Using the Avocado to Test the Satiety Effects of a Fat-Fiber Combination in Place of Carbohydrate Energy in a Breakfast Meal in Overweight and Obese Men and Women: A Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics and Study Design
2.2. Subjects
2.3. Study Meals and Intervention
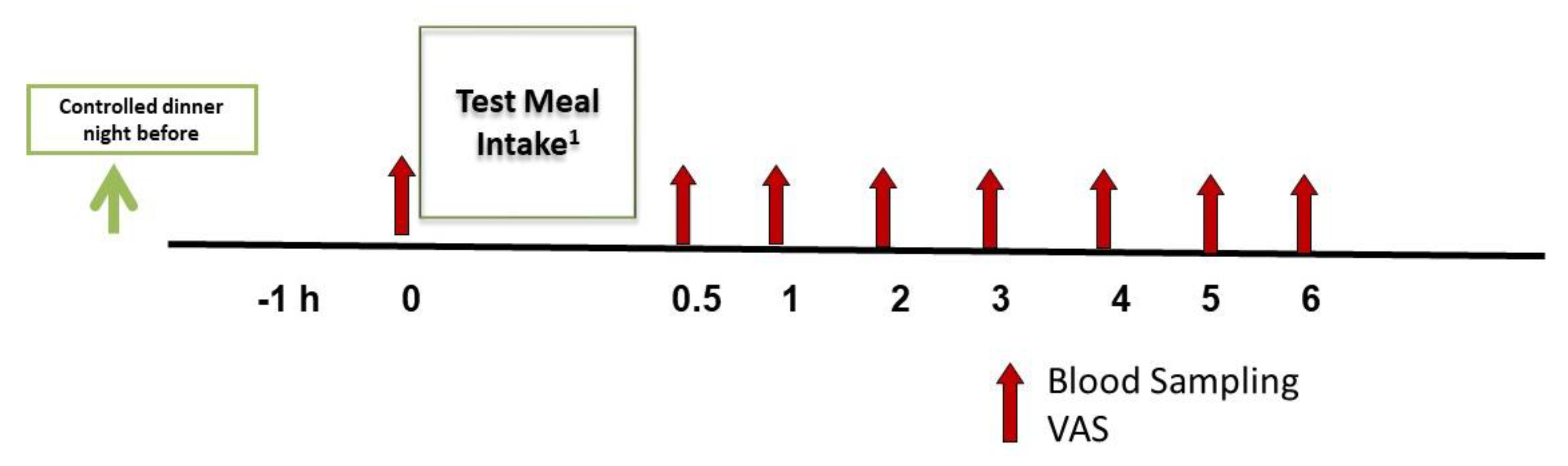
2.4. Assessments: Visual Analog Scale (VAS)
2.5. Assessments: Blood Collection and Analysis
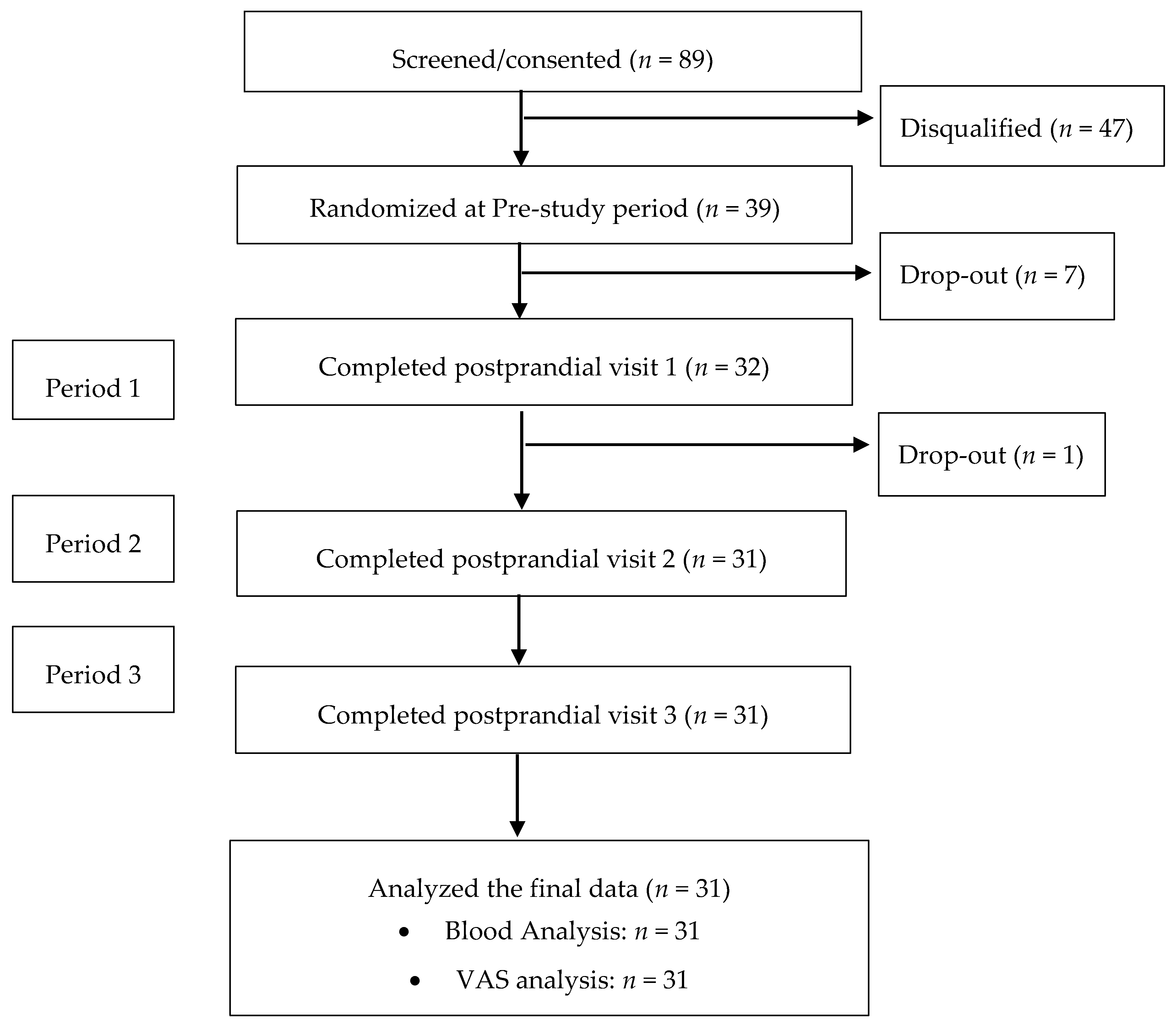
2.6. Summary of Study Procedures
2.7. Statistical Analysis
3. Results
3.1. Subjects and Test meals
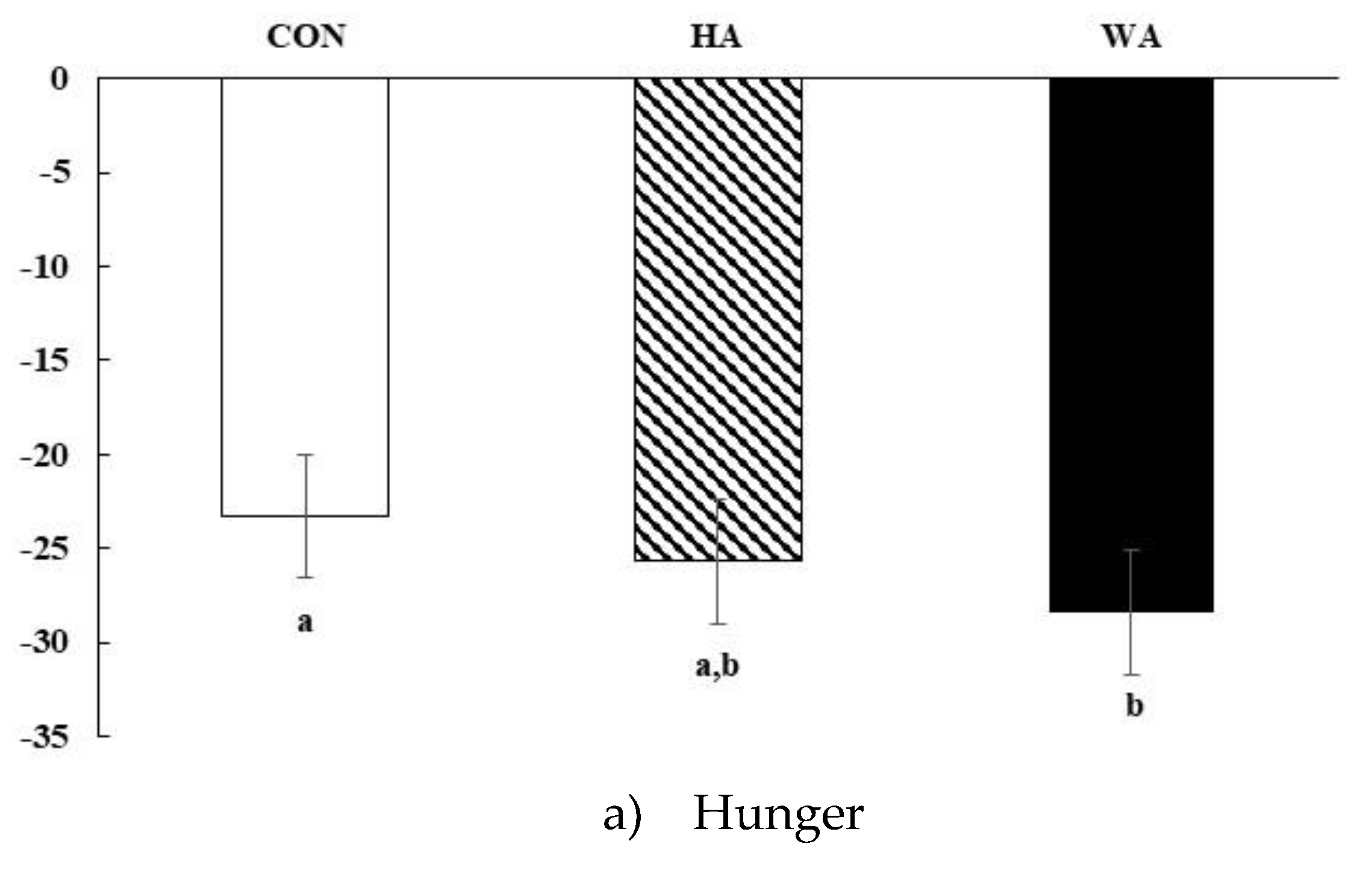
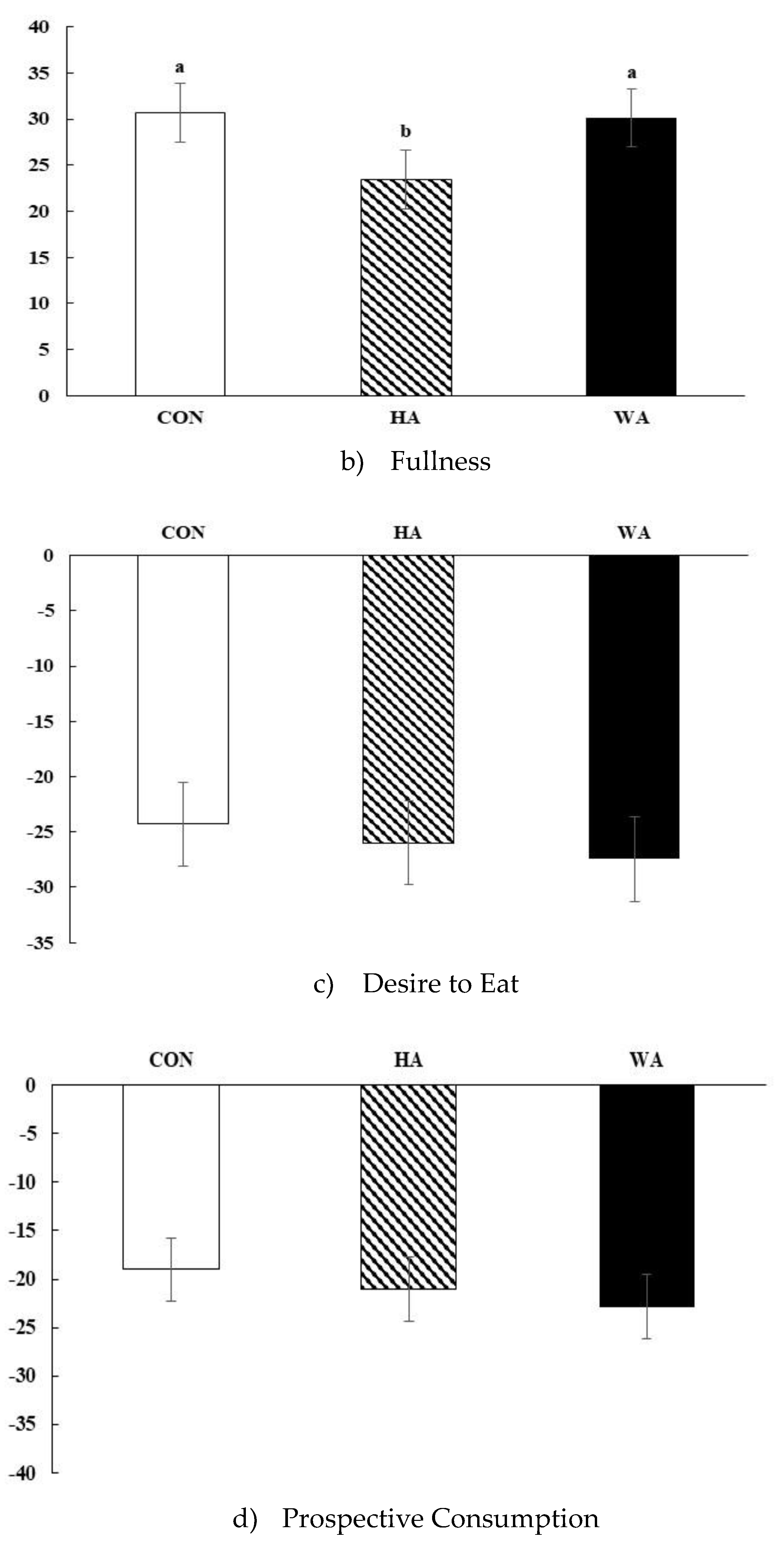
3.2. Subjective Satiety
3.3. Gut Peptide and Insulin Responses
3.4. Relationship of Hormones and Subjective Satiety
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Arroyo-Johnson, C.; Mincey, K.D. Obesity epidemiology trends by race/ethnicity, gender, and education: National Health Interview Survey, 1997–2012. Gastroenterol. Clin. North Am. 2016, 45, 571–579. [Google Scholar] [CrossRef]
- Flegal, K.M.; Carroll, M.D.; Kit, B.K.; Ogden, C.L. Prevalence of Obesity and Trends in the Distribution of Body Mass Index Among US Adults, 1999–2010. JAMA 2012, 307, 491. [Google Scholar] [CrossRef]
- Hales, C.M.; Carroll, M.D.; Fryar, C.D.; Ogden, C.L. Prevalence of Obesity Among Adults and Youth: United States, 2015–2016. NCHS Data Brief 2017, 288, 1–8. [Google Scholar]
- Ogden, C.L.; Fakhouri, T.H.; Carroll, M.D.; Hales, C.M.; Fryar, C.D.; Li, X.; Freedman, D.S. Prevalence of Obesity Among Adults, by Household Income and Education — United States, 2011–2014. MMWR 2017, 66, 1369–1373. [Google Scholar] [CrossRef]
- Ogden, C.L.; Carroll, M.D.; Curtin, L.R.; Lamb, M.M.; Flegal, K.M. Prevalence of high body mass index in US children and adolescents, 2007–2008. JAMA 2010, 303, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Shiller, J.S.; Lucas, J.W.; Ward, B.W.; Peregoy, J.A. Summary health statistics for U.S. adults: National Health Interview Survey, 2011. Vital Health Stat. 2012, 252, 1–207. [Google Scholar]
- Burton-Freeman, B. Dietary Fiber and Energy Regulation. J. Nutr. 2000, 130, 272S–275S. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.J.; Slavin, J.L. The Effect of Fiber on Satiety and Food Intake: A Systematic Review. J. Am. Nutr. 2013, 32, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Fallaize, R.; Wilson, L.; Gray, J.; Morgan, L.M.; Griffin, B.A. Variation in the effects of three different breakfast meals on subjective satiety and subsequent intake of energy at lunch and evening meal. Eur. J. Nutr. 2013, 52, 1353–1359. [Google Scholar] [CrossRef]
- Holt, S.; Delargy, H.; Lawton, C.; Blundell, J. The effects of high-carbohydrate vs high-fat breakfasts on feelings of fullness and alertness, and subsequent food intake. Int. J. Food Sci. Nutr. 1999, 50, 13–28. [Google Scholar] [CrossRef]
- Latner, J.; Schwartz, M. The Effects of a High-carbohydrate, High-protein or Balanced Lunch upon Later Food Intake and Hunger Ratings. Appetite 1999, 33, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Cecil, J.E.; Francis, J.; Read, N.W. Comparison of the Effects of a High-Fat and High-Carbohydrate Soup Delivered Orally and Intragastrically on Gastric Emptying, Appetite, and Eating Behaviour. Physiol. Behav. 1999, 67, 299–306. [Google Scholar] [CrossRef]
- Rolls, B.J.; A Bell, E. Intake of fat and carbohydrate: role of energy density. Eur. J. Clin. Nutr. 1999, 53, s166–s173. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, A.M.; Shannon, E.; Whybrow, S.; Reid, C.A.; Stubbs, R.J. Altering the temporal distribution of energy intake with isoenergetically dense foods given as snacks does not affect total daily energy intake in normal-weight men. Br. J. Nutr. 2000, 83, 7–14. [Google Scholar]
- Kaviani, S.; A Cooper, J. Appetite responses to high-fat meals or diets of varying fatty acid composition: A comprehensive review. Eur. J. Clin. Nutr. 2017, 71, 1154–1165. [Google Scholar] [CrossRef]
- Poutanen, K.S.; Dussort, P.; Erkner, A.; Fiszman, S.; Karnik, K.; Kristensen, M.; Marsaux, C.F.; Miquel-Kergoat, S.; Pentikäinen, S.P.; Putz, P.; et al. A review of the characteristics of dietary fibers relevant to appetite and energy intake outcomes in human intervention trials. Am. J. Clin. Nutr. 2017, 106, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Gentilcore, D.; Chaikomin, R.; Jones, K.L.; Russo, A.; Feinle-Bisset, C.; Wishart, J.M.; Rayner, C.K.; Horowitz, M. Effects of Fat on Gastric Emptying of and the Glycemic, Insulin, and Incretin Responses to a Carbohydrate Meal in Type 2 Diabetes. J. Clin. Endocrinol. Metab. 2006, 91, 2062–2067. [Google Scholar] [CrossRef]
- Little, T.J.; Feinle-Bisset, C. Effects of dietary fat on appetite and energy intake in health and obesity—Oral and gastrointestinal sensory contributions. Physiol. Behav. 2011, 104, 613–620. [Google Scholar] [CrossRef]
- Schneema, B.O. Dietary fiber and gastrointestinal function. Nutr. Rev. 1987, 45, 129–132. [Google Scholar] [CrossRef]
- Essah, P.A.; Sistrun, S.N.; Kelly, S.M.; Nestler, J.E.; Levy, J.R. Effect of Macronutrient Composition on Postprandial Peptide YY Levels. J. Clin. Endocrinol. Metab. 2007, 92, 4052–4055. [Google Scholar] [CrossRef]
- MacIntosh, C.G.; Andrews, J.M.; Jones, K.L.; Wishart, J.M.; A Morris, H.; Jansen, J.B.; E Morley, J.; Horowitz, M.; Chapman, I.M. Effects of age on concentrations of plasma cholecystokinin, glucagon-like peptide 1, and peptide YY and their relation to appetite and pyloric motility. Am. J. Clin. Nutr. 1999, 69, 999–1006. [Google Scholar] [CrossRef]
- Paniagua, J.A.; De La Sacristana, A.G.; Sanchez, E.; Romero, I.; Vidal-Puig, A.; Berral, F.J.; Escribano, A.; Moyano, M.J.; Perez-Martinez, P.; López-Miranda, J.; et al. A MUFA-Rich Diet Improves Posprandial Glucose, Lipid and GLP-1 Responses in Insulin-Resistant Subjects. J. Am. Nutr. 2007, 26, 434–444. [Google Scholar] [CrossRef]
- Schneeman, B.O.; Burton-Freeman, B.; Davis, P. Incorporating Dairy Foods into Low and High Fat Diets Increases the Postprandial Cholecystokinin Response in Men and Women. J. Nutr. 2003, 133, 4124–4128. [Google Scholar] [CrossRef]
- Burton-Freeman, B.; A Davis, P.; O Schneeman, B. Plasma cholecystokinin is associated with subjective measures of satiety in women1–3. Am. J. Clin. Nutr. 2002, 76, 659–667. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture. Agricultural Research Service USDA National Nutrient Database for Standard Reference, Release 24. 2011. Available online: https://www.ars.usda.gov/northeast-area/beltsville-md-bhnrc/beltsville-human-nutrition-research-center/nutrient-data-laboratory/docs/usda-national-nutrient-database-for-standard-reference/ (accessed on 14 July 2017).
- Wien, M.; Haddad, E.; Oda, K.; Sabaté, J. A randomized 3x3 crossover study to evaluate the effect of Hass avocado intake on post-ingestive satiety, glucose and insulin levels, and subsequent energy intake in overweight adults. Nutr. J. 2013, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Edirisinghe, I.; Burton-Freeman, B. Avocado Fruit on Postprandial Markers of Cardio-Metabolic Risk: A Randomized Controlled Dose Response Trial in Overweight and Obese Men and Women. Nutrients 2018, 10, 1287. [Google Scholar] [CrossRef]
- Flint, A.; Raben, A.; Blundell, J.; Astrup, A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int. J. Obes. 2000, 24, 38–48. [Google Scholar] [CrossRef]
- SAS Institute Inc SAS 2015. SAS® University Edition: Installation Guide for Windows; SAS Institute Inc.: Cary, NC, USA, 2015. [Google Scholar]
- IBM SPSS Statistics for Windows; Version 24.0; IBM Corp SPSS 2016; IBM Corp.: Armonk, NY, USA, 2016.
- Paddon-Jones, D.; Westman, E.; Mattes, R.D.; Wolfe, R.R.; Astrup, A.; Westerterp-Plantenga, M. Protein, weight management, and satiety. Am. J. Clin. Nutr. 2008, 87, 1558S–1561S. [Google Scholar] [CrossRef]
- Stubbs, R.J.; Harbron, C.G.; Prentice, A.M. Covert manipulation of the dietary fat to carbohydrate ratio of isoenergetically dense diets: Effect on food intake in feeding men ad libitum. Int. J. Obes. Relat. Metab. Disord. 1996, 20, 651–660. [Google Scholar]
- Van Stratum, P.; Lussenburg, R.N.; A Van Wezel, L.; Vergroesen, A.J.; Cremer, H.D.; Agin, W.; Beule, B.; Potter, E.; Kraal, J.H. The effect of dietary carbohydrate:fat ratio on energy intake by adult women. Am. J. Clin. Nutr. 1978, 31, 206–212. [Google Scholar] [CrossRef]
- Flint, A.; Gregersen, N.T.; Gluud, L.L.; Møller, B.K.; Raben, A.; Tetens, I.; Verdich, C.; Astrup, A. Associations between postprandial insulin and blood glucose responses, appetite sensations and energy intake in normal weight and overweight individuals: A meta-analysis of test meal studies. Br. J. Nutr. 2007, 98, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, G.H. Peptide YY (1–36) and Peptide YY (3–36): Part I. Distribution, Release and Actions. Obes. Surg. 2006, 16, 651–658. [Google Scholar] [CrossRef]
- Holst, J.J. The Physiology of Glucagon-like Peptide 1. Physiol. Rev. 2007, 87, 1409–1439. [Google Scholar] [CrossRef] [PubMed]
- El Khoury, D.T.D.; Obeid, O.; Azar, S.T.; Hwalla, N. Variations in Postprandial Ghrelin Status following Ingestion of High-Carbohydrate, High-Fat, and High-Protein Meals in Males. Ann. Nutr. Metab. 2006, 50, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Monteleone, P.; Bencivenga, R.; Longobardi, N.; Serritella, C.; Maj, M. Differential Responses of Circulating Ghrelin to High-Fat or High-Carbohydrate Meal in Healthy Women. J. Clin. Endocrinol. Metab. 2003, 88, 5510–5514. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ferrannini, E. Insulin Resistance: A Multifaceted Syndrome Responsible for NIDDM, Obesity, Hypertension, Dyslipidemia, and Atherosclerotic Cardiovascular Disease. Diabetes Care. 1991, 14, 173–194. [Google Scholar] [CrossRef] [PubMed]
- Doostvandi, T.; Mozaffari-Khosravi, H.; Mirmiran, P.; Bahadoran, Z. The Association Between Dietary Patterns and Insulin Resistance: A Systematic Review. Int. J. Nutr. Food Sci. 2016, 5, 14. [Google Scholar] [CrossRef]
- Esmaillzadeh, A.; Kimiagar, M.; Mehrabi, Y.; Azadbakht, L.; Hu, F.B.; Willett, W.C. Dietary patterns, insulin resistance, and prevalence of the metabolic syndrome in women. Am. J. Clin. Nutr. 2007, 85, 910–918. [Google Scholar] [CrossRef]
- Kim, B.; Feldman, E.L. Insulin resistance as a key link for the increased risk of cognitive impairment in the metabolic syndrome. Exp. Mol. Med. 2015, 47, e149. [Google Scholar] [CrossRef]
- Stanley, M.; Macauley, S.L.; Caesar, E.E.; Koscal, L.J.; Moritz, W.; Robinson, G.O.; Roh, J.; Keyser, J.; Jiang, H.; Holtzman, D.M. The Effects of Peripheral and Central High Insulin on Brain Insulin Signaling and Amyloid-β in Young and Old APP/PS1 Mice. J. Neurosci. 2016, 36, 11704–11715. [Google Scholar] [CrossRef]
- Van Der Heide, L.P.; Ramakers, G.M.; Smidt, M.P. Insulin signaling in the central nervous system: Learning to survive. Prog. Neurobiol. 2006, 79, 205–221. [Google Scholar] [CrossRef] [PubMed]
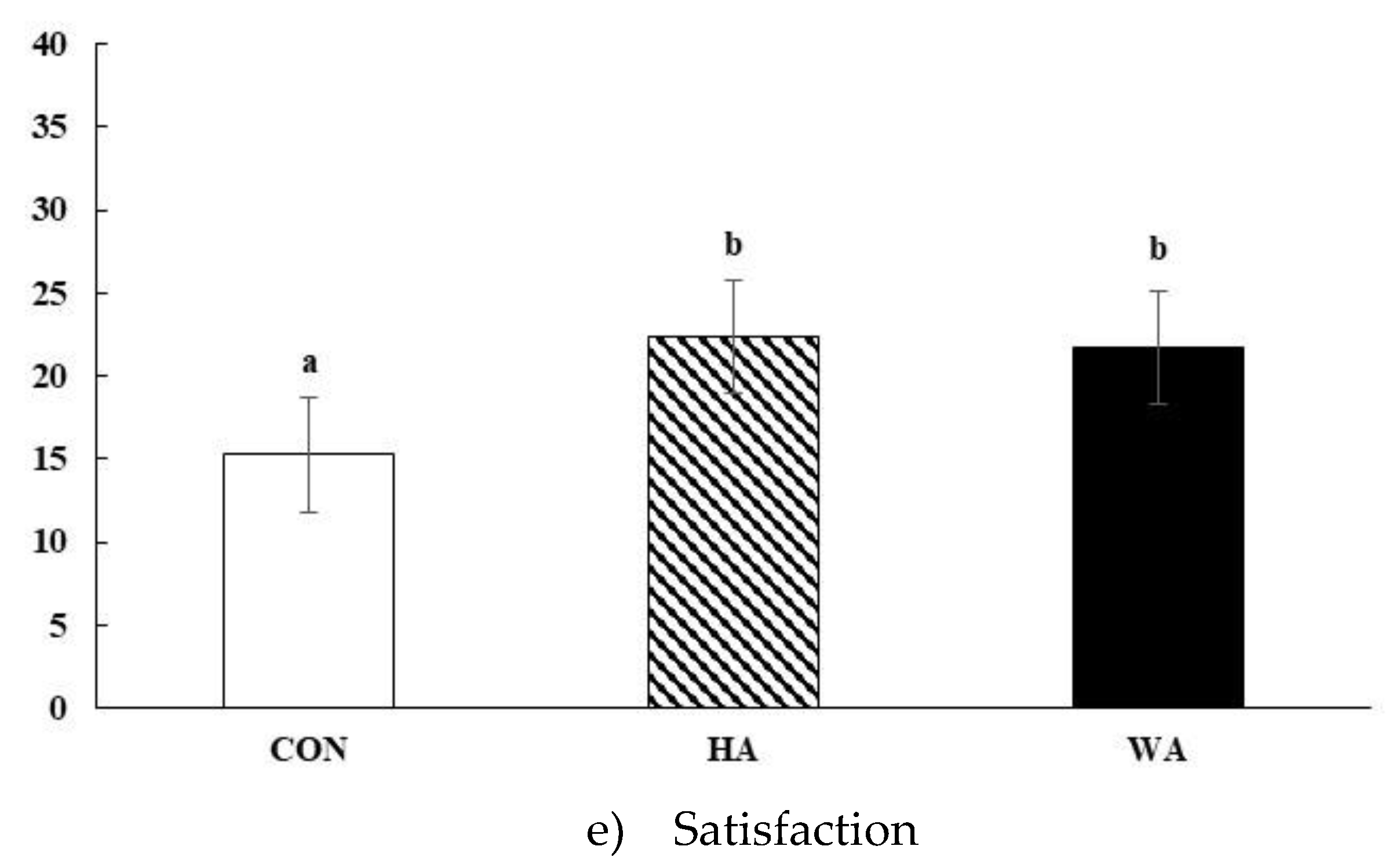
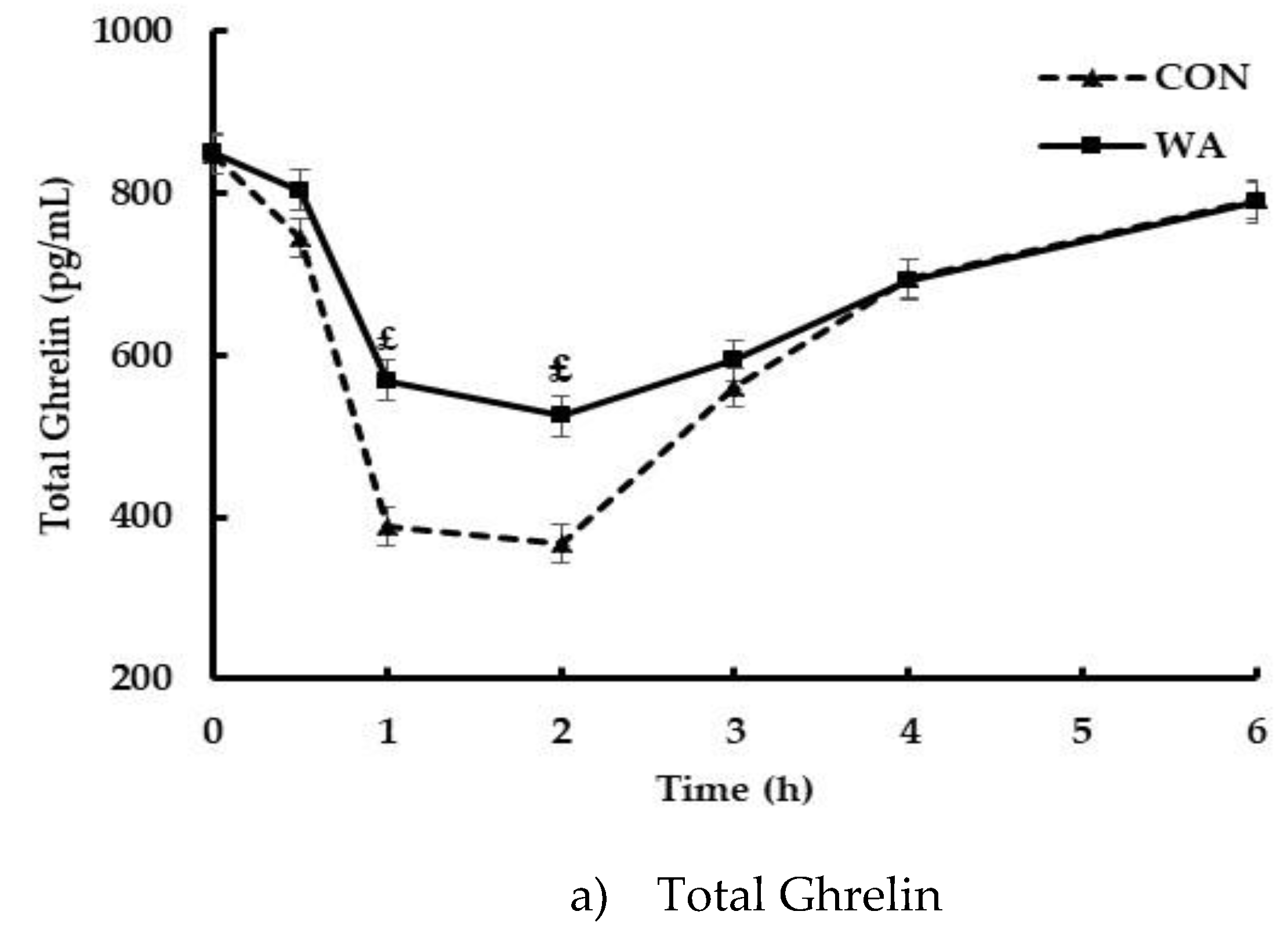
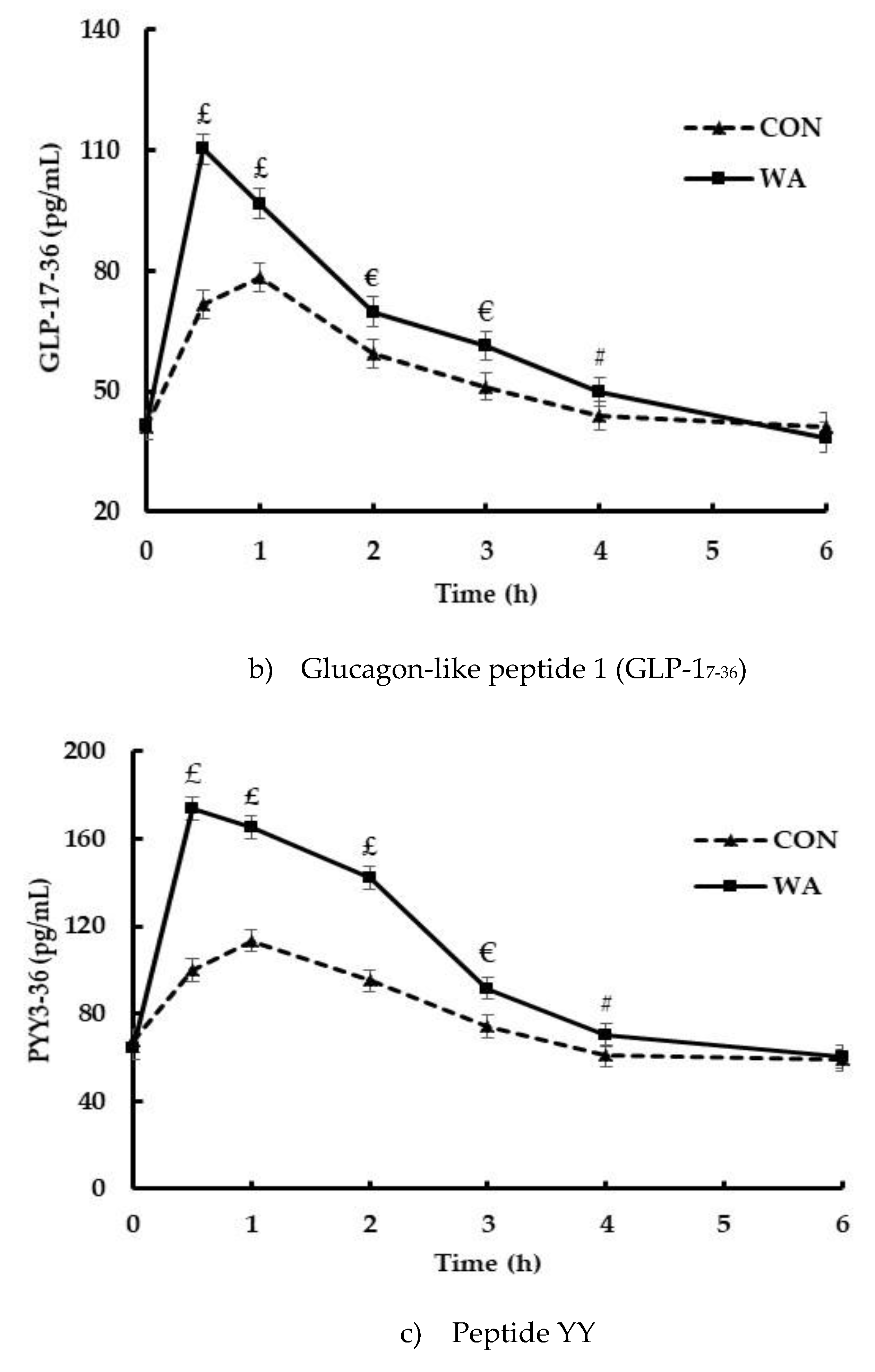
| Nutrient | Control Meal CON | Half Avocado Meal HA | Whole Avocado Meal WA |
|---|---|---|---|
| Calories (kcal) | 637 | 618 | 642 |
| % Carbohydrate | 75.7 | 50.8 | 50.3 |
| % Fat | 14.0 | 39.8 | 43.0 |
| % Protein | 11.5 | 11.8 | 10.4 |
| Dietary Fiber (g) 2 | 4.9 | 8.6 | 13.1 |
| Sugar (g) | 59.4 | 26.5 | 25.5 |
| Total Fat (g) 3 | 9.9 | 27.3 | 30.7 |
| Saturated Fat (g) | 4.9 | 11.3 | 8.6 |
| Monounsaturated Fat (g) | 2.1 | 10.8 | 15.8 |
| Polyunsaturated Fat (g) | 1.2 | 2.6 | 3.6 |
| Energy Density (kcal/g) | 2.1 | 2.0 | 2.1 |
| Characteristic | Total Subjects (n = 31) | |
|---|---|---|
| Age (years) | 38 ± 10 | |
| BMI (kg m−2) | 29 ± 2 | |
| Mid-point waist circumference (cm) | 93 ± 10 | |
| Race/Ethnicity, n | Cau:AA:AS:His | 9:13:5:4 |
| Gender, n | Male:Female | 15:16 |
| Hormones | WA | CON | p Value |
|---|---|---|---|
| Ghrelin iAUC (pg min·mL−1) | −1000.7 ± 147.3 | −1345.7 ± 142.4 | 0.01 * |
| GLP iAUC (pg min·mL−1) | 130.4 ± 12.3 | 73.5 ± 11.9 | 0.002 |
| PYY iAUC (pg min·mL−1) | 239.3 ± 19.5 | 73.8 ± 18.8 | <0.0001 |
| Insulin iAUC (pmol·L−1) | 4285.5 ± 562.8 | 6221.3 ± 557.9 | <0.0001 |
| CON | WA | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | 2 Beta | t | p | R2 | Cumulative R2 | Variable | 2 Beta | t | p | R2 | Cumulative R2 |
| Hunger | Hunger | ||||||||||
| Insulin | −0.33 | −4.40 | 0.000 | 0.22 | 0.22 | PYY | −0.36 | −3.74 | 0.000 | 0.30 | 0.30 |
| PYY | −0.22 | −2.94 | 0.004 | 0.03 | 0.25 | GLP | −0.29 | −2.92 | 0.004 | 0.02 | 0.32 |
| GLP | −0.00 | −0.04 | 0.966 | Ghrelin | −0.17 | −2.91 | 0.004 | 0.03 | 0.35 | ||
| Ghrelin | −0.11 | −1.61 | 0.109 | Insulin | −0.01 | −0.09 | 0.928 | ||||
| Fullness | Fullness | ||||||||||
| GLP | 0.39 | 6.17 | 0.000 | 0.32 | 0.32 | PYY | 0.44 | 6.96 | 0.000 | 0.20 | 0.20 |
| Insulin | 0.34 | 5.38 | 0.000 | 0.08 | 0.40 | Insulin | 0.11 | 1.03 | 0.305 | ||
| PYY | 0.12 | 1.65 | 0.100 | GLP | 0.06 | 0.55 | 0.581 | ||||
| Ghrelin | 0.06 | 1.06 | 0.292 | Ghrelin | 0.03 | 0.47 | 0.640 | ||||
| Desire to Eat | Desire to Eat | ||||||||||
| Insulin | −0.31 | −4.28 | 0.000 | 0.23 | 0.23 | PYY | −0.53 | −8.45 | 0.000 | 0.24 | 0.24 |
| PYY | −0.29 | −4.01 | 0.000 | 0.05 | 0.28 | Ghrelin | −0.16 | −2.54 | 0.012 | 0.02 | 0.27 |
| GLP | −0.01 | −0.12 | 0.907 | Insulin | −0.09 | −0.93 | 0.352 | ||||
| Ghrelin | −0.32 | −0.49 | 0.623 | GLP | −0.17 | −1.68 | 0.095 | ||||
| Prospective Consumption | Prospective Consumption | ||||||||||
| Insulin | −0.34 | −4.46 | 0.000 | 0.29 | 0.29 | PYY | −0.52 | −8.28 | 0.000 | 0.23 | 0.23 |
| PYY | −0.18 | −2.32 | 0.021 | 0.04 | 0.33 | Ghrelin | −0.16 | −2.55 | 0.012 | 0.02 | 0.26 |
| GLP | −0.16 | −2.20 | 0.029 | 0.02 | 0.34 | Insulin | −0.09 | −0.92 | 0.359 | ||
| Ghrelin | −0.08 | −1.26 | 0.210 | GLP | −0.16 | −1.51 | 0.132 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, L.; Huang, Y.; Edirisinghe, I.; Park, E.; Burton-Freeman, B. Using the Avocado to Test the Satiety Effects of a Fat-Fiber Combination in Place of Carbohydrate Energy in a Breakfast Meal in Overweight and Obese Men and Women: A Randomized Clinical Trial. Nutrients 2019, 11, 952. https://doi.org/10.3390/nu11050952
Zhu L, Huang Y, Edirisinghe I, Park E, Burton-Freeman B. Using the Avocado to Test the Satiety Effects of a Fat-Fiber Combination in Place of Carbohydrate Energy in a Breakfast Meal in Overweight and Obese Men and Women: A Randomized Clinical Trial. Nutrients. 2019; 11(5):952. https://doi.org/10.3390/nu11050952
Chicago/Turabian StyleZhu, Lanjun, Yancui Huang, Indika Edirisinghe, Eunyoung Park, and Britt Burton-Freeman. 2019. "Using the Avocado to Test the Satiety Effects of a Fat-Fiber Combination in Place of Carbohydrate Energy in a Breakfast Meal in Overweight and Obese Men and Women: A Randomized Clinical Trial" Nutrients 11, no. 5: 952. https://doi.org/10.3390/nu11050952
APA StyleZhu, L., Huang, Y., Edirisinghe, I., Park, E., & Burton-Freeman, B. (2019). Using the Avocado to Test the Satiety Effects of a Fat-Fiber Combination in Place of Carbohydrate Energy in a Breakfast Meal in Overweight and Obese Men and Women: A Randomized Clinical Trial. Nutrients, 11(5), 952. https://doi.org/10.3390/nu11050952





