Neuroprotective Effects of Diets Containing Olive Oil and DHA/EPA in a Mouse Model of Cerebral Ischemia
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Diets
2.3. Fatty Acid Composition of Dietary Fats
2.4. Experimental Design
2.5. Middle Cerebral Artery Occlusion
2.6. Infarct Volume, Infarct Severity, and the Evaluation of Haemorrhagic Transformation
2.7. Neurobehavioural Tests
2.7.1. Grip Strength Test
2.7.2. Neurological Scale
2.7.3. Footprint Test
2.8. Determination of Antioxidant Enzyme Activity
2.9. Statistical Analysis
3. Results
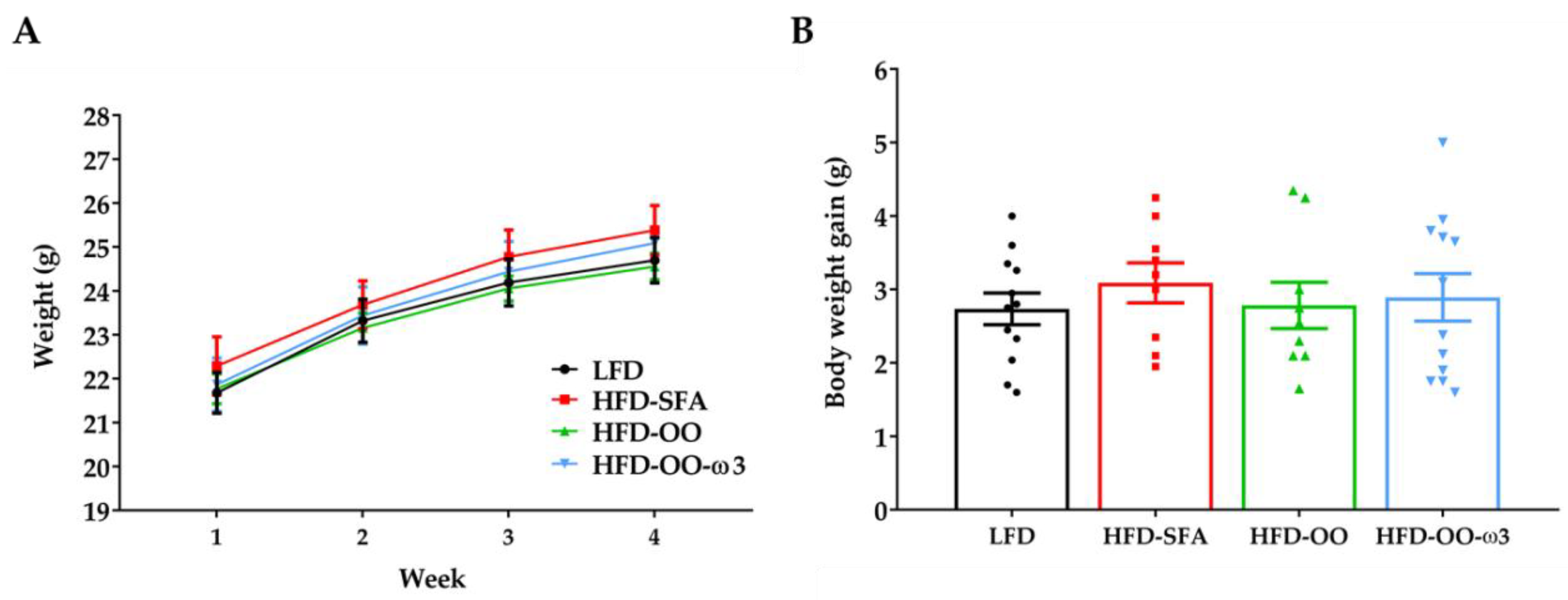
3.1. Animal Growth and Food Intake
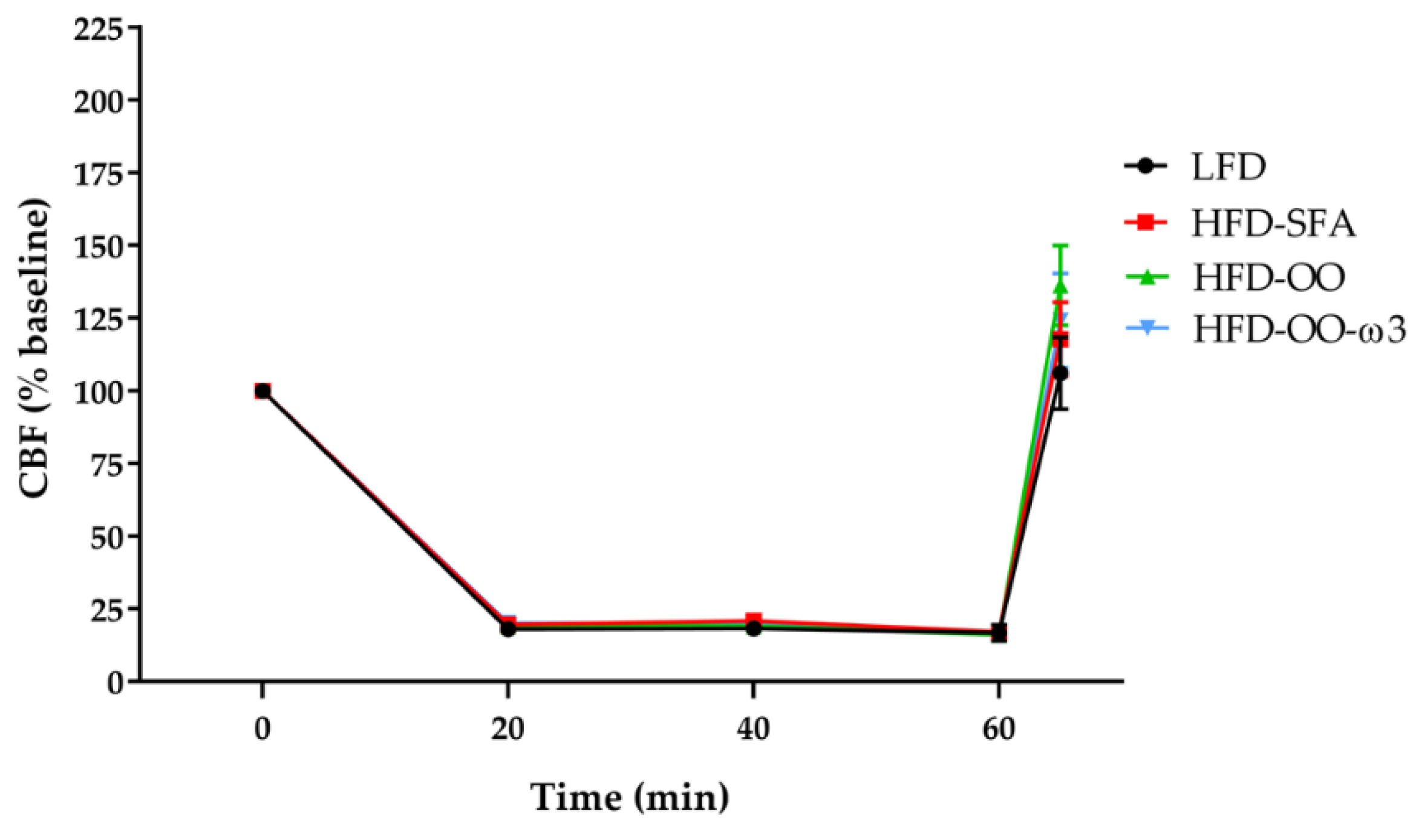
3.2. Cerebral Blood Flow (CBF)
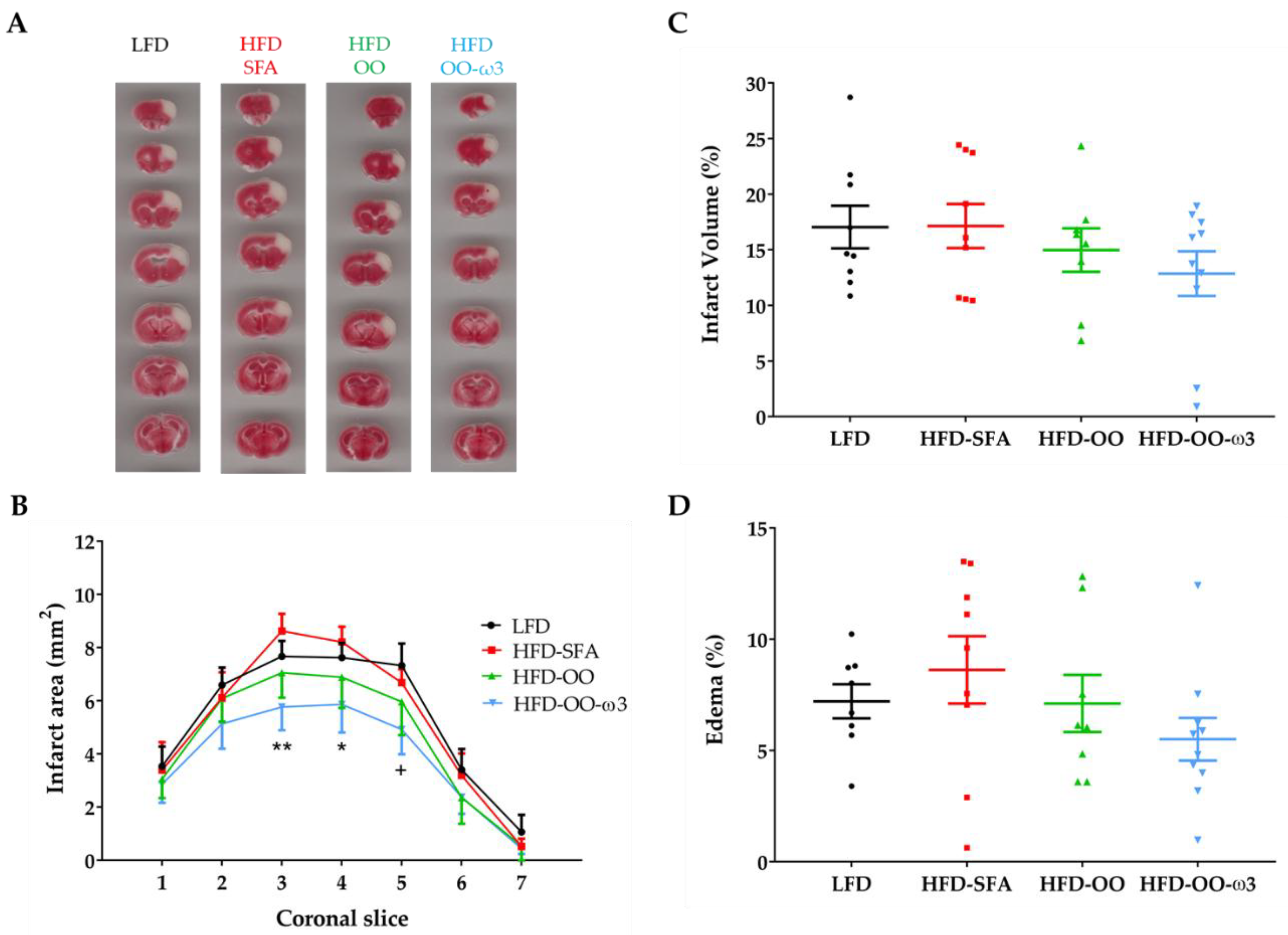
3.3. A High-Fat Diet Enriched in DHA/EPA and MUFAs from Olive Oil Reduces the Infarct Area in Mice Subjected to Transient Focal Cerebral Ischemia
3.4. Beneficial Effects of Dietary Intervention with DHA+EPA and Olive Oil-Enriched Food on Brain Infarct Severity
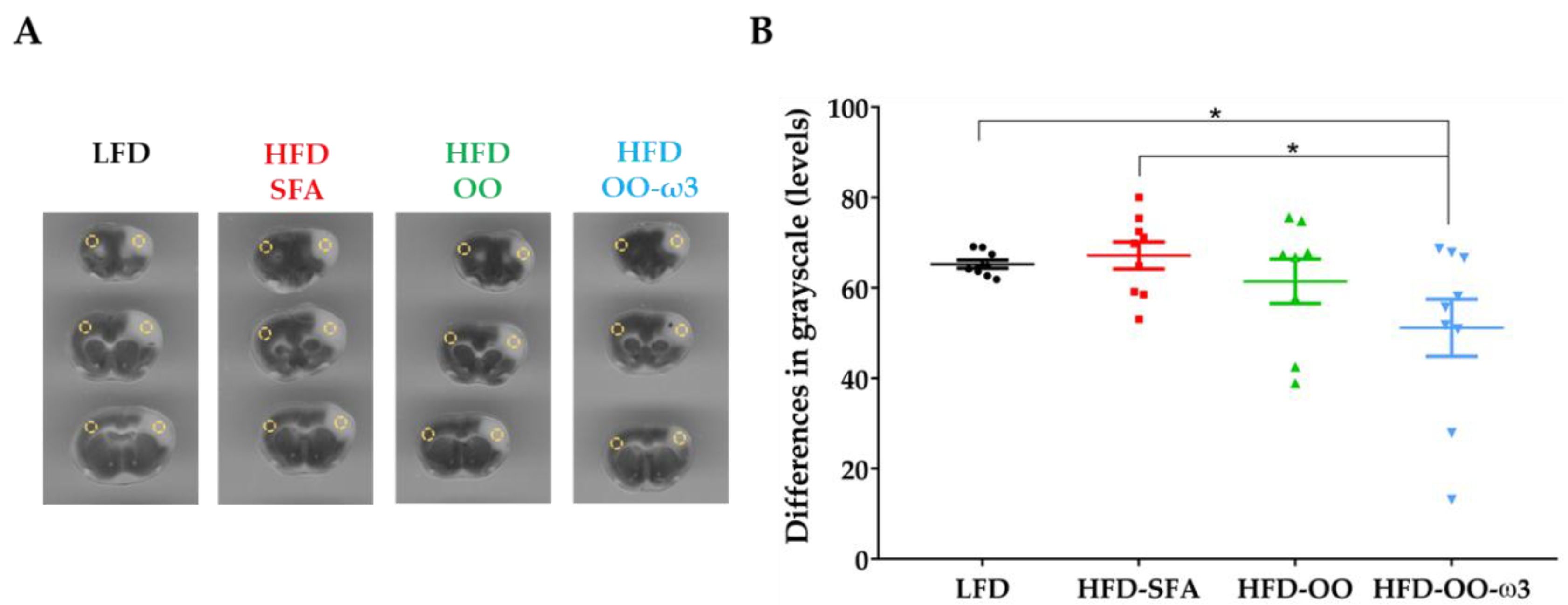
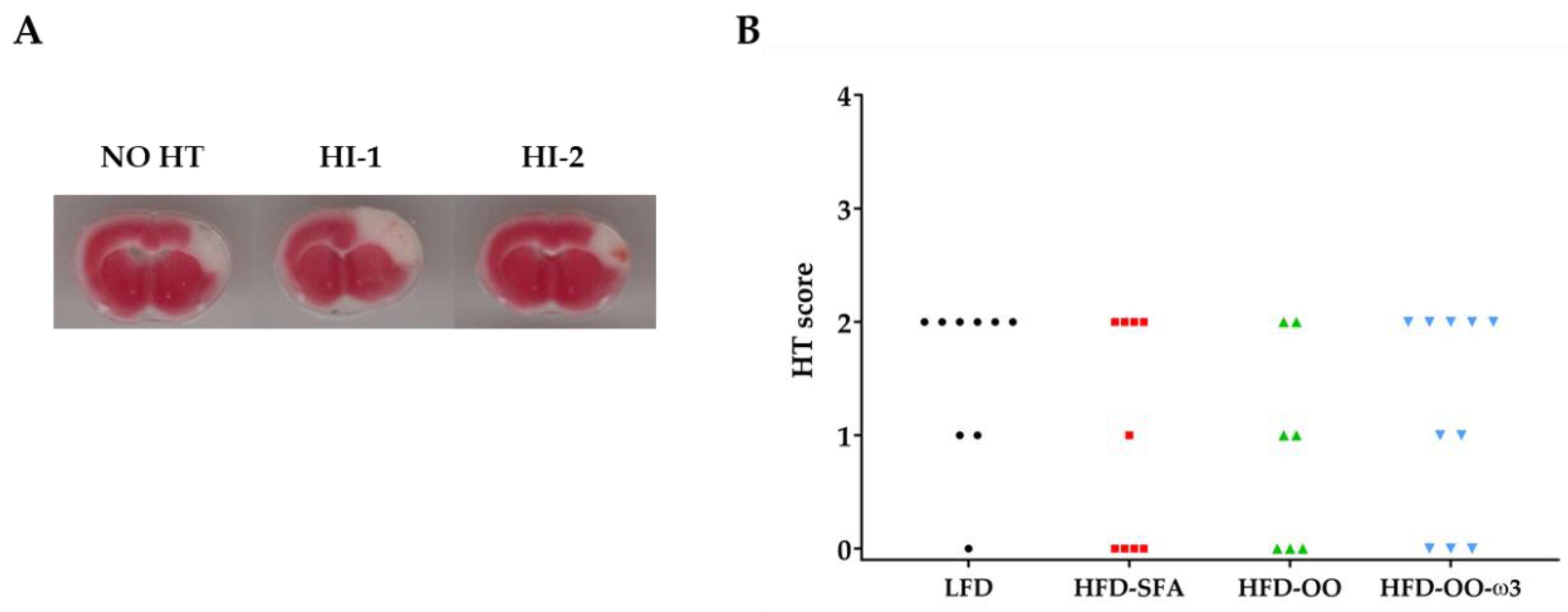
3.5. Haemorrhagic Transformation
3.6. Improvement of Post-Ischemic Neurobehavioural and Physical Outcomes in Mice Fed with Diets Rich in MUFAs from Olive Oil or in DHA/EPA + MUFAs from Olive Oil
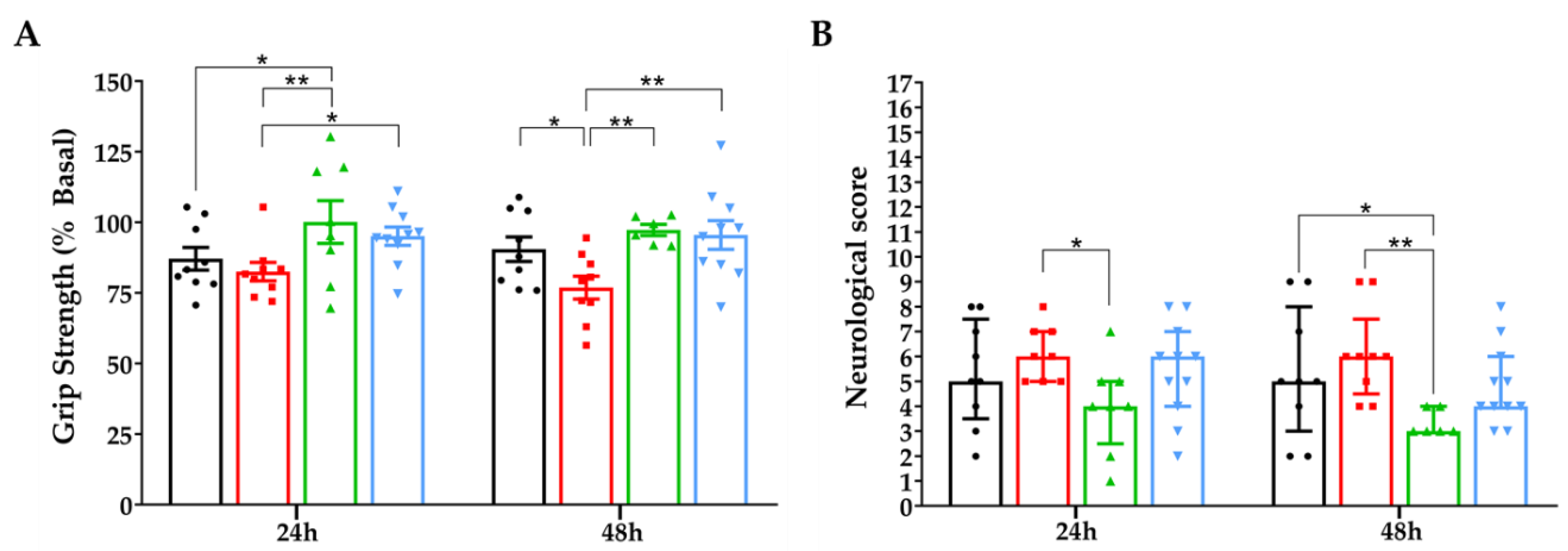
3.6.1. Grip Strength Test and Neurological Score
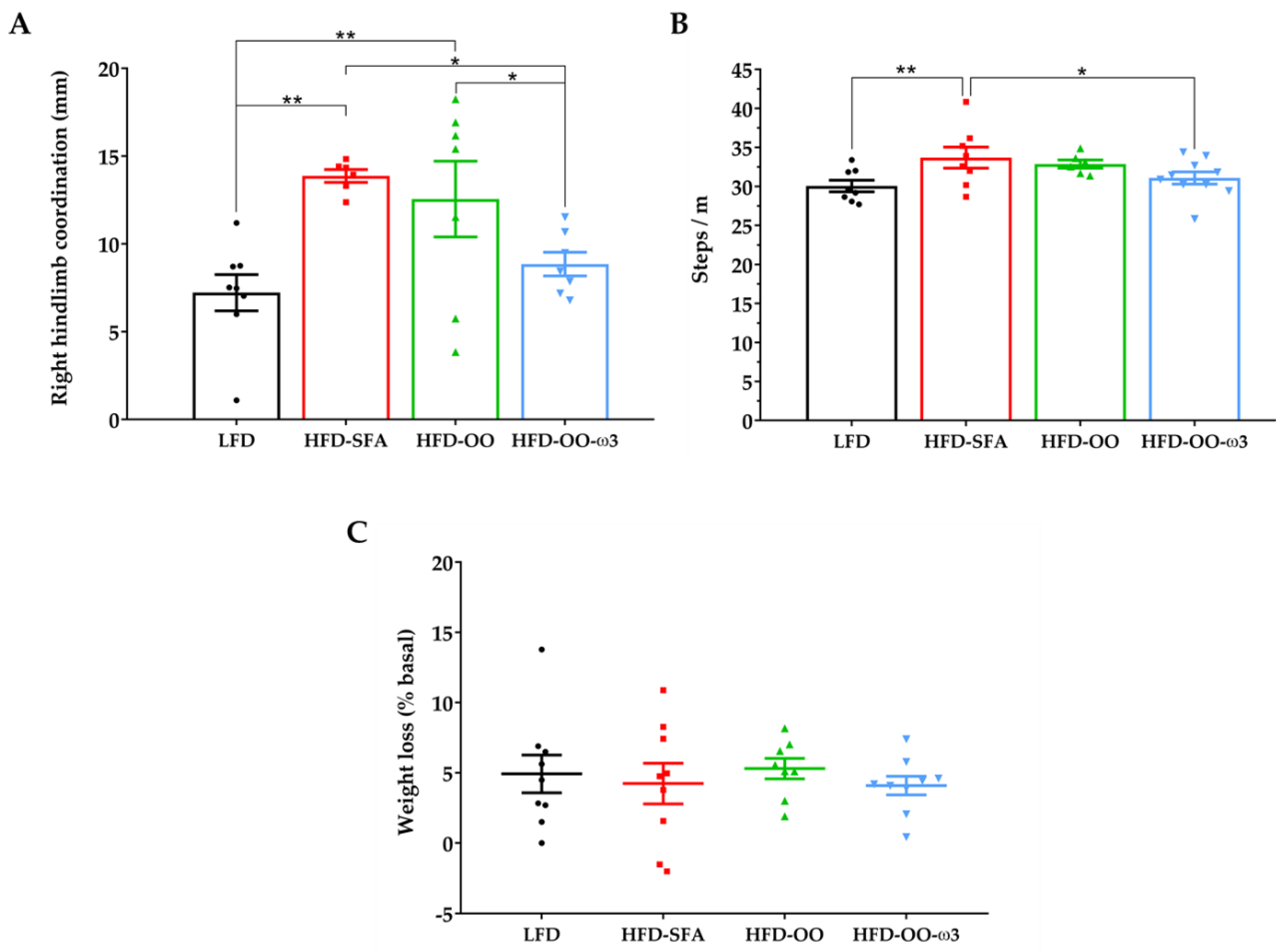
3.6.2. Gait Analysis and Weight Loss
3.7. Effect of Custom HFDs on the Antioxidant Enzyme Activities in the Plasma
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benjamin, E.J.; Virani, S.S.; Callaway, C.W.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Chiuve, S.E.; Cushman, M.; Delling, F.N.; Deo, R.; et al. Heart Disease and Stroke Statistics-2018 Update: A Report From the American Heart Association. Circulation 2018, 137, e67. [Google Scholar] [CrossRef]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef]
- World Health Organization. Available online: https://www.who.int/topics/cerebrovascular_accident/en/ (accessed on 25 February 2019).
- Ayuso, M.I.; Martinez-Alonso, E.; Cid, C.; Alonso de Lecinana, M.; Alcazar, A. The translational repressor eIF4E-binding protein 2 (4E-BP2) correlates with selective delayed neuronal death after ischemia. J. Cereb. Blood Flow Metab. 2013, 33, 1173–1181. [Google Scholar] [CrossRef]
- White, B.C.; Sullivan, J.M.; DeGracia, D.J.; O’Neil, B.J.; Neumar, R.W.; Grossman, L.I.; Rafols, J.A.; Krause, G.S. Brain ischemia and reperfusion: Molecular mechanisms of neuronal injury. J. Neurol. Sci. 2000, 179, 1–33. [Google Scholar] [CrossRef]
- Drake, C.; Boutin, H.; Jones, M.S.; Denes, A.; McColl, B.W.; Selvarajah, J.R.; Hulme, S.; Georgiou, R.F.; Hinz, R.; Gerhard, A.; et al. Brain inflammation is induced by co-morbidities and risk factors for stroke. Brain Behav. Immun. 2011, 25, 1113–1122. [Google Scholar] [CrossRef] [PubMed]
- Haley, M.J.; Krishnan, S.; Burrows, D.; de Hoog, L.; Thakrar, J.; Schiessl, I.; Allan, S.M.; Lawrence, C.B. Acute high-fat feeding leads to disruptions in glucose homeostasis and worsens stroke outcome. J. Cereb. Blood Flow Metab. 2017. [Google Scholar] [CrossRef]
- Duan, Y.; Zeng, L.; Zheng, C.; Song, B.; Li, F.; Kong, X.; Xu, K. Inflammatory Links Between High Fat Diets and Diseases. Front. Immunol. 2018, 9, 2649. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, S.; Hawken, S.; Ounpuu, S.; Dans, T.; Avezum, A.; Lanas, F.; McQueen, M.; Budaj, A.; Pais, P.; Varigos, J.; et al. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): Case-control study. Lancet 2004, 364, 937–952. [Google Scholar] [CrossRef]
- Dhungana, H.; Rolova, T.; Savchenko, E.; Wojciechowski, S.; Savolainen, K.; Ruotsalainen, A.K.; Sullivan, P.M.; Koistinaho, J.; Malm, T. Western-type diet modulates inflammatory responses and impairs functional outcome following permanent mdidle cerebral artery occlusion in aged mice expressing the human apolipoprotein E4 allele. J. Neuroinflamm. 2013, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Hoane, M.R.; Swan, A.A.; Heck, S.E. The effects of a high-fat sucrose diet on functional outcome following cortical contusion injury in the rat. Behav. Brain Res. 2011, 223, 119–124. [Google Scholar] [CrossRef][Green Version]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gomez-Gracia, E.; Ruiz-Gutierrez, V.; Fiol, M.; Lapetra, J.; et al. Primary prevention of cardiovascular disease with a Mediterranean diet. N. Engl. J. Med. 2013, 368, 1279–1290. [Google Scholar] [CrossRef]
- Rodriguez-Campello, A.; Jimenez-Conde, J.; Ois, A.; Cuadrado-Godia, E.; Giralt-Steinhauer, E.; Schroeder, H.; Romeral, G.; Llop, M.; Soriano-Tarraga, C.; Garralda-Anaya, M.; et al. Dietary habits in patients with ischemic stroke: A case-control study. PLoS ONE 2014, 9, e114716. [Google Scholar] [CrossRef]
- Li, W.; Prakash, R.; Chawla, D.; Du, W.; Didion, S.P.; Filosa, J.A.; Zhang, Q.; Brann, D.W.; Lima, V.V.; Tostes, R.C.; et al. Early effects of high-fat diet on neurovascular function and focal ischemic brain injury. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R1001–R1008. [Google Scholar] [CrossRef]
- Ayuso, M.I.; Gonzalo-Gobernado, R.; Montaner, J. Neuroprotective diets for stroke. Neurochem. Int. 2017, 107, 4–10. [Google Scholar] [CrossRef]
- Casas, R.; Sacanella, E.; Estruch, R. The immune protective effect of the Mediterranean diet against chronic low-grade inflammatory diseases. Endocr. Metab. Immune Disord. Drug Targets 2014, 14, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Bach-Faig, A.; Berry, E.M.; Lairon, D.; Reguant, J.; Trichopoulou, A.; Dernini, S.; Medina, F.X.; Battino, M.; Belahsen, R.; Miranda, G.; et al. Mediterranean diet pyramid today. Science and cultural updates. Public Health Nutr. 2011, 14, 2274–2284. [Google Scholar] [CrossRef]
- Mori, M.; Mori, H.; Hamada, A.; Yamori, Y. Taurine in morning spot urine for the useful assessment of dietary seafood intake in Japanese children and adolescents. J. Biomed. Sci. 2010, 17, S43. [Google Scholar] [CrossRef]
- Virtanen, J.K.; Siscovick, D.S.; Longstreth, W.T., Jr.; Kuller, L.H.; Mozaffarian, D. Fish consumption and risk of subclinical brain abnormalities on MRI in older adults. Neurology 2008, 71, 439–446. [Google Scholar] [CrossRef]
- Mozaffarian, D.; Wu, J.H. Omega-3 fatty acids and cardiovascular disease: Effects on risk factors, molecular pathways, and clinical events. J. Am. Coll. Cardiol. 2011, 58, 2047–2067. [Google Scholar] [CrossRef]
- Zamani, M.; Hassanshahi, J.; Soleimani, M.; Zamani, F. Neuroprotective effect of olive oil in the hippocampus CA1 neurons following ischemia: Reperfusion in mice. J. Neurosci. Rural Pract. 2013, 4, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Mohagheghi, F.; Bigdeli, M.R.; Rasoulian, B.; Zeinanloo, A.A.; Khoshbaten, A. Dietary virgin olive oil reduces blood brain barrier permeability, brain edema, and brain injury in rats subjected to ischemia-reperfusion. Sci. World J. 2010, 10, 1180–1191. [Google Scholar] [CrossRef]
- Rabiei, Z.; Bigdeli, M.R.; Rasoulian, B.; Ghassempour, A.; Mirzajani, F. The neuroprotection effect of pretreatment with olive leaf extract on brain lipidomics in rat stroke model. Phytomedicine 2012, 19, 940–946. [Google Scholar] [CrossRef]
- Montserrat-de la Paz, S.; Naranjo, M.C.; Bermudez, B.; Lopez, S.; Moreda, W.; Abia, R.; Muriana, F.J. Postprandial dietary fatty acids exert divergent inflammatory responses in retinal-pigmented epithelium cells. Food Funct. 2016, 7, 1345–1353. [Google Scholar] [CrossRef]
- Montserrat-de la Paz, S.; Naranjo, M.C.; Lopez, S.; Abia, R.; Muriana, F.J.; Bermudez, B. Niacin and olive oil promote skewing to the M2 phenotype in bone marrow-derived macrophages of mice with metabolic syndrome. Food Funct. 2016, 7, 2233–2238. [Google Scholar] [CrossRef]
- Ayuso, M.I.; Martinez-Alonso, E.; Chioua, M.; Escobar-Peso, A.; Gonzalo-Gobernado, R.; Montaner, J.; Marco-Contelles, J.; Alcazar, A. Quinolinyl Nitrone RP19 Induces Neuroprotection after Transient Brain Ischemia. ACS Chem. Neurosci. 2017, 8, 2202–2213. [Google Scholar] [CrossRef]
- Morancho, A.; Garcia-Bonilla, L.; Barcelo, V.; Giralt, D.; Campos-Martorell, M.; Garcia, S.; Montaner, J.; Rosell, A. A new method for focal transient cerebral ischaemia by distal compression of the middle cerebral artery. Neuropathol. Appl. Neurobiol. 2012, 38, 617–627. [Google Scholar] [CrossRef]
- Bederson, J.B.; Pitts, L.H.; Germano, S.M.; Nishimura, M.C.; Davis, R.L.; Bartkowski, H.M. Evaluation of 2,3,5-triphenyltetrazolium chloride as a stain for detection and quantification of experimental cerebral infarction in rats. Stroke 1986, 17, 1304–1308. [Google Scholar] [CrossRef]
- Berressem, D.; Koch, K.; Franke, N.; Klein, J.; Eckert, G.P. Intravenous Treatment with a Long-Chain Omega-3 Lipid Emulsion Provides Neuroprotection in a Murine Model of Ischemic Stroke—A Pilot Study. PLoS ONE 2016, 11, e0167329. [Google Scholar] [CrossRef]
- Horn, T.; Klein, J. Neuroprotective effects of lactate in brain ischemia: Dependence on anesthetic drugs. Neurochem. Int. 2013, 62, 251–257. [Google Scholar] [CrossRef]
- Aronowski, J.; Strong, R.; Shirzadi, A.; Grotta, J.C. Ethanol plus caffeine (caffeinol) for treatment of ischemic stroke: Preclinical experience. Stroke 2003, 34, 1246–1251. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, M.; Wan, J.; Wen, S.; Sun, J.; Zhao, H.; Lou, M. Docosahexaenoic acid attenuates hyperglycemia-enhanced hemorrhagic transformation after transient focal cerebral ischemia in rats. Neuroscience 2015, 301, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Linden, J.; Fassotte, L.; Tirelli, E.; Plumier, J.C.; Ferrara, A. Assessment of behavioral flexibility after middle cerebral artery occlusion in mice. Behav. Brain Res. 2014, 258, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Chioua, M.; Martinez-Alonso, E.; Gonzalo-Gobernado, R.; Ayuso, M.I.; Escobar-Peso, A.; Infantes, L.; Hadjipavlou-Litina, D.J.; Montoya, J.J.; Montaner, J.; Alcazar, A.; et al. New QuinolylNitrones for Stroke Therapy: Antioxidant and Neuroprotective (Z)-N-t-Butyl-1-(2-chloro-6-methoxyquinolin-3-yl)methanimine Oxide as a New Lead-Compound for Ischemic Stroke Treatment. J. Med. Chem. 2019. [Google Scholar] [CrossRef]
- Bederson, J.B.; Pitts, L.H.; Tsuji, M.; Nishimura, M.C.; Davis, R.L.; Bartkowski, H. Rat middle cerebral artery occlusion: Evaluation of the model and development of a neurologic examination. Stroke 1986, 17, 472–476. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, J.; Wang, L.; Lu, M.; Chopp, M. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology 2001, 56, 1666–1672. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.P.; Trueman, R.C.; Dunnett, S.B. Assessment of Motor Coordination and Balance in Mice Using the Rotarod, Elevated Bridge, and Footprint Tests. Curr. Protoc. Mouse Biol. 2012, 2, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Carter, R.J.; Lione, L.A.; Humby, T.; Mangiarini, L.; Mahal, A.; Bates, G.P.; Dunnett, S.B.; Morton, A.J. Characterization of progressive motor deficits in mice transgenic for the human Huntington’s disease mutation. J. Neurosci. 1999, 19, 3248–3257. [Google Scholar] [CrossRef]
- Munoz-Manchado, A.B.; Villadiego, J.; Romo-Madero, S.; Suarez-Luna, N.; Bermejo-Navas, A.; Rodriguez-Gomez, J.A.; Garrido-Gil, P.; Labandeira-Garcia, J.L.; Echevarria, M.; Lopez-Barneo, J.; et al. Chronic and progressive Parkinson’s disease MPTP model in adult and aged mice. J. Neurochem. 2016, 136, 373–387. [Google Scholar] [CrossRef]
- Cohen, G.; Somerson, N.L. Glucose-dependent secretion and destruction of hydrogen peroxide by Mycoplasma pneumoniae. J. Bacteriol. 1969, 98, 547–551. [Google Scholar] [PubMed]
- Franklin, K.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates, 3rd ed.; Academic Press: San Diego, CA, USA, 2007. [Google Scholar]
- Blondeau, N.; Widmann, C.; Lazdunski, M.; Heurteaux, C. Polyunsaturated fatty acids induce ischemic and epileptic tolerance. Neuroscience 2002, 109, 231–241. [Google Scholar] [CrossRef]
- Ueda, M.; Inaba, T.; Nito, C.; Kamiya, N.; Katayama, Y. Therapeutic impact of eicosapentaenoic acid on ischemic brain damage following transient focal cerebral ischemia in rats. Brain Res. 2013, 1519, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, H.; Zhang, H.; Leak, R.K.; Shi, Y.; Hu, X.; Gao, Y.; Chen, J. Dietary supplementation with omega-3 polyunsaturated fatty acids robustly promotes neurovascular restorative dynamics and improves neurological functions after stroke. Exp. Neurol. 2015, 272, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.Y.; Kuan, Y.H.; Li, J.R.; Chen, W.Y.; Ou, Y.C.; Pan, H.C.; Liao, S.L.; Raung, S.L.; Chang, C.J.; Chen, C.J. Docosahexaenoic acid reduces cellular inflammatory response following permanent focal cerebral ischemia in rats. J. Nutr. Biochem. 2013, 24, 2127–2137. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.C.; Kao, T.K.; Ou, Y.C.; Yang, D.Y.; Yen, Y.J.; Wang, C.C.; Chuang, Y.H.; Liao, S.L.; Raung, S.L.; Wu, C.W.; et al. Protective effect of docosahexaenoic acid against brain injury in ischemic rats. J. Nutr. Biochem. 2009, 20, 715–725. [Google Scholar] [CrossRef]
- Okabe, N.; Nakamura, T.; Toyoshima, T.; Miyamoto, O.; Lu, F.; Itano, T. Eicosapentaenoic acid prevents memory impairment after ischemia by inhibiting inflammatory response and oxidative damage. J. Stroke Cerebrovasc. Dis. 2011, 20, 188–195. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, S.; Mao, L.; Leak, R.K.; Shi, Y.; Zhang, W.; Hu, X.; Sun, B.; Cao, G.; Gao, Y.; et al. Omega-3 fatty acids protect the brain against ischemic injury by activating Nrf2 and upregulating heme oxygenase 1. J. Neurosci. 2014, 34, 1903–1915. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Najmi, A.K.; Mujeeb, M.; Akhtar, M. Protective Effect of Guggulipid in High Fat Diet and Middle Cerebral Artery Occlusion (MCAO) Induced Ischemic Cerebral Injury in Rats. Drug Res. 2016, 66, 407–414. [Google Scholar] [CrossRef]
- Maysami, S.; Haley, M.J.; Gorenkova, N.; Krishnan, S.; McColl, B.W.; Lawrence, C.B. Prolonged diet-induced obesity in mice modifies the inflammatory response and leads to worse outcome after stroke. J. Neuroinflamm. 2015, 12, 140. [Google Scholar] [CrossRef]
- Tilley, S.L.; Coffman, T.M.; Koller, B.H. Mixed messages: Modulation of inflammation and immune responses by prostaglandins and thromboxanes. J. Clin. Investig. 2001, 108, 15–23. [Google Scholar] [CrossRef]
- Lausada, N.; Arnal, N.; Astiz, M.; Marin, M.C.; Lofeudo, J.M.; Stringa, P.; Tacconi de Alaniz, M.J.; Tacconi de Gomez Dumm, N.; Hurtado de Catalfo, G.; Cristalli de Pinero, N.; et al. Dietary fats significantly influence the survival of penumbral neurons in a rat model of chronic ischemic by modifying lipid mediators, inflammatory biomarkers, NOS production, and redox-dependent apoptotic signals. Nutrition 2015, 31, 1430–1442. [Google Scholar] [CrossRef]
- Belayev, L.; Khoutorova, L.; Atkins, K.D.; Eady, T.N.; Hong, S.; Lu, Y.; Obenaus, A.; Bazan, N.G. Docosahexaenoic Acid therapy of experimental ischemic stroke. Transl. Stroke Res. 2011, 2, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Belayev, L.; Khoutorova, L.; Obenaus, A.; Bazan, N.G. Docosahexaenoic acid confers enduring neuroprotection in experimental stroke. J. Neurol. Sci. 2014, 338, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Mayurasakorn, K.; Williams, J.J.; Ten, V.S.; Deckelbaum, R.J. Docosahexaenoic acid: Brain accretion and roles in neuroprotection after brain hypoxia and ischemia. Curr. Opin. Clin. Nutr. Metab. Care 2011, 14, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Foroughi, M.; Akhavanzanjani, M.; Maghsoudi, Z.; Ghiasvand, R.; Khorvash, F.; Askari, G. Stroke and nutrition: A review of studies. Int. J. Prev. Med. 2013, 4, S165–S179. [Google Scholar]
- Ayuso, M.I.; Montaner, J. Advanced neuroprotection for brain ischemia: An alternative approach to minimize stroke damage. Expert Opin. Investig. Drugs 2015, 24, 1137–1142. [Google Scholar] [CrossRef] [PubMed]

| Macronutrient | LFD | HFD-SFA | HFD-OO | HFD-OO-ω3 |
|---|---|---|---|---|
| Fat (%) | 3 | 24 | 24 | 24 |
| Carbohydrate (%) | 77.5 | 56.5 | 56.5 | 56.5 |
| Protein (%) | 19.5 | 19.5 | 19.5 | 19.5 |
| HFD-SFA | HFD-OO | HFD-OO-ω3 | |
|---|---|---|---|
| Fatty Acid | g/100 g of Fatty Acid | ||
| 4:0, butyric | 0.83 ± 0.09 | ||
| 6:0, caproic | 0.25 ± 0.01 | ||
| 8:0, caprylic | 0.61 ± 0.04 | ||
| 10:0, capric | 2.47 ± 0.08 | ||
| 12:0, lauric | 3.09 ± 0.24 | ||
| 14:0, myristic | 10.9 ± 0.53 | ||
| 16:0, palmitic | 35.5 ± 0.47 | 20.4 ± 0.51 | 20.5 ± 0.37 |
| 16:1(ω-7), palmitoleic | 3.60 ± 0.18 | 0.97 ± 0.10 | 0.82 ± 0.07 |
| 18:0, stearic | 11.5 ± 0.43 | 5.70 ± 0.06 | 4.49 ± 0.21 |
| 18:1(ω-9), oleic | 25.3 ± 0.41 | 61.9 ± 0.71 | 61.5 ± 0.56 |
| 18:2(ω-6), linoleic | 4.27 ± 0.47 | 7.97 ± 0.38 | 8.04 ± 0.31 |
| 18:3(ω-3), α-linolenic | 0.39 ± 0.03 | 1.04 ± 0.08 | 0.94 ± 0.02 |
| 20:5(ω-3), eicosapentaenoic | 0.92 ± 0.05 | ||
| 22:6(ω-3), docosahexaenoic | 0.72 ± 0.06 | ||
| Others | 0.96 ± 0.24 | 2.05 ± 0.62 | 2.01 ± 0.51 |
| Total SFAs | 63.46 ± 1.07 | 26.11 ± 0.59*** | 25.01 ± 0.49*** |
| Total MUFAs | 28.93 ± 1.48 | 62.83 ± 0.78*** | 62.36 ± 0.59*** |
| Total PUFAs | 4.66 ± 0.46 | 9.01 ± 0.42** | 10.62 ± 0.39*** |
| Food Intake (g) | |
|---|---|
| LFD | 4.53 ± 0.20 |
| HFD-SFA | 5.26 ± 0.25 |
| HFD-OO | 5.76 ± 0.35 |
| HFD-OO-ω3 | 5.30 ± 0.40 |
| LFD | HFD-SFA | HFD-OO | HFD-OO-ω3 | |
|---|---|---|---|---|
| CAT (U/mg protein) | 5.53 ± 0.38 + | 5.19 ± 0.31 + | 8.89 ± 1.92 | 5.27 ± 0.30 + |
| GR (mU/mg protein) | 12.97 ± 1.37 | 14.02 ± 1.72 | 16.52 ± 2.61 | 14.00 ± 1.10 |
| GPx (mU/mg protein) | 10.29 ± 5.10 | 33.53 ± 14.62 | 15.26 ± 5.43 | 18.86 ± 6.49 |
| GR/GPx | 1.26 ± 0.29 ** | 0.42 ± 0.05 | 1.08 ± 0.23 ** | 0.77 ± 0.07 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonzalo-Gobernado, R.; Ayuso, M.I.; Sansone, L.; Bernal-Jiménez, J.J.; Ramos-Herrero, V.D.; Sánchez-García, E.; Ramos, T.L.; Abia, R.; Muriana, F.J.G.; Bermúdez, B.; et al. Neuroprotective Effects of Diets Containing Olive Oil and DHA/EPA in a Mouse Model of Cerebral Ischemia. Nutrients 2019, 11, 1109. https://doi.org/10.3390/nu11051109
Gonzalo-Gobernado R, Ayuso MI, Sansone L, Bernal-Jiménez JJ, Ramos-Herrero VD, Sánchez-García E, Ramos TL, Abia R, Muriana FJG, Bermúdez B, et al. Neuroprotective Effects of Diets Containing Olive Oil and DHA/EPA in a Mouse Model of Cerebral Ischemia. Nutrients. 2019; 11(5):1109. https://doi.org/10.3390/nu11051109
Chicago/Turabian StyleGonzalo-Gobernado, Rafael, María Irene Ayuso, Loredana Sansone, Juan José Bernal-Jiménez, Víctor Darío Ramos-Herrero, Enrique Sánchez-García, Teresa L. Ramos, Rocío Abia, Francisco J. G. Muriana, Beatriz Bermúdez, and et al. 2019. "Neuroprotective Effects of Diets Containing Olive Oil and DHA/EPA in a Mouse Model of Cerebral Ischemia" Nutrients 11, no. 5: 1109. https://doi.org/10.3390/nu11051109
APA StyleGonzalo-Gobernado, R., Ayuso, M. I., Sansone, L., Bernal-Jiménez, J. J., Ramos-Herrero, V. D., Sánchez-García, E., Ramos, T. L., Abia, R., Muriana, F. J. G., Bermúdez, B., & Montaner, J. (2019). Neuroprotective Effects of Diets Containing Olive Oil and DHA/EPA in a Mouse Model of Cerebral Ischemia. Nutrients, 11(5), 1109. https://doi.org/10.3390/nu11051109





