Can Resveratrol Treatment Control the Progression of Induced Periodontal Disease? A Systematic Review and Meta-Analysis of Preclinical Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Focused Question
2.2. Eligibility Criteria
2.2.1. Type of Studies
2.2.2. Research Strategy
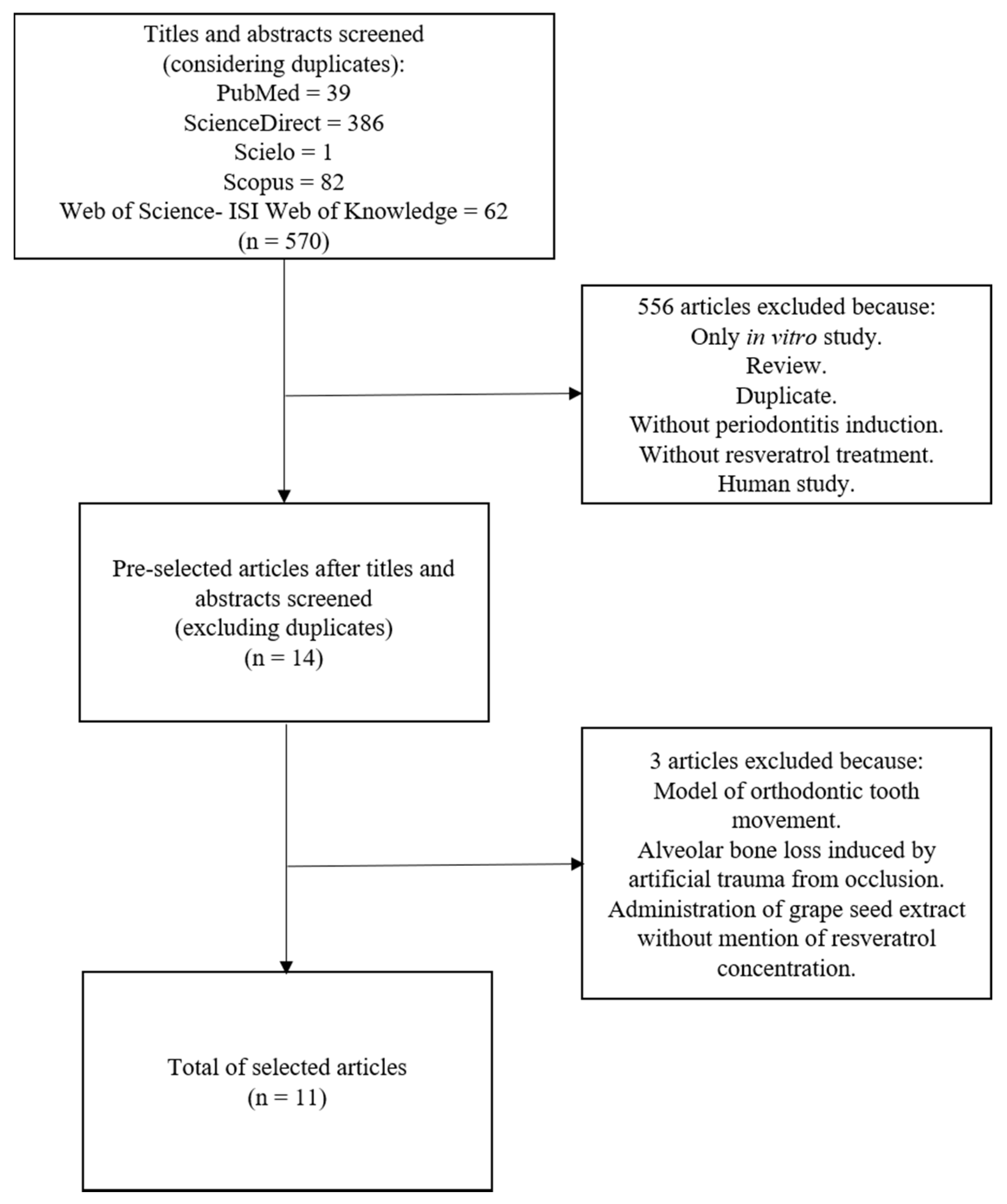
2.3. Screening Methods and Selection of Studies
2.4. Quality Criteria Assessment
2.5. Bias Risk Assessment
2.6. Statistical Analysis
3. Results
3.1. Risk of Bias in Studies
3.2. Quality Assessment of Selected Publications
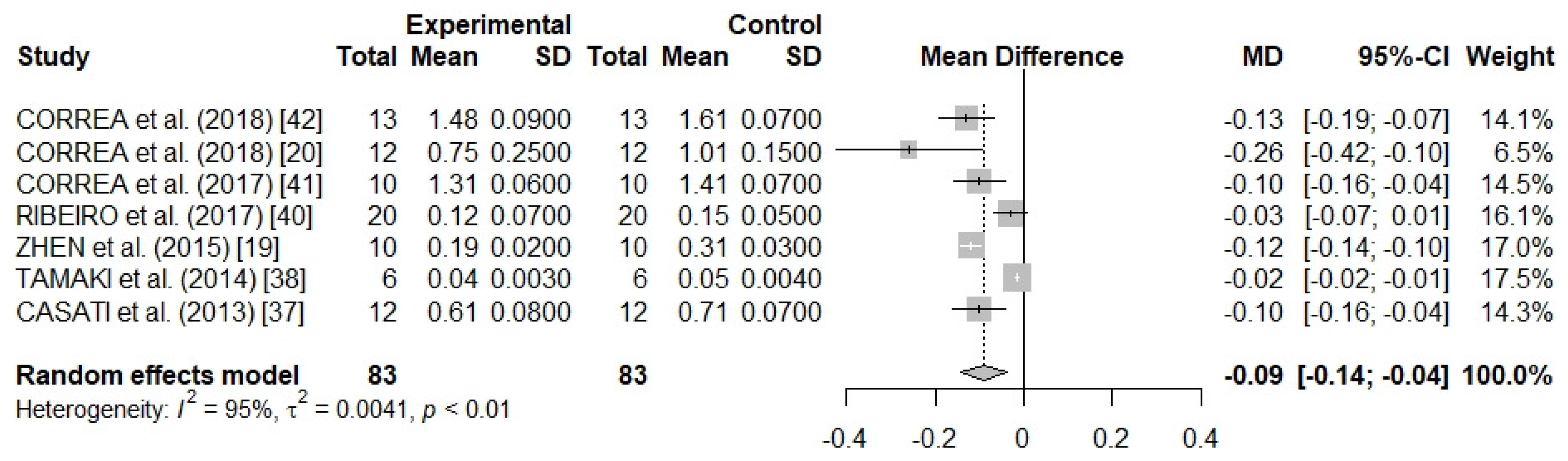
3.3. Meta-Analysis Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pastor, R.F.; Restani, P.; Di Lorenzo, C.; Orgiu, F.; Teissedre, P.-L.; Stockley, C.; Ruf, J.C.; Quini, C.I.; Garcìa Tejedor, N.; Gargantini, R.; et al. Resveratrol, human health and winemaking perspectives. Crit. Rev. Food Sci. Nutr. 2017, 5, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sales, J.M.; Resurreccion, A.V.A. Resveratrol in Peanuts. Crit. Rev. Food Sci. Nutr. 2014, 54, 734–770. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: the in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef]
- Catalgol, B.; Batirel, S.; Taga, Y.; Ozer, N.K. Resveratrol: French Paradox Revisited. Front. Pharmacol. 2012, 3, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Biagi, M.; Bertelli, A.A.E. Wine, alcohol and pills: What future for the French paradox? Life Sci. 2015, 131, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Weiskirchen, S.; Weiskirchen, R. Resveratrol: How Much Wine Do You Have to Drink to Stay Healthy? Adv. Nutr. An Int. Rev. J. 2016, 7, 706–718. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, J.; Girnary, M.S.; Jing, L.; Miao, M.Z.; Zhang, S.; Sun, L.; Morelli, T.; Schoenfisch, M.H.; Inohara, N.; Offenbacher, S.; et al. An experimental murine model to study periodontitis. Nat. Protoc. 2018, 13, 2247–2267. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.-T.; Cheng, G.-Y.; Shih, Y.-J.; Lin, C.-Y.; Lin, S.-J.; Lai, H.-Y.; Whang-Peng, J.; Chiu, H.-C.; Lee, S.-Y.; Fu, E.; et al. Therapeutic applications of resveratrol and its derivatives on periodontitis. Ann. N. Y. Acad. Sci. 2017, 1403, 101–108. [Google Scholar] [CrossRef]
- Cekici, A.; Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Inflammatory and immune pathways in the pathogenesis of periodontal disease. Periodontol. 2000 2014, 64, 57–80. [Google Scholar] [CrossRef]
- Casanova, L.; Hughes, F.J.; Preshaw, P.M. Diabetes and periodontal disease: A two-way relationship. Br. Dent. J. 2014, 217, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Winning, L.; Linden, G.J. Periodontitis and systemic disease. BDJ Team 2015, 2, 15163. [Google Scholar] [CrossRef]
- Borgnakke, W.S.; Ylöstalo, P.V.; Taylor, G.W.; Genco, R.J. Effect of periodontal disease on diabetes: Systematic review of epidemiologic observational evidence. J. Periodontol. 2013, 84, S135–S152. [Google Scholar] [CrossRef] [PubMed]
- Eke, P.I.; Lu, H.; Zhang, X.; Thornton-Evans, G.; Borgnakke, W.S.; Holt, J.B.; Croft, J.B. Geospatial distribution of periodontists and US adults with severe periodontitis. J. Am. Dent. Assoc. 2019, 150, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Smith, A.G.C.; Bernabé, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.J.L.; Marcenes, W.; GBD 2015 Oral Health Collaborators. Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J. Dent. Res. 2017, 96, 380–387. [Google Scholar] [CrossRef]
- Dumitrescu, A.L. Editorial: Periodontal Disease—A Public Health Problem. Front. Public. Heal. 2015, 3, e278. [Google Scholar] [CrossRef]
- Genco, R.J.; Borgnakke, W.S. Risk factors for periodontal disease. Periodontol. 2000 2013, 62, 59–94. [Google Scholar] [CrossRef]
- Jiang, H.; Xiong, X.; Su, Y.; Zhang, Y.; Wu, H.; Jiang, Z.; Qian, X. A randomized controlled trial of pre-conception treatment for periodontal disease to improve periodontal status during pregnancy and birth outcomes. BMC Pregnancy Childbirth 2013, 13, 1–7. [Google Scholar] [CrossRef]
- Al-Zahrani, M.S.; Borawski, E.A.; Bissada, N.F. Periodontitis and three health-enhancing behaviors: maintaining normal weight, engaging in recommended level of exercise, and consuming a high-quality diet. J. Periodontol. 2005, 76, 1362–1366. [Google Scholar] [CrossRef]
- Zhen, L.; Fan, D.; Zhang, Y.; Cao, X.; Wang, L. Resveratrol ameliorates experimental periodontitis in diabetic mice through negative regulation of TLR4 signaling. Acta Pharmacol. Sin. 2015, 36, 221–228. [Google Scholar] [CrossRef]
- Corrêa, M.G.; Absy, S.; Tenenbaum, H.; Ribeiro, F.V.; Cirano, F.R.; Casati, M.Z.; Pimentel, S.P. Resveratrol attenuates oxidative stress during experimental periodontitis in rats exposed to cigarette smoke inhalation. J. Periodontal Res. 2018. [Google Scholar] [CrossRef]
- Bhattarai, G.; Poudel, S.B.; Kook, S.-H.; Lee, J.-C. Resveratrol prevents alveolar bone loss in an experimental rat model of periodontitis. Acta Biomater. 2016, 29, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.S.; Kunz, R.; Kleijnen, J.; Antes, G. Five steps to conducting a systematic review. J. R. Soc. Med. 2003, 96, 118–121. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, T.P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- de Vries, R.B.M.; Hooijmans, C.R.; Langendam, M.W.; van Luijk, J.; Leenaars, M.; Ritskes-Hoitinga, M.; Wever, K.E. A protocol format for the preparation, registration and publication of systematic reviews of animal intervention studies. Evidence-based Preclin. Med. 2015, 2, e00007. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef]
- Javed, F.; Kellesarian, S.V.; Abduljabbar, T.; Abduljabbar, A.T.; Akram, Z.; Vohra, F.; Rahman, I.; Romanos, G.E. Influence of involuntary cigarette smoke inhalation on osseointegration: A systematic review and meta-analysis of preclinical studies. Int. J. Oral Maxillofac. Surg. 2018, 47, 764–772. [Google Scholar] [CrossRef]
- Delgado-Ruiz, R.A.; Calvo-Guirado, J.L.; Romanos, G.E. Critical size defects for bone regeneration experiments in rabbit calvariae: Systematic review and quality evaluation using ARRIVE guidelines. Clin. Oral Implants Res. 2015, 26, 915–930. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, e43. [Google Scholar] [CrossRef]
- Schwarzer, G. meta: An R Package for Meta-Analysis. R News 2007, 7, 40–45. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Sterne, J.A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef]
- Chae, H.S.; Park, H.-J.; Hwang, H.R.; Kwon, A.; Lim, W.-H.; Yi, W.J.; Han, D.-H.; Kim, Y.H.; Baek, J.-H. The effect of antioxidants on the production of pro-inflammatory cytokines and orthodontic tooth movement. Mol. Cells 2011, 32, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, Y.; Minagawa, T.; Okui, T.; Yamazaki, K. Resveratrol suppresses the alveolar bone resorption induced by artificial trauma from occlusion in mice. Oral Dis. 2018, 24, 412–421. [Google Scholar] [CrossRef]
- Kara, M.; Kesim, S.; Aral, C.A.; Elmalı, F. Effect of Grape Seed Extract Upon Plasma Oxidative Status and Alveolar Bone, in Ligature Induced Periodontitis. Biotechnol. Biotechnol. Equip. 2013, 27, 4131–4136. [Google Scholar] [CrossRef][Green Version]
- Zare Javid, A.; Hormoznejad, R.; Yousefimanesh, H.A.; Zakerkish, M.; Haghighi-zadeh, M.H.; Dehghan, P.; Ravanbakhsh, M. The Impact of Resveratrol Supplementation on Blood Glucose, Insulin, Insulin Resistance, Triglyceride, and Periodontal Markers in Type 2 Diabetic Patients with Chronic Periodontitis. Phyther. Res. 2017, 31, 108–114. [Google Scholar] [CrossRef]
- Casati, M.Z.; Algayer, C.; Cardoso da Cruz, G.; Ribeiro, F.V.; Casarin, R.C.V.; Pimentel, S.P.; Cirano, F.R. Resveratrol Decreases Periodontal Breakdown and Modulates Local Levels of Cytokines During Periodontitis in Rats. J. Periodontol. 2013, 84, e58–e64. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, N.; Cristina Orihuela-Campos, R.; Inagaki, Y.; Fukui, M.; Nagata, T.; Ito, H.-O. Resveratrol improves oxidative stress and prevents the progression of periodontitis via the activation of the Sirt1/AMPK and the Nrf2/antioxidant defense pathways in a rat periodontitis model. Free Radic. Biol. Med. 2014, 75, 222–229. [Google Scholar] [CrossRef]
- Cirano, F.R.; Casarin, R.C.V.; Ribeiro, F.V.; Casati, M.Z.; Pimentel, S.P.; Taiete, T.; Bernardi, M.M. Effect of Resveratrol on periodontal pathogens during experimental periodontitis in rats. Braz. Oral Res. 2016, 30, e128. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, F.V.; Pino, D.S.; Franck, F.C.; Benatti, B.B.; Tenenbaum, H.; Davies, J.E.; Pimentel, S.P.; Casarin, R.C.; Cirano, F.R.; Casati, M.Z. Resveratrol Inhibits Periodontitis-Related Bone Loss in Rats Subjected to Cigarette Smoke Inhalation. J. Periodontol. 2017, 88, 788–798. [Google Scholar] [CrossRef]
- Corrêa, M.G.; Pires, P.R.; Ribeiro, F.V.; Pimentel, S.Z.; Casarin, R.C.V.; Cirano, F.R.; Tenenbaum, H.T.; Casati, M.Z. Systemic treatment with resveratrol and/or curcumin reduces the progression of experimental periodontitis in rats. J. Periodontal Res. 2017, 52, 201–209. [Google Scholar] [CrossRef]
- Corrêa, M.G.; Pires, P.R.; Ribeiro, F.V.; Pimentel, S.P.; Cirano, F.R.; Napimoga, M.H.; Casati, M.Z.; Casarin, R.C.V. Systemic treatment with resveratrol reduces the progression of experimental periodontitis and arthritis in rats. PLoS One 2018, 13, e0204414. [Google Scholar] [CrossRef] [PubMed]
- Chin, Y.-T.; Hsieh, M.-T.; Lin, C.-Y.; Kuo, P.-J.; Yang, Y.-C.S.H.; Shih, Y.-J.; Lai, H.-Y.; Cheng, G.-Y.; Tang, H.-Y.; Lee, C.-C.; et al. 2,3,5,4’-Tetrahydroxystilbene-2-O-β-glucoside Isolated from Polygoni Multiflori Ameliorates the Development of Periodontitis. Mediators Inflamm. 2016, 2016, 6953459. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, E.; Ikeda, Y.; Wang, Y.; Fine, N.; Sheikh, Z.; Viniegra, A.; Barzilay, O.; Ganss, B.; Tenenbaum, H.C.; Glogauer, M. Resveratrol derivative-rich melinjo seed extract induces healing in a murine model of established periodontitis. J. Periodontol. 2018, 89, 586–595. [Google Scholar] [CrossRef]
- Mokni, M.; Elkahoui, S.; Limam, F.; Amri, M.; Aouani, E. Effect of Resveratrol on Antioxidant Enzyme Activities in the Brain of Healthy Rat. Neurochem. Res. 2007, 32, 981–987. [Google Scholar] [CrossRef]
- Cochran, D.L. Inflammation and Bone Loss in Periodontal Disease. J. Periodontol. 2008, 79, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Mo, L.; Niu, Y.; Li, X.; Zhou, X.; Xu, X. The Role of Reactive Oxygen Species and Autophagy in Periodontitis and Their Potential Linkage. Front. Physiol. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Walle, T.; Hsieh, F.; DeLegge, M.H.; Oatis, J.E.; Walle, U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab Dispos. 2004, 32, 1377–1382. [Google Scholar] [CrossRef] [PubMed]
- Ilango, P.; Mahalingam, A.; Parthasarathy, H.; Katamreddy, V.; Subbareddy, V. Evaluation of TLR2 and 4 in Chronic Periodontitis. J. Clin. Diagn. Res. 2016, 10, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Dessaune Neto, N.; Porpino, M.T.M.; Antunes, H.d.S.; Rodrigues, R.C.V.; Perez, A.R.; Pires, F.R.; Siqueira, J.F., Jr.; Armada, L.; Dessaune Neto, N.; Porpino, M.T.M.; et al. Pro-inflammatory and anti-inflammatory cytokine expression in post-treatment apical periodontitis. J. Appl. Oral Sci. 2018, 26, e20170455. [Google Scholar] [CrossRef]
- Oz, H.S.; Puleo, D.A. Animal Models for Periodontal Disease. J. Biomed. Biotechnol. 2011, 2011, 1–8. [Google Scholar] [CrossRef]
- Theodoro, L.H.; Pires, J.R.; Fernandes, L.A.; Gualberto Júnior, E.C.; Longo, M.; de Almeida, J.M.; Garcia, V.G. Effect of antimicrobial photodynamic therapy on periodontally infected tooth sockets in rats. Lasers Med. Sci. 2015, 30, 677–683. [Google Scholar] [CrossRef]
- Rovin, S.; Costich, E.R.; Gordon, H.A. The influence of bacteria and irritation in the initiation of periodontal disease in germfree and conventional rats. J. Periodontal Res. 1966, 1, 193–204. [Google Scholar] [CrossRef]
- Arora, H.; Ivanovski, S. Melatonin as a pro-osteogenic agent in oral implantology: a systematic review of histomorphometric outcomes in animals and quality evaluation using ARRIVE guidelines. J. Periodontal Res. 2017, 52, 151–161. [Google Scholar] [CrossRef]
- Dereka, X.; Calciolari, E.; Donos, N.; Mardas, N. Osseointegration in osteoporotic-like condition: A systematic review of preclinical studies. J. Periodontal Res. 2018, 53, 933–940. [Google Scholar] [CrossRef]
- Osorio Parra, M.M.; Elangovan, S.; Lee, C.-T. Specialized pro-resolving lipid mediators in experimental periodontitis: A systematic review. Oral Dis. 2018. [Google Scholar] [CrossRef]
- Ma, B.; Xu, J.; Wu, W.; Liu, H.; Kou, C.; Liu, N.; Zhao, L. Survey of basic medical researchers on the awareness of animal experimental designs and reporting standards in China. PLoS One 2017, 12, e0174530. [Google Scholar] [CrossRef]
| Author and Year of Publication | Animal Model (Age at Beginning of Study) | Groups (n/Group) | Characteristics of Periodontitis Induction Protocol | Time of PD Induction | Ligature Permanence | Route of Resveratrol Administration | Resveratrol Dose | Main Outcomes |
|---|---|---|---|---|---|---|---|---|
| CORRÊA et al. (2018) [42] | Male Wistar rats (NC) | 1: PD + smoke inhalation + placebo (13) 2: PD + smoke inhalation + resveratrol (13) | Cotton ligature around both mandibular first and second maxillar molars. | 26 days after beginning experiment. | 11 days | Gavage | 10 mg/kg of body weight 30 days (entire experiment). | Resveratrol reduced both ABL and gingival NADPH oxidase as well increased tissue levels of SIRT1 and SOD1. |
| CORRÊA et al. (2018) [20] | Male Wistar rats (NC) | 1: PD + experimental arthritis + placebo (12). 2: PD + experimental arthritis + ibuprofen (12). 3: PD + experimental arthritis + resveratrol (12). | Cotton ligature around mandibular first molars. | At beginning of experiment | 30 days | Gavage | 10 mg/kg of body weight during 30 days (entire experiment). | Resveratrol treatment decreased ABL and increased serum IL-4 levels. |
| IKEDA et al. (2018) [44] | Male C57BL/6J wild-type mice (6–7 weeks old) | 1: PD + placebo (NC) 2: PD treatment + placebo (NC) 3: PD + resveratrol (NC) 4: PD treatment + resveratrol (NC) | Silk suture around the gingival sulcus of the maxillar second molar. In PD treatment group ligature was removed 15 days before finishing the experiment. | At beginning of experiment | 15 days in PD treatment group. In the other groups ligature remained 17, 20, and 22 days. | Intraperitoneal | Single dose 0.001% (w/w) of body weight on day 14. | Resveratrol decreased ABL, IL-1β, and oxidative stress Production of osteoclasts was inhibited by resveratrol. |
| CORRÊA et al. (2017) [41] | Male Wistar Rats (10 weeks old) | 1: PD + placebo (10). 2: PD + resveratrol (10). 3: PD + curcumin (10). 4: PD + resveratrol + curcumin (10). | Cotton ligature around mandibular first molars. | At beginning of experiment | 30 days | Gavage | 10 mg/kg of body weight during 30 days (entire experiment). | Resveratrol decreased ABL and gingival IFN-γ. Resveratrol increased gingival IL-4. |
| RIBEIRO et al. (2017) [40] | Male Wistar rats (10 weeks old) | 1: Control + placebo (20) 2: PD + smoke inhalation + placebo (20) 3: PD + smoke inhalation + resveratrol (20) | Cotton ligature around both first mandibular molar and second maxillar molar. | 19 days after beginning experiment | 11 days | Gavage | 10 mg/kg of body weight during 30 days (entire experiment). | Resveratrol reduced linear ABL and increased interradicular bone density. It also reduced expression of RANKL and Th17/Th2 levels whereas increased serum levels of IL-4. |
| BHATTARAI et al. (2016) [21] | Male Sprague–DawleyRats (NC) | 1: Sham (5) 2: Control + DMSO (5) 3: PD + DMSO (5) 4: PD + Resveratrol (5) | Elastic ligature between first and second maxillary molars and received 20 µl of 1 mg/mL LPS three times/week into the palatal gingivae | At beginning of experiment | 14 days | Subcutaneous | 5 mg/kg of body weight during 14 days (entire experiment). | Resveratrol attenuated ABL soft tissue damage and inhibited osteoclast formation. It also reduced COX-2, MMP-2, and MMP-9 levels. Resveratrol increased bone mineral density and SOD activity. |
| CHIN et al. (2016) [43] | Male Sprague–Dawley rats (8 weeks old) | 1: control (10) 2: PD (10) 3: PD + 0.1 mg/kg/day of THSG (5) 4: PD + 10 mg/kg/day of THSG (5) 5: PD + resveratrol (5) 6: PD + 12.5 mg/kg/day of P. multiflora ethanol extracts (5) 7: PD + 25 mg/kg/day of P. multiflora ethanol extracts (5) 8: PD + 50 mg/kg/d of P. multiflora ethanol extracts (5) | Silk sutures around mandibular first molars | At beginning of experiment | 8 days | Gavage | 25mg/kg of body weight during 7 days. | Resveratrol treatment did not alter significantly (p = 0.054) periodontal bone-supporting ratio. |
| CIRANO et al. (2016) [39] | Male Wistar rats (10 weeks old) | 1: Control (PD) + placebo (12) 2: PD + resveratrol (12) | Cotton ligature around first mandibular molar. | 19 days after beginning experiment | 11 days | Gavage | 10 mg/kg of body weight during 30 days (entire experiment). | Resveratrol treatment did not alter concentrations of Aggregatibacter Actinomycetemcomitans, Porphyromonas gingivalis, and Tannerella forsythia in rats’ ligatures. |
| ZHEN et al. (2015) [19] | C57BLKS/J-db/db male mice (6–8 weeks old) | 1: Untreated control (10) 2: PD + placebo (10) 3: PD + resveratrol (10) | Cotton ligature presoaked in a medium containing Porphyromonas gingivalis (108/mL) around maxillar first molars. | At beginning of experiment | 28 days | Gavage | 20 mg/kg of body weight during 28 days (entire experiment). | Resveratrol decreased ABL and decreased IL-1β, IL-6, IL-8, and TNF-α levels. The expression downstream signaling activation of TLR4 was attenuated. |
| TAMAKI et al. (2014) [38] | Male Wistar Rats (8 weeks old) | 1: Control + water (6) 2: PD + water (6) 3: PD + resveratrol (6) | Ligature of thread placed around the right second molar of maxilla. | At beginning of experiment | 20 days | Oral | 10 mg/kg of body weight of melinjo resveratrol during 20 days (entire experiment). | Resveratrol ABL and activated the Sirt1/AMPK and the Nrf2/antioxidant defense pathways in inflamed gingival tissues. Resveratrol inhibits the NF-κB/MAPK pathway and lowered both serum IL-6 and TNF-α. |
| CASATI et al. (2013) [37] | Male Wistar Rats (10 weeks old) | 1: PD + placebo (12) 2: PD + resveratrol (12) | Cotton ligature around mandibular first molar. | 19 days after beginning experiment | 11 days | Gavage | 10 mg/Kg of body weight during 30 days (entire experiment). | Lower ABL and lower levels of IL-1β and IL-17 in resveratrol treated group. |
| Study | Strain | Resveratrol Dose | ABL Evaluation Method | ABL | % Reduction | p Value | |
|---|---|---|---|---|---|---|---|
| Ligated with Resveratrol | Ligated without Resveratrol | ||||||
| CORRÊA et al. (2018) [42] # | Wistar rats | 10 mg/kg | Measurement of cementoenamel junction distance in methylene blue stain specimens | 1.48 mm | 1.61 mm | 8.07% | 0.0001 |
| CORRÊA et al. (2018) [20] ¥ | Wistar rats | 10 mg/kg | Measurement of cementoenamel junction distance in methylene blue stain specimens | 0.75 mm * | 1.01 mm * | 25.45% | <0.05 |
| IKEDA et al. (2018) [44] | C57BL/6J wild-type mice | Single dose (intraperitoneal) | Measurement of cementoenamel junction distance in methylene blue stain specimens | 65.00 µm * | 165.00 µm * | 60.60% | <0.01 |
| CORRÊA et al. (2017) [41] | Wistar rats | 10 mg/kg | Measurement of cementoenamel junction distance in methylene blue stain specimens | 1.31 mm | 1.41 mm | 7.09% | <0.05 |
| RIBEIRO et al. (2017) [40] ¥ | Wistar rats | 10 mg/kg | Measurement of cementoenamel junction distance in methylene blue stain specimens | 0.12 mm * | 0.15 mm * | 20.00% | <0.05 |
| BHATTARAI et al. (2016) [21] | Sprague–Dawley rats | 5 mg/kg (subcutaneous) | Measurement of cementoenamel junction distance in hematoxylin and eosin stained slices. | 55.00 µm * | 130.00 µm * | 57.69% | <0.05 |
| Measurement of bone mineral density in Micro CT specimens. | 0.24 g/cm3 * | 0.29 g/cm3 * | 17.24% | ||||
| CHIN et al. (2016) [43] | Sprague–Dawley rats | 25 mg/kg | Measurement of loss of periodontal bone-supporting ratio along the distal root surface junction in radiographic images of mandibles. | 60.00% * | 70.00%* | 10.00% | <0.05 |
| CIRANO et al. (2016) [39] | Wistar Rats | 10 mg/kg | Don’t evaluated bone loss | - | - | - | - |
| ZHEN et al. (2015) [19] | C57BLKS/J-db/db mice | 20 mg/kg | Measurement of cementoenamel junction distance in methylene blue stain specimens | 0.19 mm * | 0.31 mm * | −38.70% | <0.05 |
| TAMAKI et al. (2014) [38] | Wistar rats | 10 mg/kg | Measurement of distance from the cementoenamel junction to the alveolar bone crest in Micro CT specimens. | 0.038 mm * | 0.054 mm * | −29.63% | <0.001 |
| CASATI et al. (2013) [37] | Wistar rats | 10 mg/kg | Measurement of cementoenamel junction distance in methylene blue stain specimens. | 0.61 mm * | 0.71 mm * | −14.08% | <0.05 |
| STUDIES | A | B | C | D | E | F | G | H | I | J |
|---|---|---|---|---|---|---|---|---|---|---|
| CORRÊA et al. (2018) [42] | - | + | - | - | ? | ? | + | + | + | + |
| CORRÊA et al. (2018) [20] | + | + | - | ? | ? | ? | + | ? | + | + |
| IKEDA et al. (2018) [44] | + | ? | - | ? | - | - | - | ? | + | + |
| CORRÊA et al. (2017) [41] | + | + | - | ? | ? | ? | + | + | + | + |
| RIBEIRO et al. (2017) [40] | - | + | - | - | - | - | - | ? | + | ? |
| BHATTARAI et al. (2016) [21] | + | + | - | ? | - | - | - | + | + | + |
| CHIN et al. (2016) [43] | - | - | - | ? | - | - | - | ? | ? | ? |
| CIRANO et al. (2016) [39] | - | + | - | - | + | ? | + | ? | + | + |
| ZHEN et al. (2015) [19] | + | + | - | ? | - | - | - | ? | + | + |
| TAMAKI et al. (2014) [38] | + | + | - | ? | - | - | - | + | + | + |
| CASATI et al. (2013) [37] | + | + | - | ? | ? | ? | + | + | + | + |
| Studies | ARRIVE Items | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | K | L | M | N | O | P | Q | R | S | T | Total | |
| CORRÊA et al. (2018) [42] | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 32 |
| CORRÊA et al. (2018) [20] | 1 | 2 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 2 | 0 | 30 |
| IKEDA et al. (2018) [44] | 1 | 1 | 1 | 0 | 2 | 1 | 1 | 2 | 0 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 1 | 1 | 2 | 1 | 26 |
| CORRÊA et al. (2017) [41] | 1 | 2 | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 31 |
| RIBEIRO et al. (2017) [40] | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 2 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 29 |
| BHATTARAI et al. (2016) [21] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 29 |
| CHIN et al. (2016) [43] | 0 | 1 | 1 | 0 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 2 | 18 |
| CIRANO et al. (2016) [39] | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 1 | 0 | 1 | 2 | 2 | 2 | 29 |
| ZHEN et al. (2015) [19] | 1 | 2 | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 1 | 1 | 2 | 2 | 31 |
| TAMAKI et al. (2014) [38] | 1 | 2 | 1 | 1 | 2 | 1 | 1 | 2 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 1 | 1 | 2 | 2 | 31 |
| CASATI et al. (2013) [37] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 2 | 2 | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 28 |
| Category Score (Quality Obtained) | 10 | 15 | 10 | 9 | 20 | 15 | 14 | 20 | 15 | 12 | 10 | 20 | 20 | 10 | 15 | 18 | 16 | 11 | 20 | 17 | - |
| Maximum Score Expected (Quality Expected) | 11 | 22 | 22 | 11 | 22 | 22 | 22 | 22 | 22 | 22 | 11 | 22 | 22 | 11 | 22 | 22 | 22 | 22 | 22 | 22 | - |
| Ratio Quality Score/Maximum Score | 0.91 | 0.68 | 0.50 | 0.82 | 1.0 | 0.72 | 0.68 | 1.0 | 0.72 | 0.59 | 1.0 | 0.95 | 0.95 | 0.91 | 0.72 | 0.82 | 0.72 | 0.54 | 0.95 | 0.86 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, E.F.; Orlando, D.R.; Araújo, A.M.S.; de Andrade, J.N.B.M.; Azzi, D.V.; de Lima, R.R.; Lobo-Júnior, A.R.; Pereira, L.J. Can Resveratrol Treatment Control the Progression of Induced Periodontal Disease? A Systematic Review and Meta-Analysis of Preclinical Studies. Nutrients 2019, 11, 953. https://doi.org/10.3390/nu11050953
Andrade EF, Orlando DR, Araújo AMS, de Andrade JNBM, Azzi DV, de Lima RR, Lobo-Júnior AR, Pereira LJ. Can Resveratrol Treatment Control the Progression of Induced Periodontal Disease? A Systematic Review and Meta-Analysis of Preclinical Studies. Nutrients. 2019; 11(5):953. https://doi.org/10.3390/nu11050953
Chicago/Turabian StyleAndrade, Eric Francelino, Débora Ribeiro Orlando, Amanda Melo Sant’Anna Araújo, James Newton Bizetto Meira de Andrade, Diana Vilela Azzi, Renato Ribeiro de Lima, Adalfredo Rocha Lobo-Júnior, and Luciano José Pereira. 2019. "Can Resveratrol Treatment Control the Progression of Induced Periodontal Disease? A Systematic Review and Meta-Analysis of Preclinical Studies" Nutrients 11, no. 5: 953. https://doi.org/10.3390/nu11050953
APA StyleAndrade, E. F., Orlando, D. R., Araújo, A. M. S., de Andrade, J. N. B. M., Azzi, D. V., de Lima, R. R., Lobo-Júnior, A. R., & Pereira, L. J. (2019). Can Resveratrol Treatment Control the Progression of Induced Periodontal Disease? A Systematic Review and Meta-Analysis of Preclinical Studies. Nutrients, 11(5), 953. https://doi.org/10.3390/nu11050953





