC Publ. Health. 2014, 14, S6. [Google Scholar] [CrossRef]
- Nutritient Composition of Malaysian Foods. Available online: http://www.nutriscene.org.my/books/Tee%20et%20al%201997%20-%20Nutr%20Comp%20of%20Malaysian%20Foods.pdf (accessed on 26 April 2019).
- Mohd Yusoff, N.A.; Safii, N.A.; Ghazali, R.; Ahmed, R.; Shahar, S. Atlas of Food Exchanges and Portion Sizes, 2nd ed.; MDC Publisher Sdn Bhd: Kuala Lumpur, Malaysia, 2009. [Google Scholar]
- Abdul Majid, H.; Ramli, L.; Ying, S.P.; Su, T.T.; Jalaludin, M.Y.; Abdul Mohsein, N.A. Dietary Intake among Adolescents in a Middle-Income Country: An Outcome from the Malaysian Health and Adolescents Longitudinal Research Team Study (the MyHeARTs Study). PLoS ONE 2016, 11, e0155447. [Google Scholar] [CrossRef]
- Johnson, L.; Toumpakari, Z.; Papadaki, A. Social Gradients and Physical Activity Trends in an Obesogenic Dietary Pattern: Cross-Sectional Analysis of the UK National Diet and Nutrition Survey 2008–2014. 2018, 10, 388. [Google Scholar] [CrossRef]
- Timlin, M.T.; Pereira, M.A.; Story, M.; Neumark-Sztainer, D. Breakfast Eating and Weight Change in a 5-Year Prospective Analysis of Adolescents: Project EAT (Eating Among Teens). Pediatrics 2008, 121, e638–e645. [Google Scholar] [CrossRef]
- O’Neil, C.E.; Byrd-Bredbenner, C.; Hayes, D.; Jana, L.; Klinger, S.E.; Stephenson-Martin, S. The Role of Breakfast in Health: Definition and Criteria for a Quality Breakfast. J. Acad. Nutr. Diet. 2014, 114, S8–S26. [Google Scholar] [CrossRef]
- Soran, H.; Dent, R.; Durrington, P. Evidence-based goals in LDL-C reduction. Clin. Res. Cardiol. 2017, 106, 237–248. [Google Scholar] [CrossRef]
- Shafiee, G.; Kelishadi, R.; Qorbani, M.; Motlagh, M.E.; Taheri, M.; Ardalan, G.; Taslimi, M.; Poursafa, P.; Heshmat, R.; Larijani, B. Association of breakfast intake with cardiometabolic risk factors. J. Pediatr. (Rio. J.) 2013, 89, 575–582. [Google Scholar] [CrossRef]
- Marz, W.; Kleber, M.E.; Scharnagl, H.; Speer, T.; Zewinger, S.; Ritsch, A.; Parhofer, K.G.; von Eckardstein, A.; Landmesser, U.; Laufs, U. HDL cholesterol: reappraisal of its clinical relevance. Clin. Res. Cardiol. 2017, 106, 663–675. [Google Scholar] [CrossRef] [PubMed]
- Silva, F.A.; Padez, C.; Sartorelli, D.S.; Oliveira, R.M.S.; Netto, M.P.; Mendes, L.L.; Candido, A.P.C. Cross-sectional study showed that breakfast consumption was associated with demographic, clinical and biochemical factors in children and adolescents. Acta. Paediatr. 2018. [Google Scholar] [CrossRef] [PubMed]
- Farshchi, H.R.; Taylor, M.A.; Macdonald, I.A. Regular meal frequency creates more appropriate insulin sensitivity and lipid profiles compared with irregular meal frequency in healthy lean women. Eur. J. Clin. Nutr. 2004, 58, 1071–1077. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Lopez, E.; Grijalva-Haro, M.I.; Valencia, M.E.; Antonio Ponce, J.; Artalejo, E. Effect of a School Breakfast Program on the prevalence of obesity and cardiovascular risk factors in children. Salud Publica Mex 2005, 47, 126–133. [Google Scholar]
- Dialektakou, K.D.; Vranas, P.B. Breakfast skipping and body mass index among adolescents in Greece: Whether an association exists depends on how breakfast skipping is defined. J. Am. Diet. Assoc. 2008, 108, 1517–1525. [Google Scholar] [CrossRef]
- Bayham, B.E.; Greenway, F.L.; Johnson, W.D.; Dhurandhar, N.V. A randomized trial to manipulate the quality instead of quantity of dietary proteins to influence the markers of satiety. J. Diabetes Complic. 2014, 28, 547–552. [Google Scholar] [CrossRef]
- Rebello, C.J.; Johnson, W.D.; Martin, C.K.; Xie, W.; O’Shea, M.; Kurilich, A.; Bordenave, N.; Andler, S.; Klinken, B.J.W.v.; Chu, Y.-F.; et al. Acute Effect of Oatmeal on Subjective Measures of Appetite and Satiety Compared to a Ready-to-Eat Breakfast Cereal: A Randomized Crossover Trial. J. Am. Coll. Nutr. 2013, 32, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Alexy, U.; Wicher, M.; Kersting, M. Breakfast trends in children and adolescents: frequency and quality. Publ. Health Nutr. 2010, 13, 1795–1802. [Google Scholar] [CrossRef] [PubMed]
- Randler, C. Association between morningness-eveningness and mental and physical health in adolescents. Psychol. Health Med. 2011, 16, 29–38. [Google Scholar] [CrossRef]
- Scheer, F.A.; Hilton, M.F.; Mantzoros, C.S.; Shea, S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef]
- Kong, A.P.; Wing, Y.K.; Choi, K.C.; Li, A.M.; Ko, G.T.; Ma, R.C.; Tong, P.C.; Ho, C.S.; Chan, M.H.; Ng, M.H.; et al. Associations of sleep duration with obesity and serum lipid profile in children and adolescents. Sleep Med. 2011, 12, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Van Cauter, E.; Polonsky, K.S.; Scheen, A.J. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr. Rev. 1997, 18, 716–738. [Google Scholar] [CrossRef] [PubMed]
- Mendoza, J. Circadian clocks: Setting time by food. J. Neuroendocrinol. 2007, 19, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Scheer, F. Circadian System and Glucose Metabolism: Implications for Physiology and Disease. Trends. Endocrinol. Metab. 2016, 27, 282–293. [Google Scholar] [CrossRef] [PubMed]
- Woolhead, C.; Gibney, M.J.; Walsh, M.C.; Brennan, L.; Gibney, E.R. A generic coding approach for the examination of meal patterns. Am. J. Clin. Nutr. 2015, 102, 316–323. [Google Scholar] [CrossRef]
- Murakami, K.; Livingstone, M.B.E.; Sasaki, S. Establishment of a Meal Coding System for the Characterization of Meal-Based Dietary Patterns in Japan. J. Nutr. 2017, 147, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.; Mander, A.P.; Jones, L.R.; Emmett, P.M.; Jebb, S.A. Energy-dense, low-fiber, high-fat dietary pattern is associated with increased fatness in childhood. Am. J. Clin. Nutr. 2008, 87, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Matthys, C.; De Henauw, S.; Bellemans, M.; De Maeyer, M.; De Backer, G. Breakfast habits affect overall nutrient profiles in adolescents. Publ. Health Nutr. 2007, 10, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Berkey, C.S.; Rockett, H.R.; Gillman, M.W.; Field, A.E.; Colditz, G.A. Longitudinal study of skipping breakfast and weight change in adolescents. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
| Breakfast Frequency | ||||||
|---|---|---|---|---|---|---|
| Total | 0 days/week | 1–3 days/week | 4–6 days/week | Daily | p-Value | |
| All children, n (%) | 795 (100) | 76 (10) | 115 (15) | 196 (25) | 408 (51) | - |
| Age (years), mean (SD) | 12.9 (0.3) | 12.9 (0.3) | 12.9 (0.4) | 12.8 (0.3) | 12.9 (0.3) | - |
| Sex | ||||||
| Boys, n (%) | 298 (37) | 32 (10) | 36 (12) | 58 (19) | 172 (58) | 0.01 a |
| Girls, n (%) | 497 (63) | 44 (9) | 79 (16) | 138 (28) | 236 (47) | |
| Urbanicity | ||||||
| Urban, n (%) | 401 (50) | 17 (4) | 53 (13) | 114 (28) | 217 (54) | <0.001 a |
| Rural, n (%) | 394 (50) | 59 (15) | 62 (16) | 82 (21) | 191 (48) | |
| Ethnicity | ||||||
| Malay, n (%) | 650 (82) | 68 (10) | 100 (15) | 162 (25) | 320 (49) | 0.03 a |
| Chinese, n (%) | 44 (6) | 0 (0) | 8 (18) | 12 (27) | 24 (55) | |
| Indian, n (%) | 69 (14) | 5 (7) | 3 (4) | 12 (17) | 49 (71) | |
| Others, n (%) | 32 (6) | 3 (9) | 4 (13) | 10 (31) | 15 (47) | |
| Smoking status | ||||||
| Yes, n (%) | 73 (9) | 11 (15) | 12 (16) | 13 (18) | 37 (51) | 0.23 a |
| No, n (%) | 722 (91) | 65 (9) | 103 (14) | 183 (25) | 371 (51) | |
| Alcohol intake | ||||||
| Yes, n (%) | 23 (3) | 3 (13) | 5 (22) | 4 (17) | 11 (48) | 0.55 a |
| No, n (%) | 772 (97) | 73 (9) | 110 (14) | 192 (25) | 397 (51) | |
| Physical activity (in last 7 days) | ||||||
| Never n (%) | 237 (30) | 27 (11) | 35 (15) | 55 (23) | 120 (51) | 0.35 a |
| 1–2 times last week n (%) | 361 (45) | 32 (8) | 58 (16) | 89 (25) | 182 (50) | |
| 3–4 times last week n (%) | 113 (14) | 8 (7) | 13 (12) | 31 (27) | 61 (54) | |
| 5–6 times last week n (%) | 34 (4) | 2 (6) | 8 (24) | 8 (24) | 16 (47) | |
| 7+ times last week n (%) | 50 (6) | 7 (14) | 1 (2) | 13 (26) | 29 (58) | |
| Total daily intake | ||||||
| Energy (kcal/day) | 1673 (332) | 1744 (433) | 1573 (276) | 1594 (267) | 1726 (339) | <0.001 b |
| Protein (% of total energy) | 15 (2) | 15 (2) | 15 (2) | 15 (2) | 15 (2) | 0.63 b |
| Fat (% of total energy) | 29.930 (5) | 30 (5) | 30 (4) | 30 (5) | 30 (4) | 0.87 b |
| Carbohydrate (% of total energy) | 55 (5) | 55 (5) | 56 (5) | 55 (5) | 55 (6) | 0.62 b |
| Cholesterol (mg/1000 kcal) | 133 (52) | 137 (58) | 139 (50) | 131(47) | 132 (54) | 0.53 b |
| SFA (% of total energy) | 6 (2) | 6 (2) | 5 (2) | 6 (3) | 6 (2) | 0.14 b |
| Sodium (mg/1000 kcal) | 1387 (345) | 1328 (380) | 1363 (352) | 1400 (329) | 1399 (344) | 0.32 b |
| Calcium (mg/1000 kcal) | 226 (91) | 229 (116) | 195 (827) | 222 (81) | 236 (92) | <0.001 b |
| Iron (mg/1000 kcal) | 9 (3) | 9 (3) | 8 (6) | 8 (2) | 9 (2) | 0.42 b |
| Crude fiber (g/1000 kcal) | 2 (1) | 2 (1) | 2 (1) | 2 (11) | 2 (1) | 0.51 b |
| Sugar (% of total energy) | 8 (4) | 8 (4) | 7 (3) | 9 (4) | 8 (4) | 0.04 b |
| Dietary pattern score (SD units) | 0.01 (1.10) | −0.06 (1.18) | −0.09 (1.02) | −0.12 (1.081) | 0.12 (1.10) | 0.05 b |
| Breakfast Frequency | p-Value (ANOVA) | ptrend | ||||
|---|---|---|---|---|---|---|
| 0 days/week (n = 76) | 1–3 days/week (n = 115) | 4–6 days/week (n = 196) | Daily (n = 408) | |||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| BMI (kg/m2) | 19.9 (5.7) | 20.9 (5.3) | 20.0 (4.4) | 19.2 (4.7) | 0.003 | 0.06 |
| WC (cm) | 70.1 (12.9) | 71.0 (13.4) | 69.0 (10.6) | 67.9 (11.6) | 0.06 | 0.91 |
| FBG (mmol/L) | 4.9 (0.4) | 4.9 (0.4) | 4.9 (0.4) | 4.9 (0.8) | 0.79 | 0.61 |
| TC (mmol/L) | 4.8 (1.0) | 4.6 (0.9) | 4.7 (0.8) | 4.6 (0.8) | 0.01 | 0.32 |
| HDL(mmol/L) | 1.5 (0.3) | 1.5 (0.3) | 1.4 (0.3) | 1.5 (0.3) | 0.56 | 0.38 |
| LDL (mmol/L) | 2.9 (0.9) | 2.8 (0.8) | 2.8 (0.7) | 2.7 (0.7) | 0.01 | 0.41 |
| SBP (mmHg) | 109 (13) | 111 (11) | 109 (11) | 109 (11) | 0.32 | 0.31 |
| DBP (mmHg) | 67 (13) | 68 (10) | 67 (10) | 68 (10) | 0.45 | 0.34 |
| TAG (mmol/L) | 0.98 (0.60) | 0.92 (0.45) | 0.99 (0.47) | 0.91 (0.46) | 0.24 | 0.23 |
| β (95% CI) | p-Value | |
|---|---|---|
| Body mass index (kg/m2) | ||
| Model 1 a | −0.21 (−0.35, −0.07) | 0.004 |
| Model 2 b | −0.20 (−0.34, −0.06) | 0.01 |
| Model 4 d | −0.20 (−0.34, −0.05) | 0.01 |
| Model 5 f | −0.18 (−0.33, −0.04) | 0.01 |
| Waist circumference (cm) | ||
| Model 1 a | −0.44 (−0.78, −0.10) | 0.01 |
| Model 2 b | −0.50 (−0.84, −0.16) | 0.004 |
| Model 3c | −0.06 (−0.21, 0.09) | 0.40 |
| Model 4 e | −0.07 (−0.22, 0.08) | 0.36 |
| Model 5 g | −0.06 (−0.21, 0.09) | 0.43 |
| Fasting glucose concentration (mmol/L) | ||
| Model 1 a | 0.00 (−0.02, 0.02) | 0.80 |
| Model 2 b | 0.00 (−0.02, 0.02) | 0.88 |
| Model 3 c | 0.00 (−0.02. 0.02) | 0.79 |
| Model 4 e | 0.00 (−0.02, 0.02) | 086 |
| Model 5 g | 0.00 (−0.02, 0.02) | 0.82 |
| Total cholesterol concentration (mmol/L) | ||
| Model 1 a | −0.04 (−0.06, −0.01) | 0.004 |
| Model 2 b | −0.04 (−0.06, −0.01) | 0.003 |
| Model 3 c | −0.04 (−0.06, −0.01) | 0.01 |
| Model 4 e | −0.03 (−0.06, −0.01) | 0.01 |
| Model 5 g | −0.03 (−0.06, −0.01) | 0.01 |
| High-density lipoprotein cholesterol concentration (mmol/L) | ||
| Model 1 a | 0.00 (−0.01, 0.01) | 0.68 |
| Model 2 b | 0.00 (−0.01, 0.01) | 0.66 |
| Model 3 c | 0.00 (−0.01, 0.01) | 0.57 |
| Model 4 e | 0.00 (−0.01, 0.01) | 0.80 |
| Model 5 g | 0.00 (−0.01, 0.01) | 0.82 |
| Low-density lipoprotein cholesterol concentration (mmol/L) | ||
| Model 1 a | −0.03 (−0.06, −0.01) | 0.002 |
| Model 2 b | −0.04 (−0.06, −0.01) | 0.001 |
| Model 3 c | −0.03 (−0.05, −0.01) | 0.03 |
| Model 4 e | −0.03 (−0.05, −0.01) | 0.01 |
| Model 5 g | −0.03 (−0.05, −0.01) | 0.01 |
| Systolic blood pressure (mmHg) | ||
| Model 1 a | −0.18 (−0.51, 0.15) | 0.28 |
| Model 2 b | −0.14 (−0.46, 0.18) | 0.389 |
| Model 3 c | 0.04 (−0.25, 0.34) | 0.78 |
| Model 4 e | 0.07 (−0.23, 0.37) | 0.65 |
| Model 5 g | 0.07 (−0.23, 0.37) | 0.65 |
| Diastolic blood pressure (mmHg) | ||
| Model 1 a | 0.11 (−0.19, 0.41) | 0.48 |
| Model 2 b | 0.12 (−0.18, 0.42) | 0.44 |
| Model 3 c | 0.26 (−0.03, 0.55) | 0.08 |
| Model 4 e | 0.27 (−0.03, 0.56) | 0.08 |
| Model 5 g | 0.27 (−0.02, 0.57) | 0.07 |
| Triacylglycerol concentration (mmol/L) | ||
| Model 1 a | −0.01 (−0.02, 0.01) | 0.26 |
| Model 2 b | −0.01 (−0.02, 0.01) | 0.34 |
| Model 3 c | 0.00 (−0.01, 0.01) | 0.92 |
| Model 4 e | 0.00 (−0.02, 0.01) | 0.82 |
| Model 5 g | 0.00 (−0.01, 0.01) | 0.90 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafa, N.; Abd Majid, H.; Toumpakari, Z.; Carroll, H.A.; Yazid Jalaludin, M.; Al Sadat, N.; Johnson, L. The Association of Breakfast Frequency and Cardiovascular Disease (CVD) Risk Factors among Adolescents in Malaysia. Nutrients 2019, 11, 973. https://doi.org/10.3390/nu11050973
Mustafa N, Abd Majid H, Toumpakari Z, Carroll HA, Yazid Jalaludin M, Al Sadat N, Johnson L. The Association of Breakfast Frequency and Cardiovascular Disease (CVD) Risk Factors among Adolescents in Malaysia. Nutrients. 2019; 11(5):973. https://doi.org/10.3390/nu11050973
Chicago/Turabian StyleMustafa, Norashikin, Hazreen Abd Majid, Zoi Toumpakari, Harriet Amy Carroll, Muhammad Yazid Jalaludin, Nabilla Al Sadat, and Laura Johnson. 2019. "The Association of Breakfast Frequency and Cardiovascular Disease (CVD) Risk Factors among Adolescents in Malaysia" Nutrients 11, no. 5: 973. https://doi.org/10.3390/nu11050973
APA StyleMustafa, N., Abd Majid, H., Toumpakari, Z., Carroll, H. A., Yazid Jalaludin, M., Al Sadat, N., & Johnson, L. (2019). The Association of Breakfast Frequency and Cardiovascular Disease (CVD) Risk Factors among Adolescents in Malaysia. Nutrients, 11(5), 973. https://doi.org/10.3390/nu11050973





