Hepatoprotective Potential of Partially Hydrolyzed Guar Gum against Acute Alcohol-Induced Liver Injury in Vitro and Vivo
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. PHGG Preparation and Structural Features
2.3. Cell Culture and Membrane Integrity Evaluation
2.4. Mitochondrial Membrane Potential Determination
2.5. Preparation of Mitochondria
2.6. Animals and Grouping
2.7. Serum Biochemical Analysis
2.8. Lipid Peroxidation and Antioxidant Enzyme Activities
2.9. RNA Isolation and Gene Expression Quantification
2.10. Western Blot Analysis
2.11. Histopathological and Apoptosis Analysis
2.12. Statistical Analysis
3. Results
3.1. Attenuation of Alcohol-Induced Cytotoxicity by PHGG in Vitro
3.2. Protection against Alcohol-Induced Liver Injury by PHGG in Vivo
3.3. Regulation on Lipid Metabolism by PHGG in Alcoholic Fatty Liver
3.4. Alleviation of TLR4 Mediated Inflammatory Responses by PHGG
3.5. Suppression of Alcohol-Induced Hepatocyte Apoptosis by PHGG
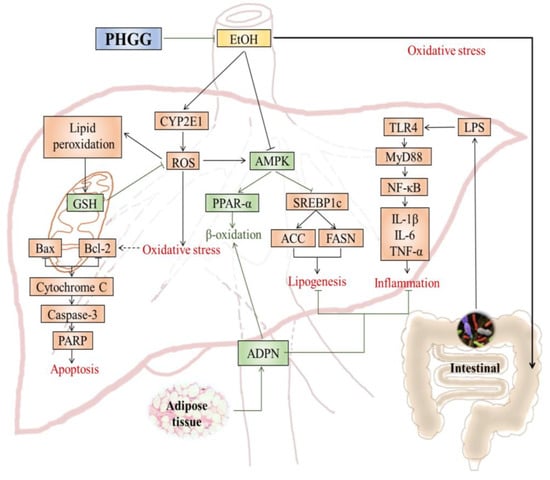
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Liu, Y.; Wang, J.; Li, L.; Hu, W.; Qu, Y.; Ding, Y.; Meng, L.; Teng, L.; Wang, D. Hepatoprotective effects of Antrodia cinnamomea: The modulation of oxidative stress signaling in a mouse model of alcohol-induced acute liver injury. Oxid. Med. Cell Longev. 2017, 7841823. [Google Scholar] [CrossRef]
- Stickel, F.; Datz, C.; Hampe, J.; Bataller, R. Pathophysiology and management of alcoholic liver disease: Update. Gut Liver 2017, 11, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Zakhari, S. Overview: How is alcohol metabolized by the body? Alcohol Res. 2006, 29, 245−254. [Google Scholar] [CrossRef]
- Bergheim, I.; Guo, L.; Davis, M.A.; Lambert, J.; Beier, J.; Duveau, I.; Luyendyk, J.; Roth, R.; Arteel, G. Metformin prevents alcohol-induced liver injury in the mouse: Critical role of plasminogen activator inhibitor-1. Gastroenterology 2006, 130, 2099–2112. [Google Scholar] [CrossRef] [PubMed]
- Sha, L.; Tan, H.Y.; Ning, W.; Zhang, Z.; Lao, L.; Wong, C.W.; Feng, Y. The role of oxidative stress and antioxidants in liver diseases. Int. J. Mol. Sci. 2015, 16, 26087–26124. [Google Scholar] [CrossRef]
- Kang, K.H.; Qian, Z.J.; Ryu, B.M.; Kim, D.; Kim, S.K. Protective effects of protein hydrolysate from marine microalgae Navicula incerta on ethanol-induced toxicity in HepG2/CYP2E1 cells. Food Chem. 2012, 132, 677–685. [Google Scholar] [CrossRef]
- Gaschler, M.M.; Stockwell, B.R. Lipid peroxidation in cell death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef]
- Jeon, S.M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2015, 48, 1–13. [Google Scholar] [CrossRef]
- Prieto, I.; Monsalve, M. ROS homeostasis, a key determinant in liver ischemic-preconditioning. Redox Biol. 2017, 12, 1020–1025. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jin, Q.; Zhang, Y.; Wu, Y.; Jin, C.; Cui, B.; Li, Y.; Jin, M.; Shang, Y.; Jiang, M.; et al. Inhibition of P2x7R-NLRP3 inflammasome activation by Pleurotus citrinopileatus: A possible protective role in alcoholic hepatosteatosis. J. Agric Food Chem. 2018, 66, 13183–13190. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Chen, H.; Li, Y. Protective effect of bicyclol on acute alcohol-induced liver injury in mice. Eur. J. Pharmacol. 2008, 586, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Abenavoli, L.; Capasso, R.; Milic, N.; Capasso, F. Milk thistle in liver diseases: Past, present, future. Phytother. Res. 2010, 24, 1423–1432. [Google Scholar] [CrossRef]
- Pan, S.Y.; Yang, R.; Dong, H.; Yu, Z.L.; Ko, K.M. Bifendate treatment attenuates hepatic steatosis in cholesterol/bile salt- and high-fat diet-induced hypercholesterolemia in mice. Eur. J. Pharmaco. 2006, 552, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.D.; Lee, S.R.; Kim, T.; Jang, S.; Kang, S.; Koo, H.J.; Sohn, E.; Bak, J.; Namkoong, S.; Kim, H.; Song, I.; et al. Fucoidan from Fucus vesiculosus protects against alcohol-induced liver damage by modulating inflammatory mediators in mice and HepG2 cells. Marine Drugs 2015, 13, 1051–1067. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Ma, H.; Tong, C.; Qu, M.; Jin, Q.; Li, W. Hepatoprotective effect of a polysaccharide from Crassostrea gigas on acute and chronic models of liver injury. Int. J. Biol. Macromol. 2015, 78, 142–148. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, P.; Jiang, C.; Ma, L.; Zhang, Z.; Zeng, X. Preliminary characterization, antioxidant activity in vitro and hepatoprotective effect on acute alcohol-induced liver injury in mice of polysaccharides from the peduncles of Hovenia dulcis. Food Chem. Toxicol. 2012, 50, 2964–2970. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.; Zhu, Y.; Liu, Y.X.; Tipoe, G.L.; Xing, F.Y.; So, K.F. Lycium barbarum polysaccharide attenuates alcoholic cellular injury through TXNIP-NLRP3 inflammasome pathway. Int. J. Biol. Macromol. 2014, 69, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.Y.; Hwang, H.J.; Nam, T.J. Protective effect of a polysaccharide from Hizikia fusiformis against ethanol-induced cytotoxicity in IEC-6 cells. Toxicol. Vitro 2010, 24, 79–84. [Google Scholar] [CrossRef]
- Yoon, S.J.; Chu, D.C.; Juneja, L.R. Chemical and physical properties, safety and application of partially hydrolized guar gum as dietary fiber. J. Clin. Biochem. Nutr. 2008, 42, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Slavin, J.L.; Greenberg, N.A. Partially hydrolyzed guar gum: Clinical nutrition uses. Nutrition 2003, 19, 549–552. [Google Scholar] [CrossRef]
- Li, Y.; Yi, P.; Wang, N.; Liu, J.; Liu, X.; Yan, Q.; Jiang, Z. High level expression of beta-mannanase (RmMan5A) in Pichia pastoris for partially hydrolyzed guar gum production. Int. J. Biol. Macromol. 2017, 105, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Ikegami, S.; Tsuchihashi, F.; Harada, H.; Tsuchihashi, N.; Nishide, E.; Innami, S. Effect of viscous indigestible polysaccharides on pancreatic-biliary secretion and digestive organs in rats. J. Nutr. 1990, 120, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Giannini, E.G.; Mansi, C.; Dulbecco, P.; Savarino, V. Role of partially hydrolyzed guar gum in the treatment of irritable bowel syndrome. Nutrition 2006, 22, 334–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, J.; Zhang, J.; Zhao, B.; Yao, J.; Wang, Y. Structure-antioxidant relationships of sulfated galactomannan from guar gum. Int. J. Biol. Macromol. 2010, 46, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Santas, J.; Espadaler, J.; Cune, J.; Rafecas, M. Partially hydrolyzed guar gums reduce dietary fatty acid and sterol absorption in guinea pigs independent of viscosity. Lipids 2012, 47, 697–705. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, D.; Barak, S.; Patel, A.; Shah, N. Partially hydrolyzed guar gum as a potential prebiotic source. Int. J. Biol. Macromol. 2018, 112, 207–210. [Google Scholar] [CrossRef]
- Carlson, J.; Gould, T.; Slavin, J. In vitro analysis of partially hydrolyzed guar gum fermentation on identified gut microbiota. Anaerobe 2016, 42, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Carlson, J.; Hospattankar, A.; Deng, P.; Swanson, K.; Slavin, J. Prebiotic effects and fermentation kinetics of wheat dextrin and partially hydrolyzed guar gum in an in vitro batch fermentation system. Foods 2015, 4, 349–358. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Y.; Huang, G. Preparation and antioxidant activities of important traditional plant polysaccharides. Int. J. Biol. Macromol. 2018, 111, 780–786. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, Q.; Wang, L.; Zhao, M.; Zhao, B. Protective effect of polysaccharide from maca (Lepidium meyenii) on HepG2 cells and alcoholic liver oxidative injury in mice. Int. J. Biol. Macromol. 2017, 99, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lan, Y.; Zhu, Y.; Li, S.; Liu, M.; Song, X.; Zhao, H.; Liu, W.; Zhang, J.; Wang, S.; et al. Hepatoprotective effects of Auricularia cornea var. Li. polysaccharides against the alcoholic liver diseases through different metabolic pathways. Sci. Rep. 2018, 8, 7574. [Google Scholar] [CrossRef]
- Song, X.; Shen, Q.; Liu, M.; Zhang, C.; Zhang, L.; Ren, Z.; Wang, W.; Dong, Y.; Wang, X.; Zhang, J.; et al. Antioxidant and hepatoprotective effects of intracellular mycelium polysaccharides from Pleurotus geesteranus against alcoholic liver diseases. Int. J. Biol. Macromol. 2018, 114, 979–988. [Google Scholar] [CrossRef]
- Yuan, R.; Tao, X.; Liang, S.; Pan, Y.; Li, H.; Sun, J.; Ju, W.; Li, X.; Chen, J.; Wang, C. Protective effect of acidic polysaccharide from Schisandra chinensis on acute ethanol-induced liver injury through reducing CYP2E1-dependent oxidative stress. Biomed. Pharmacother. 2018, 99, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Lim, Y.; Kim, J.; Heo, W.; Lee, K.; Shin, H.; Kim, J.; Lee, J.; Kim, Y. Root bark of Ulmus davidiana var. japonica restrains acute alcohol-induced hepatic steatosis onset in mice by inhibiting ROS accumulation. PLoS ONE 2017, 12, e0188381. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Scott, G.; Ren, J. Involvement of AMPK in alcohol dehydrogenase accentuated myocardial dysfunction following acute ethanol challenge in mice. PLoS ONE 2010, 5, E11268. [Google Scholar] [CrossRef]
- Wada, S.; Yamazaki, T.; Kawano, Y.; Miura, S.; Ezaki, O. Fish oil fed prior to ethanol administration prevents acute ethanol-induced fatty liver in mice. J. Hepatol. 2008, 49, 441–450. [Google Scholar] [CrossRef]
- Yao, Y.; Han, X.; Li, Z.; Lian, L.; Nan, J.; Wu, Y. Acanthoic acid can partially prevent alcohol exposure-induced liver lipid deposition and inflammation. Front. Pharmacol. 2017, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wu, X.; Chen, X.; Wu, J.; Su, Z.; Liang, J.; Li, Y.; Lai, X.; Chen, J.; Liu, Y. Patchouli oil isolated from the leaves of Pogostemon cablin ameliorates ethanol-induced acute liver injury in rats via inhibition of oxidative stress and lipid accumulation. RSC Adv. 2018, 8, 24399–24410. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Wang, Z.; Han, Y.; Tian, Y.; Zhang, G.; Sun, Y.; Wang, Y. Platycodin D isolated from the aerial parts of Platycodon grandiflorum protects alcohol-induced liver injury in mice. Food Funct. 2015, 6, 1418–1427. [Google Scholar] [CrossRef]
- Chen, W.; Xu, C.; Chen, S.; Xu, G.; Ye, H. Citric acid reduces the decline in P300 amplitude induced by acute alcohol consumption in healthy adults. J. Zhejiang Univ. Sci. B. 2012, 13, 395–401. [Google Scholar] [CrossRef]
- Seberg, S.; Andersen, E.S.; Dalgaard, N.B.; Jarlheft, I.; Hansen, N.L.; Hoffmann, N.; Vilsboll, T.; Chenchar, A.; Jensen, M.; Grevengoed, T.J.; et al. FGF21, a liver hormone that inhibits alcohol intake in mice, increases in human circulation after acute alcohol ingestion and sustained binge drinking at Oktoberfest. Mol. Metab. 2018, 11, 96–103. [Google Scholar] [CrossRef]
- Rivera, C.A.; Bradford, B.U.; Seabra, V.; Thurman, R.G. Role of endotoxin in the hypermetabolic state after acute ethanol exposure. Am. J. Physiol. 1998, 275, G1252–G1258. [Google Scholar] [CrossRef] [PubMed]
- Giridhar, K.; Sabine, W.; Marianne, L.; Eva, P.; Claus, H.; Stephan, C.B.; Ina, B. Effect of acute beer ingestion on the liver: Studies in female mice. Eur. J. Nutr. 2015, 3, 465–474. [Google Scholar] [CrossRef]
- Liu, S.; Tian, L.; Chai, G.; Wen, B.; Wang, B. Targeting heme oxygenase-1 by quercetin ameliorates alcohol-induced acute liver injury via inhibiting NLRP3 inflammasome activation. Food Funct. 2018, 9, 4184–4193. [Google Scholar] [CrossRef]
- Neyrinck, A.; Etxeberria, U.; Taminiau, B.; Daube, G.; Van, H.M.; Everard, A.; Cani, P.; Bindels, L.; Delzenne, N.M. Rhubarb extract prevents hepatic inflammation induced by acute alcohol intake, an effect related to the modulation of the gut microbiota. Mol. Nutr. Food Res. 2017, 61, 1500899. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Miyamoto, Y.; Mazagova, M.; Lee, K.; Eckmann, L.; Schnabl, B. Microbiota protects mice against acute alcohol-induced liver injury. Alcohol Clin. Exp. Res. 2015, 39, 2313–2323. [Google Scholar] [CrossRef] [PubMed]
- Katada, K.; Naito, Y.; Takagi, T.; Mizushima, K.; Higashimura, Y.; Okayama, T.; Yoshida, N.; Kamada, K.; Uchiyama, K.; Takeshi, I.; et al. Su1467 Partially hydrolyzed guar gum (PHGG) attenuates nonalcoholic steatohepatitis (NASH) in mice through the gut-liver axis. Gastroenterology 2014, 146, S477. [Google Scholar] [CrossRef]
- Isabelle, M.; Moreel, X.; Gagné, J.P.; Rouleau, M.; Ethier, C.; Gagné, P.; Hendzel, M.J.; Poirier, G.G. Investigation of PARP-1, PARP-2, and PARG interactomes by affinity-purification mass spectrometry. Proteome Sci. 2010, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Thomadaki, H.; Scorilas, A. Bcl-2 family of apoptosis-related genes: Functions and clinical implications in cancer. Crit. Rev. Clin. Lab. Sci. 2006, 43, 1–67. [Google Scholar] [CrossRef] [PubMed]





| Genes | Primer Sequences (5′−3′) |
|---|---|
| PPARα | Forward: CTGAGGAAGCCATTCTGCGACATC |
| Reverse: GCGTCTGACTCGGTCTTCTTGATG | |
| SREBP1 | Forward: AAGCAAATCACTGAAGGACCTGG |
| Reverse: AAAGACAAGGGGCTACTCTGGGAG | |
| TLR-4 | Forward: CTGTATTCCCTCAGCACTCTTGATT |
| Reverse: TGCTTCTGTTCCTTGACCCACT | |
| MyD88 | Forward: ATGGTGGTGGTTGTTTCTGACG |
| Reverse: GTCGCATATAGTGATGAACCGCA | |
| IκBα | Forward: AATCCTGACCTGGTTTCGCTCTT |
| Reverse: ATCCTCGCTCTCGGGTAGCAT | |
| NOS2 | Forward: GGAGCGAGTTGTGGATTG |
| Reverse: CCAGGCAGTAGGTGAGGG | |
| GAPDH | Forward: TGGAGAAACCTGCCAAGTATGA |
| Reverse: TGGAAGAATGGGAGTTGCTGT |
| Group | SOD (U/mg protein) | CAT (U/mg protein) | GSH-Px (U/mg protein) | MDA (nmol/mg protein) |
|---|---|---|---|---|
| Control | 245.8 ± 15.5 | 34.0 ± 3.0 | 269.9 ± 10.1 | 1.56 ± 0.18 |
| Alcohol | 154.2 ±10.1 ## | 21.4 ± 1.8 ## | 143.8 ± 10.1 ## | 6.64 ± 2.56 ## |
| Bifendate + Alcohol | 194.4 ± 11.1 ** | 27.6 ± 2.6 * | 215.7 ± 17.4 ** | 2.63 ± 1.11 ** |
| PHGG-L + Alcohol | 163.1 ± 14.3 | 27.7 ± 2.3 * | 186.2 ± 11.0 ** | 4.43 ± 2.60 |
| PHGG-M + Alcohol | 198.8 ± 10.2 ** | 34.7 ± 1.9 ** | 236.1 ± 5.8 ** | 1.57 ± 0.50 ** |
| PHGG-H + Alcohol | 225.6 ± 10.4 ** | 43.9 ± 2.9 ** | 336.7 ± 17.3 ** | 2.26 |
| Group | TC (mmol/L) | TG (mmol/L) | LDL-C (mmol/L) | FFA (mmol/L) | ADPN (mg/L) |
|---|---|---|---|---|---|
| Control | 2.62 ± 0.42 | 2.05 ± 0.04 | 0.63 ± 0.10 | 0.27 ± 0.01 | 13.6 ± 2.2 |
| Alcohol | 4.01 ± 0.24 ## | 2.37 ± 0.13 ## | 0.95 ± 0.04 ## | 0.48 ± 0.05 ## | 6.99 ± 0.86 ## |
| Bifendate + Alcohol | 2.60 ± 0.27 ** | 2.03 ± 0.13 ** | 0.60 ± 0.06 ** | 0.31 ± 0.05 ** | 11.3 ± 1.4 ** |
| PHGG-L + Alcohol | 2.78 ± 0.43 ** | 2.10 ± 0.04 ** | 0.72 ± 0.08 ** | 0.37 ± 0.06 ** | 8.75 ± 0.86 |
| PHGG-M + Alcohol | 2.69 ± 0.33 ** | 2.11 ± 0.07 ** | 0.67 ± 0.08 ** | 0.31 ± 0.05 ** | 10.6 ± 1.4 ** |
| PHGG-H + Alcohol | 2.53 ± 0.21 ** | 2.09 ± 0.08 ** | 0.69 ± 0.04 ** | 0.27 ± 0.03 ** | 9.15 ± 1.20 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Liu, J.; Tang, Y.; Li, Y.; Yan, Q.; Jiang, Z. Hepatoprotective Potential of Partially Hydrolyzed Guar Gum against Acute Alcohol-Induced Liver Injury in Vitro and Vivo. Nutrients 2019, 11, 963. https://doi.org/10.3390/nu11050963
Wu C, Liu J, Tang Y, Li Y, Yan Q, Jiang Z. Hepatoprotective Potential of Partially Hydrolyzed Guar Gum against Acute Alcohol-Induced Liver Injury in Vitro and Vivo. Nutrients. 2019; 11(5):963. https://doi.org/10.3390/nu11050963
Chicago/Turabian StyleWu, Chenxuan, Jun Liu, Yanbin Tang, Yanxiao Li, Qiaojuan Yan, and Zhengqiang Jiang. 2019. "Hepatoprotective Potential of Partially Hydrolyzed Guar Gum against Acute Alcohol-Induced Liver Injury in Vitro and Vivo" Nutrients 11, no. 5: 963. https://doi.org/10.3390/nu11050963
APA StyleWu, C., Liu, J., Tang, Y., Li, Y., Yan, Q., & Jiang, Z. (2019). Hepatoprotective Potential of Partially Hydrolyzed Guar Gum against Acute Alcohol-Induced Liver Injury in Vitro and Vivo. Nutrients, 11(5), 963. https://doi.org/10.3390/nu11050963