Helianthus annuus Seed Extract Affects Weight and Body Composition of Healthy Obese Adults during 12 Weeks of Consumption: A Randomized, Double-Blind, Placebo-Controlled Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Subjects
2.3. Intervention Treatments and Composition of the Interventional Product
2.4. Study Protocol
2.5. Primary/Secondary Outcomes
2.6. Anthropometric Measurements
2.7. Blood Sampling and Biochemical Analysis
2.8. Safety Assessment
2.9. Statistical Analysis
3. Results
3.1. Study Flow, Participants Characteristics and Safety
3.2. Effect of Sunflower Extract Supplementation on Anthropometric and Biochemical Parameters
3.3. Optimization via Age and Sexes
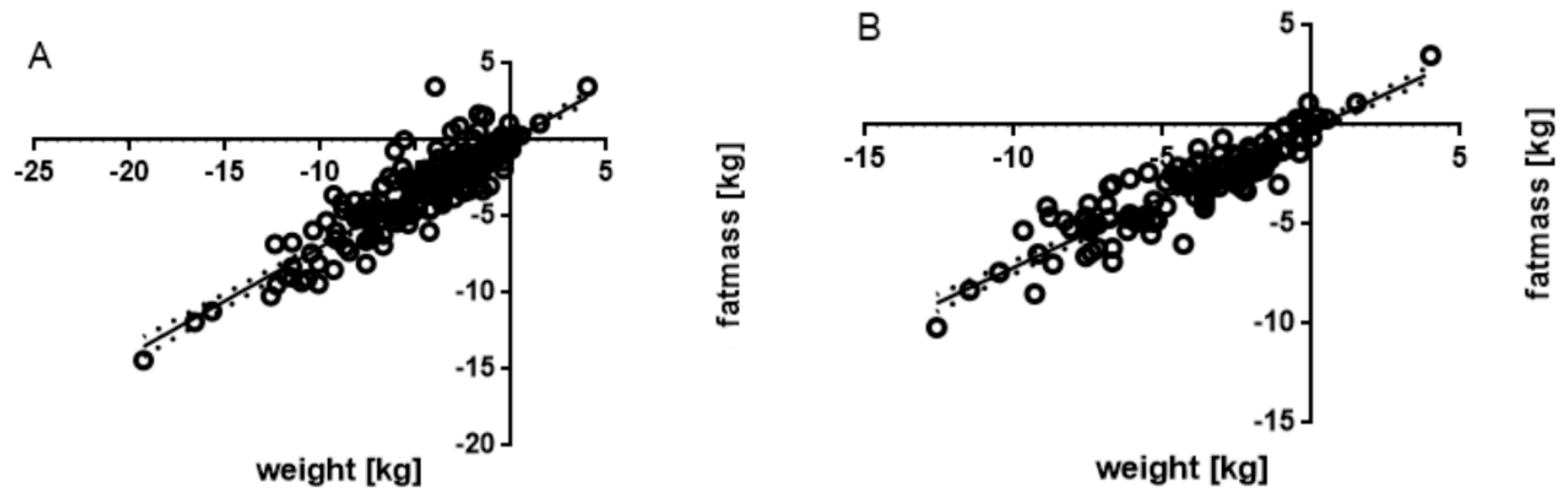
3.4. Associated Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HbA1c | Glycated Haemoglobin |
| HDL | High Density Lipoproteins |
| LDL | Low Density Lipoproteins |
| AMPK | Adenosine monophosphate-activated protein kinase |
| PPAR | Peroxisome proliferator-activated receptor |
References
- WHO. Obesity and Overweight. Updated on 16/02/2018. Available online: http://www.who.int/en/news-room/fact-sheets/detail/obesity-and-overweight/ (accessed on 9 October 2018).
- Pi-Sunyer, X. The medical risks of obesity. Postgrad. Med. 2009, 121, 21–33. [Google Scholar] [CrossRef]
- WHO/Fact Sheet N°311. Obesity. Updated on August 2014. Available online: http://www.wpro.who.int/mediacentre/factsheets/obesity/en/ (accessed on 9 October 2018).
- Kakker, K.; Dahiya, N. Drug treatment of obesity: Current status and future prospects. Eur. J. Intern. Med. 2015, 26, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Daneschvar, H.L.; Aronson, M.D.; Smetana, G.W. FDA-approved anti-obesity drugs in the United States. Am. J. Med. 2016, 129, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.W. Possible anti-obesity therapeutics from nature—A review. Phytochemistry 2010, 71, 1625–1641. [Google Scholar] [CrossRef] [PubMed]
- Kazemipoor, M.; Wan Mohamed Radzi, C.W.J.; Cordell, G.A.; Yaze, I. Safety, Efficacy and Metabolism of Traditional Medicinal Plants in the Management of Obesity: A Review. Int. J. Chem. Eng. Appl. 2012, 3, 288–292. [Google Scholar] [CrossRef]
- Guo, S.; Ge, Y.; Na Jom, K. A review of phytochemistry, metabolite changes, and medicinal uses of the common sunflower seed and sprouts (Helianthus annuus L.). Chem. Cent. J. 2017, 11, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Weisz, G.M.; Kammerer, D.R.; Carle, R. Identification and quantification of phenolic compounds from sunflower (Helianthus annuus L.) kernels and shells by HPLC-DAD/ESI-MSn. Food Chem. 2009, 115, 758–765. [Google Scholar] [CrossRef]
- Farah, A.; Monteiro, M.; Donangelo, C.M.; Lafay, S. Chlorogenic Acids from Green Coffee Extract are Highly Bioavailable in Humans. J. Nutr. 2008, 138, 2309–2315. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of Chlorogenic Acid on regulating glucose and lipids metabolism: A Review. Evid. Based Complement. Altern. Med. 2013, 2013, 801457. [Google Scholar] [CrossRef] [PubMed]
- Saini, S.; Sharma, S. Antidiabetic effect of Helianthus annuus L. seeds ethanolic extract in streptozotocin-nicotinamide induced type 2 diabetes mellitus. Int. J. Pharm. Pharm. Sci. 2013, 5, 382–387. [Google Scholar]
- Aqeel, Z.; Truong, D.; Preston, J.; Lazzaron, S.; Baugh, S. Chlorogenic Acids from Green Coffee by HPLC; Application Note ChromaDex/Phenomenex, TN-1134; Phenomenex: Irvine, CA, USA, 2012. [Google Scholar]
- Gouthamchandra, K.; Sudeep, H.V.; Venkatesh, B.J.; Shyam Prasad, K. Chlorogenic acid complex (CGA7), standardized extract from green coffee beans exerts anticancer effects against cultured human colon cancer HCT-116 cells. Food Sci. Hum. Wellness 2017, 6, 147–153. [Google Scholar] [CrossRef]
- Balkau, B.; Deanfield, J.E.; Després, J.P.; Bassand, J.P.; Fox, K.A.A.; Smith, S.C., Jr.; Barter, P.; Tan, C.E.; Van Gaal, L.; Wittchen, H.U.; et al. International Day for the Evaluation of Abdominal Obesity (IDEA) A Study of Waist Circumference, Cardiovascular Disease, and Diabetes Mellitus in 168,000 Primary Care Patients in 63 Countries. Circulation 2007, 116, 1942–1951. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Guideline on Clinical Evaluation of Medicinal Products Used in Weight Control; EMA/CHMP/311805/2014; European Medicines Agency: London, UK, 2016. [Google Scholar]
- Onakpoya, I.; Terry, R.; Ernst, E. The Use of Green Coffee Extract as a Weight Loss Supplement: A systematic Review and Meta-Analysis of Randomised Clinical Trials. Gastroenterol. Res. Pract. 2011, 6, 382852. [Google Scholar] [CrossRef] [PubMed]
- Dellalibera, O.; Lemaire, B.; Lafay, S. Svetol®, green coffee extract, induces weight loss and increases the lean-to fat mass ratio in volunteers with overweight problem. Phytotherapy 2006, 4, 194–197. [Google Scholar] [CrossRef]
- Shimoda, H.; Seki, E.; Aitani, M. Inhibitory effect of green coffee bean extract on fat accumulation and body weight gain in mice. BMC Complement. Altern. Med. 2006, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Wan, C.W.; Wong, C.N.Y.; Pin, W.K.; Wong, M.H.Y.; Kwok, C.Y.; Chan, R.Y.K.; Yu, P.H.F.; Chan, S.W. Chlorogenic acid exhibits cholesterol lowering and fatty liver attenuating properties by up-regulating the gene expression of PPAR-α in hypercholesterolemic rats induced with a High-Cholesterol Diet. Phytother. Res. 2012, 27, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Castelli, W.P. Lipids, risk factors and ischaemic heart disease. Atherosclerosis 1996, 124, 1–9. [Google Scholar] [CrossRef]
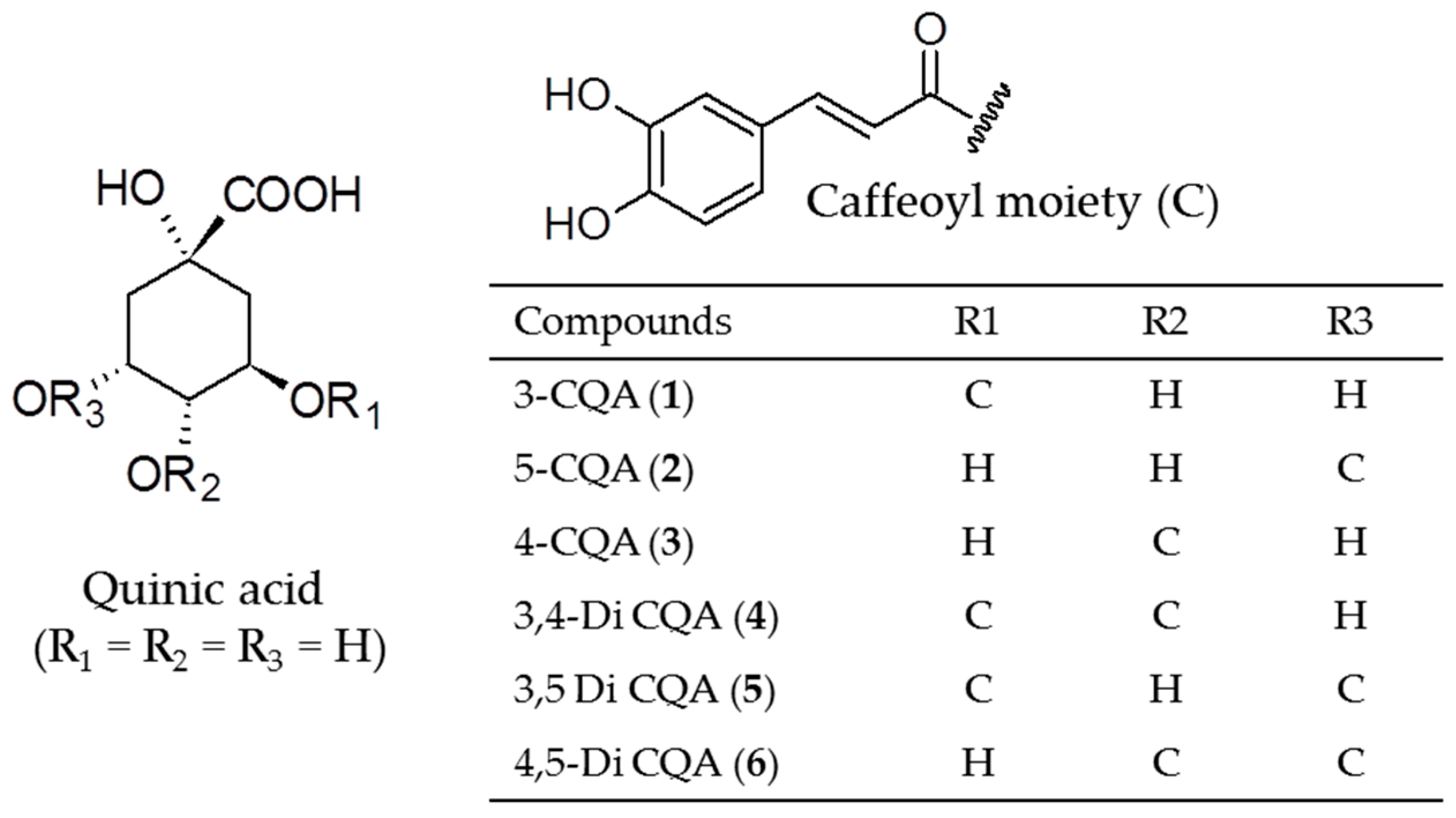


| Compound | Retention Time (min) | % |
|---|---|---|
| 3-Caffeoyl quinic acid (3-CQA) (1) | 3.64 | 5.72 |
| 5-Caffeoyl quinic acid (5-CQA) (2) | 5.33 | 26.66 |
| 4-Caffeoyl quinic acid (4-CQA) (3) | 5.50 | 8.51 |
| 3,4-Di-caffeoyl quinic acid (3,4-Di CQA) (4) | 10.76 | 1.19 |
| 3,5-Di-caffeoyl quinic acid (3,5-Di CQA) (5) | 11.22 | 1.07 |
| 4,5-Di-caffeoyl quinic acid (4,5-Di CQA) (6) | 12.29 | 1.47 |
| Total | 44.62 |
| Variable (Unit) | Placebo Group (N = 20) (5 Men and 15 Women) | Sunflower Extract Group (N = 30) (13 Men and 17 Women) | p Value (Intergroup) | ||
|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | ||
| Age (years) | 40.5 ± 9.57 | 21–51 | 44.5 ± 9.69 | 23–64 | 0.15 |
| Weight (kg) | 93.8 ± 11.86 | 73.8–122.2 | 101.0 ± 14.46 | 74.2–129.9 | 0.07 |
| Height (cm) | 165.9 ± 7.58 | 150–183 | 168.7 ± 9.91 | 154–192 | 0.28 |
| BMI (kg/m2) | 34.0 ± 2.91 | 30.4–39.1 | 35.3 ± 2.93 | 30.1–39.9 | 0.12 |
| Waist circumference (cm) | 109.1 ± 9.16 | 95–129 | 114.0 ± 8.48 | 95–136 | 0.06 |
| Lean mass (kg) | 55.0 ± 10.09 | 43.9–84.3 | 60.9 ± 13.01 | 44.9–88.5 | 0.09 |
| Fat mass (kg) | 38.8 ± 7.31 | 24.9–53.6 | 40.0 ± 7.22 | 27.5–58.3 | 0.57 |
| MM/FM (ratio) | 1.5 ± 0.47 | 0.99–2.67 | 1.6 ± 0.45 | 0.96–2.61 | 0.47 |
| Total body water (kg) | 40.3 ± 7.39 | 32.1–61.7 | 44.1 ± 9.31 | 32.9–64.8 | 0.12 |
| Fat (%) | 41.4 ± 6.29 | 27.2–50.0 | 40.0 ± 6.58 | 27.7–51.1 | 0.47 |
| Glucose (mg/dl) | 92.9 ± 14.32 | 73–143 | 96.5 ± 18.18 | 76–177 | 0.37 |
| HbA1c (%) | 5.7 ± 0.54 | 4.7–7.4 | 5.7 ± 0.55 | 5.0–7.7 | 0.83 |
| Cholesterol (mg/dL) | 203.7 ± 42.20 | 131.0–291.0 | 207.7 ± 41.69 | 129.0–299.0 | 0.74 |
| Triglycerides (mg/dL) | 116.3 ± 63.53 | 60.0–331.0 | 130.1 ± 74.06 | 33.0–322.0 | 0.45 |
| LDL (mg/dL) | 132.3 ± 33.87 | 75–216 | 132.7 ± 35.83 | 54.0–195.0 | 0.97 |
| HDL (mg/dL) | 48.7 ± 14.31 | 31.0–96.0 | 46.7 ± 11.74 | 26.0–73.0 | 0.66 |
| LDL/HDL (ratio) | 2.7± 0.68 | 1.8–4.2 | 2.9 ± 0.97 | 1.6–5.1 | 0.46 |
| Free Fatty Acids (nmol/dL) | 0.4 ± 0.15 | 0.24–0.82 | 0.4 ± 0.15 | 0.09–0.73 | 0.90 |
| Variable (Unit) | All Subjects (N = 50) | Subjects > 30 Years (N = 44) | Women > 30 years (N = 30) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo (N = 18) | Sunflower Extract (N = 28) | p-Value | Placebo (N = 17) | Sunflower Extract (N = 26) | p-Value | Placebo (N = 13) | Sunflower Extract (N = 15) | p-Value | |
| Anthropometric measurements | |||||||||
| Weight (kg) | −5.53 ± 1.30 | −6.90 ± 1.30 | 0.092 | −5.71 ± 1.38 | −7.28 ± 1.43 | 0.043 | −4.93 ± 0.68 | −6.01 ± 0.94 | 0.167 |
| BMI (kg/m2) | −1.88 ± 0.32 | −2.60 ± 0.33 | 0.011 | −1.94 ± 0.33 | −2.75 ± 0.33 | 0.004 | −1.81 ± 0.25 | −2.31 ± 0.35 | 0.109 |
| WC (cm) | −4.75 ± 1.76 | −8.44 ± 0.81 | 0.000 | −4.91 ± 1.86 | −8.82 ± 0.81 | 0.000 | −3.23 ± 2.11 | −9.15 ± 0.87 | 0.000 |
| Bioimpedance analysis | |||||||||
| MM (kg) | −1.54 ± 0.43 | −1.88 ± 0.31 | 0.403 | −1.69 ± 0.43 | −1.86 ± 0.32 | 0.265 | −1.37 ± 0.42 | −1.22 ± 0.37 | 0.219 |
| FM (kg) | −3.98 ± 0.71 | −4.87 ± 0.69 | 0.095 | −4.02 ± 0.75 | −5.26 ± 0.68 | 0.018 | −3.56 ± 0.44 | −4.72 ± 0.74 | 0.026 |
| MM/FM (ratio) | 0.19 ± 0.06 | 0.20 ± 0.04 | 0.543 | 0.19 ± 0.07 | 0.22 ± 0.04 | 0.511 | 0.09 ± 0.02 | 0.14 ± 0.03 | 0.004 |
| TBW (kg) | −1.13 ± 0.32 | −0.90 ± 0.52 | 0.376 | −1.24 ± 0.32 | −0.85 ± 0.56 | 0.258 | −1.00 ± 0.32 | −0.89 ± 0.27 | 0.157 |
| Fat (%) | −2.03 ± 0.47 | −2.44 ± 0.40 | 0.255 | −2.00 ± 0.49 | −2.69 ± 0.39 | 0.047 | −1.62 ± 0.31 | −2.41 ± 0.44 | 0.014 |
| Biochemical analysis | |||||||||
| Glucose (mg/dl) | −2.61 ± 2.61 | −3.71 ± 1.80 | 0.132 | −2.41 ± 2.76 | −4.35 ± 1.88 | 0.227 | −5.00 ± 1.78 | −0.53 ± 1.82 | 0.046 |
| HbA1c (%) | −0.13 ± 0.03 | −0.13 ± 0.05 | 0.664 | −0.12 ± 0.03 | −0.14 ± 0.06 | 0.700 | −0.11 ± 0.04 | −0.07 ± 0.04 | 0.624 |
| Cholesterol (mg/dL) | −8.72 ± 5.45 | −18.43 ± 4.86 | 0.018 | −8.65 ± 5.78 | −18.31 ± 5.07 | 0.028 | −4.85 ± 5.97 | −15.53 ± 4.76 | 0.578 |
| Triglycerides (TG) (mg/dL) | −23.94 ± 14.45 | −25.46 ± 9.22 | 0.411 | −23.29 ± 15.31 | −26.08 ± 9.85 | 0.342 | −6.54 ± 8.53 | −25.00 ± 7.33 | 0.065 |
| LDL (mg/dL) | −3.53 ± 4.37 | −9.69 ± 4.02 | 0.150 | −3.69 ± 4.65 | −9.71 ± 4.19 | 0.164 | −2.00 ± 4.91 | −8.20 ± 4.62 | 0.350 |
| HDL (mg/dL) | −0.78 ± 2.15 | −2.75 ± 0.85 | 0.327 | −0.71 ± 2.28 | −2.42 ± 0.88 | 0.427 | −1.69 ± 2.90 | −2.27 ± 1.42 | 0.112 |
| LDL/HDL (ratio) | −0.03 ± 0.12 | −0.02 ± 0.10 | 0.465 | −0.04 ± 0.13 | − 0.03 ± 0.10 | 0.433 | 0.06 ± 0.14 | 0.00 ± 0.10 | 0.265 |
| FFA (nmol/dL) | 0.14 ± 0.06 | 0.09 ± 0.05 | 0.370 | 0.15 ± 0.06 | 0.07 ± 0.04 | 0.310 | 0.15 ± 0.08 | 0.04 ± 0.05 | 0.255 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leverrier, A.; Daguet, D.; Calame, W.; Dhoye, P.; Kodimule, S.P. Helianthus annuus Seed Extract Affects Weight and Body Composition of Healthy Obese Adults during 12 Weeks of Consumption: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. Nutrients 2019, 11, 1080. https://doi.org/10.3390/nu11051080
Leverrier A, Daguet D, Calame W, Dhoye P, Kodimule SP. Helianthus annuus Seed Extract Affects Weight and Body Composition of Healthy Obese Adults during 12 Weeks of Consumption: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. Nutrients. 2019; 11(5):1080. https://doi.org/10.3390/nu11051080
Chicago/Turabian StyleLeverrier, Aurélie, David Daguet, Wim Calame, Pierre Dhoye, and Shyam Prasad Kodimule. 2019. "Helianthus annuus Seed Extract Affects Weight and Body Composition of Healthy Obese Adults during 12 Weeks of Consumption: A Randomized, Double-Blind, Placebo-Controlled Pilot Study" Nutrients 11, no. 5: 1080. https://doi.org/10.3390/nu11051080
APA StyleLeverrier, A., Daguet, D., Calame, W., Dhoye, P., & Kodimule, S. P. (2019). Helianthus annuus Seed Extract Affects Weight and Body Composition of Healthy Obese Adults during 12 Weeks of Consumption: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. Nutrients, 11(5), 1080. https://doi.org/10.3390/nu11051080





