Isocaloric Substitution of Dietary Carbohydrate Intake with Fat Intake and MRI-Determined Total Volumes of Visceral, Subcutaneous and Hepatic Fat Content in Middle-Aged Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Dietary Assessment
2.3. Magnetic Resonance Imaging (MRI)
2.4. Assessment of Covariables
2.5. Clinical Chemical Measurements
2.6. Statistical Analysis
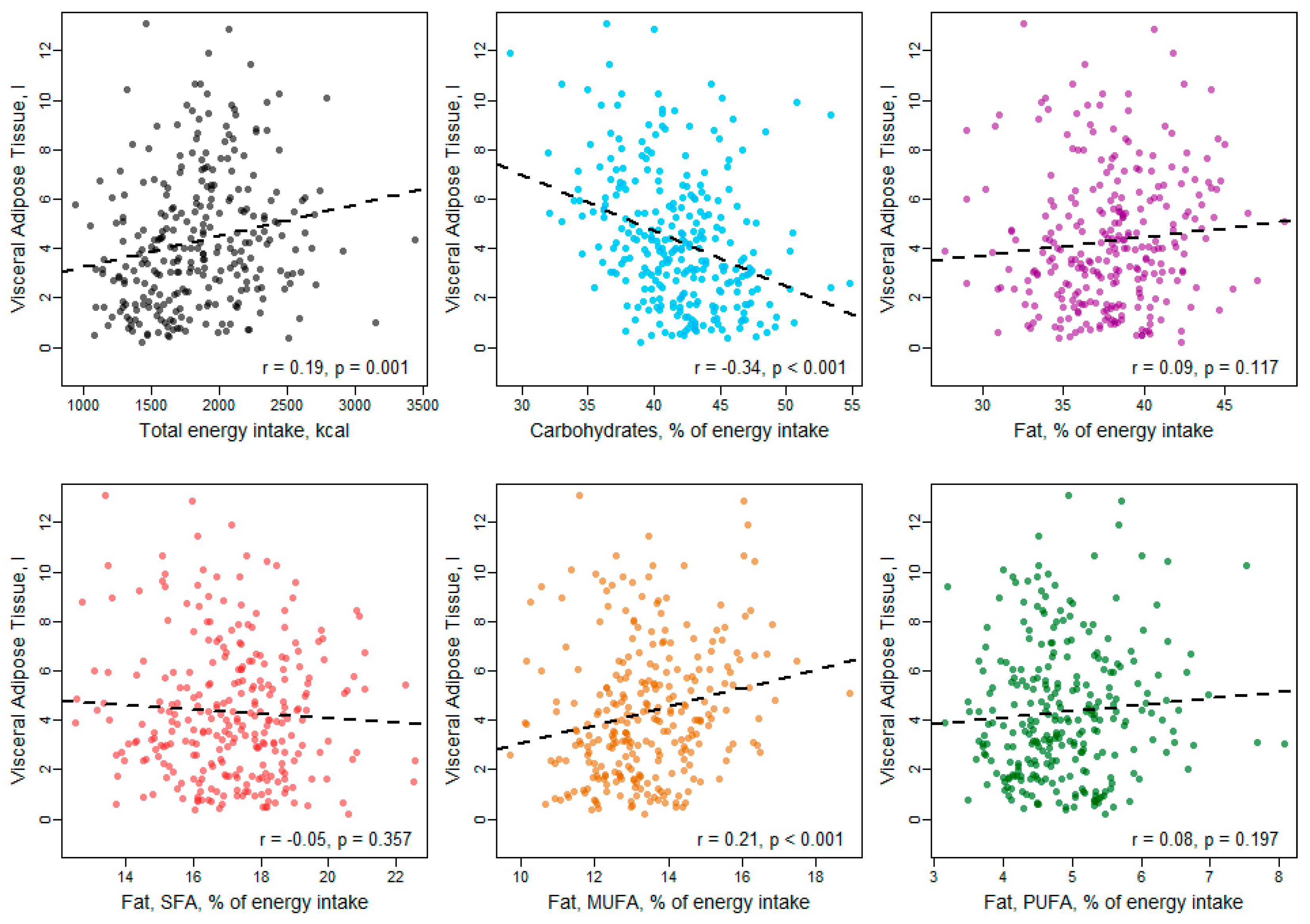
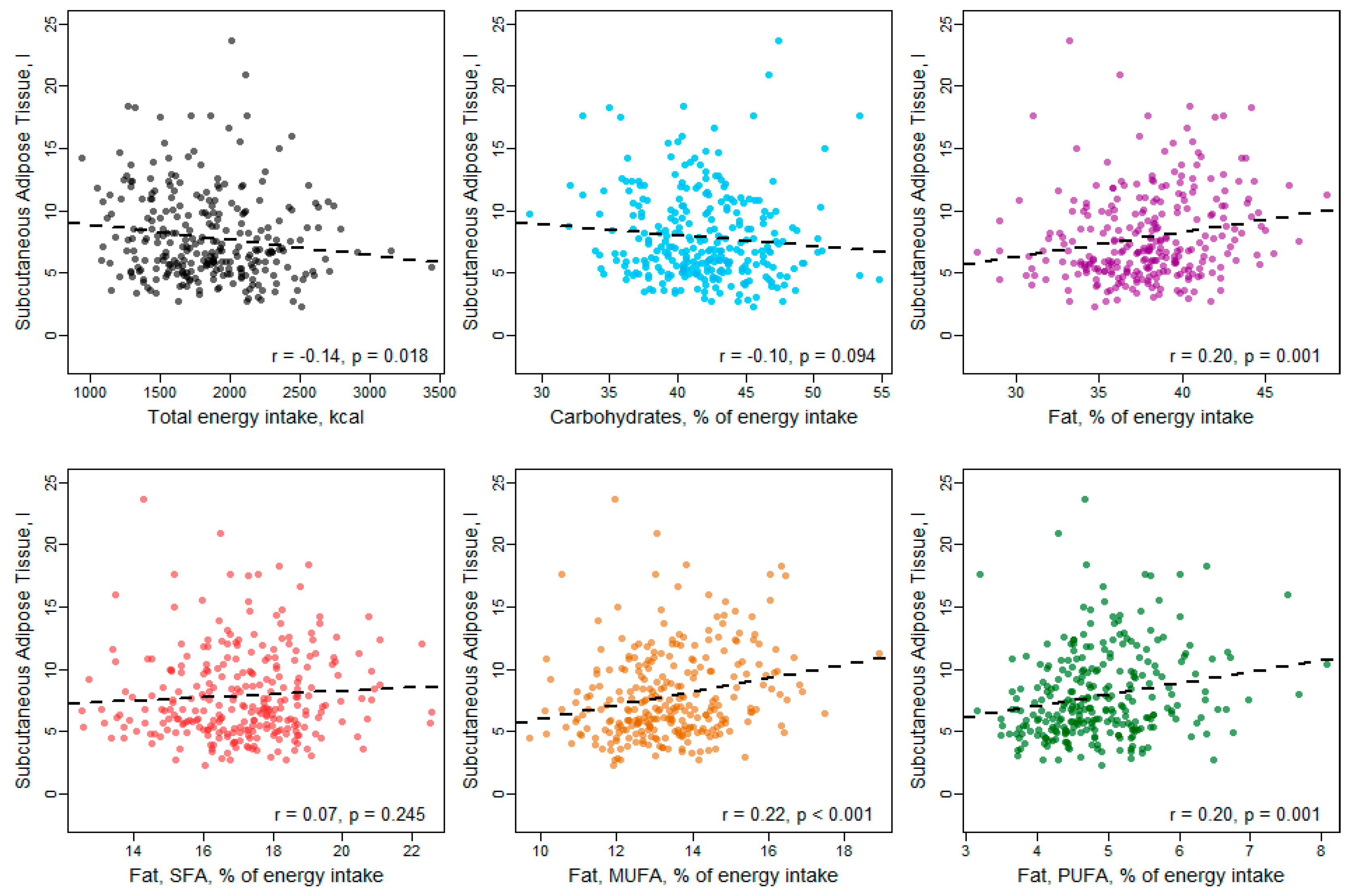
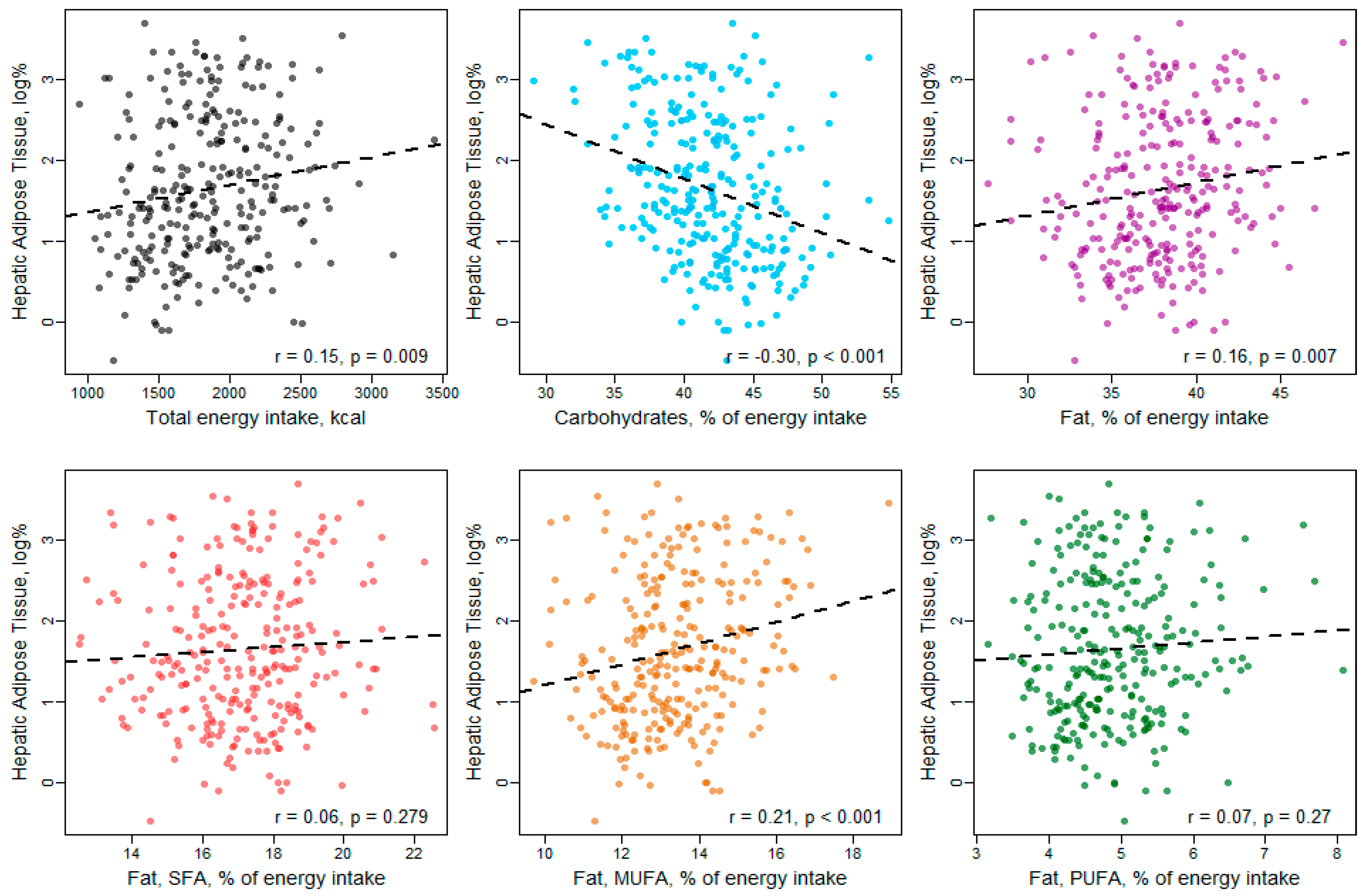
3. Results
4. Discussion
Differences between VAT and SAT
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Obesity and Overweight. 2017. Available online: http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 17 June 2018).
- Haffner, S.M. Relationship of metabolic risk factors and development of cardiovascular disease and diabetes. Obesity (Silver Spring) 2006, 14, 121–127. [Google Scholar] [CrossRef]
- Lim, S.; Meigs, J.B. Links between ectopic fat and vascular disease in humans. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1820–1826. [Google Scholar] [CrossRef] [PubMed]
- Storz, C.; Heber, S.D.; Rospleszcz, S.; Machann, J.; Sellner, S.; Nikolaou, K.; Lorbeer, R.; Gatidis, S.; Elser, S.; Peters, A.; et al. The role of visceral and subcutaneous adipose tissue measurements and their ratio by magnetic resonance imaging in subjects with prediabetes, diabetes and healthy controls from a general population without cardiovascular disease. Br. J. Radiol. 2018, 91, 20170808. [Google Scholar] [CrossRef]
- Austin, G.L.; Ogden, L.G.; Hill, J.O. Trends in carbohydrate, fat and protein intakes and association with energy intake in normal.-weight, overweight and obese individuals: 1971–2006. Am. J. Clin. Nutr. 2011, 93, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, D.S.; Willett, W.C.; Volek, J.S.; Neuhouser, M.L. Dietary fat: From foe to friend? Science 2018, 362, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.; Cragg, M.; deFonseka, J.; Hite, A.; Rosenberg, M.; Zhou, B. Statistical review of US macronutrient consumption data, 1965–2011: Americans have been following dietary guidelines, coincident with the rise in obesity. Nutrition 2015, 31, 727–732. [Google Scholar] [CrossRef]
- Boqué, N.; Campion, J.; Paternain, L.; Garcia-Diaz, D.F.; Galarraga, M.; Portillo, M.P.; Milagro, F.I.; Ortiz de Solorzano, C.; Martinez, J.A. Influence of dietary macronutrient composition on adiposity and cellularity of different fat depots in Wistar rats. J. Physiol. Biochem. 2009, 65, 387–395. [Google Scholar] [CrossRef]
- Fischer, K.; Moewes, D.; Koch, M.; Müller, H.P.; Jacobs, G.; Kassubek, J.; Lieb, W.; Nöthlings, U. MRI-determined total volumes of visceral and subcutaneous abdominal and trunk adipose tissue are differentially and sex-dependently associated with patterns of estimated usual nutrient intake in a northern German population. Am. J. Clin. Nutr. 2015, 101, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Laxy, M.; Knoll, G.; Schunk, M.; Meisinger, C.; Huth, C.; Holle, R. Quality of diabetes care in Germany improved from 2000 to 2007 to 2014 but improvements diminished since 2007. Evidence from the population-based KORA studies. PLoS ONE 2016, 11, e0164704. [Google Scholar] [CrossRef]
- Bamberg, F.; Hetterich, H.; Rospleszcz, S.; Lorbeer, R.; Auweter, S.D.; Schlett, C.L.; Schafnitzel, A.; Bayerl, C.; Schindler, A.; Saam, T.; et al. Subclinical disease burden as assessed by whole-body MRI in subjects with prediabetes, subjects with diabetes and normal control subjects from the general population: The KORA-MRI study. Diabetes 2017, 66, 158–169. [Google Scholar] [CrossRef]
- Freese, J.; Feller, S.; Harttig, U.; Kleiser, C.; Linseisen, J.; Fischer, B.; Leitzmann, M.F.; Six-Merker, J.; Michels, K.B.; Nimptsch, K.; et al. Development and evaluation of a short 24-h food list as part of a blended dietary assessment strategy in large-scale cohort studies. Eur. J. Clin. Nutr. 2014, 68, 324–329. [Google Scholar] [CrossRef]
- Tooze, J.A.; Kipnis, V.; Buckman, D.W.; Carroll, R.J.; Freedman, L.S.; Guenther, P.M.; Krebs-Smith, S.M.; Subar, A.F.; Dodd, K.W. A mixed-effects model approach for estimating the distribution of usual intake of nutrients: The NCI method. Stat. Med. 2010, 29, 2857–2868. [Google Scholar] [CrossRef] [PubMed]
- Haubrock, J.; Nöthlings, U.; Volatier, J.L.; Dekkers, A.; Ocké, M.; Harttig, U.; Illner, A.K.; Knüppel, S.; Andersen, L.F.; Boeing, H.; et al. Estimating usual food intake distributions by using the multiple source method in the EPIC-Potsdam Calibration Study. J. Nutr. 2011, 141, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Hetterich, H.; Bayerl, C.; Peters, A.; Heier, M.; Linkohr, B.; Meisinger, C.; Auweter, S.; Kannengießer, S.A.; Kramer, H.; Ertl-Wagner, B.; et al. Feasibility of a three-step magnetic resonance imaging approach for the assessment of hepatic steatosis in an asymptomatic study population. Eur. Radiol. 2016, 26, 1895–1904. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications. Part 1: Diagnosis and Classification of Diabetes Mellitus; Report of a WHO Consultation; WHO: Geneva, Switzerland, 1999. [Google Scholar]
- Meisinger, C.; Thorand, B.; Schneider, A.; Stieber, J.; Döring, A.; Löwel, H. Sex differences in risk factors for incident type 2 diabetes mellitus: The MONICA Augsburg cohort study. JAMA Intern. Med. 2002, 162, 82–89. [Google Scholar] [CrossRef]
- Meisinger, C.; Löwel, H.; Thorand, B.; Döring, A. Leisure time physical activity and the risk of type 2 diabetes in men and women from the general population. The MONICA/KORA Augsburg Cohort Study. Diabetologia 2005, 48, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Westerbacka, J.; Lammi, K.; Häkkinen, A.M.; Rissanen, A.; Salminen, I.; Aro, A.; Yki-Järvinen, H. Dietary fat content modifies liver fat in overweight nondiabetic subjects. J. Clin. Endocrinol. Metab. 2005, 90, 2804–2809. [Google Scholar] [CrossRef]
- Rosqvist, F.; Iggman, D.; Kullberg, J.; Cedernaes, J.; Johansson, H.E.; Larsson, A.; Johansson, L.; Ahlström, H.; Arner, P.; Dahlman, I.; et al. Overfeeding polyunsaturated and saturated fat causes distinct effects on liver and visceral fat accumulation in humans. Diabetes 2014, 63, 2356–2368. [Google Scholar] [CrossRef]
- Petersson, H.; Arnlöv, J.; Zethelius, B.; Riserus, U. Serum fatty acid composition and insulin resistance are independently associated with liver fat markers in elderly men. Diabetes Res. Clin. Pract. 2010, 87, 379–384. [Google Scholar] [CrossRef]
- Allard, J.P.; Aghdassi, E.; Mohammed, S.; Raman, M.; Avand, G.; Arendt, B.M.; Jalali, P.; Kandasamy, T.; Prayitno, N.; Sherman, M.; et al. Nutritional assessment and hepatic fatty acid composition in non-alcoholic fatty liver disease (NAFLD): A cross-sectional study. J. Hepatol. 2008, 48, 300–307. [Google Scholar] [CrossRef]
- Bray, G.A.; Krauss, R.M. Overfeeding of polyunsaturated versus saturated fatty acids reduces ectopic fat. Diabetes 2014, 63, 2222–2224. [Google Scholar] [CrossRef][Green Version]
- Leamy, A.K.; Egnatchik, R.A.; Young, J.D. Molecular mechanisms and the role of saturated fatty acids in the progression of non-alcoholic fatty liver disease. Prog. Lipid Res. 2013, 52, 165–174. [Google Scholar] [CrossRef]
- Van Herpen, N.A.; Schrauwen-Hinderling, V.B.; Schaart, G.; Mensink, R.P.; Schrauwen, P. Three weeks on a high-fat diet increases intrahepatic lipid accumulation and decreases metabolic flexibility in healthy overweight men. J. Clin. Endocrinol. Metab. 2011, 96, E691–E695. [Google Scholar] [CrossRef]
- De Meijer, V.E.; Le, H.D.; Meisel, J.A.; Akhavan Sharif, M.R.; Pan, A.; Nose, V.; Puder, M. Dietary fat intake promotes the development of hepatic steatosis independently from excess caloric consumption in a murine model. Metabolism 2010, 59, 1092–1105. [Google Scholar] [CrossRef]
- Greenfield, J.R.; Samaras, K.; Jenkins, A.B.; Kelly, P.J.; Spector, T.D.; Campbell, L.V. Moderate alcohol consumption, dietary fat composition and abdominal obesity in women: Evidence for gene-environment interaction. J. Clin. Endocrinol. Metab. 2003, 88, 5381–5386. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Carroll, R.J.; Midthune, D.; Subar, A.F.; Shumakovich, M.; Freedman, L.S.; Thompson, F.E.; Kipnis, V. Taking advantage of the strengths of 2 different dietary assessment instruments to improve intake estimates for nutritional epidemiology. Am. J. Epidemiol. 2012, 175, 340–347. [Google Scholar] [CrossRef]
- Valdearcos, M.; Xu, A.W.; Koliwad, S.K. Hypothalamic inflammation in the control of metabolic function. Annu. Rev. Physiol. 2015, 77, 131–160. [Google Scholar] [CrossRef] [PubMed]
- Perfilyev, A.; Dahlman, I.; Gillberg, L.; Rosqvist, F.; Iggman, D.; Volkov, P.; Nilsson, E.; Risérus, U.; Ling, C. Impact of polyunsaturated and saturated fat overfeeding on the DNA-methylation pattern in human adipose tissue: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 105, 991–1000. [Google Scholar] [CrossRef]
- Martinez, J.A.; Milagro, F.I.; Claycombe, K.J.; Schalinske, K.L. Epigenetics in adipose tissue, obesity, weight loss and diabetes. Adv. Nutr. 2014, 5, 71–81. [Google Scholar] [CrossRef]
- Ocvirk, S.; O’Keefe, S.J. Influence of bile acids on colorectal cancer risk: Potential mechanisms mediated by diet—Gut microbiota interactions. Curr. Nutr. Rep. 2017, 6, 315–322. [Google Scholar] [CrossRef]
- Ou, J.; Carbonero, F.; Zoetendal, E.G.; DeLany, J.P.; Wang, M.; Newton, K.; Gaskins, H.R.; O’Keefe, S.J. Diet, microbiota and microbial metabolites in colon cancer risk in rural Africans and African Americans. Am. J. Clin. Nutr. 2013, 98, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.C.; Itsiopoulos, C.; Thodis, T.; Ward, G.; Trost, N.; Hofferberth, S.; O’Dea, K.; Desmond, P.V.; Johnson, N.A.; Wilson, A.M. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J. Hepatol. 2013, 59, 138–143. [Google Scholar] [CrossRef]
- Schwarz, J.M.; Noworolski, S.M.; Wen, M.J.; Dyachenko, A.; Prior, J.L.; Weinberg, M.E.; Herraiz, L.A.; Tai, V.W.; Bergeron, N.; Bersot, T.P.; et al. Effect of a high-fructose weight-maintaining diet on lipogenesis and liver fat. J. Clin. Endocrinol. Metab. 2015, 100, 2434–2442. [Google Scholar] [CrossRef]
- Augustin, L.S.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Willett, W.C.; Astrup, A.; Barclay, A.W.; Björck, I.; Brand-Miller, J.C.; Brighenti, F.; Buyken, A.E.; et al. Glycemic index, glycemic load and glycemic response: An international scientific consensus summit from the International Carbohydrate Quality Consortium (ICQC). Nutr. Metab. Cardiovasc. 2015, 25, 795–815. [Google Scholar] [CrossRef] [PubMed]
- Bawden, S.; Stephenson, M.; Falcone, Y.; Lingava, M.; Ciampi, E.; Hunter, K.; Bligh, F.; Schirra, J.; Taylor, M.; Morris, P.; et al. Increased liver fat and glycogen stores after consumption of high versus low glycaemic index food: A randomized crossover study. Diabetes Obes. Metab. 2017, 19, 70–77. [Google Scholar] [CrossRef]
- Zierath, J.R.; Livingston, J.N.; Thorne, A.; Bolinder, J.; Reynisdottir, S.; Lonnqvist, F.; Arner, P. Regional difference in insulin inhibition of non-esterified fatty acid release from human adipocytes: Relation to insulin receptor phosphorylation and intracellular signalling through the insulin receptor substrate-I pathway. Diabetologia 1998, 41, 1343–1354. [Google Scholar] [CrossRef]
- Wagenknecht, L.E.; Langefeld, C.D.; Scherzinger, A.L.; Norris, J.M.; Haffner, S.M.; Saad, M.F.; Bergman, R.N. Insulin sensitivity, insulin secretion and abdominal fat: The Insulin Resistance Atherosclerosis Study (IRAS) Family Study. Diabetes 2003, 52, 2490–2496. [Google Scholar] [CrossRef]
- Bjorntorp, P. “Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis 1990, 10, 493–496. [Google Scholar] [CrossRef]
- Despres, J.P.; Lemieux, I. Abdominal obesity and metabolic syndrome. Nature 2006, 444, 881–887. [Google Scholar] [CrossRef]
- Van der Poorten, D.; Milner, K.L.; Hui, J.; Hodge, A.; Trenell, M.I.; Kench, J.G.; London, R.; Peduto, T.; Chisholm, D.J.; George, J. Visceral fat: A key mediator of steatohepatitis in metabolic liver disease. Hepatology 2008, 48, 449–457. [Google Scholar] [CrossRef]
- Hauner, H.; Wabitsch, M.; Pfeiffer, E.F. Differentiation of adipocyte precursor cells from obese and nonobese adult women and from different adipose tissue sites. Horm. Metab. Res. Suppl. 1988, 19, 35–39. [Google Scholar] [PubMed]
- Freedland, E.S. Role of critical visceral adipose tissue threshold in metabolic syndrome: Implications for controlling dietary carbohydrates: A review. Nutr. Metab. 2004, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Frayn, K.N. Adipose tissue as a buffer for daily lipid flux. Diabetologia 2002, 45, 1201–1210. [Google Scholar] [CrossRef]
- Nazare, J.A.; Smith, J.D.; Borel, A.L.; Haffner, S.M.; Balkau, B.; Ross, R.; Massien, C.; Almeras, N.; Despres, J.P. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat and cardiometabolic risk profile: The international study of prediction of intra-abdominal adiposity and its relationship with cardiometabolic risk/intra-abdominal adiposity. Am. J. Clin. Nutr. 2012, 96, 714–726. [Google Scholar]
- Lee, M.J.; Wu, Y.; Fried, S.K. Adipose tissue heterogeneity: Implication of depot differences in adipose tissue for obesity complications. Mol. Asp. Med. 2013, 34, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Conrad, J.; Nöthlings, U. Innovative approaches to estimate individual usual dietary intake in large-scale epidemiological studies. Proc. Nutr. Soc. 2017, 76, 213–219. [Google Scholar] [CrossRef]
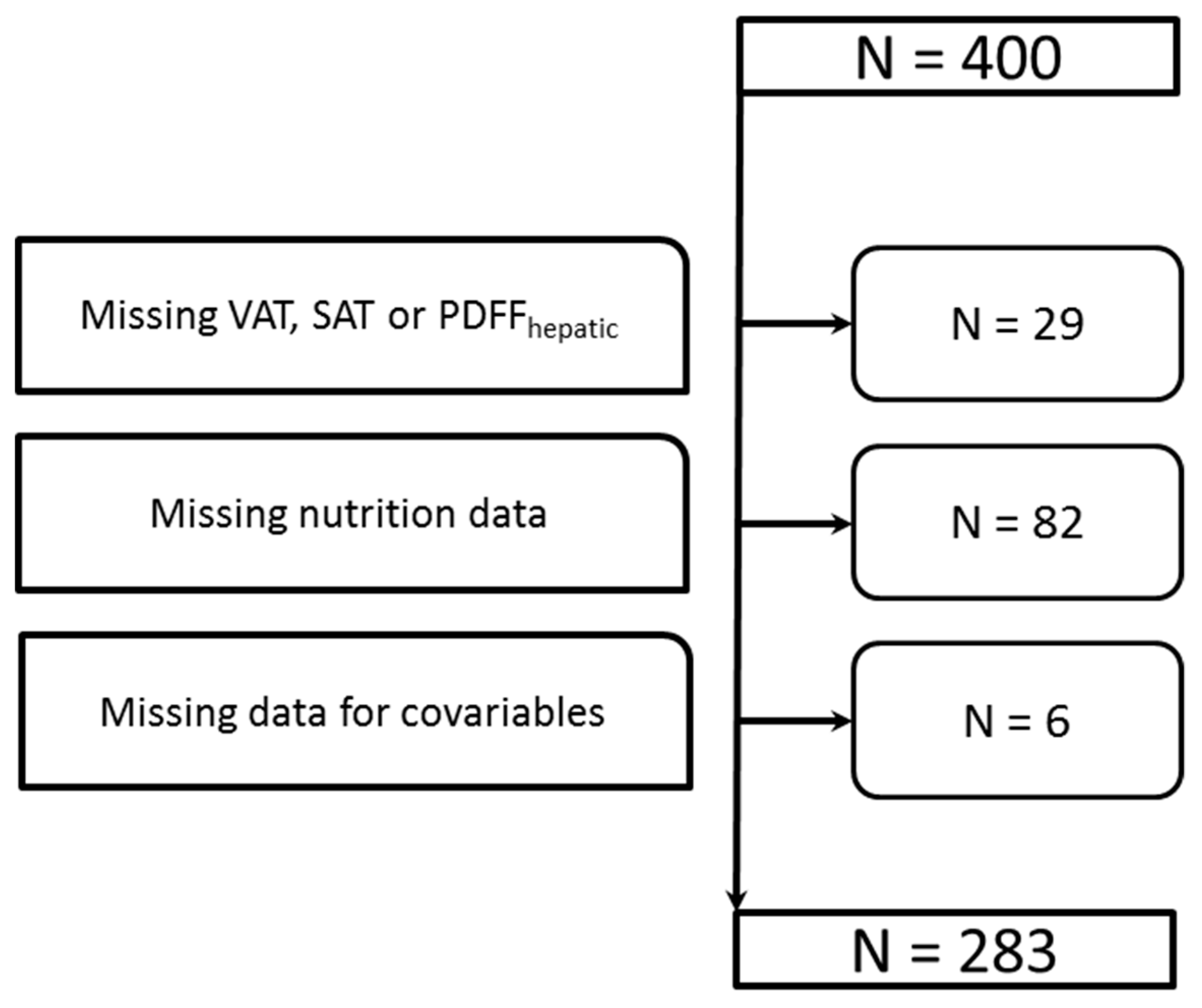
| All | Men | Women | ||
|---|---|---|---|---|
| N = 283 | N = 161 | N = 122 | p-value Men vs. Women | |
| Sociodemographics | ||||
| Age, years | 56.1 ± 9.0 | 56.1 ± 9.4 | 56.1 ± 8.6 | 0.976 |
| Education, years of schooling | 12.4 ± 2.6 | 12.9 ± 2.8 | 11.8 ± 2.4 | 0.001 |
| Anthropometric measurements | ||||
| Height, cm | 171.7 ± 9.7 | 177.9 ± 6.7 | 163.5 ± 6.4 | <0.001 |
| Weight, kg | 81.4 ± 15.7 | 88.4 ± 13.1 | 72.2 ± 14.1 | <0.001 |
| BMI, kg/m2 | 27.6 ± 4.6 | 27.9 ± 4.1 | 27.0 ± 5.2 | 0.107 |
| Waist circumference, cm | 97.0 ± 13.7 | 102.2 ± 11.7 | 90.2 ± 13.3 | <0.001 |
| Hip circumference, cm | 106.1 ± 8.3 | 106.4 ± 6.9 | 105.7 ± 9.9 | 0.483 |
| Waist-To-Hip Ratio | 0.9 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 | <0.001 |
| Blood Pressure | ||||
| Systolic BP, mmHg | 119.9 ± 15.9 | 125.1 ± 15.4 | 113.0 ± 13.9 | <0.001 |
| Diastolic BP, mmHg | 74.8 ± 9.6 | 76.9 ± 10.0 | 71.9 ± 8.4 | <0.001 |
| Hypertension | 94 (33.2%) | 64 (39.8%) | 30 (24.6%) | 0.011 |
| Antihypertensive medication | 71 (25.1%) | 42 (26.1%) | 29 (23.8%) | 0.759 |
| Diabetes | ||||
| Glycemic Status | 0.018 | |||
| normoglycemic | 183 (64.7%) | 93 (57.8%) | 90 (73.8%) | |
| prediabetes | 68 (24.0%) | 45 (28.0%) | 23 (18.9%) | |
| diabetes | 32 (11.3%) | 23 (14.3%) | 9 (7.4%) | |
| Fasting Serum glucose, mg/dL | 102.8 ± 18.0 | 106.6 ± 19.5 | 97.8 ± 14.3 | <0.001 |
| HbA1c, % | 5.5 ± 0.6 | 5.5 ± 0.6 | 5.5 ± 0.5 | 0.671 |
| Diabetes medication | 20 (7.1%) | 13 (8.1%) | 7 (5.7%) | 0.599 |
| Lipid profile | ||||
| Total Cholesterol, mg/dL | 217.2 ± 36.6 | 216.1 ± 37.8 | 218.6 ± 35.0 | 0.577 |
| HDL Cholesterol, mg/dL | 62.8 ± 18.1 | 56.0 ± 14.9 | 71.8 ± 17.9 | <0.001 |
| LDL Cholesterol, mg/dL | 138.8 ± 33.6 | 141.7 ± 34.1 | 135.0 ± 32.6 | 0.099 |
| Triglycerides, mg/dL | 127.2 ± 80.4 | 148.1 ± 95.0 | 99.5 ± 42.1 | <0.001 |
| Lipid lowering medication | 31 (11.0%) | 16 (9.9%) | 15 (12.3%) | 0.662 |
| Liver enzymes | ||||
| GGT - Gammaglutamyltransferase, µkat/L | 0.7 ± 0.7 | 0.8 ± 0.8 | 0.5 ± 0.5 | <0.001 |
| Glutamat-Oxalat-Transaminase (GOT, AST), µkat/L | 0.4 ± 0.2 | 0.4 ± 0.2 | 0.4 ± 0.2 | 0.008 |
| Glutamat-Pyruvat-Transaminase (GPT, ALT), µkat/L | 0.5 ± 0.3 | 0.6 ± 0.3 | 0.4 ± 0.3 | <0.001 |
| Behavior | ||||
| Physical activity | ||||
| no | 69 (24.4%) | 49 (30.4%) | 20 (16.4%) | 0.041 |
| sporadically | 41 (14.5%) | 22 (13.7%) | 19 (15.6%) | |
| regularly around 1 h/week | 92 (32.5%) | 45 (28.0%) | 47 (38.5%) | |
| regularly more than 2 h/week | 81 (28.6%) | 45 (28.0%) | 36 (29.5%) | |
| Smoking | 0.249 | |||
| never-smoker | 105 (37.1%) | 54 (33.5%) | 51 (41.8%) | |
| ex-smoker | 122 (43.1%) | 76 (47.2%) | 46 (37.7%) | |
| smoker | 56 (19.8%) | 31 (19.3%) | 25 (20.5%) | |
| Alcohol consumption * | ||||
| No/very low | 72 (25.4%) | 31 (19.3%) | 41 (33.6%) | <0.001 |
| Moderate | 133 (47.0%) | 65 (40.4%) | 68 (55.7%) | |
| high | 78 (27.6%) | 65 (40.4%) | 13 (10.7%) |
| All | Men | Women | ||
|---|---|---|---|---|
| N = 283 | N = 161 | N = 122 | p-value Men vs. Women | |
| Dietary intake data | ||||
| Carbohydrates, % of total energy intake | 41.8 ± 4.0 | 41.3 ± 4.3 | 42.5 ± 3.5 | 0.015 |
| Fat, % of total energy intake | 37.9 ± 3.5 | 37.3 ± 3.5 | 38.7 ± 3.3 | 0.001 |
| Ratio fat/carbohydrates | 0.9 ± 0.2 | 0.9 ± 0.2 | 0.9 ± 0.1 | 0.895 |
| SFA, % of total energy intake | 17.1 ± 1.8 | 16.8 ± 1.8 | 17.6 ± 1.8 | 0.000 |
| MUFA, % of total energy intake | 13.4 ± 1.5 | 13.4 ± 1.5 | 13.5 ± 1.4 | 0.562 |
| PUFA, % of total energy intake | 4.9 ± 0.8 | 4.8 ± 0.8 | 5.0 ± 0.7 | 0.002 |
| Protein, % of total energy intake | 15.3 ± 1.7 | 14.8 ± 1.5 | 15.8 ± 1.7 | <0.001 |
| Alcohol, % of total energy intake | 4.3 ± 3.7 | 5.9 ± 3.7 | 2.2 ± 2.3 | <0.001 |
| Total energy intake, kcal | 2061.5 ± 351.5 | 1554.8 ± 295.4 | <0.001 | |
| MRI measurements of adipose tissue | ||||
| Hepatic fat, PDFF, % (median [1st quartile, 3rd quartile]) | 4.5 [2.5, 11.1] | 6.6 [3.7, 12.8] | 3.0 [1.9, 5.4] | <0.001 |
| Total adipose tissue, liter | 12.2 ± 5.4 | 12.8 ± 5.2 | 11.5 ± 5.5 | 0.037 |
| Visceral adipose tissue, liter | 4.3 ± 2.7 | 5.5 ± 2.6 | 2.8 ± 2.1 | <0.001 |
| subcutaneous adipose tissue, liter | 7.9 ± 3.6 | 7.3 ± 3.1 | 8.7 ± 3.9 | 0.001 |
| VAT | SAT | Hepatic | |||||||
|---|---|---|---|---|---|---|---|---|---|
| β-Coefficient | 95%-CI | p-Value | β-Coefficient | 95%-CI | p-Value | Estimate | 95%-CI | p-Value | |
| Fat | 0.42 | [0.04, 0.79] | 0.031 | 0.15 | [−0.47, 0.76] | 0.642 | 1.23 | [1.07, 1.42] | 0.004 |
| Fat * | 0.63 | [0.21, 1.05] | 0.003 | 0.19 | [−0.52, 0.91] | 0.595 | 1.2 | [1.01, 1.42] | 0.034 |
| SFA | −0.04 | [−0.95, 0.86] | 0.924 | −1.65 | [−3.11, −0.19] | 0.027 | 1.3 | [0.92, 1.80] | 0.138 |
| SFA * | 0 | [−0.99, 0.98] | 0.998 | −1.34 | [−3.02, 0.33] | 0.115 | 1.26 | [0.85, 1.88] | 0.247 |
| MUFA | 0.98 | [−0.32, 2.27] | 0.138 | 2.58 | [0.49, 4.68] | 0.016 | 1.23 | [0.76, 1.99] | 0.399 |
| MUFA * | 1.89 | [0.36, 3.42] | 0.016 | 2.58 | [−0.03, 5.19] | 0.052 | 1.14 | [0.61, 2.12] | 0.680 |
| PUFA | 0.13 | [−2.00, 2.26] | 0.905 | −1.81 | [−5.25, 1.63] | 0.302 | 1.01 | [0.45, 2.25] | 0.979 |
| PUFA * | −1.12 | [−3.69, 1.44] | 0.388 | −2.22 | [−6.58, 2.14] | 0.317 | 1.09 | [0.39, 3.06] | 0.859 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meisinger, C.; Rospleszcz, S.; Wintermeyer, E.; Lorbeer, R.; Thorand, B.; Bamberg, F.; Peters, A.; Schlett, C.L.; Linseisen, J. Isocaloric Substitution of Dietary Carbohydrate Intake with Fat Intake and MRI-Determined Total Volumes of Visceral, Subcutaneous and Hepatic Fat Content in Middle-Aged Adults. Nutrients 2019, 11, 1151. https://doi.org/10.3390/nu11051151
Meisinger C, Rospleszcz S, Wintermeyer E, Lorbeer R, Thorand B, Bamberg F, Peters A, Schlett CL, Linseisen J. Isocaloric Substitution of Dietary Carbohydrate Intake with Fat Intake and MRI-Determined Total Volumes of Visceral, Subcutaneous and Hepatic Fat Content in Middle-Aged Adults. Nutrients. 2019; 11(5):1151. https://doi.org/10.3390/nu11051151
Chicago/Turabian StyleMeisinger, Christa, Susanne Rospleszcz, Elke Wintermeyer, Roberto Lorbeer, Barbara Thorand, Fabian Bamberg, Annette Peters, Christopher L. Schlett, and Jakob Linseisen. 2019. "Isocaloric Substitution of Dietary Carbohydrate Intake with Fat Intake and MRI-Determined Total Volumes of Visceral, Subcutaneous and Hepatic Fat Content in Middle-Aged Adults" Nutrients 11, no. 5: 1151. https://doi.org/10.3390/nu11051151
APA StyleMeisinger, C., Rospleszcz, S., Wintermeyer, E., Lorbeer, R., Thorand, B., Bamberg, F., Peters, A., Schlett, C. L., & Linseisen, J. (2019). Isocaloric Substitution of Dietary Carbohydrate Intake with Fat Intake and MRI-Determined Total Volumes of Visceral, Subcutaneous and Hepatic Fat Content in Middle-Aged Adults. Nutrients, 11(5), 1151. https://doi.org/10.3390/nu11051151





