Probiotic Lactobacillus casei: Effective for Managing Childhood Diarrhea by Altering Gut Microbiota and Attenuating Fecal Inflammatory Markers
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design
2.2. Patients
2.3. Bacterial Culture and Probiotic-Bacteria Count
2.4. Taxonomy-Based and Statistical Analyses
2.4.1. 16 S rRNA Amplification
2.4.2. Library Construction and Illumina MiSeq Sequencing
2.4.3. Data-Processing Pipeline
2.5. Fecal Immunoglobulin Assay
2.6. Measurement of Inflammatory Mediators in the Intestine
2.7. Statistical Analysis
3. Results
3.1. Subject Characteristics
3.2. Parameters Related to Digestive System
3.3. Fecal Bacterial Counts
3.4. Microbiota Analysis
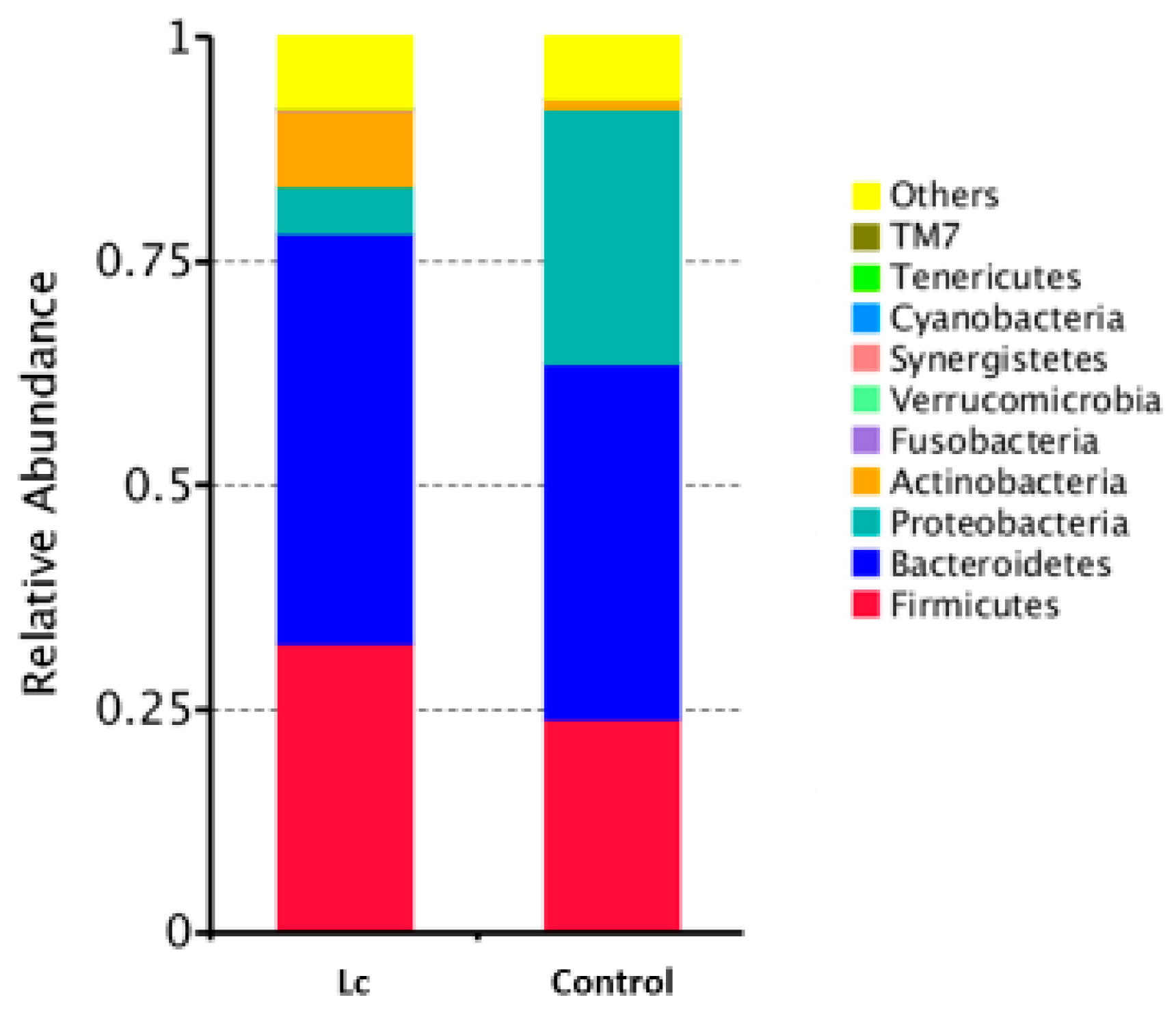
3.5. Effects at the Phylum Level
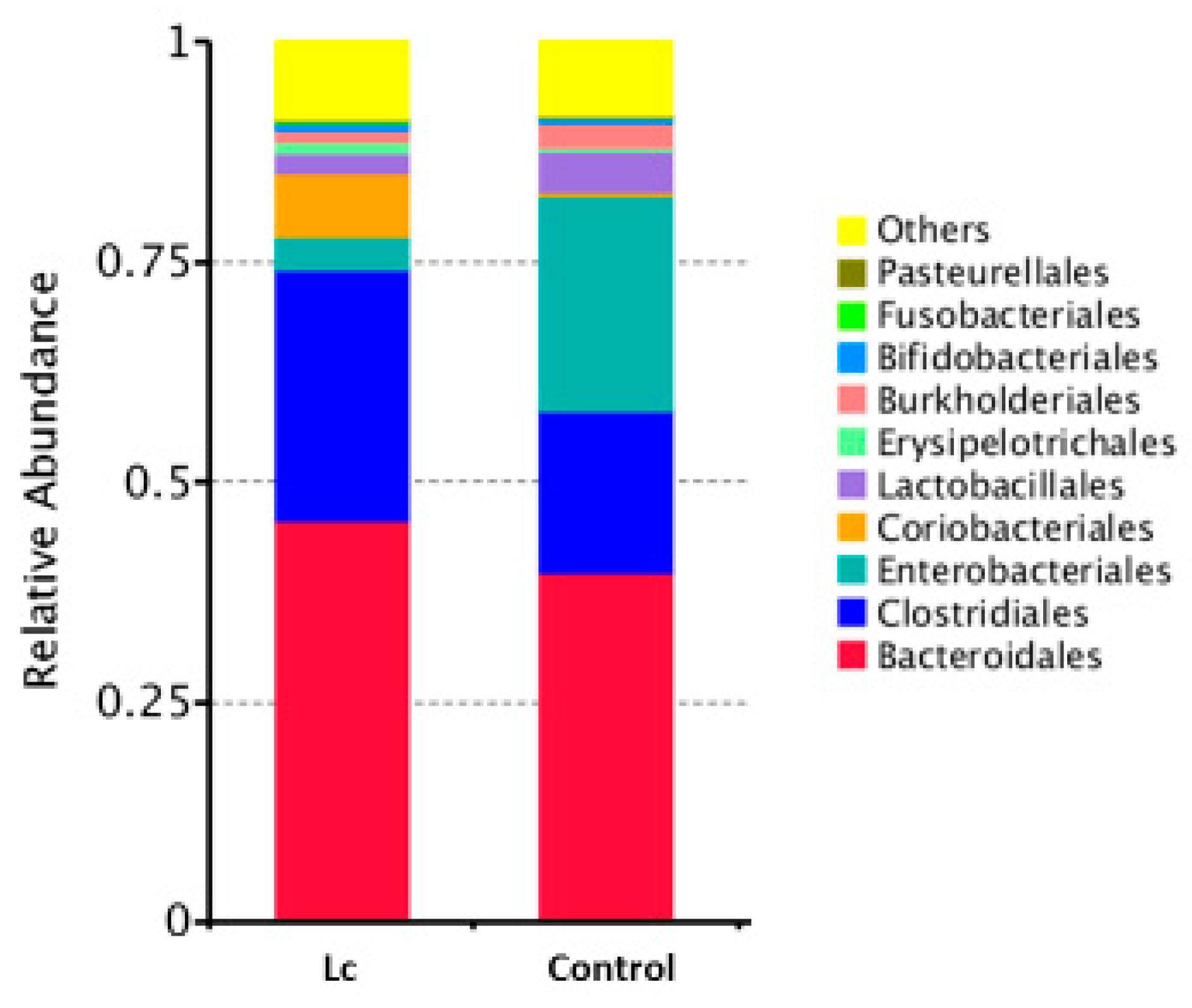
3.6. Alterations at Order Levels
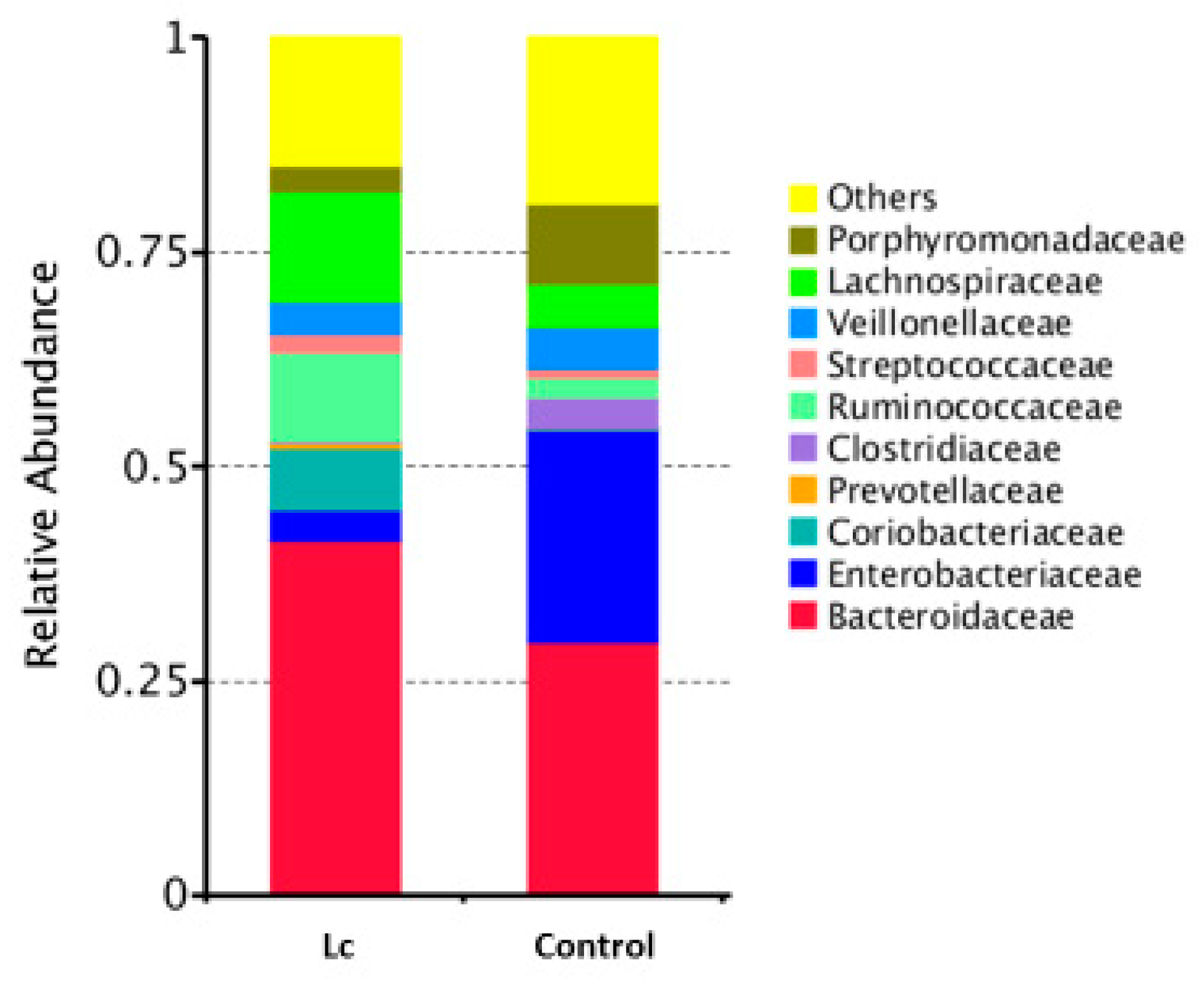
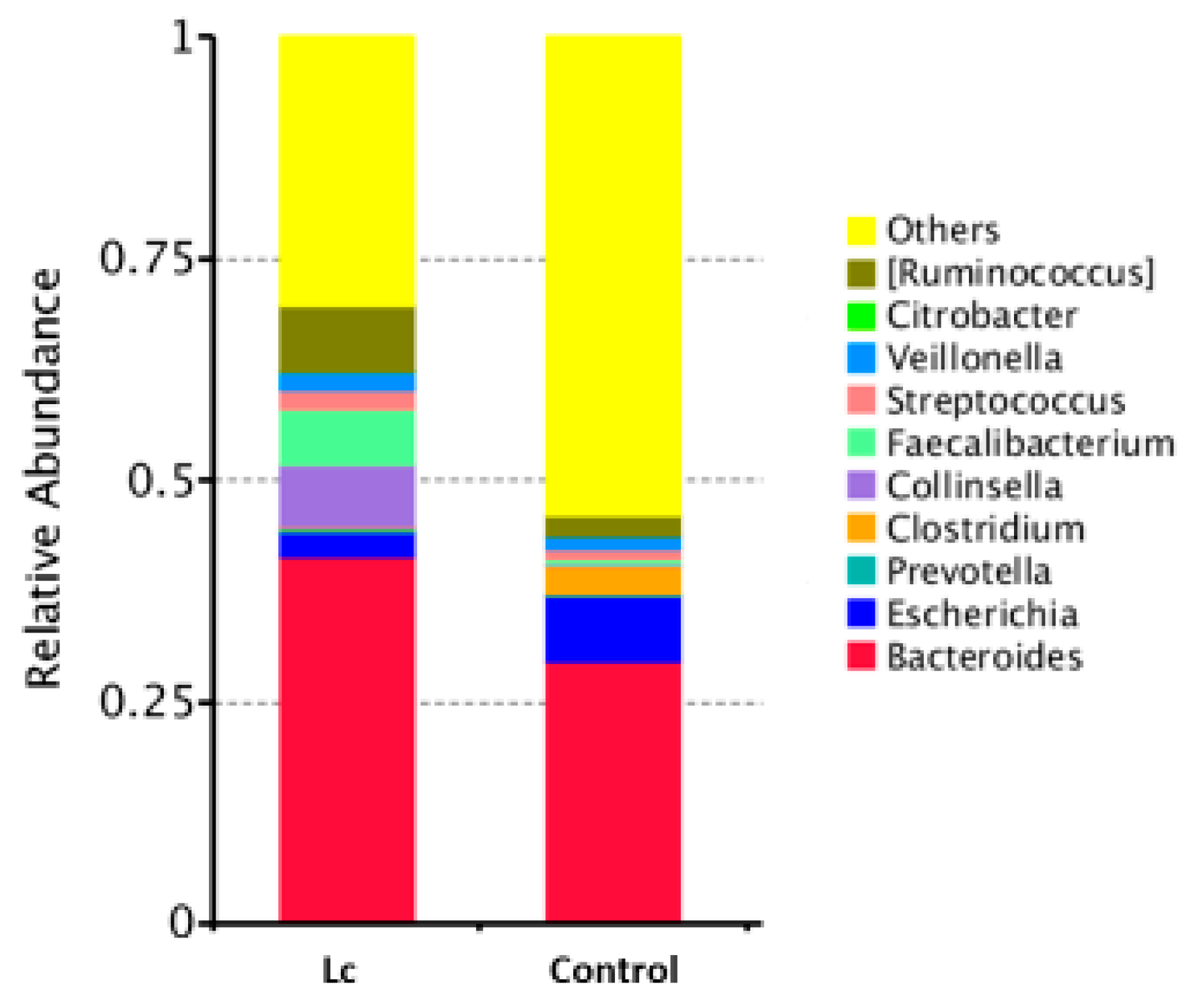
3.7. Alterations at the Family and Genus Levels
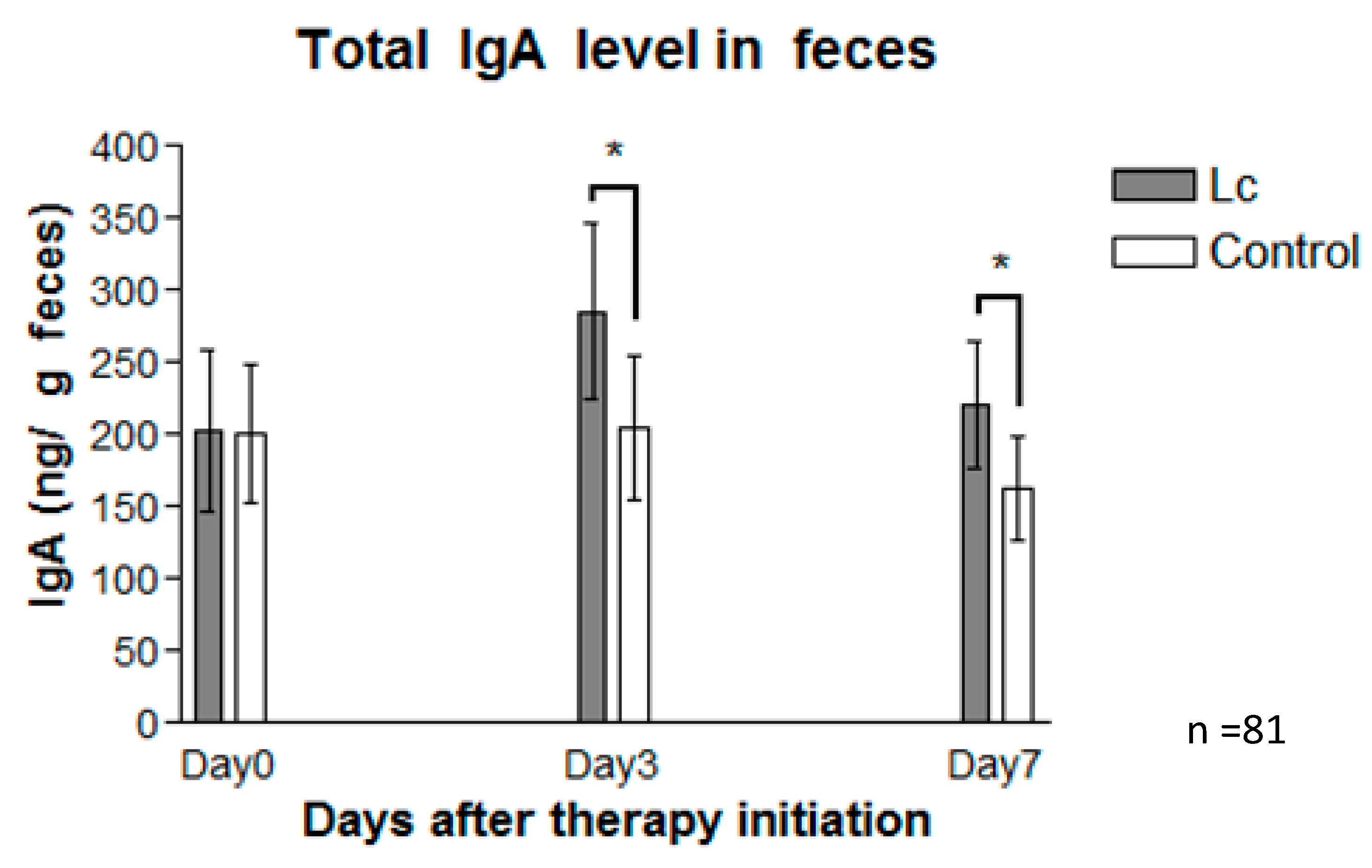
3.8. Fecal Immunoglobulin A
3.9. Measurement of Inflammatory Markers in Fecal Samples
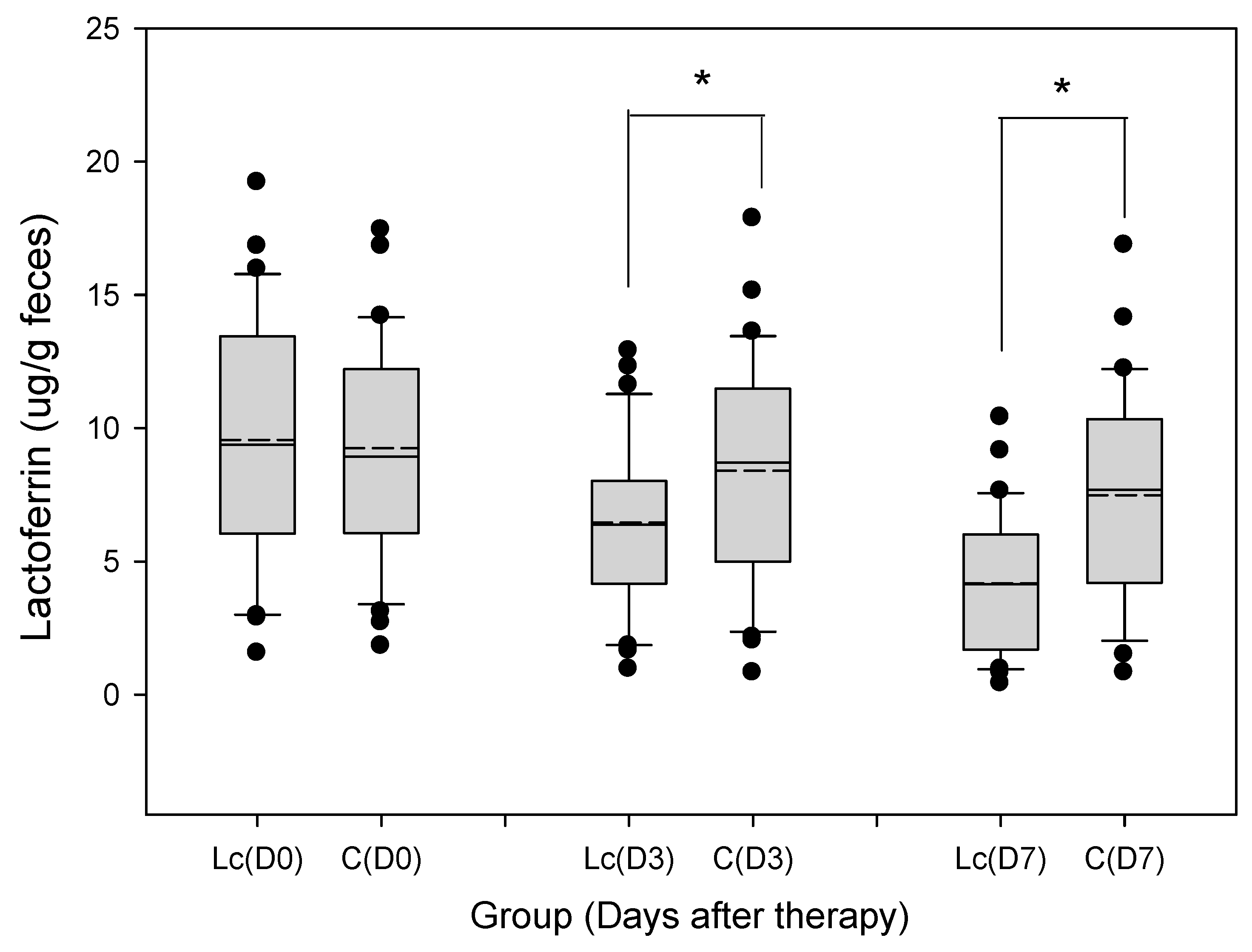
3.9.1. Fecal Lactoferrin
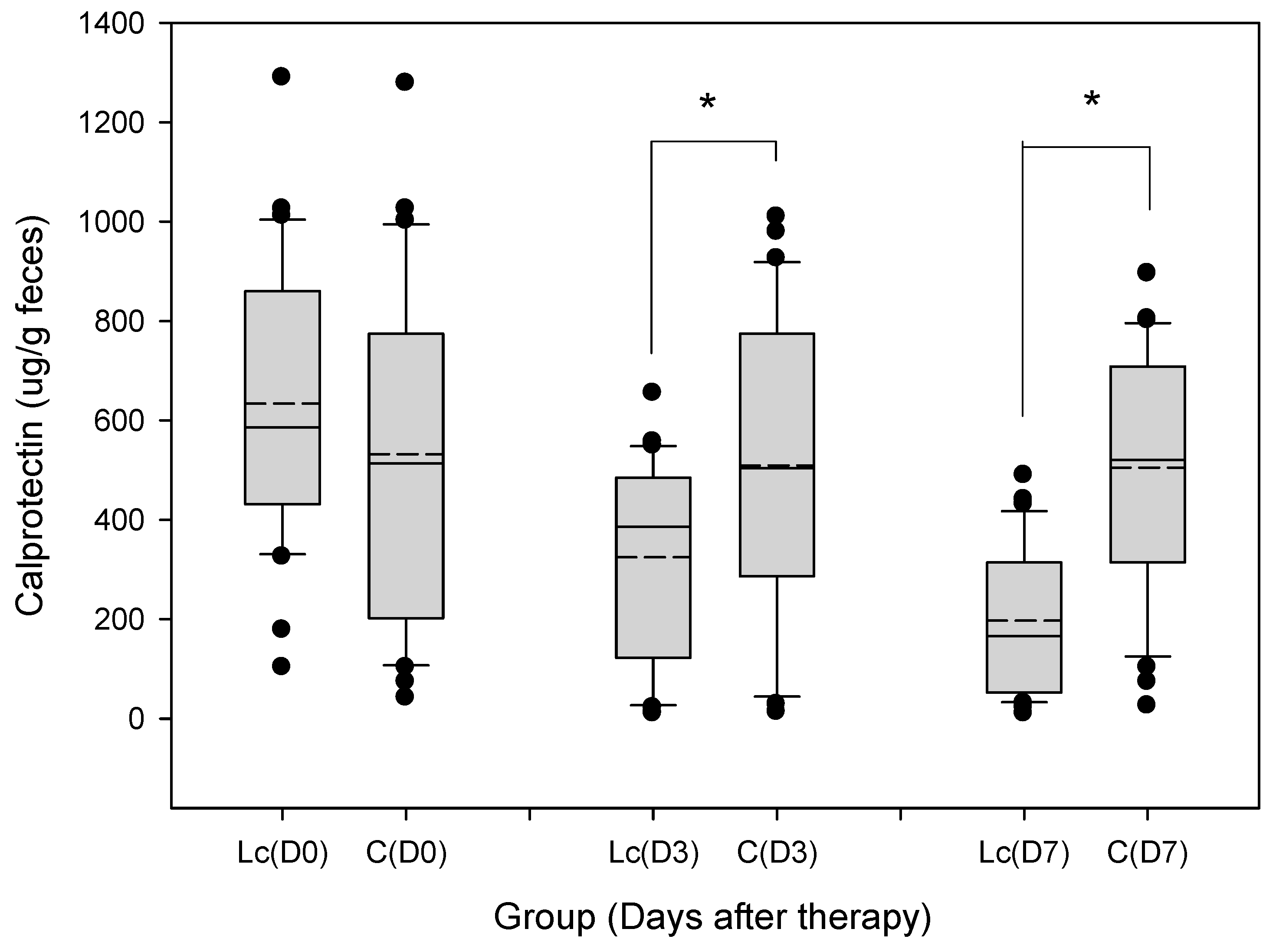
3.9.2. Fecal Calprotectin
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Pedone, C.A.; Arnaud, C.C.; Postaire, E.R.; Bouley, C.F.; Reinert, P. Multicentric study of the effect of milk fermented by Lactobacillus casei on the incidence of diarrhoea. Int. J. Clin. Pract. 2000, 54, 568–571. [Google Scholar] [PubMed]
- Gaon, D.; Garcia, H.; Winter, L.; Rodriguez, N.; Quintas, R.; Gonzalez, S.N.; Oliver, G. Effect of Lactobacillus strains and Saccharomyces boulardii on persistent diarrhea in children. Medicina (B Aires) 2003, 63, 293–298. [Google Scholar]
- Pereg, D.; Kimhi, O.; Tirosh, A.; Orr, N.; Kayouf, R.; Lishner, M. The effect of fermented yogurt on the prevention of diarrhea in a healthy adult population. Am. J. Infect. Control 2005, 33, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, R.D. The role of probiotic cultures in the control of gastrointestinal health. J. Nutr. 2000, 130, 396S–402S. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Immune-mediated mechanisms of action of probiotics and synbiotics in treating pediatric intestinal diseases. Nutrients 2018, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Marcotte, H.; Lavoie, M.C. Oral microbial ecology and the role of salivary immunoglobulin A. Microbiol. Mol. Biol. Rev. 1998, 62, 71–109. [Google Scholar]
- Walker, W.A. Role of nutrients and bacterial colonization in the development of intestinal host defense. J. Pediatr. Gastroenterol. Nutr. 2000, 30 (Suppl. 2), S2–S7. [Google Scholar] [CrossRef]
- Conlan, J.W.; North, R.J. Early pathogenesis of infection in the liver with the facultative intracellular bacteria Listeria monocytogenes, Francisella tularensis, and Salmonella typhimurium involves lysis of infected hepatocytes by leukocytes. Infect. Immun. 1992, 60, 5164–5171. [Google Scholar]
- Perdomo, J.J.; Gounon, P.; Sansonetti, P.J. Polymorphonuclear leukocyte transmigration promotes invasion of colonic epithelial monolayer by Shigella flexneri. J. Clin. Investig. 1994, 93, 633–643. [Google Scholar] [CrossRef]
- Sugi, K.; Saitoh, O.; Hirata, I.; Katsu, K. Fecal lactoferrin as a marker for disease activity in inflammatory bowel disease: Comparison with other neutrophil-derived proteins. Am. J. Gastroenterol. 1996, 91, 927–934. [Google Scholar]
- Guerrant, R.L.; Araujo, V.; Soares, E.; Kotloff, K.; Lima, A.A.; Cooper, W.H.; Lee, A.G. Measurement of fecal lactoferrin as a marker of fecal leukocytes. J. Clin. Microbiol. 1992, 30, 1238–1242. [Google Scholar]
- Olafsdottir, E.; Aksnes, L.; Fluge, G.; Berstad, A. Faecal calprotectin levels in infants with infantile colic, healthy infants, children with inflammatory bowel disease, children with recurrent abdominal pain and healthy children. Acta Paediatr. 2002, 91, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Langhorst, J.; Elsenbruch, S.; Koelzer, J.; Rueffer, A.; Michalsen, A.; Dobos, G.J. Noninvasive markers in the assessment of intestinal inflammation in inflammatory bowel diseases: Performance of fecal lactoferrin, calprotectin, and PMN-elastase, CRP, and clinical indices. Am. J. Gastroenterol. 2008, 103, 162–169. [Google Scholar] [CrossRef]
- Poullis, A.; Foster, R.; Northfield, T.C.; Mendall, M.A. Review article: Faecal markers in the assessment of activity in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2002, 16, 675–681. [Google Scholar] [CrossRef]
- Magoc, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Fagerberg, U.L.; Loof, L.; Myrdal, U.; Hansson, L.O.; Finkel, Y. Colorectal inflammation is well predicted by fecal calprotectin in children with gastrointestinal symptoms. J. Pediatr. Gastroenterol. Nutr. 2005, 40, 450–455. [Google Scholar] [CrossRef]
- Zimmerman, C.M.; Bresee, J.S.; Parashar, U.D.; Riggs, T.L.; Holman, R.C.; Glass, R.I. Cost of diarrhea-associated hospitalizations and outpatient visits in an insured population of young children in the United States. Pediatr. Infect. Dis. J. 2001, 20, 14–19. [Google Scholar] [CrossRef]
- Farthing, M.J. Novel targets for the pharmacotherapy of diarrhoea: A view for the millennium. J. Gastroenterol. Hepatol. 2000, 15, G38–G45. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeldt, V.; Michaelsen, K.F.; Jakobsen, M.; Larsen, C.N.; Moller, P.L.; Pedersen, P.; Tvede, M.; Weyrehter, H.; Valerius, N.H.; Paerregaard, A. Effect of probiotic Lactobacillus strains in young children hospitalized with acute diarrhea. Pediatr. Infect. Dis. J. 2002, 21, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Guandalini, S.; Pensabene, L.; Zikri, M.A.; Dias, J.A.; Casali, L.G.; Hoekstra, H.; Kolacek, S.; Massar, K.; Micetic-Turk, D.; Papadopoulou, A.; et al. Lactobacillus GG administered in oral rehydration solution to children with acute diarrhea: A multicenter European trial. J. Pediatr. Gastroenterol. Nutr. 2000, 30, 54–60. [Google Scholar] [CrossRef]
- Canani, R.B.; Cirillo, P.; Terrin, G.; Cesarano, L.; Spagnuolo, M.I.; De Vincenzo, A.; Albano, F.; Passariello, A.; De Marco, G.; Manguso, F.; et al. Probiotics for treatment of acute diarrhoea in children: Randomised clinical trial of five different preparations. BMJ 2007, 335, 340. [Google Scholar] [CrossRef] [PubMed]
- Corsello, G.; Carta, M.; Marinello, R.; Picca, M.; De Marco, G.; Micillo, M.; Ferrara, D.; Vigneri, P.; Cecere, G.; Ferri, P.; et al. Preventive effect of cow’s milk fermented with Lactobacillus paracasei CBA L74 on common infectious diseases in children: A multicenter randomized controlled trial. Nutrients 2017, 9, 669. [Google Scholar] [CrossRef]
- Souza, D.N.; Jorge, M.T. The effect of Lactobacillus casei and Bifidobacterium breve on antibiotic-associated diarrhea treatment: Randomized double-blind clinical trial. Rev. Soc. Bras. Med. Trop. 2012, 45, 112–116. [Google Scholar] [CrossRef]
- Salazar-Lindo, E.; Miranda-Langschwager, P.; Campos-Sanchez, M.; Chea-Woo, E.; Sack, R.B. Lactobacillus casei strain GG in the treatment of infants with acute watery diarrhea: A randomized, double-blind, placebo controlled clinical trial. BMC Pediatr. 2004, 4, 18. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Zilberman-Schapira, G.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Zur, M.; Regev-Lehavi, D.; Ben-Zeev Brik, R.; Federici, S.; et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell 2018, 174, 1406–1423.e16. [Google Scholar] [CrossRef]
- Mantegazza, C.; Molinari, P.; D’Auria, E.; Sonnino, M.; Morelli, L.; Zuccotti, G.V. Probiotics and antibiotic-associated diarrhea in children: A review and new evidence on Lactobacillus rhamnosus GG during and after antibiotic treatment. Pharmacol. Res. 2018, 128, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Sivan, A.; Corrales, L.; Hubert, N.; Williams, J.B.; Aquino-Michaels, K.; Earley, Z.M.; Benyamin, F.W.; Lei, Y.M.; Jabri, B.; Alegre, M.L.; et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015, 350, 1084–1089. [Google Scholar] [CrossRef]
- Matson, V.; Fessler, J.; Bao, R.; Chongsuwat, T.; Zha, Y.; Alegre, M.L.; Luke, J.J.; Gajewski, T.F. The commensal microbiome is associated with anti-PD-1 efficacy in metastatic melanoma patients. Science 2018, 359, 104–108. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Helmink, B.A.; Spencer, C.N.; Reuben, A.; Wargo, J.A. The influence of the gut microbiome on cancer, immunity, and cancer immunotherapy. Cancer Cell 2018, 33, 570–580. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Deng, Y.; Chu, Q.; Zhang, P. Gut microbiome and cancer immunotherapy. Cancer Lett. 2019, 447, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Rinne, M.; Kalliomaki, M.; Salminen, S.; Isolauri, E. Probiotic intervention in the first months of life: Short-term effects on gastrointestinal symptoms and long-term effects on gut microbiota. J. Pediatr. Gastroenterol. Nutr. 2006, 43, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Schrezenmeir, J.; Heller, K.; McCue, M.; Llamas, C.; Lam, W.; Burow, H.; Kindling-Rohracker, M.; Fischer, W.; Sengespeik, H.C.; Comer, G.M.; et al. Benefits of oral supplementation with and without synbiotics in young children with acute bacterial infections. Clin. Pediatr. 2004, 43, 239–249. [Google Scholar] [CrossRef]
- Berni Canani, R.; Sangwan, N.; Stefka, A.T.; Nocerino, R.; Paparo, L.; Aitoro, R.; Calignano, A.; Khan, A.A.; Gilbert, J.A.; Nagler, C.R. Lactobacillus rhamnosus GG-supplemented formula expands butyrate-producing bacterial strains in food allergic infants. ISME J. 2016, 10, 742–750. [Google Scholar] [CrossRef]
- Barcenilla, A.; Pryde, S.E.; Martin, J.C.; Duncan, S.H.; Stewart, C.S.; Henderson, C.; Flint, H.J. Phylogenetic relationships of butyrate-producing bacteria from the human gut. Appl. Environ. Microbiol. 2000, 66, 1654–1661. [Google Scholar] [CrossRef]
- Duncan, S.H.; Barcenilla, A.; Stewart, C.S.; Pryde, S.E.; Flint, H.J. Acetate utilization and butyryl coenzyme A (CoA):acetate-CoA transferase in butyrate-producing bacteria from the human large intestine. Appl. Environ. Microbiol. 2002, 68, 5186–5190. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.J.; Brummer, R.J. Review article: The role of butyrate on colonic function. Aliment. Pharmacol. There 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Zheng, L.; Kelly, C.J.; Battista, K.D.; Schaefer, R.; Lanis, J.M.; Alexeev, E.E.; Wang, R.X.; Onyiah, J.C.; Kominsky, D.J.; Colgan, S.P. Microbial-derived butyrate promotes epithelial barrier function through IL-10 receptor-dependent repression of claudin-2. J. Immunol. 2017, 199, 2976–2984. [Google Scholar] [CrossRef]
- Vancamelbeke, M.; Laeremans, T.; Vanhove, W.; Arnauts, K.; Ramalho, A.S.; Farre, R.; Cleynen, I.; Ferrante, M.; Vermeire, S. Butyrate does not protect against inflammation-induced loss of epithelial barrier function and cytokine production in primary cell monolayers from patients with ulcerative colitis. J. Crohn’s Colitis 2019. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Yeoh, B.S.; Walker, R.E.; Xiao, X.; Saha, P.; Golonka, R.M.; Cai, J.; Bretin, A.C.A.; Cheng, X.; Liu, Q.; et al. Microbiota fermentation-NLRP3 axis shapes the impact of dietary fibres on intestinal inflammation. Gut 2019. [Google Scholar] [CrossRef]
- Biddle, A.; Stewart, L.; Blanchard, J.; Leschine, S. Untangling the genetic basis of fibrolytic specialization by Lachnospiraceae and Ruminococcaceae in diverse gut communities. Diversity 2013, 5, 627–640. [Google Scholar] [CrossRef]
- Fischbach, M.A.; Sonnenburg, J.L. Eating for two: How metabolism establishes interspecies interactions in the gut. Cell Host Microbe 2011, 10, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Vael, C.; Desager, K. The importance of the development of the intestinal microbiota in infancy. Curr. Opin. Pediatr. 2009, 21, 794–800. [Google Scholar] [CrossRef]
- Shin, A.; Preidis, G.A.; Shulman, R.; Kashyap, P.C. The gut microbiome in adult and pediatric functional gastrointestinal disorders. Clin. Gastroenterol. Hepatol. 2019, 17, 256–274. [Google Scholar] [CrossRef] [PubMed]
- Lukac, P.J.; Bonomo, R.A.; Logan, L.K. Extended-spectrum beta-lactamase-producing Enterobacteriaceae in children: Old foe, emerging threat. Clin. Infect. Dis. 2015, 60, 1389–1397. [Google Scholar]
- Logan, L.K.; Medernach, R.L.; Domitrovic, T.N.; Rispens, J.R.; Hujer, A.M.; Qureshi, N.K.; Marshall, S.H.; Nguyen, D.C.; Rudin, S.D.; Zheng, X.; et al. The clinical and molecular epidemiology of CTX-M-9 group producing enterobacteriaceae infections in children. Infect. Dis. Ther. 2019, 8, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Van de Perre, P. Transfer of antibody via mother’s milk. Vaccine 2003, 21, 3374–3376. [Google Scholar] [CrossRef]
- Mazanec, M.B.; Nedrud, J.G.; Kaetzel, C.S.; Lamm, M.E. A three-tiered view of the role of IgA in mucosal defense. Immunol. Today 1993, 14, 430–435. [Google Scholar] [CrossRef]
- Bouvet, J.P.; Fischetti, V.A. Diversity of antibody-mediated immunity at the mucosal barrier. Infect. Immun. 1999, 67, 2687–2691. [Google Scholar]
- Scholtens, P.A.; Alliet, P.; Raes, M.; Alles, M.S.; Kroes, H.; Boehm, G.; Knippels, L.M.; Knol, J.; Vandenplas, Y. Fecal secretory immunoglobulin A is increased in healthy infants who receive a formula with short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides. J. Nutr. 2008, 138, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Levay, P.F.; Viljoen, M. Lactoferrin: A general review. Haematologica 1995, 80, 252–267. [Google Scholar]
- Kane, S.V.; Sandborn, W.J.; Rufo, P.A.; Zholudev, A.; Boone, J.; Lyerly, D.; Camilleri, M.; Hanauer, S.B. Fecal lactoferrin is a sensitive and specific marker in identifying intestinal inflammation. Am. J. Gastroenterol. 2003, 98, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Chang, C.J.; Lin, T.Y.; Lai, M.W.; Chao, H.C.; Kong, M.S. Usefulness of fecal lactoferrin in predicting and monitoring the clinical severity of infectious diarrhea. World J. Gastroenterol. 2011, 17, 4218–4224. [Google Scholar] [CrossRef]
- Pulimood, A.B.; Mathan, M.M.; Mathan, V.I. Quantitative and ultrastructural analysis of rectal mucosal mast cells in acute infectious diarrhea. Dig. Dis. Sci. 1998, 43, 2111–2116. [Google Scholar] [CrossRef]
- Poullis, A.; Foster, R.; Mendall, M.A.; Fagerhol, M.K. Emerging role of calprotectin in gastroenterology. J. Gastroenterol. Hepatol. 2003, 18, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Sykora, J.; Siala, K.; Huml, M.; Varvarovska, J.; Schwarz, J.; Pomahacova, R. Evaluation of faecal calprotectin as a valuable non-invasive marker in distinguishing gut pathogens in young children with acute gastroenteritis. Acta Paediatr. 2010, 99, 1389–1395. [Google Scholar] [CrossRef] [PubMed]
- Shastri, Y.M.; Bergis, D.; Povse, N.; Schafer, V.; Shastri, S.; Weindel, M.; Ackermann, H.; Stein, J. Prospective multicenter study evaluating fecal calprotectin in adult acute bacterial diarrhea. Am. J. Med. 2008, 121, 1099–1106. [Google Scholar] [CrossRef]
| Variables | Probiotics Group (n = 42) | Control Group (n = 39) | p-Value |
|---|---|---|---|
| Age at entry (months) | 27.9 ± 15.6 | 27.6 ± 16.1 | 0.672 |
| Male/Female | 24:18 | 22:17 | 0.418 |
| Duration of diarrhea before intervention (h) | 37.3 ± 22.9 | 38.1 ± 21.8 | 0.621 |
| Vomiting | 13 (30.9%) | 12 (30.7%) | 0.868 |
| Fever on admission (>38.5 °C) | 20 (47.6%) | 19 (48.7%) | 0.514 |
| White blood cells (WBC) counts (106 cells/L) | 12,354 ± 2865 | 12,075 ± 2742 | 0.426 |
| Abdominal pain/irritable crying on admission | 14 (33.3%) | 13 (33.3%) | 0.563 |
| Parameter | Day 0 | Day 3 | Day 7 | Day 14 |
|---|---|---|---|---|
| Appetite/intake (score) # | ||||
| Lc group | 1.2 ± 0.4 | * 3.5 ± 0.7 | *4.1 ± 0.7 | * 4.3 ± 0.6 |
| Control group | 1.2 ± 0.4 | * 2.8 ± 0.6 | *3.1 ± 0.6 | * 3.8 ± 0.5 |
| Bloating/abdominal distension, n (%) | ||||
| Lc group | 20 (47.6%) | 12 (28.6%) | * 6 (14.3%) | * 2 (4.8%) |
| Control group | 17 (43.6%) | 13 (33.3%) | * 11 (28.2%) | * 6 (15.4%) |
| Abdominal pain/colic, n (%) | ||||
| Lc group | 17 (40.5%) | * 8 (19.0%) | * 4 (9.5%) | * 2 (4.8%) |
| Control group | 16 (41.0%) | * 12 (30.8%) | * 9 (23.1%) | * 7 (17.9%) |
| Diarrhea, n (%) | ||||
| Lc group | 42 (100.0%) | *29 (69.0%) | *8 (19.0%) | *3 (7.1%) |
| Control group | 39 (100.0%) | *33 (84.6%) | *16 (41.0%) | *6 (15.4%) |
| Vomiting, n (%) | ||||
| Lc group | 20 (47.6%) | 6 (14.3%) | 3 (7.1%) | 2 (4.8%) |
| Control group | 18 (46.2%) | 7 (17.9%) | 4 (10.3%) | 3 (7.7%) |
| Fever, n (%) | ||||
| Lc group | 29 (69.0%) | 13 (31.0%) | 1 (2.4%) | 0 (0.0%) |
| Control group | 26 (66.7%) | 13 (33.3%) | 1 (2.6%) | 0 (0.0%) |
| Fecal Bacteria log10 CFU/g of Stool | Day 0 | Day 3 | Day 7 | |||
|---|---|---|---|---|---|---|
| Lc | Control | Lc | Control | Lc | Control | |
| Lactobacilli | 8.2 (7.6–9.0) | 8.3 (7.6–9.1) | * 8.6 (8.0–9.4) | * 8.1 (7.4–8.7) | * 9.1 (8.3–9.8) | * 8.2 (7.6–8.8) |
| Bifidobacterium | 9.3 (8.5–10.1) | 9.4 (8.6–10.2) | 9.5 (8.8–10.2) | 9.1 (8.5–9.7) | * 9.7 (9.1–10.4) | * 9.0 (8.4–9.6) |
| Gram-negative bacilli | 9.4 (8.8–9.7) | 9.3 (8.7–9.6) | 9.1 (8.6–9.5) | 9.4 (8.8–9.7) | 8.9 (8.4–9.2) | 9.3 (8.8–9.6) |
| Anaerobic bacteria | 9.5 (9.0–9.8) | 9.5 (9.1–9.8) | 9.4 (9.0–9.7) | 9.5 (9.0–9.8) | 9.4 (9.0–9.7) | 9.5 (9.1–9.8) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, H.-H.; Chiu, C.-H.; Kong, M.-S.; Chang, C.-J.; Chen, C.-C. Probiotic Lactobacillus casei: Effective for Managing Childhood Diarrhea by Altering Gut Microbiota and Attenuating Fecal Inflammatory Markers. Nutrients 2019, 11, 1150. https://doi.org/10.3390/nu11051150
Lai H-H, Chiu C-H, Kong M-S, Chang C-J, Chen C-C. Probiotic Lactobacillus casei: Effective for Managing Childhood Diarrhea by Altering Gut Microbiota and Attenuating Fecal Inflammatory Markers. Nutrients. 2019; 11(5):1150. https://doi.org/10.3390/nu11051150
Chicago/Turabian StyleLai, Hung-Hsiang, Cheng-Hsun Chiu, Man-Shan Kong, Chee-Jen Chang, and Chien-Chang Chen. 2019. "Probiotic Lactobacillus casei: Effective for Managing Childhood Diarrhea by Altering Gut Microbiota and Attenuating Fecal Inflammatory Markers" Nutrients 11, no. 5: 1150. https://doi.org/10.3390/nu11051150
APA StyleLai, H.-H., Chiu, C.-H., Kong, M.-S., Chang, C.-J., & Chen, C.-C. (2019). Probiotic Lactobacillus casei: Effective for Managing Childhood Diarrhea by Altering Gut Microbiota and Attenuating Fecal Inflammatory Markers. Nutrients, 11(5), 1150. https://doi.org/10.3390/nu11051150






