Dietary Walnut Supplementation Alters Mucosal Metabolite Profiles During DSS-Induced Colonic Ulceration
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Treatment
2.2. Tissue Processing and Analyses of Ulceration and Restitution
2.3. Measurement of Fecal and Tissue Metabolites
2.4. Statistical Analyses
3. Results
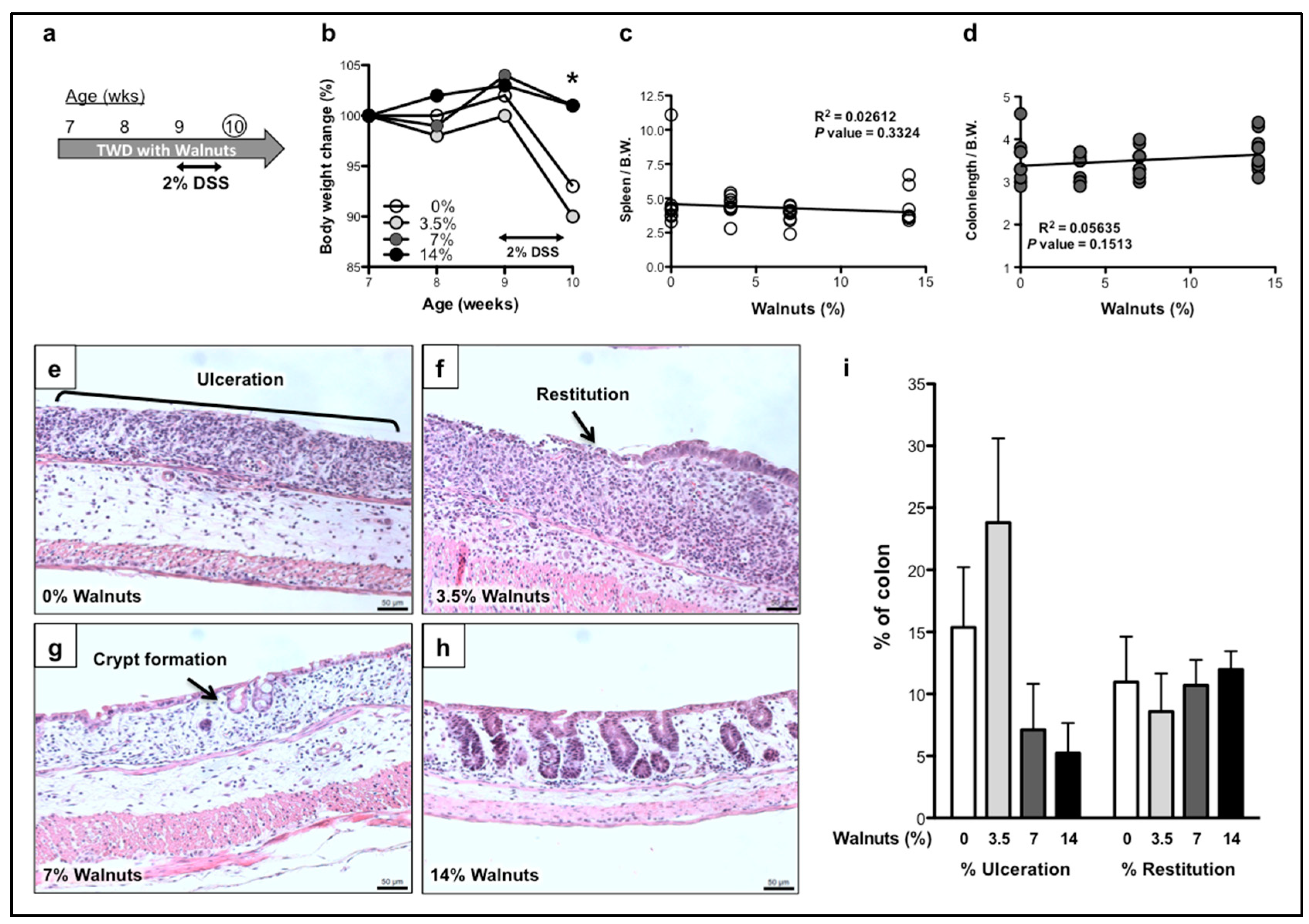
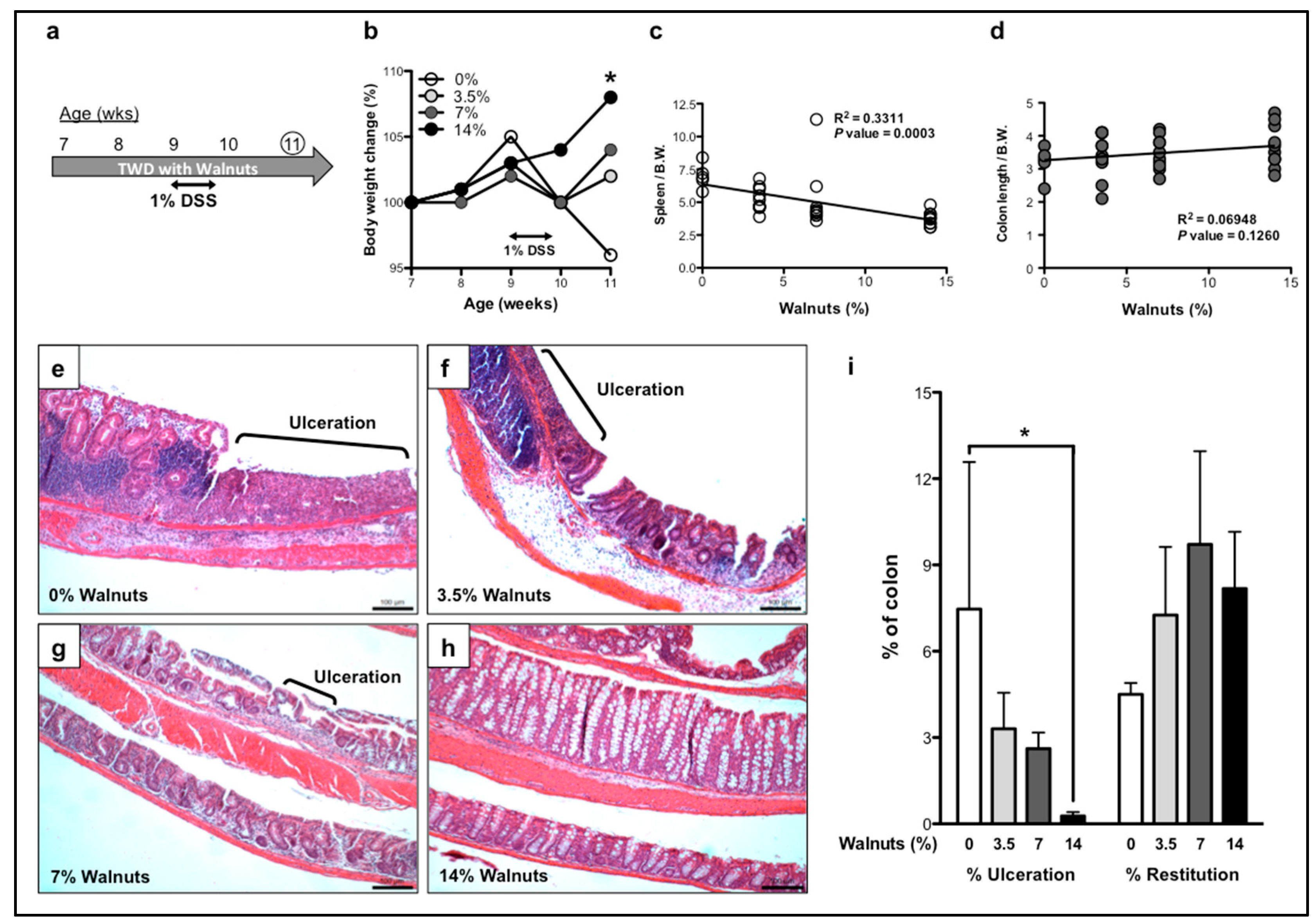
3.1. Dietary Walnut Supplementation Protects Mice From DSS-Induced Intestinal Injury
3.1.1. Effects of Walnut Supplementation in Acute Phase of DSS-Induced Colitis
3.1.2. Effects of Walnut Supplementation in Recovery Phase of DSS-Induced Colitis
3.2. Walnut Consumption Alters Fecal Metabolite Composition
3.3. Accumulation of Phytochemicals in Colon Tissue Associated with Walnut Supplementation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hayes, D.; Angove, M.J.; Tucci, J.; Dennis, C. Walnuts (Juglans regia) Chemical Composition and Research in Human Health. Crit. Rev. Food Sci. Nutr. 2016, 56, 1231–1241. [Google Scholar] [CrossRef]
- Nakanishi, M.; Chen, Y.; Qendro, V.; Miyamoto, S.; Weinstock, E.; Weinstock, G.M.; Rosenberg, D.W. Effects of Walnut Consumption on Colon Carcinogenesis and Microbial Community Structure. Cancer Prev. Res. (Phila) 2016, 9, 692–703. [Google Scholar] [CrossRef]
- Yang, C.S.; Suh, N. Cancer prevention by different forms of tocopherols. Top. Curr. Chem. 2013, 329, 21–33. [Google Scholar] [CrossRef]
- Wedlake, L.; Slack, N.; Andreyev, H.J.; Whelan, K. Fiber in the treatment and maintenance of inflammatory bowel disease: A systematic review of randomized controlled trials. Inflamm. Bowel Dis. 2014, 20, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef]
- Ordas, I.; Eckmann, L.; Talamini, M.; Baumgart, D.C.; Sandborn, W.J. Ulcerative colitis. Lancet 2012, 380, 1606–1619. [Google Scholar] [CrossRef]
- Haskey, N.; Gibson, D.L. An Examination of Diet for the Maintenance of Remission in Inflammatory Bowel Disease. Nutrients 2017, 9, 259. [Google Scholar] [CrossRef]
- Knight-Sepulveda, K.; Kais, S.; Santaolalla, R.; Abreu, M.T. Diet and Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2015, 11, 511–520. [Google Scholar]
- Sousa Guerreiro, C.; Cravo, M.; Costa, A.R.; Miranda, A.; Tavares, L.; Moura-Santos, P.; MarquesVidal, P.; Nobre Leitao, C. A comprehensive approach to evaluate nutritional status in Crohn’s patients in the era of biologic therapy: A case-control study. Am. J. Gastroenterol. 2007, 102, 2551–2556. [Google Scholar] [CrossRef]
- Hou, J.K.; Abraham, B.; El-Serag, H. Dietary intake and risk of developing inflammatory bowel disease: A systematic review of the literature. Am. J. Gastroenterol. 2011, 106, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Muzaffer, U.; Paul, V.I.; Prasad, N.R.; Karthikeyan, R.; Agilan, B. Protective effect of Juglans regia L. against ultraviolet B radiation induced inflammatory responses in human epidermal keratinocytes. Phytomedicine 2018, 42, 100–111. [Google Scholar] [CrossRef]
- Choi, Y.; Abdelmegeed, M.A.; Akbar, M.; Song, B.J. Dietary walnut reduces hepatic triglyceride content in high-fat-fed mice via modulation of hepatic fatty acid metabolism and adipose tissue inflammation. J. Nutr. Biochem. 2016, 30, 116–125. [Google Scholar] [CrossRef]
- Poulose, S.M.; Bielinski, D.F.; Shukitt-Hale, B. Walnut diet reduces accumulation of polyubiquitinated proteins and inflammation in the brain of aged rats. J. Nutr. Biochem. 2013, 24, 912–919. [Google Scholar] [CrossRef] [PubMed]
- Hintze, K.J.; Benninghoff, A.D.; Ward, R.E. Formulation of the Total Western Diet (TWD) as a basal diet for rodent cancer studies. J. Agric. Food Chem. 2012, 60, 6736–6742. [Google Scholar] [CrossRef]
- Hardman, W.E.; Ion, G. Suppression of implanted MDA-MB 231 human breast cancer growth in nude mice by dietary walnut. Nutr. Cancer 2008, 60, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Nagel, J.M.; Brinkoetter, M.; Magkos, F.; Liu, X.; Chamberland, J.P.; Shah, S.; Zhou, J.; Blackburn, G.; Mantzoros, C.S. Dietary walnuts inhibit colorectal cancer growth in mice by suppressing angiogenesis. Nutrition 2012, 28, 67–75. [Google Scholar] [CrossRef]
- Nakanishi, M.; Perret, C.; Meuillet, E.J.; Rosenberg, D.W. Non-cell autonomous effects of targeting inducible PGE2 synthesis during inflammation-associated colon carcinogenesis. Carcinogenesis 2015, 36, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, G.V.; Choi, K.; Klemashevich, C.; Wu, C.; Prabakaran, D.; Pan, L.B.; Steinmeyer, S.; Mueller, C.; Yousofshahi, M.; Alaniz, R.C.; et al. Prediction and quantification of bioactive microbiota metabolites in the mouse gut. Nat. Commun. 2014, 5, 5492. [Google Scholar] [CrossRef]
- Sheldon, M.T.; Mistrik, R.; Croley, T.R. Determination of ion structures in structurally related compounds using precursor ion fingerprinting. J. Am. Soc. Mass Spectrom. 2009, 20, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.I.; Hirai, S.; Takahashi, H.; Goto, T.; Ohyane, C.; Tsugane, T.; Konishi, C.; Fujii, T.; Inai, S.; Iijima, Y.; et al. 9-oxo-10(E),12(E)-Octadecadienoic acid derived from tomato is a potent PPAR alpha agonist to decrease triglyceride accumulation in mouse primary hepatocytes. Mol. Nutr. Food Res. 2011, 55, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Wirthgen, E.; Hoeflich, A.; Rebl, A.; Gunther, J. Kynurenic Acid: The Janus-Faced Role of an Immunomodulatory Tryptophan Metabolite and Its Link to Pathological Conditions. Front. Immunol. 2017, 8, 1957. [Google Scholar] [CrossRef] [PubMed]
- Reina-San-Martin, B.; Degrave, W.; Rougeot, C.; Cosson, A.; Chamond, N.; Cordeiro-Da-Silva, A.; Arala-Chaves, M.; Coutinho, A.; Minoprio, P. A B-cell mitogen from a pathogenic trypanosome is a eukaryotic proline racemase. Nat. Med. 2000, 6, 890–897. [Google Scholar] [CrossRef] [PubMed]
- Graf, M.; Mardirossian, M.; Nguyen, F.; Seefeldt, A.C.; Guichard, G.; Scocchi, M.; Innis, C.A.; Wilson, D.N. Proline-rich antimicrobial peptides targeting protein synthesis. Nat. Prod. Rep. 2017, 34, 702–711. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.S.; Lee, J.; Heo, S.C.; Lee, K.L.; Choi, S.W.; Kim, Y. Walnut Phenolic Extract and Its Bioactive Compounds Suppress Colon Cancer Cell Growth by Regulating Colon Cancer Stemness. Nutrients 2016, 8, 439. [Google Scholar] [CrossRef]
- Fukuda, T.; Ito, H.; Yoshida, T. Antioxidative polyphenols from walnuts (Juglans regia L.). Phytochemistry 2003, 63, 795–801. [Google Scholar] [CrossRef]
- Abraham, R.T.; Acquarone, M.; Andersen, A.; Asensi, A.; Belle, R.; Berger, F.; Bergounioux, C.; Brunn, G.; Buquet-Fagot, C.; Fagot, D.; et al. Cellular effects of olomoucine, an inhibitor of cyclin-dependent kinases. Biol. Cell 1995, 83, 105–120. [Google Scholar] [CrossRef]
- Craig, S.A. Betaine in human nutrition. Am. J. Clin. Nutr. 2004, 80, 539–549. [Google Scholar] [CrossRef]
- Sanchez-Gonzalez, C.; Ciudad, C.J.; Noe, V.; Izquierdo-Pulido, M. Health benefits of walnut polyphenols: An exploration beyond their lipid profile. Crit. Rev. Food Sci. Nutr. 2017, 57, 3373–3383. [Google Scholar] [CrossRef]
- Sturm, A.; Dignass, A.U. Epithelial restitution and wound healing in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 348–353. [Google Scholar] [CrossRef]
- Beigh, S.; Rashid, H.; Sharma, S.; Parvez, S.; Raisuddin, S. Bleomycin-induced pulmonary toxicopathological changes in rats and its prevention by walnut extract. Biomed. Pharmacother. 2017, 94, 418–429. [Google Scholar] [CrossRef]
- Koh, S.J.; Choi, Y.I.; Kim, Y.; Kim, Y.S.; Choi, S.W.; Kim, J.W.; Kim, B.G.; Lee, K.L. Walnut phenolic extract inhibits nuclear factor kappaB signaling in intestinal epithelial cells and ameliorates experimental colitis and colitis-associated colon cancer in mice. Eur. J. Nutr. 2018. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 fatty acids and inflammatory processes: From molecules to man. Biochem. Soc. Trans. 2017, 45, 1105–1115. [Google Scholar] [CrossRef] [PubMed]
- Zarate, R.; El Jaber-Vazdekis, N.; Tejera, N.; Perez, J.A.; Rodriguez, C. Significance of long chain polyunsaturated fatty acids in human health. Clin. Transl. Med. 2017, 6, 25. [Google Scholar] [CrossRef]
- Blum, A.; Monir, M.; Wirsansky, I.; Ben-Arzi, S. The beneficial effects of tomatoes. Eur. J. Intern. Med. 2005, 16, 402–404. [Google Scholar] [CrossRef] [PubMed]
- Desvergne, B.; Wahli, W. Peroxisome proliferator-activated receptors: Nuclear control of metabolism. Endocr. Rev. 1999, 20, 649–688. [Google Scholar] [CrossRef]
- Varga, T.; Czimmerer, Z.; Nagy, L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta 2011, 1812, 1007–1022. [Google Scholar] [CrossRef]
- Roepstorff, C.; Halberg, N.; Hillig, T.; Saha, A.K.; Ruderman, N.B.; Wojtaszewski, J.F.; Richter, E.A.; Kiens, B. Malonyl-CoA and carnitine in regulation of fat oxidation in human skeletal muscle during exercise. Am. J. Physiol. Endocrinol. Metab. 2005, 288, 133–142. [Google Scholar] [CrossRef]
- Adeva-Andany, M.M.; Carneiro-Freire, N.; Seco-Filgueira, M.; Fernandez-Fernandez, C.; Mourino-Bayolo, D. Mitochondrial beta-oxidation of saturated fatty acids in humans. Mitochondrion 2018, 46, 73–90. [Google Scholar] [CrossRef]
- Rinaldo, P.; Matern, D.; Bennett, M.J. Fatty acid oxidation disorders. Ann. Rev. Physiol. 2002, 64, 477–502. [Google Scholar] [CrossRef]
- Shekhawat, P.S.; Srinivas, S.R.; Matern, D.; Bennett, M.J.; Boriack, R.; George, V.; Xu, H.; Prasad, P.D.; Roon, P.; Ganapathy, V. Spontaneous development of intestinal and colonic atrophy and inflammation in the carnitine-deficient jvs (OCTN2(-/-)) mice. Mol. Genet. Metab. 2007, 92, 315–324. [Google Scholar] [CrossRef]
- Scioli, M.G.; Stasi, M.A.; Passeri, D.; Doldo, E.; Costanza, G.; Camerini, R.; Fociani, P.; Arcuri, G.; Lombardo, K.; Pace, S.; et al. Propionyl-L-Carnitine is Efficacious in Ulcerative Colitis Through its Action on the Immune Function and Microvasculature. Clin. Transl. Gastroenterol. 2014, 5, e55. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, T.A.; Ito, M.K.; Maki, K.C.; Orringer, C.E.; Bays, H.E.; Jones, P.H.; McKenney, J.M.; Grundy, S.M.; Gill, E.A.; Wild, R.A.; et al. National Lipid Association recommendations for patient-centered management of dyslipidemia: Part 1—Executive summary. J. Clin. Lipidol. 2014, 8, 473–488. [Google Scholar] [CrossRef]
- Shimoda, H.; Tanaka, J.; Kikuchi, M.; Fukuda, T.; Ito, H.; Hatano, T.; Yoshida, T. Effect of polyphenol-rich extract from walnut on diet-induced hypertriglyceridemia in mice via enhancement of fatty acid oxidation in the liver. J. Agric. Food Chem. 2009, 57, 1786–1792. [Google Scholar] [CrossRef]
- Kilic, I.; Yesiloglu, Y.; Bayrak, Y. Spectroscopic studies on the antioxidant activity of ellagic acid. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 130, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Banerjee, N.; Sirven, M.A.; Minamoto, Y.; Markel, M.E.; Suchodolski, J.S.; Talcott, S.T.; Mertens-Talcott, S.U. Pomegranate polyphenolics reduce inflammation and ulceration in intestinal colitis-involvement of the miR-145/p70S6K1/HIF1alpha axis in vivo and in vitro. J. Nutr. Biochem. 2017, 43, 107–115. [Google Scholar] [CrossRef]
- Marin, M.; Maria Giner, R.; Rios, J.L.; Recio, M.C. Intestinal anti-inflammatory activity of ellagic acid in the acute and chronic dextrane sulfate sodium models of mice colitis. J. Ethnopharmacol. 2013, 150, 925–934. [Google Scholar] [CrossRef]
- Ogawa, Y.; Kanatsu, K.; Iino, T.; Kato, S.; Jeong, Y.I.; Shibata, N.; Takada, K.; Takeuchi, K. Protection against dextran sulfate sodium-induced colitis by microspheres of ellagic acid in rats. Life Sci. 2002, 71, 827–839. [Google Scholar] [CrossRef]
- Cerda, B.; Espin, J.C.; Parra, S.; Martinez, P.; Tomas-Barberan, F.A. The potent in vitro antioxidant ellagitannins from pomegranate juice are metabolised into bioavailable but poor antioxidant hydroxy-6H-dibenzopyran-6-one derivatives by the colonic microflora of healthy humans. Eur. J. Nutr. 2004, 43, 205–220. [Google Scholar] [CrossRef]
- Espin, J.C.; Larrosa, M.; Garcia-Conesa, M.T.; Tomas-Barberan, F. Biological significance of urolithins, the gut microbial ellagic Acid-derived metabolites: The evidence so far. Evid. Based Complement. Alternat. Med. 2013, 2013, 270418. [Google Scholar] [CrossRef]
- Muku, G.E.; Murray, I.A.; Espin, J.C.; Perdew, G.H. Urolithin A Is a Dietary Microbiota-Derived Human Aryl Hydrocarbon Receptor Antagonist. Metabolites 2018, 8, 86. [Google Scholar] [CrossRef]
- Cortes-Martin, A.; Garcia-Villalba, R.; Gonzalez-Sarrias, A.; Romo-Vaquero, M.; Loria-Kohen, V.; Ramirez-de-Molina, A.; Tomas-Barberan, F.A.; Selma, M.V.; Espin, J.C. The gut microbiota urolithin metabotypes revisited: The human metabolism of ellagic acid is mainly determined by aging. Food Funct. 2018, 9, 4100–4106. [Google Scholar] [CrossRef] [PubMed]
- Nishiguchi, G.A.; Burger, M.T.; Han, W.; Lan, J.; Atallah, G.; Tamez, V.; Lindvall, M.; Bellamacina, C.; Garcia, P.; Feucht, P.; et al. Design, synthesis and structure activity relationship of potent pan-PIM kinase inhibitors derived from the pyridyl carboxamide scaffold. Bioorg. Med. Chem. Lett. 2016, 26, 2328–2332. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef] [PubMed]
| Name | Formula | Molecular Weight | Ratio: Day 14/Day 0 | p-Value: Day 14/Day 0 |
|---|---|---|---|---|
| 9-oxo-10(E),12(E)-octadecadienoic acid | C18 H30 O3 | 294.2195 | 29.29 | 0.0001 |
| Corticosterone | C21 H30 O4 | 346.2144 | 11.99 | 0.0894 |
| Docosahexaenoic acid (DHA) | C22 H32 O2 | 328.2401 | 8.33 | 0.0090 |
| Ellagic acid | C14 H6 O8 | 302.0061 | 8.25 | 0.0883 |
| Arachidonic acid (AA) | C20 H32 O2 | 304.2402 | 7.42 | 0.0283 |
| Kynurenic acid | C10 H7 N O3 | 189.0426 | 6.39 | 0.0042 |
| D-(+)-Proline | C5 H9 N O2 | 115.0634 | 3.42 | 0.0078 |
| Spectinomycin | C14 H24 N2 O7 | 332.1598 | 3.22 | 0.0501 |
| Bis(methylbenzylidene)sorbitol | C22 H26 O6 | 386.1727 | 2.59 | 0.0196 |
| N-Acetylserotonin | C12 H14 N2 O2 | 218.1055 | 2.44 | 0.0225 |
| Benzophenone | C13 H10 O | 182.0733 | 2.36 | 0.0081 |
| Sulcatol | C8 H16 O | 128.1204 | 2.31 | 0.0999 |
| L-Methionine sulfoxide | C5 H11 N O3 S | 165.0460 | 1.77 | 0.0898 |
| Dodecyltrimethylammonium | C15 H33 N | 227.2614 | 0.53 | 0.0310 |
| Name | Formula | Molecular Weight | Ratio: Day 14/Day 0 | p-Value: Day 14/Day 0 |
|---|---|---|---|---|
| Olomoucine | C15 H18 N6 O | 298.1545 | 1.73 | 0.0098 |
| Adenosine 5’-monophosphate | C10 H14 N5 O7 P | 347.0628 | 1.67 | 0.0951 |
| L-Tyrosine | C9 H11 N O3 | 181.0741 | 1.53 | 0.0823 |
| Adenosine 5’-monophosphate | C10 H14 N5 O7 P | 347.0628 | 1.52 | 0.0787 |
| S-Adenosylhomocysteine | C14 H20 N6 O5 S | 384.1213 | 1.50 | 0.0685 |
| Triisopropanolamine | C9 H21 N O3 | 191.1521 | 1.38 | 0.0009 |
| Flurandrenolide | C24 H33 F O6 | 436.2281 | 1.35 | 0.1114 |
| DL-Glutamine | C5 H10 N2 O3 | 146.0691 | 1.31 | 0.0122 |
| D-Serine | C3 H7 N O3 | 105.0426 | 1.31 | 0.1979 |
| Betaine | C5 H11 N O2 | 117.0789 | 1.26 | 0.1157 |
| Bis(4-ethylbenzylidene)sorbitol | C24 H30 O6 | 414.2040 | 1.23 | 0.1477 |
| Bis(methylbenzylidene)sorbitol | C22 H26 O6 | 386.1726 | 0.79 | 0.0069 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakanishi, M.; Matz, A.; Klemashevich, C.; Rosenberg, D.W. Dietary Walnut Supplementation Alters Mucosal Metabolite Profiles During DSS-Induced Colonic Ulceration. Nutrients 2019, 11, 1118. https://doi.org/10.3390/nu11051118
Nakanishi M, Matz A, Klemashevich C, Rosenberg DW. Dietary Walnut Supplementation Alters Mucosal Metabolite Profiles During DSS-Induced Colonic Ulceration. Nutrients. 2019; 11(5):1118. https://doi.org/10.3390/nu11051118
Chicago/Turabian StyleNakanishi, Masako, Alyssa Matz, Cory Klemashevich, and Daniel W. Rosenberg. 2019. "Dietary Walnut Supplementation Alters Mucosal Metabolite Profiles During DSS-Induced Colonic Ulceration" Nutrients 11, no. 5: 1118. https://doi.org/10.3390/nu11051118
APA StyleNakanishi, M., Matz, A., Klemashevich, C., & Rosenberg, D. W. (2019). Dietary Walnut Supplementation Alters Mucosal Metabolite Profiles During DSS-Induced Colonic Ulceration. Nutrients, 11(5), 1118. https://doi.org/10.3390/nu11051118




