Tart Cherry Concentrate Does Not Alter the Gut Microbiome, Glycaemic Control or Systemic Inflammation in a Middle-Aged Population
Abstract
1. Introduction
2. Materials and Methods
2.1. Participant Recruitment
2.2. Experimental Design
2.3. Dietary and Physical Activity Analysis
2.4. Blood Sampling and Analysis
2.5. Faecal Microbiome Analysis
2.6. Statistical Analysis
3. Results
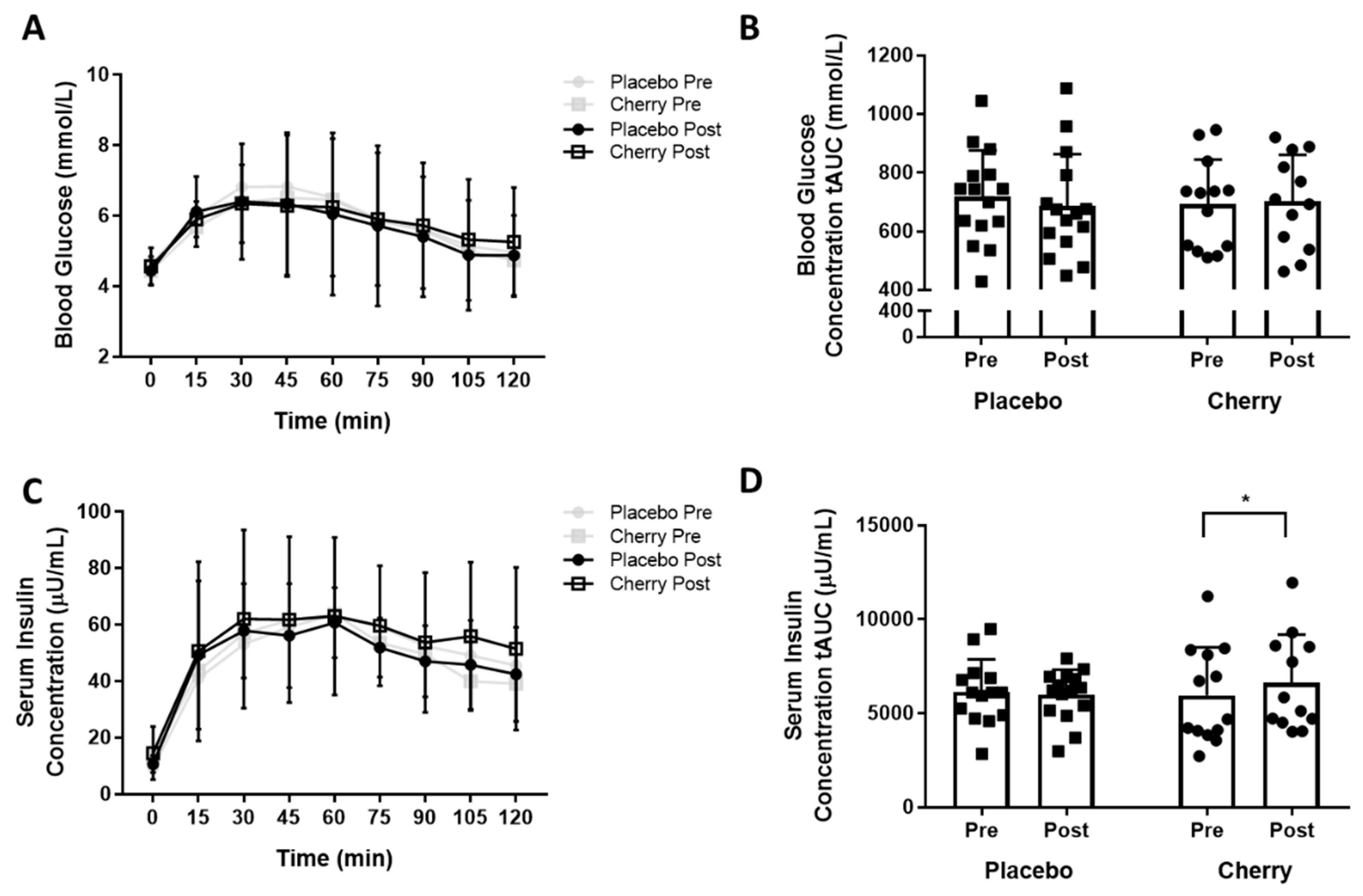
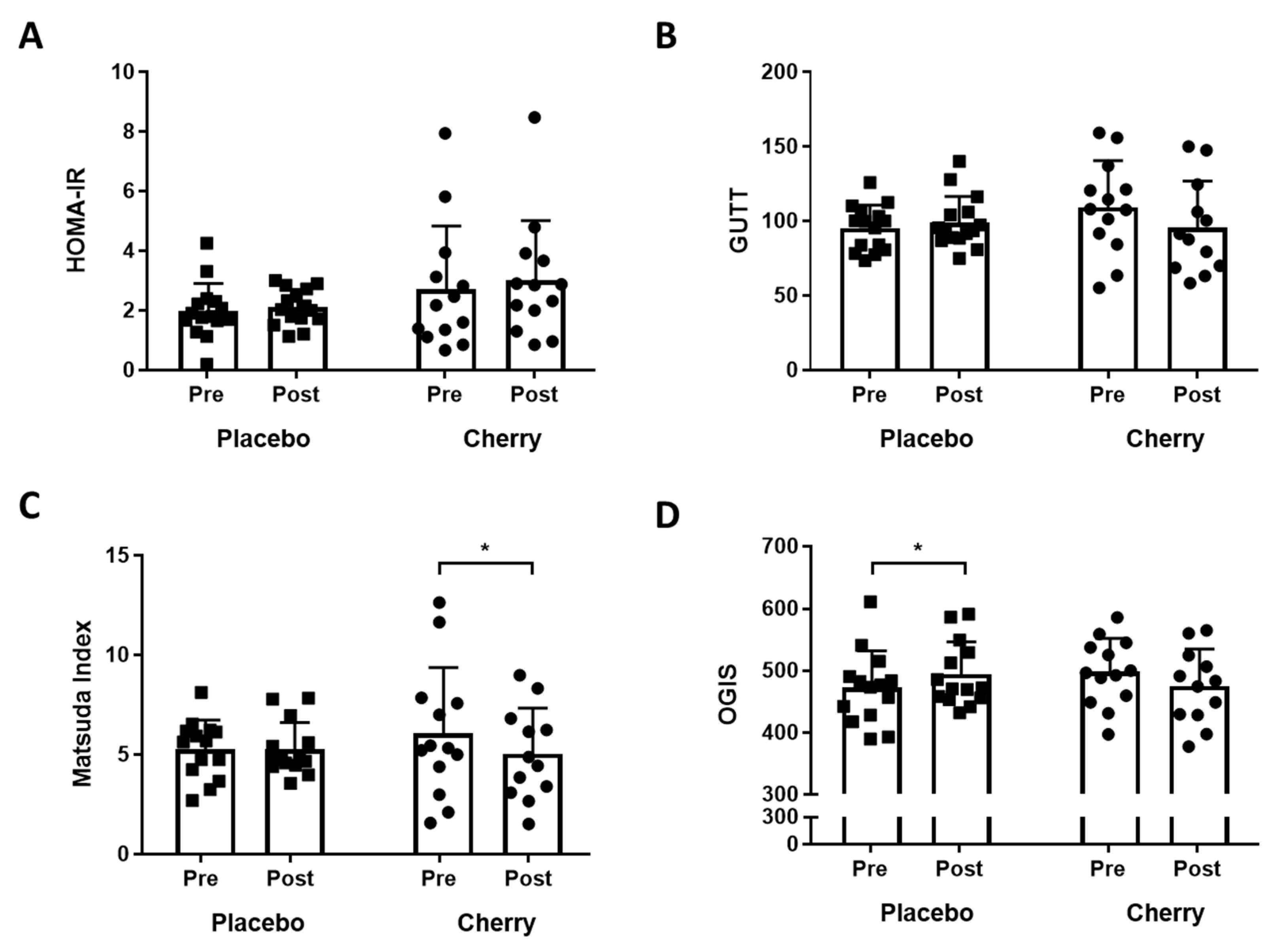
3.1. Indices of Glucose Tolerance and Insulin Sensitivity
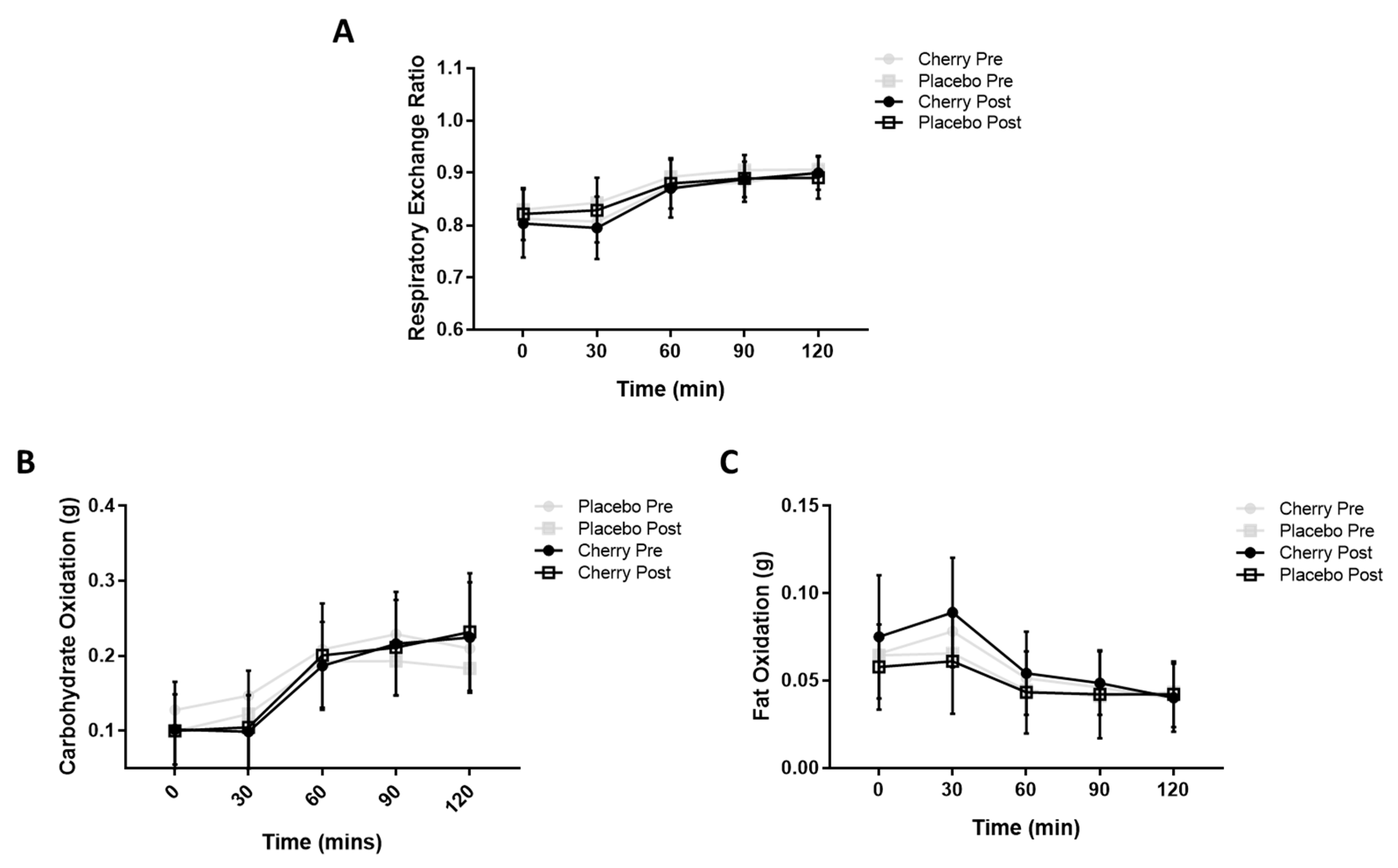
3.2. Metabolic Flexibility
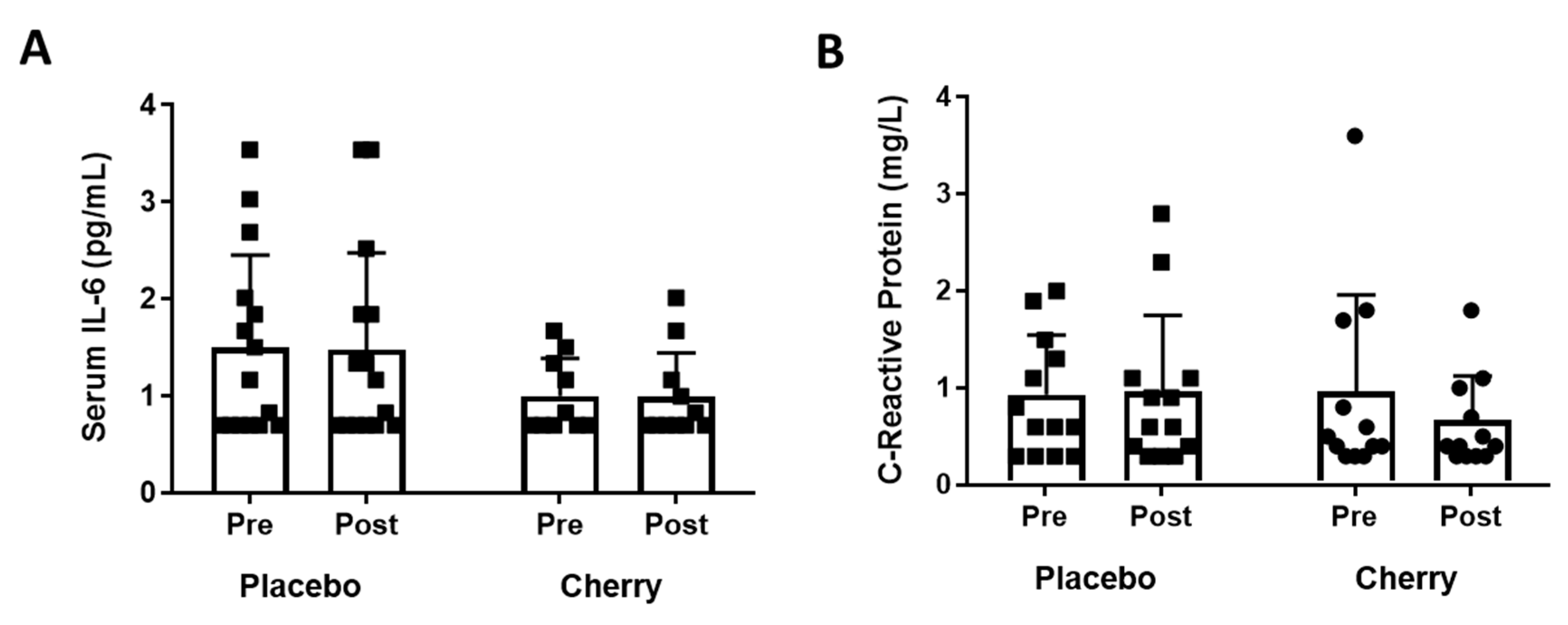
3.3. Plasma Inflammatory Markers
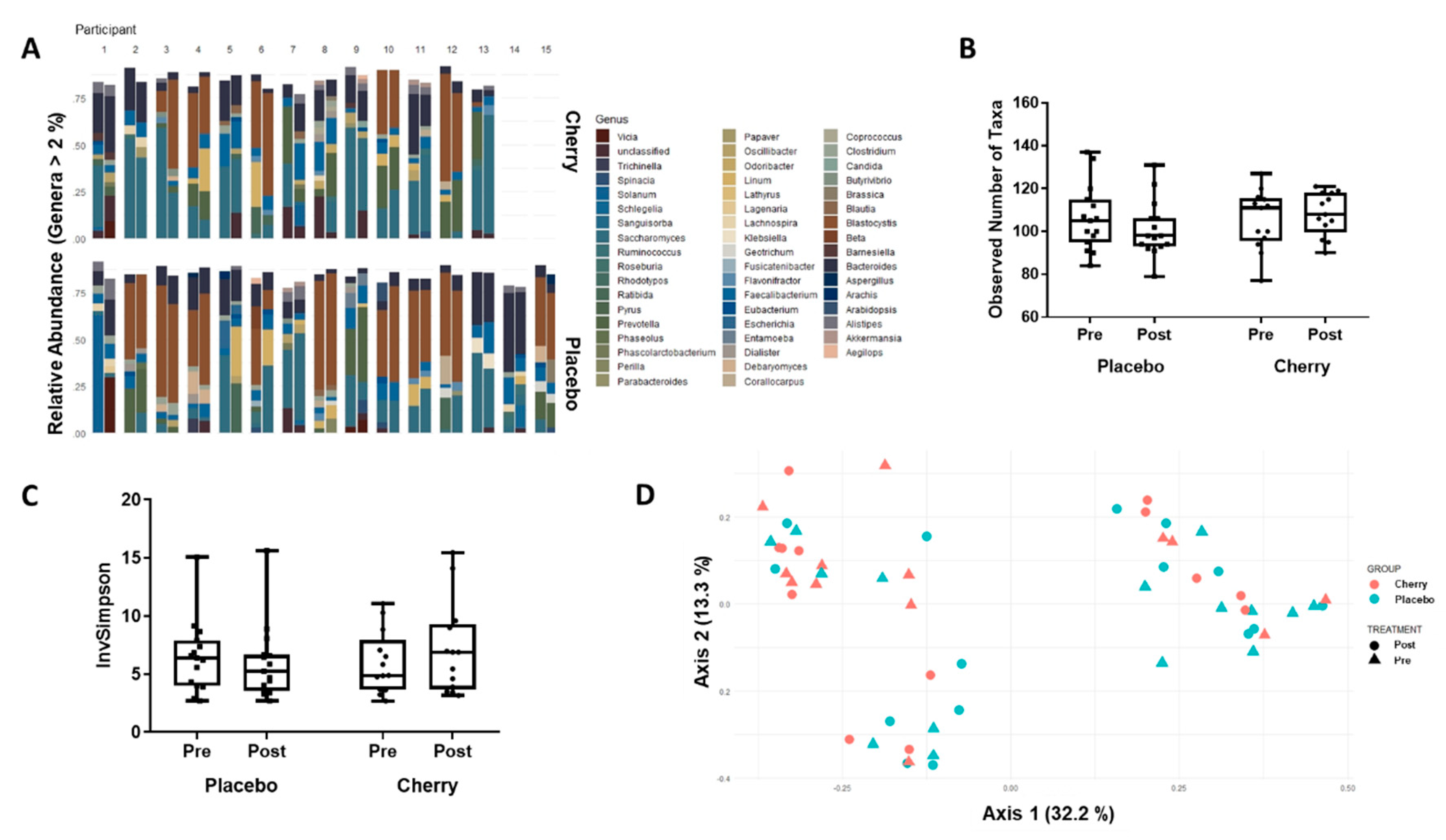
3.4. Gut Microbiome
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Rienks, J.; Barbaresko, J.; Oluwagbemigun, K.; Schmid, M.; Nöthlings, U. Polyphenol exposure and risk of type 2 diabetes: Dose-response meta-analyses and systematic review of prospective cohort studies. Am. J. Clin. Nutr. 2018, 108, 49–61. [Google Scholar] [CrossRef]
- Stull, A.J.; Cash, K.C.; Johnson, W.D.; Champagne, C.M.; Cefalu, W.T. Bioactives in blueberries improve insulin sensitivity in obese, insulin-resistant men and women. J. Nutr. 2010, 140, 1764–1768. [Google Scholar] [CrossRef]
- Kim, Y.; Keogh, J.B.; Clifton, P.M. Polyphenols and Glycemic Control. Nutrients 2016, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef] [PubMed]
- de Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef]
- Zhang, Z.F.; Li, Q.; Liang, J.; Dai, X.Q.; Ding, Y.; Wang, J.B.; Li, Y. Epigallocatechin-3-O-gallate (EGCG) protects the insulin sensitivity in rat L6 muscle cells exposed to dexamethasone condition. Phytomed. Int. J. Phytother. Phytopharm. 2010, 17, 14–18. [Google Scholar] [CrossRef]
- Cao, H.; Polansky, M.M.; Anderson, R.A. Cinnamon extract and polyphenols affect the expression of tristetraprolin, insulin receptor, and glucose transporter 4 in mouse 3T3-L1 adipocytes. Arch. Biochem. Biophys. 2007, 459, 214–222. [Google Scholar] [CrossRef] [PubMed]
- Ferrante, A.W. Macrophages, fat, and the emergence of immunometabolism. J. Clin. Investig. 2013, 123, 4992–4993. [Google Scholar] [CrossRef]
- Carvalheira, J.B.C.; Qiu, Y.; Chawla, A. Blood spotlight on leukocytes and obesity. Blood 2013, 122, 3263–3267. [Google Scholar] [CrossRef]
- Han, M.S.; Jung, D.Y.; Morel, C.; Lakhani, S.A.; Kim, J.K.; Flavell, R.A.; Davis, R.J. JNK expression by macrophages promotes obesity-induced insulin resistance and inflammation. Science 2013, 339, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Kelley, D.S.; Rasooly, R.; Jacob, R.A.; Kader, A.A.; Mackey, B.E. Consumption of Bing sweet cherries lowers circulating concentrations of inflammation markers in healthy men and women. J. Nutr. 2006, 136, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Connolly, D.A.J.; McHugh, M.P.; Padilla-Zakour, O.I.; Carlson, L.; Sayers, S.P. Efficacy of a tart cherry juice blend in preventing the symptoms of muscle damage. Br. J. Sports Med. 2006, 40, 679–683; discussion 683. [Google Scholar] [CrossRef] [PubMed]
- Howatson, G.; McHugh, M.P.; Hill, J.A.; Brouner, J.; Jewell, A.P.; van Someren, K.A.; Shave, R.E.; Howatson, S.A. Influence of tart cherry juice on indices of recovery following marathon running. Scand. J. Med. Sci. Sports 2010, 20, 843–852. [Google Scholar] [CrossRef] [PubMed]
- Bowtell, J.L.; Sumners, D.P.; Dyer, A.; Fox, P.; Mileva, K.N. Montmorency cherry juice reduces muscle damage caused by intensive strength exercise. Med. Sci. Sports Exerc. 2011, 43, 1544–1551. [Google Scholar] [CrossRef]
- Traustadóttir, T.; Davies, S.S.; Stock, A.A.; Su, Y.; Heward, C.B.; Roberts, L.J.; Harman, S.M. Tart cherry juice decreases oxidative stress in healthy older men and women. J. Nutr. 2009, 139, 1896–1900. [Google Scholar] [CrossRef]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef]
- Kanamoto, Y.; Yamashita, Y.; Nanba, F.; Yoshida, T.; Tsuda, T.; Fukuda, I.; Nakamura-Tsuruta, S.; Ashida, H. A black soybean seed coat extract prevents obesity and glucose intolerance by up-regulating uncoupling proteins and down-regulating inflammatory cytokines in high-fat diet-fed mice. J. Agric. Food Chem. 2011, 59, 8985–8993. [Google Scholar] [CrossRef]
- Terra, X.; Pallarés, V.; Ardèvol, A.; Bladé, C.; Fernández-Larrea, J.; Pujadas, G.; Salvadó, J.; Arola, L.; Blay, M. Modulatory effect of grape-seed procyanidins on local and systemic inflammation in diet-induced obesity rats. J. Nutr. Biochem. 2011, 22, 380–387. [Google Scholar] [CrossRef]
- Yokozawa, T.; Kim, H.J.; Cho, E.J. Gravinol ameliorates high-fructose-induced metabolic syndrome through regulation of lipid metabolism and proinflammatory state in rats. J. Agric. Food Chem. 2008, 56, 5026–5032. [Google Scholar] [CrossRef]
- Lee, Y.A.; Cho, E.J.; Yokozawa, T. Effects of proanthocyanidin preparations on hyperlipidemia and other biomarkers in mouse model of type 2 diabetes. J. Agric. Food Chem. 2008, 56, 7781–7789. [Google Scholar] [CrossRef]
- Cani, P.D.; Neyrinck, A.M.; Fava, F.; Knauf, C.; Burcelin, R.G.; Tuohy, K.M.; Gibson, G.R.; Delzenne, N.M. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia 2007, 50, 2374–2383. [Google Scholar] [CrossRef]
- Espley, R.V.; Butts, C.A.; Laing, W.A.; Martell, S.; Smith, H.; McGhie, T.K.; Zhang, J.; Paturi, G.; Hedderley, D.; Bovy, A.; et al. Dietary flavonoids from modified apple reduce inflammation markers and modulate gut microbiota in mice. J. Nutr. 2014, 144, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Tzounis, X.; Rodriguez-Mateos, A.; Vulevic, J.; Gibson, G.R.; Kwik-Uribe, C.; Spencer, J.P.E. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011, 93, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Queipo-Ortuño, M.I.; Boto-Ordóñez, M.; Murri, M.; Gomez-Zumaquero, J.M.; Clemente-Postigo, M.; Estruch, R.; Cardona Diaz, F.; Andrés-Lacueva, C.; Tinahones, F.J. Influence of red wine polyphenols and ethanol on the gut microbiota ecology and biochemical biomarkers. Am. J. Clin. Nutr. 2012, 95, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, J.; Tokutake, S.; Kikuchi, M.; Kubota, Y.; Konishi, H.; Mitsuoka, T. Effect of Proanthocyanidin-Rich Extract from Grape Seeds on Human Fecal Flora and Fecal Odor. Microb. Ecol. Health Dis. 2001, 13, 25–31. [Google Scholar] [CrossRef]
- Vendrame, S.; Guglielmetti, S.; Riso, P.; Arioli, S.; Klimis-Zacas, D.; Porrini, M. Six-week consumption of a wild blueberry powder drink increases bifidobacteria in the human gut. J. Agric. Food Chem. 2011, 59, 12815–12820. [Google Scholar] [CrossRef]
- Mulligan, A.A.; Luben, R.N.; Bhaniani, A.; Parry-Smith, D.J.; O’Connor, L.; Khawaja, A.P.; Forouhi, N.G.; Khaw, K.-T.; EPIC-Norfolk FFQ Study. A new tool for converting food frequency questionnaire data into nutrient and food group values: FETA research methods and availability. BMJ Open 2014, 4, e004503. [Google Scholar] [CrossRef] [PubMed]
- Milton, K.; Bull, F.C.; Bauman, A. Reliability and validity testing of a single-item physical activity measure. Br. J. Sports Med. 2011, 45, 203–208. [Google Scholar] [CrossRef]
- Ma, W.-Y.; Yang, C.-Y.; Shih, S.-R.; Hsieh, H.-J.; Hung, C.S.; Chiu, F.-C.; Lin, M.-S.; Liu, P.-H.; Hua, C.-H.; Hsein, Y.-C.; et al. Measurement of Waist Circumference. Diabetes Care 2013, 36, 1660–1666. [Google Scholar] [CrossRef]
- Bartoli, E.; Fra, G.P.; Carnevale Schianca, G.P. The oral glucose tolerance test (OGTT) revisited. Eur. J. Intern. Med. 2011, 22, 8–12. [Google Scholar] [CrossRef]
- Bell, P.G.; Walshe, I.H.; Davison, G.W.; Stevenson, E.J.; Howatson, G. Recovery facilitation with Montmorency cherries following high-intensity, metabolically challenging exercise. Appl. Physiol. Nutr. Metab. Physiol. Appl. Nutr. Metab. 2015, 40, 414–423. [Google Scholar] [CrossRef]
- Bell, P.G.; Stevenson, E.; Davison, G.W.; Howatson, G. The Effects of Montmorency Tart Cherry Concentrate Supplementation on Recovery Following Prolonged, Intermittent Exercise. Nutrients 2016, 8, 441. [Google Scholar] [CrossRef]
- Dillon, C.B.; Fitzgerald, A.P.; Kearney, P.M.; Perry, I.J.; Rennie, K.L.; Kozarski, R.; Phillips, C.M. Number of Days Required to Estimate Habitual Activity Using Wrist-Worn GENEActiv Accelerometer: A Cross-Sectional Study. PLoS ONE 2016, 11, e0109913. [Google Scholar] [CrossRef]
- Jennings, A.; MacGregor, A.; Spector, T.; Cassidy, A. Higher dietary flavonoid intakes are associated with lower objectively measured body composition in women: Evidence from discordant monozygotic twins12. Am. J. Clin. Nutr. 2017, 105, 626–634. [Google Scholar] [CrossRef]
- Jennings, A.; Welch, A.A.; Fairweather-Tait, S.J.; Kay, C.; Minihane, A.-M.; Chowienczyk, P.; Jiang, B.; Cecelja, M.; Spector, T.; Macgregor, A.; et al. Higher anthocyanin intake is associated with lower arterial stiffness and central blood pressure in women. Am. J. Clin. Nutr. 2012, 96, 781–788. [Google Scholar] [CrossRef]
- Welch, A.; MacGregor, A.; Jennings, A.; Fairweather-Tait, S.; Spector, T.; Cassidy, A. Habitual flavonoid intakes are positively associated with bone mineral density in women. J. Bone Miner. Res. 2012, 27, 1872–1878. [Google Scholar] [CrossRef]
- Hildebrand, M.; VAN Hees, V.T.; Hansen, B.H.; Ekelund, U. Age group comparability of raw accelerometer output from wrist- and hip-worn monitors. Med. Sci. Sports Exerc. 2014, 46, 1816–1824. [Google Scholar] [CrossRef]
- Kelley, D.E.; Mandarino, L.J. Fuel selection in human skeletal muscle in insulin resistance: A reexamination. Diabetes 2000, 49, 677–683. [Google Scholar] [CrossRef]
- Færch, K.; Vaag, A. Metabolic inflexibility is a common feature of impaired fasting glycaemia and impaired glucose tolerance. Acta Diabetol. 2011, 48, 349–353. [Google Scholar] [CrossRef]
- Frayn, K.N. Calculation of substrate oxidation rates in vivo from gaseous exchange. J. Appl. Physiol. 1983, 55, 628–634. [Google Scholar] [CrossRef]
- Otten, J.; Ahrén, B.; Olsson, T. Surrogate measures of insulin sensitivity vs. the hyperinsulinaemic-euglycaemic clamp: A meta-analysis. Diabetologia 2014, 57, 1781–1788. [Google Scholar] [CrossRef]
- Yu, Y.; Lee, C.; Kim, J.; Hwang, S. Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol. Bioeng. 2005, 89, 670–679. [Google Scholar] [CrossRef]
- Krogsgaard, L.R.; Andersen, L.O.; Johannesen, T.B.; Engsbro, A.L.; Stensvold, C.R.; Nielsen, H.V.; Bytzer, P. Characteristics of the bacterial microbiome in association with common intestinal parasites in irritable bowel syndrome. Clin. Transl. Gastroenterol. 2018, 9, 161. [Google Scholar] [CrossRef]
- Gilbertson, N.M.; Eichner, N.Z.M.; Francois, M.; Gaitán, J.M.; Heiston, E.M.; Weltman, A.; Malin, S.K. Glucose Tolerance is Linked to Postprandial Fuel Use Independent of Exercise Dose. Med. Sci. Sports Exerc. 2018, 50, 2058–2066. [Google Scholar] [CrossRef]
- Standards of Medical Care in Diabetes—2011. Diabetes Care 2011, 34, S11–S61. [CrossRef]
- Amiot, M.J.; Riva, C.; Vinet, A. Effects of dietary polyphenols on metabolic syndrome features in humans: A systematic review. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2016, 17, 573–586. [Google Scholar] [CrossRef]
- Association, A.D. 4. Lifestyle Management: Standards of Medical Care in Diabetes—2018. Diabetes Care 2018, 41, S38–S50. [Google Scholar] [CrossRef]
- Bell, P.G.; Walshe, I.H.; Davison, G.W.; Stevenson, E.; Howatson, G. Montmorency cherries reduce the oxidative stress and inflammatory responses to repeated days high-intensity stochastic cycling. Nutrients 2014, 6, 829–843. [Google Scholar] [CrossRef]
- Beals, K.; Allison, K.F.; Darnell, M.; Lovalekar, M.; Baker, R.; Nieman, D.C.; Vodovotz, Y.; Lephart, S.M. The effects of a tart cherry beverage on reducing exercise-induced muscle soreness. Isokinet. Exerc. Sci. 2017, 25, 53–63. [Google Scholar] [CrossRef]
- McCormick, R.; Peeling, P.; Binnie, M.; Dawson, B.; Sim, M. Effect of tart cherry juice on recovery and next day performance in well-trained Water Polo players. J. Int. Soc. Sports Nutr. 2016, 13, 41. [Google Scholar] [CrossRef]
- Himmerich, H.; Fulda, S.; Linseisen, J.; Seiler, H.; Wolfram, G.; Himmerich, S.; Gedrich, K.; Pollmächer, T. TNF-alpha, soluble TNF receptor and interleukin-6 plasma levels in the general population. Eur. Cytokine Netw. 2006, 17, 196–201. [Google Scholar] [PubMed]
- Maggio, M.; Guralnik, J.M.; Longo, D.L.; Ferrucci, L. Interleukin-6 in Aging and Chronic Disease: A Magnificent Pathway. J. Gerontol. Ser. A 2006, 61, 575–584. [Google Scholar] [CrossRef]
- Glund, S.; Krook, A. Role of interleukin-6 signalling in glucose and lipid metabolism. Acta Physiol. 2008, 192, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K. Muscles and their myokines. J. Exp. Biol. 2011, 214, 337–346. [Google Scholar] [CrossRef]
- Pedersen, B.K. Exercise-induced myokines and their role in chronic diseases. Brain. Behav. Immun. 2011, 25, 811–816. [Google Scholar] [CrossRef]
- Pedersen, B.K. Muscular interleukin-6 and its role as an energy sensor. Med. Sci. Sports Exerc. 2012, 44, 392–396. [Google Scholar] [CrossRef]
- Woloshin, S.; Schwartz, L.M. Distribution of C-Reactive Protein Values in the United States. N. Engl. J. Med. 2005, 352, 1611–1613. [Google Scholar] [CrossRef]
- Roopchand, D.E.; Carmody, R.N.; Kuhn, P.; Moskal, K.; Rojas-Silva, P.; Turnbaugh, P.J.; Raskin, I. Dietary Polyphenols Promote Growth of the Gut Bacterium Akkermansia muciniphila and Attenuate High-Fat Diet-Induced Metabolic Syndrome. Diabetes 2015, 64, 2847–2858. [Google Scholar] [CrossRef]
- Weisberg, S.P.; Leibel, R.; Tortoriello, D.V. Dietary curcumin significantly improves obesity-associated inflammation and diabetes in mouse models of diabesity. Endocrinology 2008, 149, 3549–3558. [Google Scholar] [CrossRef]
- Bo, S.; Ponzo, V.; Ciccone, G.; Evangelista, A.; Saba, F.; Goitre, I.; Procopio, M.; Pagano, G.F.; Cassader, M.; Gambino, R. Six months of resveratrol supplementation has no measurable effect in type 2 diabetic patients. A randomized, double blind, placebo-controlled trial. Pharmacol. Res. 2016, 111, 896–905. [Google Scholar] [CrossRef]
- Panahi, Y.; Khalili, N.; Sahebi, E.; Namazi, S.; Simental-Mendía, L.E.; Majeed, M.; Sahebkar, A. Effects of Curcuminoids Plus Piperine on Glycemic, Hepatic and Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Placebo-Controlled Trial. Drug Res. 2018, 68, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Sarriá, B.; Martínez-López, S.; Sierra-Cinos, J.L.; García-Diz, L.; Mateos, R.; Bravo, L. Regular consumption of a cocoa product improves the cardiometabolic profile in healthy and moderately hypercholesterolaemic adults. Br. J. Nutr. 2014, 111, 122–134. [Google Scholar] [CrossRef]
- Schumacher, H.R.; Pullman-Mooar, S.; Gupta, S.R.; Dinnella, J.E.; Kim, R.; McHugh, M.P. Randomized double-blind crossover study of the efficacy of a tart cherry juice blend in treatment of osteoarthritis (OA) of the knee. Osteoarthr. Cartil. 2013, 21, 1035–1041. [Google Scholar] [CrossRef]
- Philp, A.M.; Davis, E.T.; Jones, S.W. Developing anti-inflammatory therapeutics for patients with osteoarthritis. Rheumatol. Oxf. Engl. 2017, 56, 869–881. [Google Scholar] [CrossRef]
- Almonacid, D.E.; Kraal, L.; Ossandon, F.J.; Budovskaya, Y.V.; Cardenas, J.P.; Bik, E.M.; Goddard, A.D.; Richman, J.; Apte, Z.S. 16S rRNA gene sequencing and healthy reference ranges for 28 clinically relevant microbial taxa from the human gut microbiome. PLoS ONE 2017, 12, e0176555. [Google Scholar] [CrossRef]
- Flores, R.; Shi, J.; Yu, G.; Ma, B.; Ravel, J.; Goedert, J.J.; Sinha, R. Collection media and delayed freezing effects on microbial composition of human stool. Microbiome 2015, 3, 33. [Google Scholar] [CrossRef]
| Placebo | Cherry | p Value | |
|---|---|---|---|
| Age (yr) | 53.2 ± 5.6 | 48.9 ± 5.4 | 0.05 |
| Sex | 8 M/7 F | 8 M/5 F | |
| Body Mass Index | 24.1 ± 2.1 | 25.8 ± 2.3 | 0.05 |
| Waist Circumference (cm) | 84.6 ± 10.6 | 86.2 ± 9.4 | 0.41 ^ |
| Habitual Physical Activity (days/week >30 min activity) | 3.8 ± 2.0 | 4.5 ± 1.9 | 0.38 |
| Flavonoid Intake (mg) | 1086 ± 514 | 1353 ± 535 | 0.19 |
| Anthocyanin Intake (mg) | 26.4 ± 23.2 | 27.2 ± 16.3 | 0.59 ^ |
| Proanthocyanin Intake (mg) | 326 ± 147 | 310 ± 128 | 0.77 |
| Insulin tAUC | Glucose tAUC | Matsuda | GUTT | HOMA-IR | OGIS | ||
|---|---|---|---|---|---|---|---|
| Bacteroides | r | −0.35 | −0.10 | 0.08 | 0.07 | −0.17 | 0.03 |
| 95% CI | −0.67 to 0.09 | −0.49 to 0.32 | −0.33 to 0.47 | −0.34 to 0.46 | −0.54 to 0.25 | 0.38 to 0.43 | |
| p value | 0.10 | 0.63 | 0.69 | 0.73 | 0.41 | 0.89 | |
| Faecalibacterium | r | −0.05 | 0.03 | 0.08 | −0.12 | 0.01 | −0.16 |
| 95% CI | −0.44 to 0.37 | −0.37 to 0.41 | −0.32 to 0.46 | −0.48 to 0.29 | −0.38 to 0.40 | −0.52 to 0.24 | |
| p value | 0.82 | 0.89 | 0.68 | 0.57 | 0.97 | 0.41 | |
| Richness | r | −0.03 | 0.15 | −0.16 | −0.20 | 0.06 | −0.27 |
| 95% CI | −0.41 to 0.35 | −0.25 to 0.50 | −0.51 to 0.23 | −0.54 to 0.20 | −0.33 to 0.44 | −0.59 to 0.13 | |
| p value | 0.87 | 0.45 | 0.41 | 0.31 | 0.75 | 0.17 | |
| Diversity | r | −0.21 | 0.04 | 0.30 | 0.20 | −0.11 | 0.16 |
| 95% CI | −0.55 to 0.19 | −0.35 to 0.42 | −0.09 to 0.61 | −0.20 to 0.54 | −0.47 to 0.28 | −0.24 to 0.51 | |
| p value | 0.29 | 0.85 | 0.12 | 0.31 | 0.57 | 0.43 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lear, R.; O’Leary, M.; O’Brien Andersen, L.; Holt, C.C.; Stensvold, C.R.; van der Giezen, M.; Bowtell, J.L. Tart Cherry Concentrate Does Not Alter the Gut Microbiome, Glycaemic Control or Systemic Inflammation in a Middle-Aged Population. Nutrients 2019, 11, 1063. https://doi.org/10.3390/nu11051063
Lear R, O’Leary M, O’Brien Andersen L, Holt CC, Stensvold CR, van der Giezen M, Bowtell JL. Tart Cherry Concentrate Does Not Alter the Gut Microbiome, Glycaemic Control or Systemic Inflammation in a Middle-Aged Population. Nutrients. 2019; 11(5):1063. https://doi.org/10.3390/nu11051063
Chicago/Turabian StyleLear, Rebecca, Mary O’Leary, Lee O’Brien Andersen, Corey Carrington Holt, Christen Rune Stensvold, Mark van der Giezen, and Joanna L. Bowtell. 2019. "Tart Cherry Concentrate Does Not Alter the Gut Microbiome, Glycaemic Control or Systemic Inflammation in a Middle-Aged Population" Nutrients 11, no. 5: 1063. https://doi.org/10.3390/nu11051063
APA StyleLear, R., O’Leary, M., O’Brien Andersen, L., Holt, C. C., Stensvold, C. R., van der Giezen, M., & Bowtell, J. L. (2019). Tart Cherry Concentrate Does Not Alter the Gut Microbiome, Glycaemic Control or Systemic Inflammation in a Middle-Aged Population. Nutrients, 11(5), 1063. https://doi.org/10.3390/nu11051063





