Ganoderma lucidum Extract Reduces the Motility of Breast Cancer Cells Mediated by the RAC–Lamellipodin Axis
Abstract
1. Introduction
2. Materials and Methods
2.1. Whole Mushroom Ganoderma Lucidum Extract (GLE)
2.2. Cell Culture
2.3. Cell Viability
2.4. Wash Out Assays
2.5. Wound Healing Assay
2.6. Invasion Assay
2.7. Rac Activity Assay
2.8. Western Blot Analysis
2.9. Immunofluorescence
2.10. Statistical Analysis
3. Results
3.1. GLE Selectively Inhibits Cancer Cell Viability
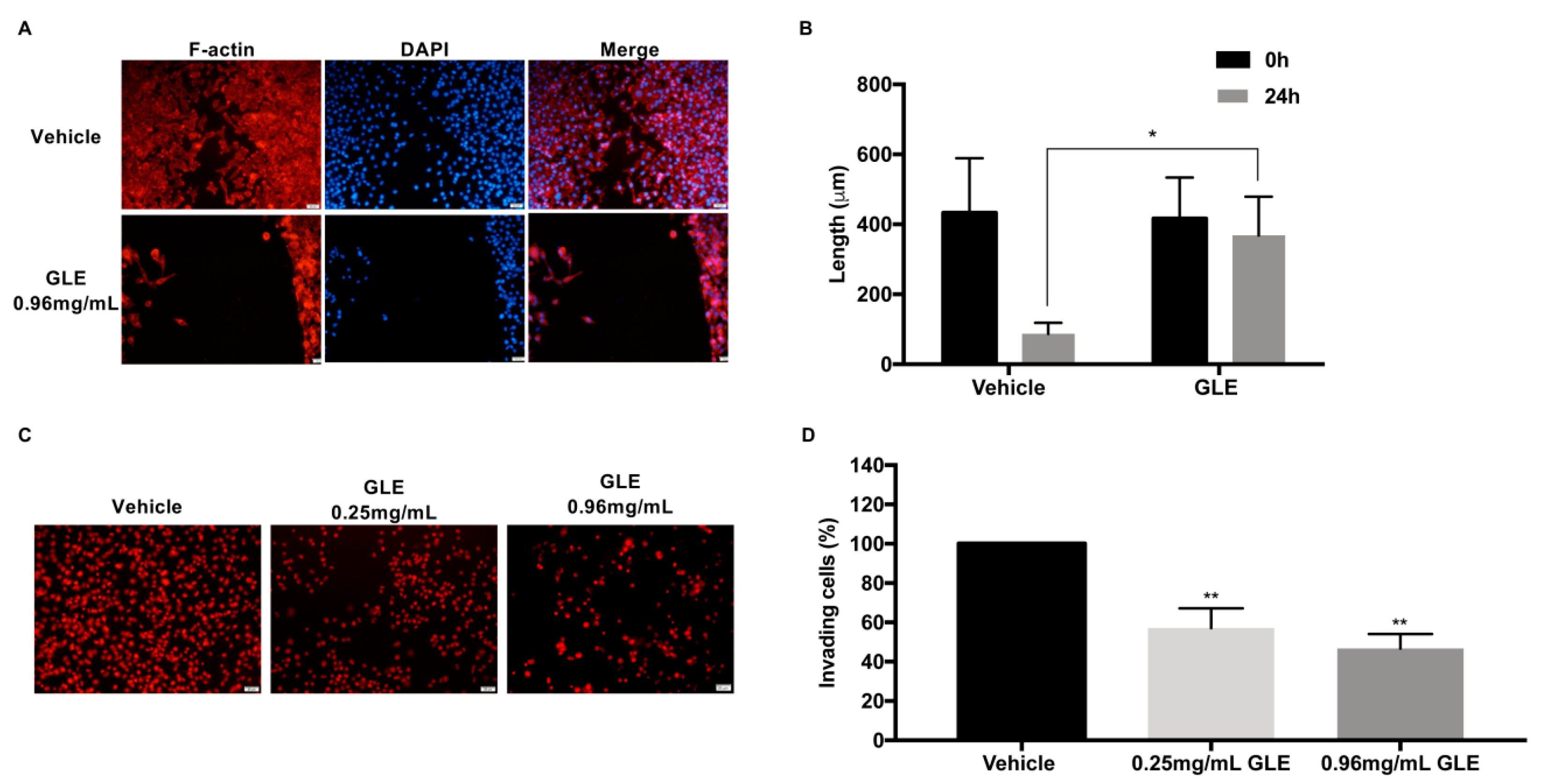
3.2. GLE inhibits Cancer Cell Migration and Invasion
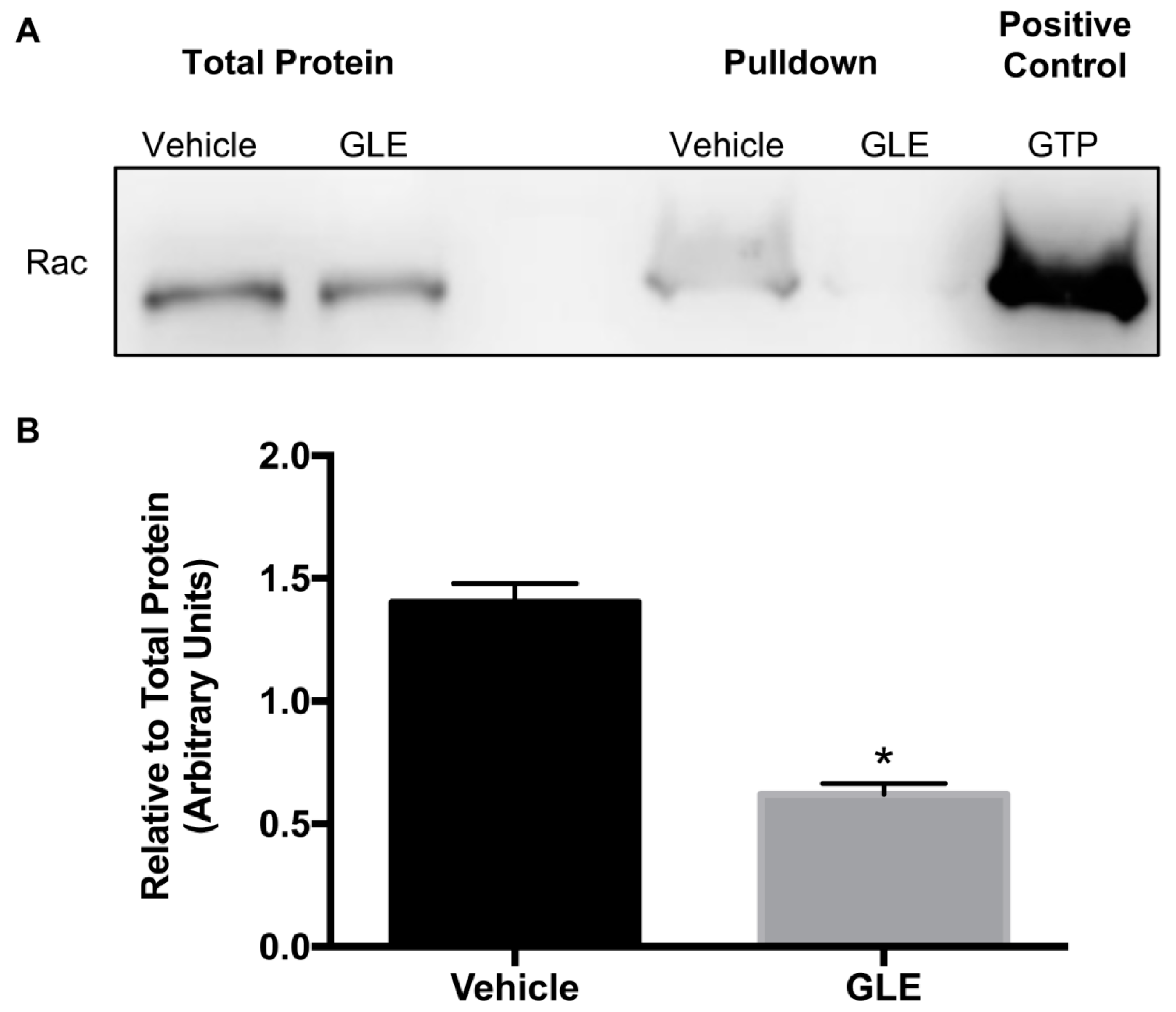
3.3. GLE Affects Rac Activity
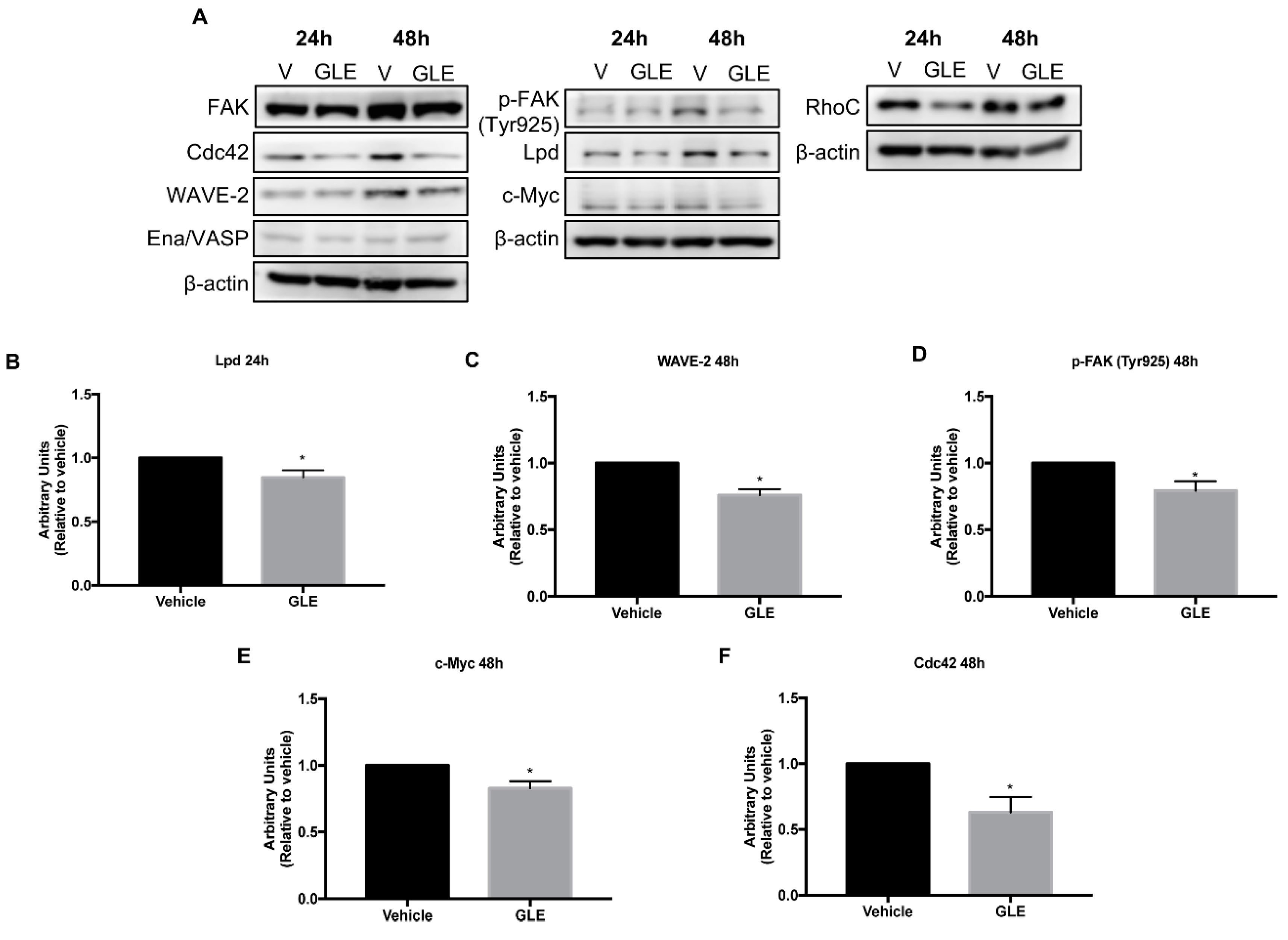
3.4. GLE Decreases Expression of Proteins Involved in Cell Migration
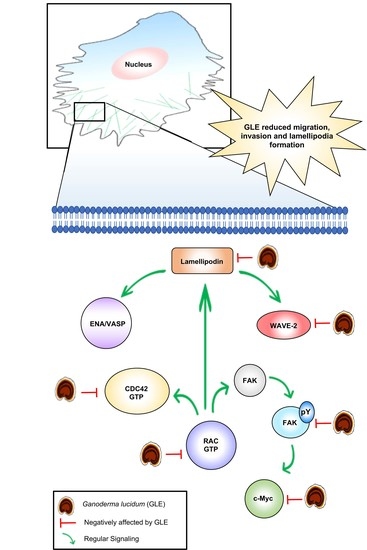
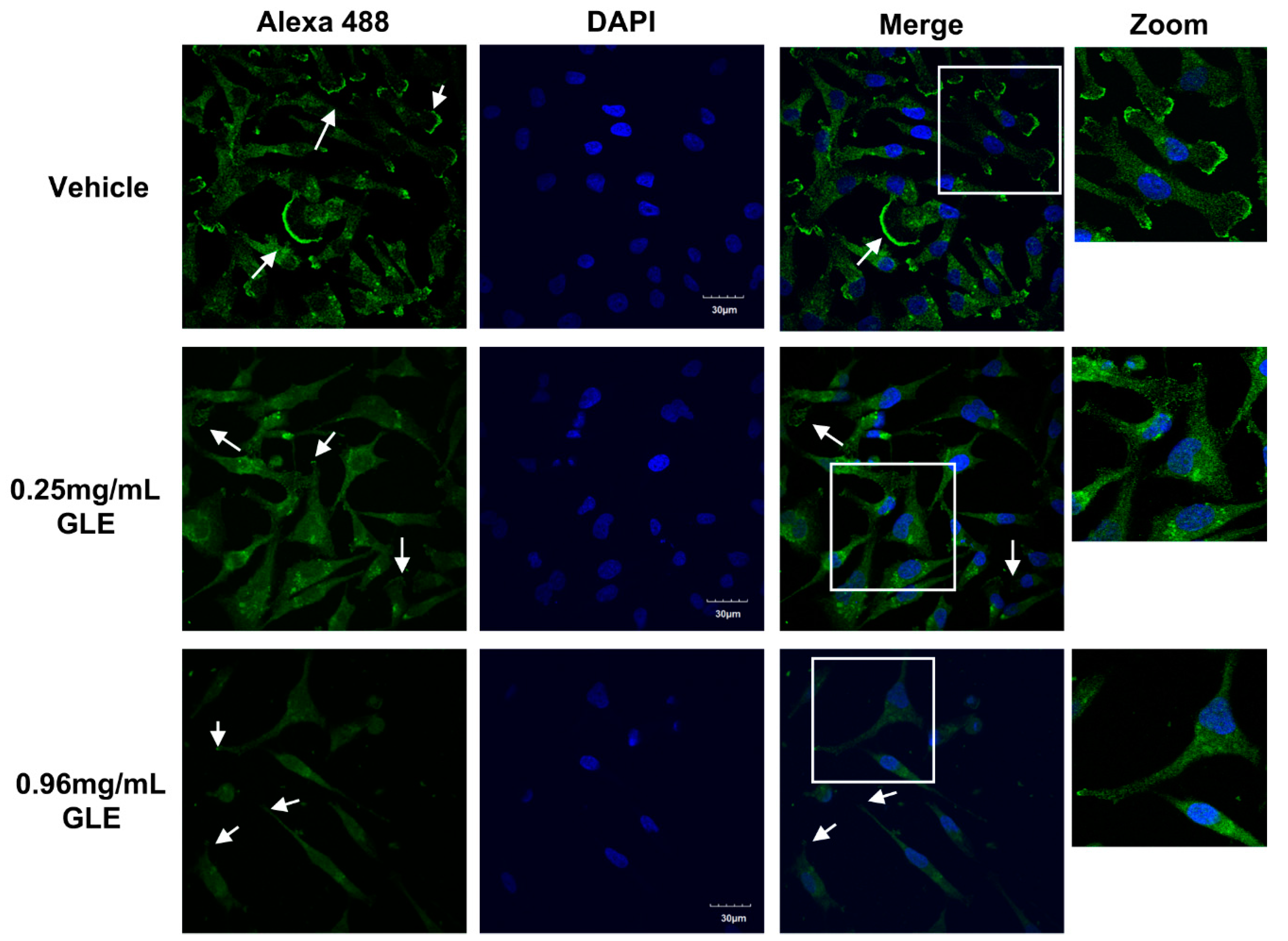
3.5. GLE Affects Lamellipodia Formation in MDA-MB-231 Cells
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- DeSantis, C.E.; Ma, J.; Goding Sauer, A.; Newman, L.A.; Jemal, A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J. Clin. 2017, 67, 439–448. [Google Scholar] [CrossRef]
- Chambers, A.F.; Groom, A.C.; MacDonald, I.C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2002, 2, 563–572. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Condeelis, J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim. Biophys. Acta 2007, 1773, 642–652. [Google Scholar] [CrossRef]
- Polacheck, W.J.; Zervantonakis, I.K.; Kamm, R.D. Tumor cell migration in complex microenvironments. Cell Mol. Life Sci. 2013, 70, 1335–1356. [Google Scholar] [CrossRef]
- Bozzuto, G.; Ruggieri, P.; Fau-Molinari, A.; Molinari, A. Molecular aspects of tumor cell migration and invasion. Ann. Ist Super Sanita 2010, 46, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.; Christofori, G. Mechanisms of Motility in Metastasizing Cells. Mol. Cancer Res. 2010, 8, 629–642. [Google Scholar] [CrossRef]
- Wertheimer, E.; Gutierrez-Uzquiza, A.; Rosemblit, C.; Lopez-Haber, C.; Sosa, M.S.; Kazanietz, M.G. Rac signaling in breast cancer: A tale of GEFs and GAPs. Cell Signal 2012, 24, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Ridley, A.J. Rho GTPases and actin dynamics in membrane protrusions and vesicle trafficking. Trends Cell Biol. 2006, 16, 522–529. [Google Scholar] [CrossRef]
- Kazanietz, M.G.; Caloca, M.J. The Rac GTPase in Cancer: From Old Concepts to New Paradigms. Cancer Res. 2017, 77, 5445–5451. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.R.; Rossman, K.L.; Der, C.J. Rho guanine nucleotide exchange factors: Regulators of Rho GTPase activity in development and disease. Oncogene 2013, 33, 4021. [Google Scholar] [CrossRef] [PubMed]
- Azios, N.G.; Krishnamoorthy, L.; Harris, M.; Cubano, L.A.; Cammer, M.; Dharmawardhane, S.F. Estrogen and resveratrol regulate Rac and Cdc42 signaling to the actin cytoskeleton of metastatic breast cancer cells. Neoplasia 2007, 9, 147–158. [Google Scholar] [CrossRef]
- Bae, Y.H.; Mui, K.L.; Hsu, B.Y.; Liu, S.L.; Cretu, A.; Razinia, Z.; Xu, T.; Pure, E.; Assoian, R.K. A FAK-Cas-Rac-lamellipodin signaling module transduces extracellular matrix stiffness into mechanosensitive cell cycling. Sci. Signal 2014, 7, ra57. [Google Scholar] [CrossRef] [PubMed]
- Law, A.L.; Vehlow, A.; Kotini, M.; Dodgson, L.; Soong, D.; Theveneau, E.; Bodo, C.; Taylor, E.; Navarro, C.; Perera, U.; et al. Lamellipodin and the Scar/WAVE complex cooperate to promote cell migration in vivo. J. Cell Biol. 2013, 203, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Leslie, J.D.; Stewart, M.; Lafuente, E.M.; Valderrama, F.; Jagannathan, R.; Strasser, G.A.; Rubinson, D.A.; Liu, H.; Way, M.; et al. Lamellipodin, an Ena/VASP ligand, is implicated in the regulation of lamellipodial dynamics. Dev. Cell 2004, 7, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Gautreau, A. Steering cell migration: Lamellipodium dynamics and the regulation of directional persistence. Nat. Rev. Mol. Cell Biol. 2014, 15, 577–590. [Google Scholar] [CrossRef]
- Vehlow, A.; Soong, D.; Vizcay-Barrena, G.; Bodo, C.; Law, A.L.; Perera, U.; Krause, M. Endophilin, Lamellipodin, and Mena cooperate to regulate F-actin-dependent EGF-receptor endocytosis. EMBO J. 2013, 32, 2722–2734. [Google Scholar] [CrossRef]
- Carmona, G.; Perera, U.; Gillett, C.; Naba, A.; Law, A.L.; Sharma, V.P.; Wang, J.; Wyckoff, J.; Balsamo, M.; Mosis, F.; et al. Lamellipodin promotes invasive 3D cancer cell migration via regulated interactions with Ena/VASP and SCAR/WAVE. Oncogene 2016, 35, 5155–5169. [Google Scholar] [CrossRef]
- Suarez-Arroyo, I.J.; Loperena-Alvarez, Y.; Rosario-Acevedo, R.; Martinez-Montemayor, M.M. Ganoderma spp.: A Promising Adjuvant Treatment for Breast Cancer. Medicines 2017, 4, 15. [Google Scholar] [CrossRef]
- Rossi, P.; Difrancia, R.; Quagliariello, V.; Savino, E.; Tralongo, P.; Randazzo, C.L.; Berretta, M. B-glucans from Grifola frondosa and Ganoderma lucidum in breast cancer: An example of complementary and integrative medicine. Oncotarget 2018, 9, 24837–24856. [Google Scholar] [CrossRef]
- Smina, T.P.; Nitha, B.; Devasagayam, T.P.; Janardhanan, K.K. Ganoderma lucidum total triterpenes induce apoptosis in MCF-7 cells and attenuate DMBA induced mammary and skin carcinomas in experimental animals. Mutat. Res. 2017, 813, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Na, K.; Chen, D.; Wang, Y.; Pan, H.; Wang, X. Effects of non-steroidal anti-inflammatory drug-activated gene-1 on Ganoderma lucidum polysaccharides-induced apoptosis of human prostate cancer PC-3 cells. Int. J. Oncol. 2018, 53, 2356–2368. [Google Scholar] [CrossRef]
- Martinez-Montemayor, M.M.; Ling, T.; Suarez-Arroyo, I.J.; Ortiz-Soto, G.; Santiago-Negron, C.L.; Lacourt-Ventura, M.Y.; Valentin-Acevedo, A.; Lang, W.H.; Rivas, F. Identification of Biologically Active Ganoderma lucidum Compounds and Synthesis of Improved Derivatives That Confer Anti-cancer Activities in vitro. Front. Pharmacol. 2019, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Sliva, D. Ganoderma lucidum (Reishi) in cancer treatment. Integr. Cancer Ther. 2003, 2, 358–364. [Google Scholar] [CrossRef]
- Martinez-Montemayor, M.M.; Acevedo, R.R.; Otero-Franqui, E.; Cubano, L.A.; Dharmawardhane, S.F. Ganoderma lucidum (Reishi) inhibits cancer cell growth and expression of key molecules in inflammatory breast cancer. Nutr. Cancer 2011, 63, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Slivova, V.; Harvey, K.; Valachovicova, T.; Sliva, D. Ganoderma lucidum Suppresses Growth of Breast Cancer Cells Through the Inhibition of Akt/NF-κB Signaling. Nutr. Cancer 2004, 49, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Slivova, V.; Fau-Sliva, D.; Sliva, D. Ganoderma lucidum inhibits proliferation of human breast cancer cells by down-regulation of estrogen receptor and NF-kappaB signaling. Int. J. Oncol. 2006, 29, 695–703. [Google Scholar]
- Loganathan, J.; Jiang, J.; Smith, A.; Jedinak, A.; Thyagarajan-Sahu, A.; Sandusky, G.E.; Nakshatri, H.; Sliva, D. The mushroom Ganoderma lucidum suppresses breast-to-lung cancer metastasis through the inhibition of pro-invasive genes. Int. J. Oncol. 2014, 44, 2009–2015. [Google Scholar] [CrossRef]
- Sliva, D.; Labarrere, C.; Slivova, V.; Sedlak, M.; Lloyd, F.P.; Ho, N.W.Y. Ganoderma lucidum suppresses motility of highly invasive breast and prostate cancer cells. Biochem. Biophys. Res. Commun. 2002, 298, 603–612. [Google Scholar] [CrossRef]
- Suarez-Arroyo, I.J.; Rios-Fuller, T.J.; Feliz-Mosquea, Y.R.; Lacourt-Ventura, M.; Leal-Alviarez, D.J.; Maldonado-Martinez, G.; Cubano, L.A.; Martinez-Montemayor, M.M. Ganoderma lucidum Combined with the EGFR Tyrosine Kinase Inhibitor, Erlotinib Synergize to Reduce Inflammatory Breast Cancer Progression. J. Cancer 2016, 7, 500–511. [Google Scholar] [CrossRef]
- Suarez-Arroyo, I.J.; Rosario-Acevedo, R.; Aguilar-Perez, A.; Clemente, P.L.; Cubano, L.A.; Serrano, J.; Schneider, R.J.; Martinez-Montemayor, M.M. Anti-tumor effects of Ganoderma lucidum (reishi) in inflammatory breast cancer in in vivo and in vitro models. PLoS ONE 2013, 8, e57431. [Google Scholar] [CrossRef]
- Rios-Fuller, T.J.; Ortiz-Soto, G.; Lacourt-Ventura, M.; Maldonado-Martinez, G.; Cubano, L.A.; Schneider, R.J.; Martinez-Montemayor, M.M. Ganoderma lucidum extract (GLE) impairs breast cancer stem cells by targeting the STAT3 pathway. Oncotarget 2018, 9, 35907–35921. [Google Scholar] [CrossRef]
- Baugher, P.J.; Krishnamoorthy, L.; Price, J.E.; Dharmawardhane, S.F. Rac1 and Rac3 isoform activation is involved in the invasive and metastatic phenotype of human breast cancer cells. Breast Cancer Res. 2005, 7, R965–R974. [Google Scholar] [CrossRef]
- Deramaudt, T.B.; Dujardin, D.; Hamadi, A.; Noulet, F.; Kolli, K.; Mey, J.D.; Takeda, K.; Rondé, P.; Assoian, R.K. FAK phosphorylation at Tyr-925 regulates cross-talk between focal adhesion turnover and cell protrusion. Mol. Biol. Cell 2011, 22, 964–975. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Lefringhouse, J.; Liu, Z.; West, D.; Baldwin, L.A.; Ou, C.; Chen, L.; Napier, D.; Chaiswing, L.; Brewer, L.D.; et al. Inhibition of the integrin/FAK signaling axis and c-Myc synergistically disrupts ovarian cancer malignancy. Oncogenesis 2017, 6, e295. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.S.; Song, Y.L.; Yin, Z.Q.; Guo, J.J.; Wang, S.P.; Zhao, W.W.; Chen, X.P.; Zhang, Q.W.; Lu, J.J.; Wang, Y.T. Ganoderiol A-enriched extract suppresses migration and adhesion of MDA-MB-231 cells by inhibiting FAK-SRC-paxillin cascade pathway. PLoS ONE 2013, 8, e76620. [Google Scholar] [CrossRef]
- Gotzmann, J.; Mikula, M.; Eger, A.; Schulte-Hermann, R.; Foisner, R.; Beug, H.; Mikulits, W. Molecular aspects of epithelial cell plasticity: Implications for local tumor invasion and metastasis. Mutat. Res. 2004, 566, 9–20. [Google Scholar] [CrossRef]
- Hall, A. Rho GTPases and the Actin Cytoskeleton. Science 1998, 279, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Kolsch, V.; Charest, P.G.; Firtel, R.A. The regulation of cell motility and chemotaxis by phospholipid signaling. J. Cell Sci. 2008, 121, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Hampsch, R.A.; Shee, K.; Bates, D.; Lewis, L.D.; Désiré, L.; Leblond, B.; Demidenko, E.; Stefan, K.; Huang, Y.H.; Miller, T.W. Therapeutic sensitivity to Rac GTPase inhibition requires consequential suppression of mTORC1, AKT, and MEK signaling in breast cancer. Oncotarget 2017, 8, 21806–21817. [Google Scholar] [CrossRef]
- Fernando, H.S.; Davies, S.R.; Chhabra, A.; Watkins, G.; Douglas-Jones, A.; Kynaston, H.; Mansel, R.E.; Jiang, W.G. Expression of the WASP Verprolin-Homologues (WAVE Members) in Human Breast Cancer. Oncology 2007, 73, 376–383. [Google Scholar] [CrossRef]
- Krause, M.; Dent, E.W.; Bear, J.E.; Loureiro, J.J.; Gertler, F.B. Ena/VASP Proteins: Regulators of the Actin Cytoskeleton and Cell Migration. Annu. Rev. Cell Dev. Biol. 2003, 19, 541–564. [Google Scholar] [CrossRef] [PubMed]
- Duperret, E.K.; Dahal, A.; Ridky, T.W. Focal-adhesion-independent integrin-αv regulation of FAK and c-Myc is necessary for 3D skin formation and tumor invasion. J. Cell Sci. 2015, 128, 3997–4013. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, K.; Itoh, R.E.; Yoshizaki, H.; Nakamura, Y.O.T.; Matsuda, M. Coactivation of Rac1 and Cdc42 at Lamellipodia and Membrane Ruffles Induced by Epidermal Growth Factor. Mol. Biol. Cell 2004, 15, 1003–1010. [Google Scholar] [CrossRef]
- Bray, K.; Gillette, M.; Young, J.; Loughran, E.; Hwang, M.; Sears, J.C.; Vargo-Gogola, T. Cdc42 overexpression induces hyperbranching in the developing mammary gland by enhancing cell migration. Breast Cancer Res. 2013, 15, R91. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acevedo-Díaz, A.; Ortiz-Soto, G.; Suárez-Arroyo, I.J.; Zayas-Santiago, A.; Martínez Montemayor, M.M. Ganoderma lucidum Extract Reduces the Motility of Breast Cancer Cells Mediated by the RAC–Lamellipodin Axis. Nutrients 2019, 11, 1116. https://doi.org/10.3390/nu11051116
Acevedo-Díaz A, Ortiz-Soto G, Suárez-Arroyo IJ, Zayas-Santiago A, Martínez Montemayor MM. Ganoderma lucidum Extract Reduces the Motility of Breast Cancer Cells Mediated by the RAC–Lamellipodin Axis. Nutrients. 2019; 11(5):1116. https://doi.org/10.3390/nu11051116
Chicago/Turabian StyleAcevedo-Díaz, Ariana, Gabriela Ortiz-Soto, Ivette J. Suárez-Arroyo, Astrid Zayas-Santiago, and Michelle M. Martínez Montemayor. 2019. "Ganoderma lucidum Extract Reduces the Motility of Breast Cancer Cells Mediated by the RAC–Lamellipodin Axis" Nutrients 11, no. 5: 1116. https://doi.org/10.3390/nu11051116
APA StyleAcevedo-Díaz, A., Ortiz-Soto, G., Suárez-Arroyo, I. J., Zayas-Santiago, A., & Martínez Montemayor, M. M. (2019). Ganoderma lucidum Extract Reduces the Motility of Breast Cancer Cells Mediated by the RAC–Lamellipodin Axis. Nutrients, 11(5), 1116. https://doi.org/10.3390/nu11051116