Effect of Maternal Obesity and Preconceptional Weight Loss on Male and Female Offspring Metabolism and Olfactory Performance in Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Experiment Procedures
2.2. Assessment of Glucose Metabolism
2.3. Assessment of Lipid Metabolism
2.4. Assessment of Body Composition
2.5. Olfactory Behavioural Experiments
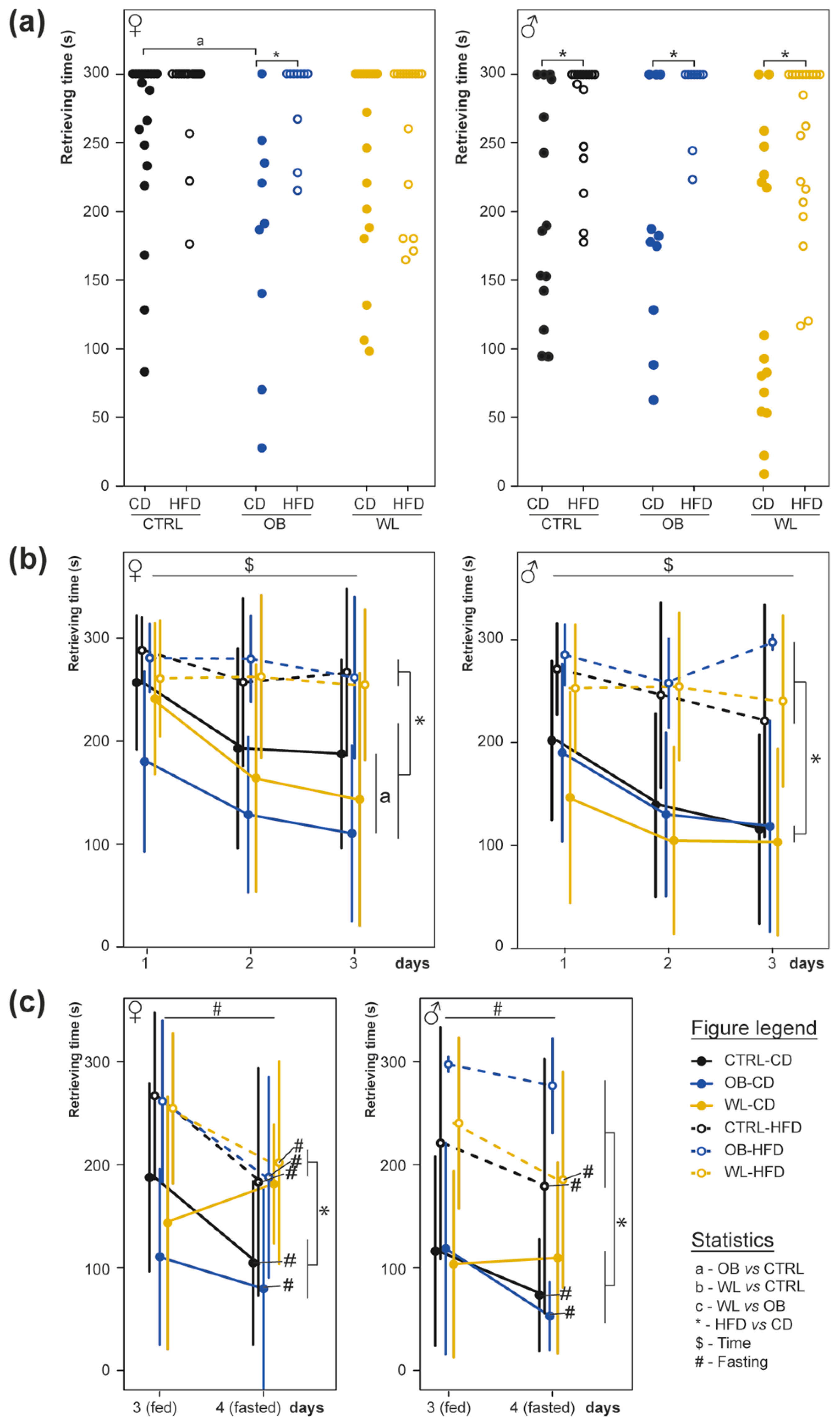
2.5.1. The Olfactory Sensitivity Test (Hidden Cookie Test)
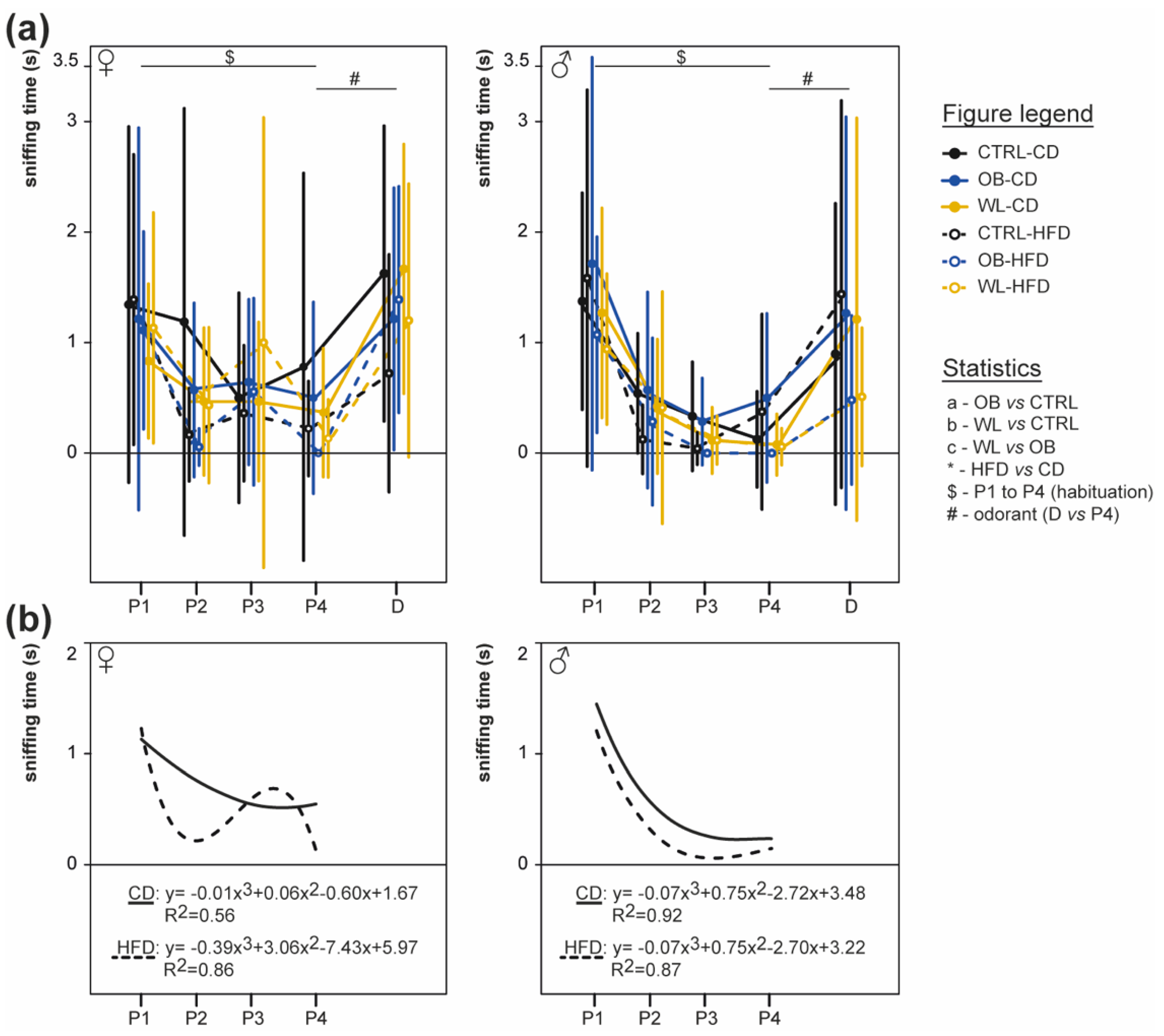
2.5.2. Habituation/Discrimination Test
2.6. Electro-Olfactogram (EOG)
2.7. Statistics
3. Results
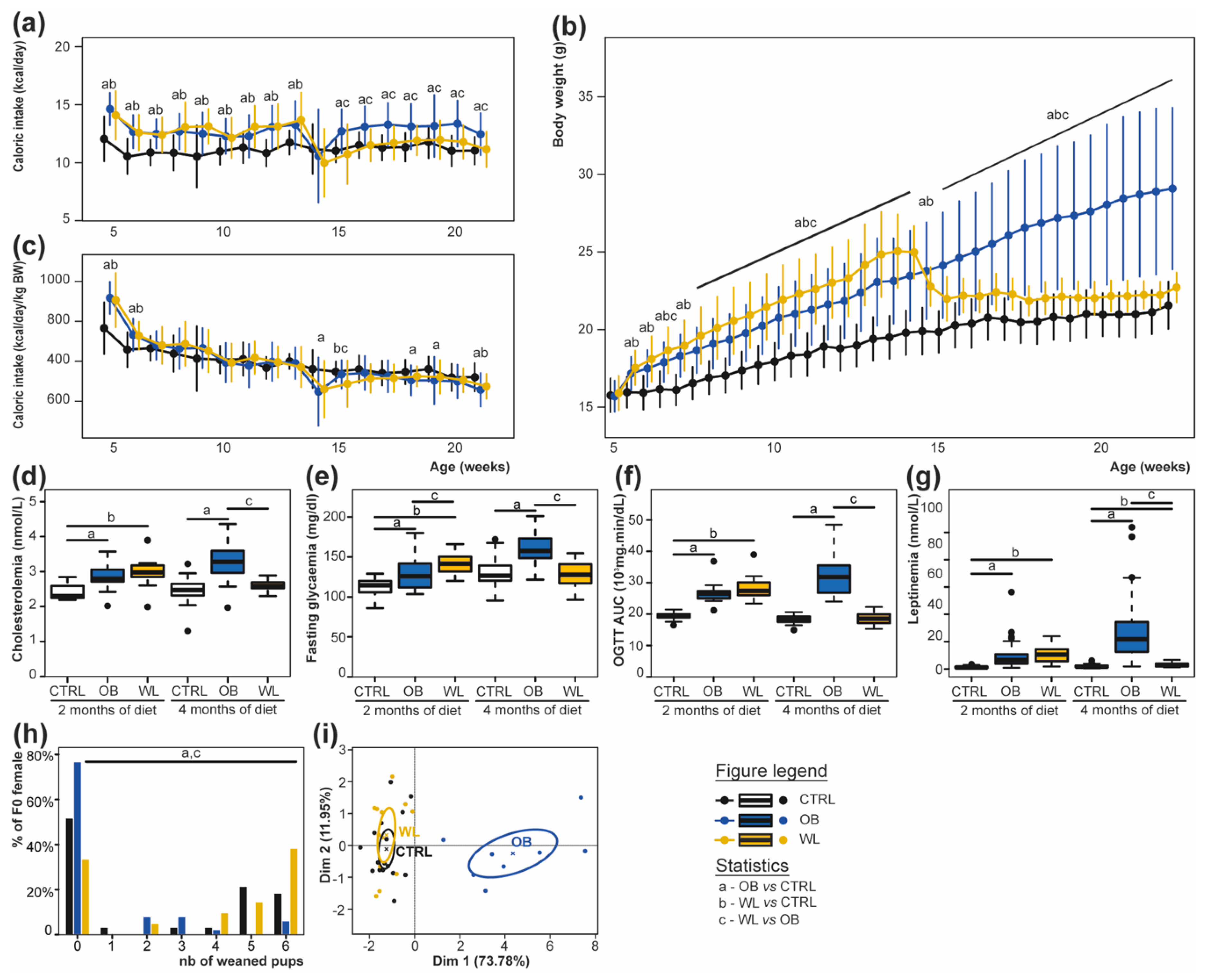
3.1. Preconceptional Weight Loss Normalizes the Metabolic Profile of Obese Dams
3.2. Pup Survival Is Impaired by Maternal Obesity and Normalized by Maternal Weight Loss
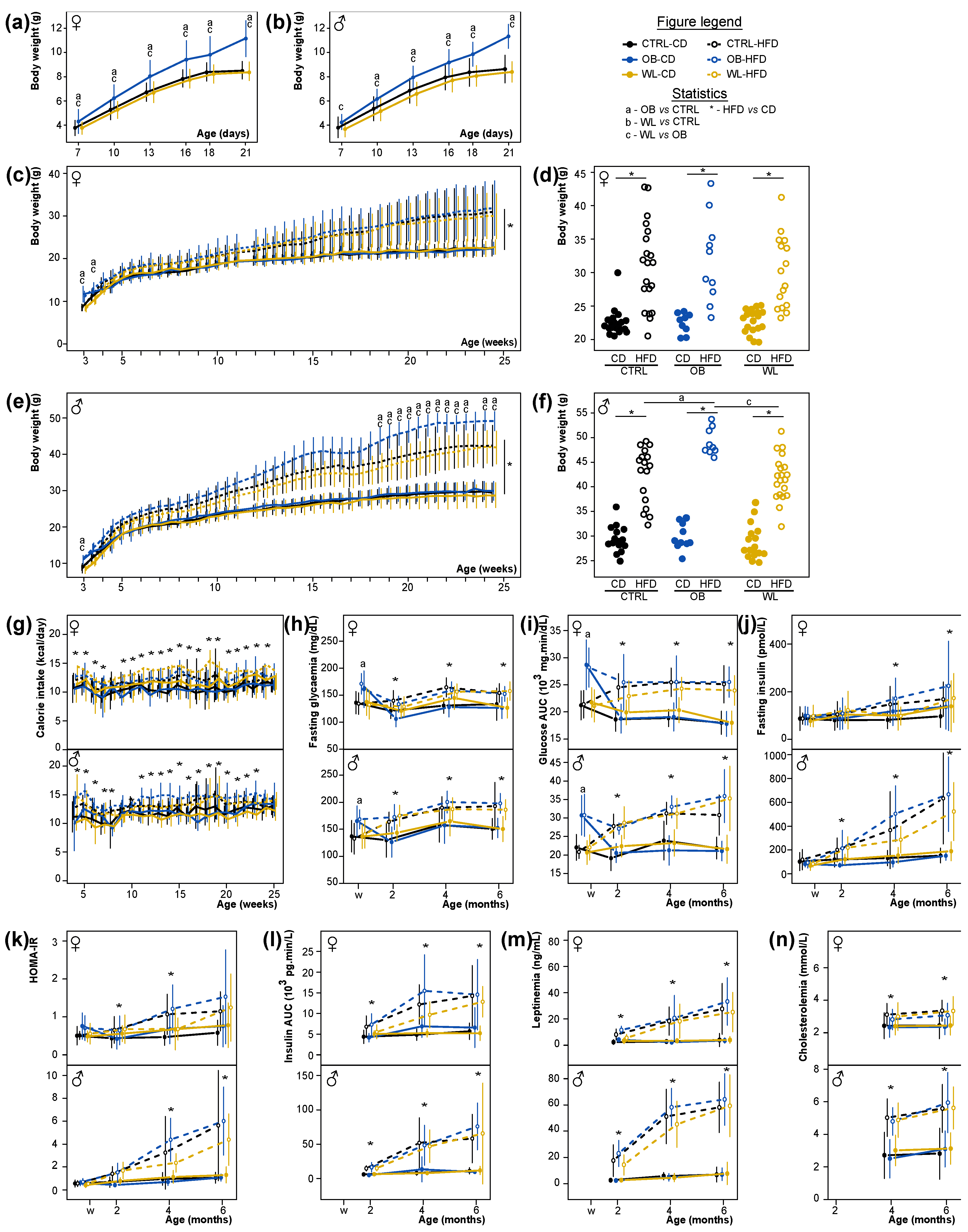
3.3. Early Post-Natal Growth Curve Is Normalized by Maternal Preconceptional Weight Loss
3.4. Maternal Obesity Has Few Persistent Effects after Weaning and Preconceptional Weight-Loss Does Not Induce a Specific Metabolic Phenotype in the Offspring
3.5. Post-Weaning HFD Offspring Display Poor Olfactory Performance
3.6. Post-Weaning Diet Influences Odour Habituation Capacities in Females
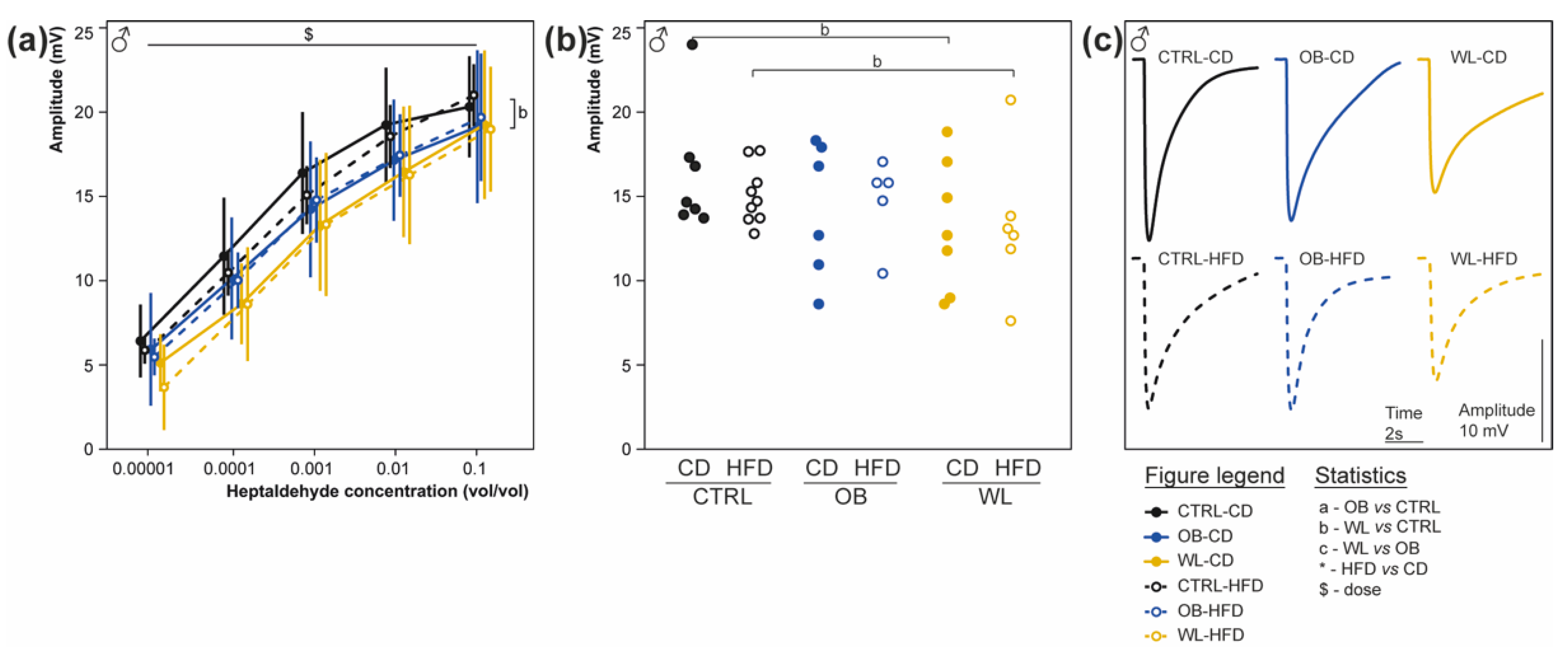
3.7. Maternal Weight Loss Decreases the Amplitude of the Electrical Response of Olfactory Epithelium to a Non-Food Odorant
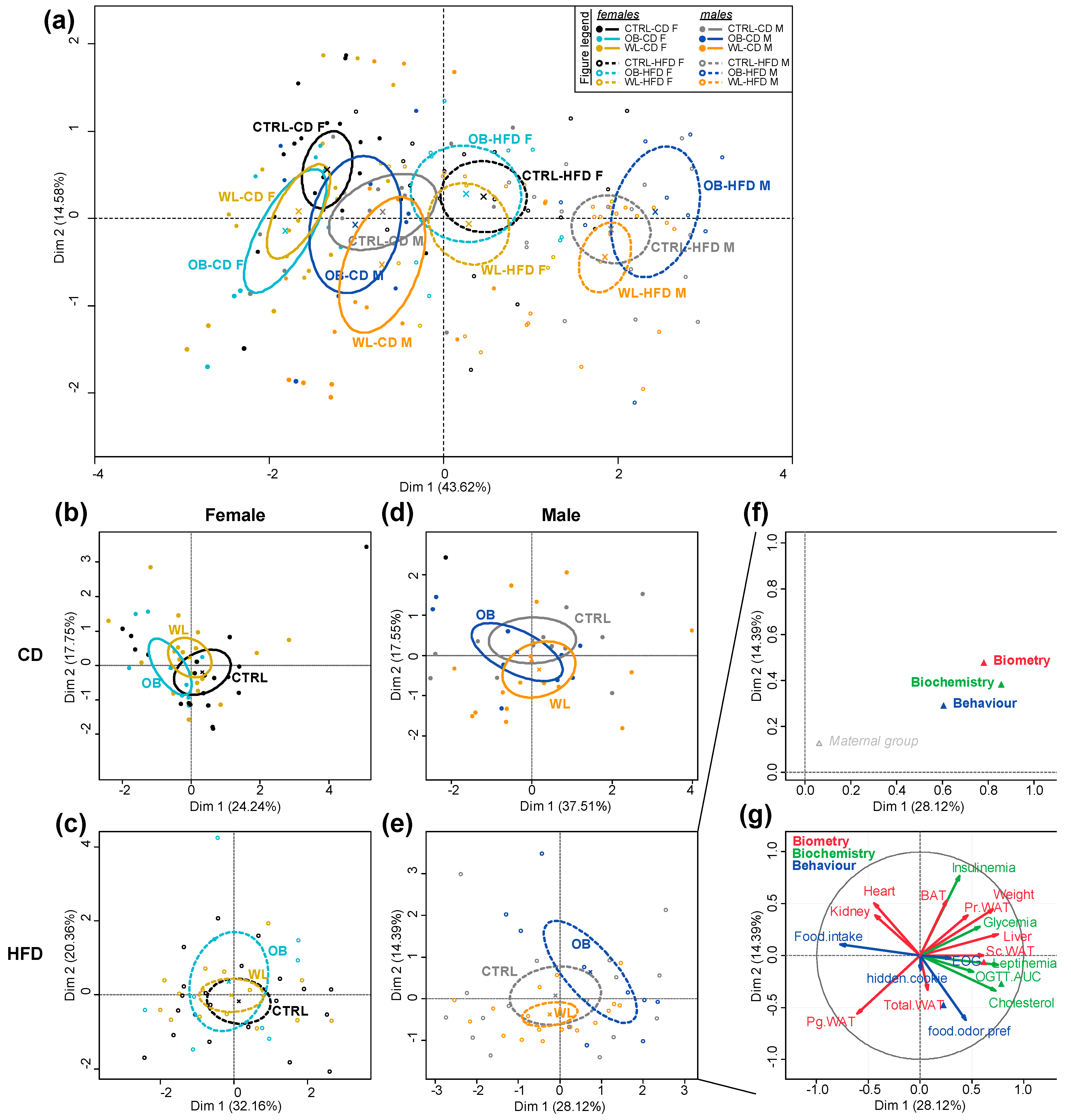
3.8. Post-Weaning Diet Has a Major Effect on F1 Phenotype and HFD-Fed Males Are Additionally Influenced by Maternal Group
4. Discussion
- The main effects on metabolic and olfactory phenotypes are linked to the post-weaning diet.
- There is a noticeable influence of the maternal group: OB-HFD are more susceptible to develop obesity than CTRL-HFD males, OB-CD females are the best performing in the cookie test, fasting does not improve the performance of WL-CD. In HFD-fed offspring, MFA reveals differences between OB and WL mice.
- While maternal WL has positive effects on offspring metabolism, there is a programming effect of the preconceptional intervention on olfactory sensitivity.
- Sex differences are observed: maternal obesity impairs male metabolism, and improves olfactory performances in female fed a CD. Odorant habituation is sensitive to the postweaning diet in female only.
4.1. Post-Weaning Diet Determined Metabolic and Olfactory Behavioural Risk in Mice
4.2. Maternal Obesity Affects Male Offspring Weight and Male and Female Olfactory Behaviour
4.3. Maternal Weight Loss Normalized Metabolic Parameters But Led to Impaired Olfactory Sensitivity
4.4. Sex Differences in the Offspring Phenotype
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seidell, J.C.; Halberstadt, J. The global burden of obesity and the challenges of prevention. ANM 2015, 66, 7–12. [Google Scholar] [CrossRef]
- Reichelt, A.C.; Stoeckel, L.E.; Reagan, L.P.; Winstanley, C.A.; Page, K.A. Dietary influences on cognition. Physiol. Behav. 2018, 192, 118–126. [Google Scholar] [CrossRef]
- Islam, M.A.; Fagundo, A.B.; Arcelus, J.; Agüera, Z.; Jiménez-Murcia, S.; Fernández-Real, J.M.; Tinahones, F.J.; de la Torre, R.; Botella, C.; Frühbeck, G.; et al. Olfaction in eating disorders and abnormal eating behavior: A systematic review. Front. Psychol. 2015, 6, 1431. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, M.-C.; Caillol, M.; Durieux, D.; Monnerie, R.; Grebert, D.; Pellerin, L.; Repond, C.; Tolle, V.; Zizzari, P.; Baly, C. Long-lasting metabolic imbalance related to obesity alters olfactory tissue homeostasis and impairs olfactory-driven behaviors. Chem. Senses 2015, 40, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Thiebaud, N.; Johnson, M.C.; Butler, J.L.; Bell, G.A.; Ferguson, K.L.; Fadool, A.R.; Fadool, J.C.; Gale, A.M.; Gale, D.S.; Fadool, D.A. Hyperlipidemic diet causes loss of olfactory sensory neurons, reduces olfactory discrimination, and disrupts odor-reversal learning. J. Neurosci. 2014, 34, 6970–6984. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.R.; Godbey, S.J.; Thiebaud, N.; Fadool, D.A. Olfactory ability and object memory in three mouse models of varying body weight, metabolic hormones, and adiposity. Physiol. Behav. 2012, 107, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Palouzier-Paulignan, B.; Lacroix, M.-C.; Aimé, P.; Baly, C.; Caillol, M.; Congar, P.; Julliard, A.K.; Tucker, K.; Fadool, D.A. Olfaction under metabolic influences. Chem. Senses 2012, 37, 769–797. [Google Scholar] [PubMed]
- Fernandez-Garcia, J.C.; Alcaide, J.; Santiago-Fernandez, C.; Roca-Rodriguez, M.M.; Aguera, Z.; Baños, R.; Botella, C.; de la Torre, R.; Fernandez-Real, J.M.; Fruhbeck, G.; et al. An increase in visceral fat is associated with a decrease in the taste and olfactory capacity. PLoS ONE 2017, 12, e0171204. [Google Scholar]
- Patel, Z.M.; DelGaudio, J.M.; Wise, S.K. Higher body mass index is associated with subjective olfactory dysfunction. Behav. Neurol. 2015, 2015, 675635. [Google Scholar] [CrossRef]
- Simchen, U.; Koebnick, C.; Hoyer, S.; Issanchou, S.; Zunft, H.-J.F. Odour and taste sensitivity is associated with body weight and extent of misreporting of body weight. Eur. J. Clin. Nutr. 2006, 60, 698–705. [Google Scholar] [CrossRef]
- Barker, D.J. The fetal and infant origins of adult disease. BMJ 1990, 301, 1111. [Google Scholar] [CrossRef]
- Hanson, M.A.; Gluckman, P.D. Early developmental conditioning of later health and disease: Physiology or pathophysiology? Physiol. Rev. 2014, 94, 1027–1076. [Google Scholar] [CrossRef] [PubMed]
- Portha, B.; Grandjean, V.; Movassat, J. Mother or father: Who is in the front line? Mechanisms underlying the non-genomic transmission of obesity/diabetes via the maternal or the paternal line. Nutrients 2019, 11, 233. [Google Scholar] [CrossRef]
- Coppola, D.M.; Millar, L.C. Olfaction in utero: Behavioral studies of the mouse fetus. Behav. Process. 1997, 39, 53–68. [Google Scholar] [CrossRef]
- Carreon, M.; Torrero, C.; Regalado, M.; Rubio, L.; Salas, M. Olfactory discrimination in adult male rats undernourished during the pre- and neonatal period. J. Behav. Brain Sci. 2012, 2, 283–290. [Google Scholar] [CrossRef]
- Frias, C.; Torrero, C.; Regalado, M.; Salas, M. Development of mitral cells and olfactory bulb layers in neonatally undernourished rats. Nutr. Neurosci. 2009, 12, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-López, M.T.; Vázquez, M.; Lomazzo, E.; Hofmann, C.; Blanco, R.N.; Alén, F.; Antón, M.; Decara, J.; Arco, R.; Orio, L.; et al. A moderate diet restriction during pregnancy alters the levels of endocannabinoids and endocannabinoid-related lipids in the hypothalamus, hippocampus and olfactory bulb of rat offspring in a sex-specific manner. PLoS ONE 2017, 12, e0174307. [Google Scholar]
- Merle, L.; Person, O.; Bonnet, P.; Grégoire, S.; Soubeyre, V.; Grosmaitre, X.; Jarriault, D. Maternal high fat high sugar diet disrupts olfactory behavior but not mucosa sensitivity in the offspring. Psychoneuroendocrinology 2019, 104, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Carlin, G.; Chaumontet, C.; Blachier, F.; Barbillon, P.; Darcel, N.; Blais, A.; Delteil, C.; Guillin, F.M.; Blat, S.; van der Beek, E.M.; et al. Maternal high-protein diet during pregnancy modifies rat offspring body weight and insulin signaling but not macronutrient preference in adulthood. Nutrients 2019, 11. [Google Scholar] [CrossRef]
- Brenseke, B.; Bahamonde, J.; Talanian, M.; Kornfeind, E.; Daly, J.; Cobb, G.; Zhang, J.; Prater, M.R.; Davis, G.C.; Good, D.J. Mitigating or exacerbating effects of maternal-fetal programming of female mice through the food choice environment. Endocrinology 2015, 156, 182–192. [Google Scholar] [CrossRef]
- Mariona, F.G. Perspectives in obesity and pregnancy. Womens Health (Lond.) 2016, 12, 523–532. [Google Scholar] [CrossRef]
- Catalano, P.; deMouzon, S.H. Maternal obesity and metabolic risk to the offspring: Why lifestyle interventions may have not achieved the desired outcomes. Int. J. Obes. 2015, 39, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Hanson, M.; Barker, M.; Dodd, J.M.; Kumanyika, S.; Norris, S.; Steegers, E.; Stephenson, J.; Thangaratinam, S.; Yang, H. Interventions to prevent maternal obesity before conception, during pregnancy, and post partum. Lancet Diabetes Endocrinol. 2017, 5, 65–76. [Google Scholar] [CrossRef]
- Van Oers, A.M.; Mutsaerts, M.A.Q.; Burggraaff, J.M.; Kuchenbecker, W.K.H.; Perquin, D.A.M.; Koks, C.A.M.; van Golde, R.; Kaaijk, E.M.; Broekmans, F.J.; de Bruin, J.P.; et al. Association between periconceptional weight loss and maternal and neonatal outcomes in obese infertile women. PLoS ONE 2018, 13, e0192670. [Google Scholar] [CrossRef] [PubMed]
- Kjaer, M.M.; Nilas, L. Pregnancy after bariatric surgery—A review of benefits and risks. Acta Obstet. Gynecol. Scand. 2013, 92, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.; Cianflone, K.; Biron, S.; Hould, F.S.; Lebel, S.; Marceau, S.; Lescelleur, O.; Biertho, L.; Simard, S.; Kral, J.G.; et al. Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. J. Clin. Endocrinol. Metab. 2009, 94, 4275–4283. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.P.; Gavard, J.A.; Rice, J.J.; Catanzaro, R.B.; Artal, R.; Hopkins, S.A. The impact of interpregnancy weight change on birthweight in obese women. Am. J. Obstet. Gynecol. 2013, 208, 205. [Google Scholar] [CrossRef]
- McBain, R.D.; Dekker, G.A.; Clifton, V.L.; Mol, B.W.; Grzeskowiak, L.E. Impact of inter-pregnancy BMI change on perinatal outcomes: A retrospective cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2016, 205, 98–104. [Google Scholar] [CrossRef]
- Diouf, I.; Charles, M.A.; Thiebaugeorges, O.; Forhan, A.; Kaminski, M.; Heude, B.; EDEN Mother-Child Cohort Study Group. Maternal weight change before pregnancy in relation to birthweight and risks of adverse pregnancy outcomes. Eur. J. Epidemiol. 2011, 26, 789–796. [Google Scholar] [CrossRef]
- Oteng-Ntim, E.; Mononen, S.; Sawicki, O.; Seed, P.T.; Bick, D.; Poston, L. Interpregnancy weight change and adverse pregnancy outcomes: A systematic review and meta-analysis. BMJ Open 2018, 8, e018778. [Google Scholar] [CrossRef]
- Forsum, E.; Brantsæter, A.L.; Olafsdottir, A.-S.; Olsen, S.F.; Thorsdottir, I. Weight loss before conception: A systematic literature review. Food Nutr. Res. 2013, 57. [Google Scholar] [CrossRef][Green Version]
- Matusiak, K.; Barrett, H.L.; Callaway, L.K.; Nitert, M.D. Periconception weight loss: Common sense for mothers, but what about for babies? J. Obes. 2014, 2014, 204295. [Google Scholar] [CrossRef]
- Nathanielsz, P.W.; Ford, S.P.; Long, N.M.; Vega, C.C.; Reyes-Castro, L.A.; Zambrano, E. Interventions to prevent adverse fetal programming due to maternal obesity during pregnancy. Nutr. Rev. 2013, 71, S78–S87. [Google Scholar] [CrossRef]
- Dennison, C.A.; Eslinger, A.J.; Reimer, R.A. Preconception prebiotic and sitagliptin treatment in obese rats affects pregnancy outcomes and offspring microbiota, adiposity, and glycemia. Front. Endocrinol. 2017, 8, 301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Rattanatray, L.; Morrison, J.L.; Nicholas, L.M.; Lie, S.; McMillen, I.C. Maternal obesity and the early origins of childhood obesity: Weighing up the benefits and costs of maternal weight loss in the periconceptional period for the offspring. Exp. Diabetes Res. 2011, 2011, 585749. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, L.M.; Morrison, J.L.; Rattanatray, L.; Ozanne, S.E.; Kleemann, D.O.; Walker, S.K.; MacLaughlin, S.M.; Zhang, S.; Martin-Gronert, M.S.; McMillen, I.C. Differential effects of exposure to maternal obesity or maternal weight loss during the periconceptional period in the sheep on insulin signalling molecules in skeletal muscle of the offspring at 4 months of age. PLoS ONE 2013, 8, e84594. [Google Scholar]
- Holinski, F.; Menenakos, C.; Haber, G.; Olze, H.; Ordemann, J. Olfactory and gustatory function after bariatric surgery. Obes. Surg. 2015, 25, 2314–2320. [Google Scholar] [CrossRef] [PubMed]
- Richardson, B.E.; Vanderwoude, E.A.; Sudan, R.; Leopold, D.A.; Thompson, J.S. Gastric bypass does not influence olfactory function in obese patients. Obes. Surg. 2012, 22, 283–286. [Google Scholar] [CrossRef]
- Zerrweck, C.; Gallardo, V.C.; Calleja, C.; Sepúlveda, E.; Guilber, L. Gross olfaction before and after laparoscopic gastric bypass. Obes. Surg. 2017, 27, 2988–2992. [Google Scholar] [CrossRef] [PubMed]
- Panchenko, P.E.; Voisin, S.; Jouin, M.; Jouneau, L.; Prézelin, A.; Lecoutre, S.; Breton, C.; Jammes, H.; Junien, C.; Gabory, A. Expression of epigenetic machinery genes is sensitive to maternal obesity and weight loss in relation to fetal growth in mice. Clin. Epigenetics 2016, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Gallou-Kabani, C.; Vigé, A.; Gross, M.-S.; Boileau, C.; Rabes, J.-P.; Fruchart-Najib, J.; Jais, J.-P.; Junien, C. Resistance to high-fat diet in the female progeny of obese mice fed a control diet during the periconceptual, gestation, and lactation periods. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E1095–E1100. [Google Scholar] [CrossRef] [PubMed]
- Mandairon, N.; Sultan, S.; Rey, N.; Kermen, F.; Moreno, M.; Busto, G.; Farget, V.; Messaoudi, B.; Thevenet, M.; Didier, A. A computer-assisted odorized hole-board for testing olfactory perception in mice. J. Neurosci. Methods 2009, 180, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, M.-C.; Badonnel, K.; Meunier, N.; Tan, F.; Schlegel-Le Poupon, C.; Durieux, D.; Monnerie, R.; Baly, C.; Congar, P.; Salesse, R.; et al. Expression of insulin system in the olfactory epithelium: First approaches to its role and regulation. J. Neuroendocrinol. 2008, 20, 1176–1190. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, K.; Gel, Y.; Brunner, E.; Konietschke, F. nparLD: An R software package for the nonparametric analysis of longitudinal data in factorial experiments. J. Stat. Softw. 2012, 50, 1–23. [Google Scholar] [CrossRef]
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R package for multivariate analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- King, V.; Norman, J.E.; Seckl, J.R.; Drake, A.J. Post-weaning diet determines metabolic risk in mice exposed to overnutrition in early life. Reprod. Biol. Endocrinol. 2014, 12, 73. [Google Scholar] [CrossRef]
- Aimé, P.; Palouzier-Paulignan, B.; Salem, R.; Al Koborssy, D.; Garcia, S.; Duchamp, C.; Romestaing, C.; Julliard, A.K. Modulation of olfactory sensitivity and glucose-sensing by the feeding state in obese Zucker rats. Front. Behav. Neurosci. 2014, 8, 326. [Google Scholar]
- Getchell, T.V.; Kwong, K.; Saunders, C.P.; Stromberg, A.J.; Getchell, M.L. Leptin regulates olfactory-mediated behavior in ob/ob mice. Physiol. Behav. 2006, 87, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Badonnel, K.; Lacroix, M.-C.; Durieux, D.; Monnerie, R.; Caillol, M.; Baly, C. Rat strains with different metabolic statuses differ in food olfactory-driven behavior. Behav. Brain Res. 2014, 270, 228–239. [Google Scholar] [CrossRef]
- Obrebowski, A.; Obrebowska-Karsznia, Z.; Gawliński, M. Smell and taste in children with simple obesity. Int. J. Pediatr. Otorhinolaryngol. 2000, 55, 191–196. [Google Scholar] [CrossRef]
- Stafford, L.D.; Whittle, A. Obese individuals have higher preference and sensitivity to odor of chocolate. Chem. Senses 2015, 40, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Harb, M.R.; Almeida, O.F.X. Altered motivation masks appetitive learning potential of obese mice. Front. Behav. Neurosci. 2014, 8, 377. [Google Scholar] [CrossRef] [PubMed]
- Rossi, H.L.; Luu, A.K.S.; Kothari, S.D.; Kuburas, A.; Neubert, J.K.; Caudle, R.M.; Recober, A. Effects of diet-induced obesity on motivation and pain behavior in an operant assay. Neuroscience 2013, 235, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Finger, B.C.; Dinan, T.G.; Cryan, J.F. Diet-induced obesity blunts the behavioural effects of ghrelin: Studies in a mouse-progressive ratio task. Psychopharmacology 2012, 220, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Shin, A.C.; Berthoud, H.-R. Food reward functions as affected by obesity and bariatric surgery. Int. J. Obes. (Lond.) 2011, 35, S40–S44. [Google Scholar] [CrossRef] [PubMed]
- Cordner, Z.A.; Tamashiro, K.L.K. Effects of high-fat diet exposure on learning & memory. Physiol. Behav. 2015, 152, 363–371. [Google Scholar]
- Martin, A.A.; Davidson, T.L. Human cognitive function and the obesogenic environment. Physiol. Behav. 2014, 136, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.J.; Knight, N.S.; Cochlin, L.E.; McAleese, S.; Deacon, R.M.J.; Rawlins, J.N.P.; Clarke, K. Deterioration of physical performance and cognitive function in rats with short-term high-fat feeding. FASEB J. 2009, 23, 4353–4360. [Google Scholar] [CrossRef]
- Del Rio, D.; Morales, L.; Ruiz-Gayo, M.; Del Olmo, N. Effect of high-fat diets on mood and learning performance in adolescent mice. Behav. Brain Res. 2016, 311, 167–172. [Google Scholar] [CrossRef]
- Dias-Rocha, C.P.; Almeida, M.M.; Santana, E.M.; Costa, J.C.B.; Franco, J.G.; Pazos-Moura, C.C.; Trevenzoli, I.H. Maternal high-fat diet induces sex-specific endocannabinoid system changes in newborn rats and programs adiposity, energy expenditure and food preference in adulthood. J. Nutr. Biochem. 2018, 51, 56–68. [Google Scholar] [CrossRef]
- Boitard, C.; Etchamendy, N.; Sauvant, J.; Aubert, A.; Tronel, S.; Marighetto, A.; Layé, S.; Ferreira, G. Juvenile, but not adult exposure to high-fat diet impairs relational memory and hippocampal neurogenesis in mice. Hippocampus 2012, 22, 2095–2100. [Google Scholar] [CrossRef] [PubMed]
- Valladolid-Acebes, I.; Fole, A.; Martín, M.; Morales, L.; Cano, M.V.; Ruiz-Gayo, M.; Del Olmo, N. Spatial memory impairment and changes in hippocampal morphology are triggered by high-fat diets in adolescent mice. Is there a role of leptin? Neurobiol. Learn. Mem. 2013, 106, 18–25. [Google Scholar] [PubMed]
- Murofushi, W.; Mori, K.; Murata, K.; Yamaguchi, M. Functional development of olfactory tubercle domains during weaning period in mice. Sci. Rep. 2018, 8, 13204. [Google Scholar] [CrossRef] [PubMed]
- Peleg-Raibstein, D.; Sarker, G.; Litwan, K.; Krämer, S.D.; Ametamey, S.M.; Schibli, R.; Wolfrum, C. Enhanced sensitivity to drugs of abuse and palatable foods following maternal overnutrition. Transl. Psychiatry 2016, 6, e911. [Google Scholar] [CrossRef]
- Wiss, D.A.; Criscitelli, K.; Gold, M.; Avena, N. Preclinical evidence for the addiction potential of highly palatable foods: Current developments related to maternal influence. Appetite 2017, 115, 19–27. [Google Scholar] [CrossRef]
- Li, M.; Sloboda, D.M.; Vickers, M.H. Maternal obesity and developmental programming of metabolic disorders in offspring: Evidence from animal models. Exp. Diabetes Res. 2011, 2011, 592408. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.; Seki, Y.; Vuguin, P.M.; Charron, M.J. Animal models of in utero exposure to a high fat diet: A review. Biochim. Biophys. Acta 2014, 1842, 507–519. [Google Scholar] [CrossRef] [PubMed]
- Wankhade, U.D.; Zhong, Y.; Kang, P.; Alfaro, M.; Chintapalli, S.V.; Thakali, K.M.; Shankar, K. Enhanced offspring predisposition to steatohepatitis with maternal high-fat diet is associated with epigenetic and microbiome alterations. PLoS ONE 2017, 12, e0175675. [Google Scholar] [CrossRef] [PubMed]
- King, V.; Hibbert, N.; Seckl, J.R.; Norman, J.E.; Drake, A.J. The effects of an obesogenic diet during pregnancy on fetal growth and placental gene expression are gestation dependent. Placenta 2013, 34, 1087–1090. [Google Scholar] [CrossRef]
- Platt, K.M.; Charnigo, R.J.; Pearson, K.J. Adult offspring of high-fat diet-fed dams can have normal glucose tolerance and body composition. J. Dev. Orig. Health Dis. 2014, 5, 229–239. [Google Scholar] [CrossRef][Green Version]
- Sasson, I.E.; Vitins, A.P.; Mainigi, M.A.; Moley, K.H.; Simmons, R.A. Pre-gestational vs. gestational exposure to maternal obesity differentially programs the offspring in mice. Diabetologia 2015, 58, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Ribaroff, G.A.; Wastnedge, E.; Drake, A.J.; Sharpe, R.M.; Chambers, T.J.G. Animal models of maternal high fat diet exposure and effects on metabolism in offspring: A meta-regression analysis. Obes. Rev. 2017, 18, 673–686. [Google Scholar] [CrossRef] [PubMed]
- Taouk, L.; Schulkin, J. Transgenerational transmission of pregestational and prenatal experience: Maternal adversity, enrichment, and underlying epigenetic and environmental mechanisms. J. Dev. Orig. Health Dis. 2016, 7, 588–601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Morrison, J.L.; Gill, A.; Rattanatray, L.; MacLaughlin, S.M.; Kleemann, D.; Walker, S.K.; McMillen, I.C. Maternal dietary restriction during the periconceptional period in normal-weight or obese ewes results in adrenocortical hypertrophy, an up-regulation of the JAK/STAT and down-regulation of the IGF1R signaling pathways in the adrenal of the postnatal lamb. Endocrinology 2013, 154, 4650–4662. [Google Scholar] [CrossRef] [PubMed]
- Ferry, B. The orexinergic system influences conditioned odor aversion learning in the rat: A theory on the processes and hypothesis on the circuit involved. Front. Behav. Neurosci. 2014, 8, 164. [Google Scholar] [CrossRef] [PubMed]
- Wankhade, U.D.; Zhong, Y.; Kang, P.; Alfaro, M.; Chintapalli, S.V.; Piccolo, B.D.; Mercer, K.E.; Andres, A.; Thakali, K.M.; Shankar, K. Maternal high-fat diet programs offspring liver steatosis in a sexually dimorphic manner in association with changes in gut microbial ecology in mice. Sci. Rep. 2018, 8, 16502. [Google Scholar] [CrossRef] [PubMed]
- Dearden, L.; Bouret, S.G.; Ozanne, S.E. Sex and gender differences in developmental programming of metabolism. Mol. Metab. 2018, 15, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Boukouvalas, G.; Antoniou, K.; Papalexi, E.; Kitraki, E. Post weaning high fat feeding affects rats’ behavior and hypothalamic pituitary adrenal axis at the onset of puberty in a sexually dimorphic manner. Neuroscience 2008, 153, 373–382. [Google Scholar] [CrossRef]

| Formula | CD | HFD | ||
|---|---|---|---|---|
| #D12450K | #D12492 | |||
| Composition | g | kcal | g | kcal |
| Protein | 19.2 | 20% | 26.2 | 20% |
| Carbohydrate | 67.3 | 70% | 26.3 | 20.1% |
| Fat | 4.3 | 10% | 34.9 | 59.9% |
| Total | 100% | 100% | ||
| Energy (kcal/g) | 3.85 | 5.24 | ||
| Ingredient | g | kcal | g | kcal |
| Casein, 80 Mesh | 200 | 800 | 200 | 800 |
| L-Cystine | 3 | 12 | 3 | 12 |
| Corn starch | 550 | 2200 | 0 | 0 |
| Maltodextrin 10 | 150 | 600 | 125 | 500 |
| Sucrose | 0 | 0 | 68.8 | 275.2 |
| Cellulose, BW200 | 50 | 0 | 50 | 0 |
| Soybean oil | 25 | 225 | 25 | 225 |
| Lard | 20 | 180 | 245 | 2205 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panchenko, P.E.; Lacroix, M.-C.; Jouin, M.; Voisin, S.; Badonnel, K.; Lemaire, M.; Meunier, N.; Safi-Stibler, S.; Persuy, M.-A.; Jouneau, L.; et al. Effect of Maternal Obesity and Preconceptional Weight Loss on Male and Female Offspring Metabolism and Olfactory Performance in Mice. Nutrients 2019, 11, 948. https://doi.org/10.3390/nu11050948
Panchenko PE, Lacroix M-C, Jouin M, Voisin S, Badonnel K, Lemaire M, Meunier N, Safi-Stibler S, Persuy M-A, Jouneau L, et al. Effect of Maternal Obesity and Preconceptional Weight Loss on Male and Female Offspring Metabolism and Olfactory Performance in Mice. Nutrients. 2019; 11(5):948. https://doi.org/10.3390/nu11050948
Chicago/Turabian StylePanchenko, Polina E., Marie-Christine Lacroix, Mélanie Jouin, Sarah Voisin, Karine Badonnel, Marion Lemaire, Nicolas Meunier, Sofiane Safi-Stibler, Marie-Annick Persuy, Luc Jouneau, and et al. 2019. "Effect of Maternal Obesity and Preconceptional Weight Loss on Male and Female Offspring Metabolism and Olfactory Performance in Mice" Nutrients 11, no. 5: 948. https://doi.org/10.3390/nu11050948
APA StylePanchenko, P. E., Lacroix, M.-C., Jouin, M., Voisin, S., Badonnel, K., Lemaire, M., Meunier, N., Safi-Stibler, S., Persuy, M.-A., Jouneau, L., Durieux, D., Lecoutre, S., Jammes, H., Rousseau-Ralliard, D., Breton, C., Junien, C., Baly, C., & Gabory, A. (2019). Effect of Maternal Obesity and Preconceptional Weight Loss on Male and Female Offspring Metabolism and Olfactory Performance in Mice. Nutrients, 11(5), 948. https://doi.org/10.3390/nu11050948





