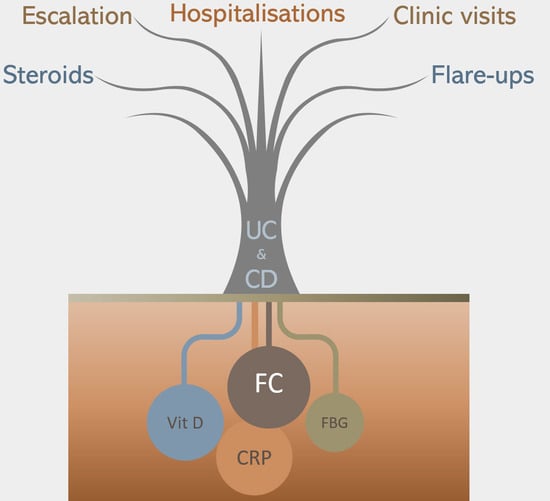
Influence of Vitamin D Deficiency on Inflammatory Markers and Clinical Disease Activity in IBD Patients
Abstract
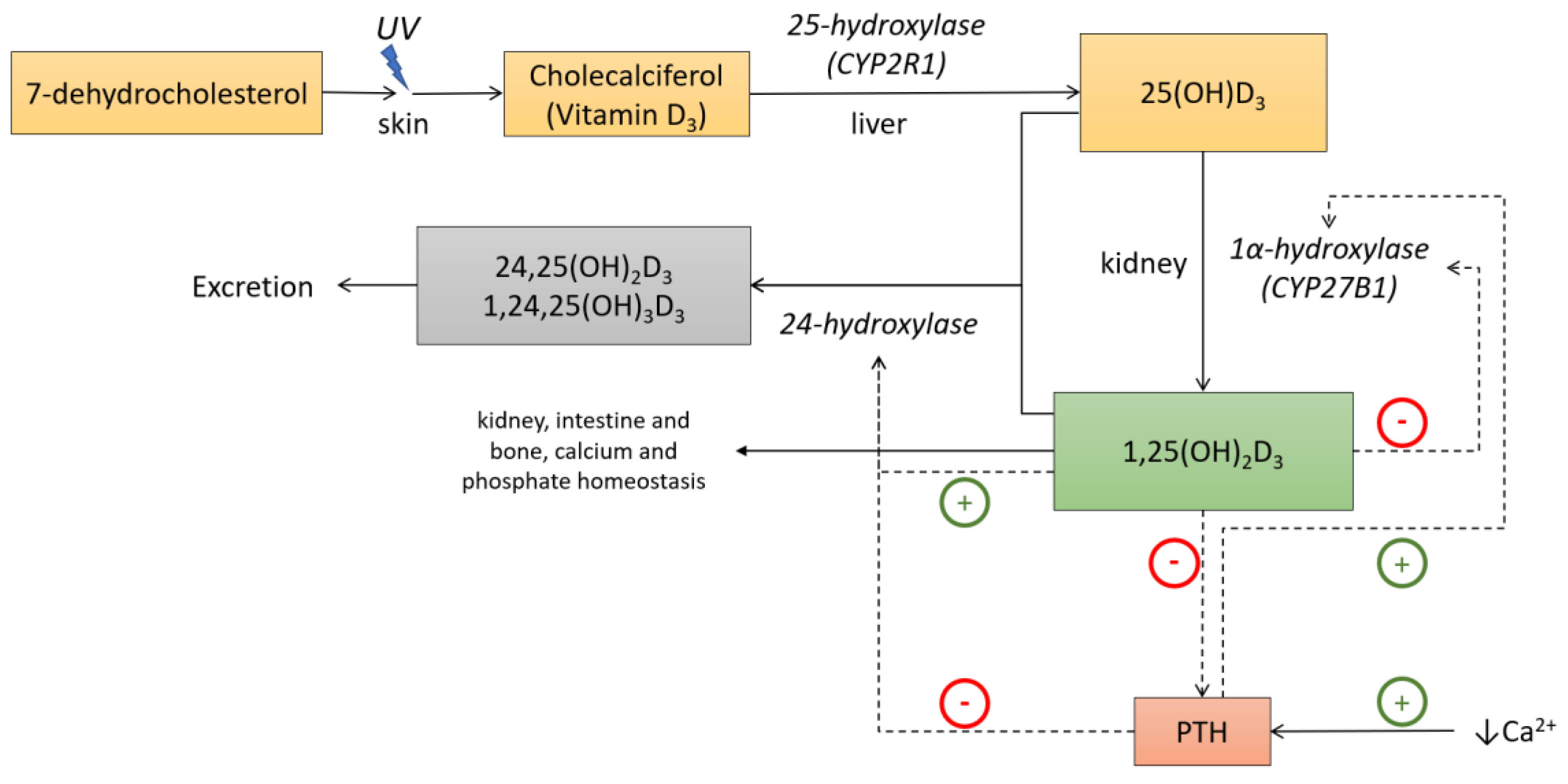
1. Introduction
2. Materials and Methods
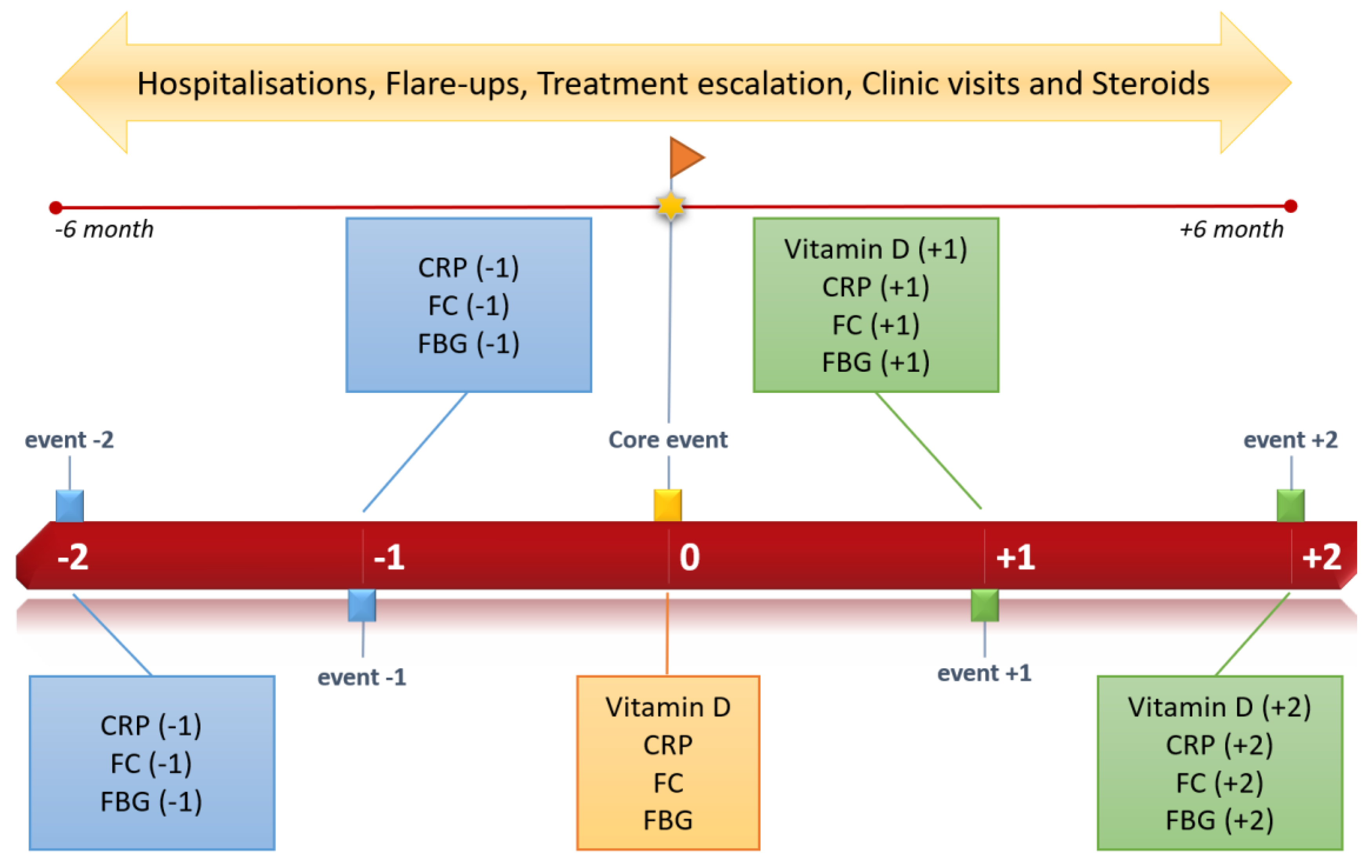
2.1. Study Design and Patient Enrolment
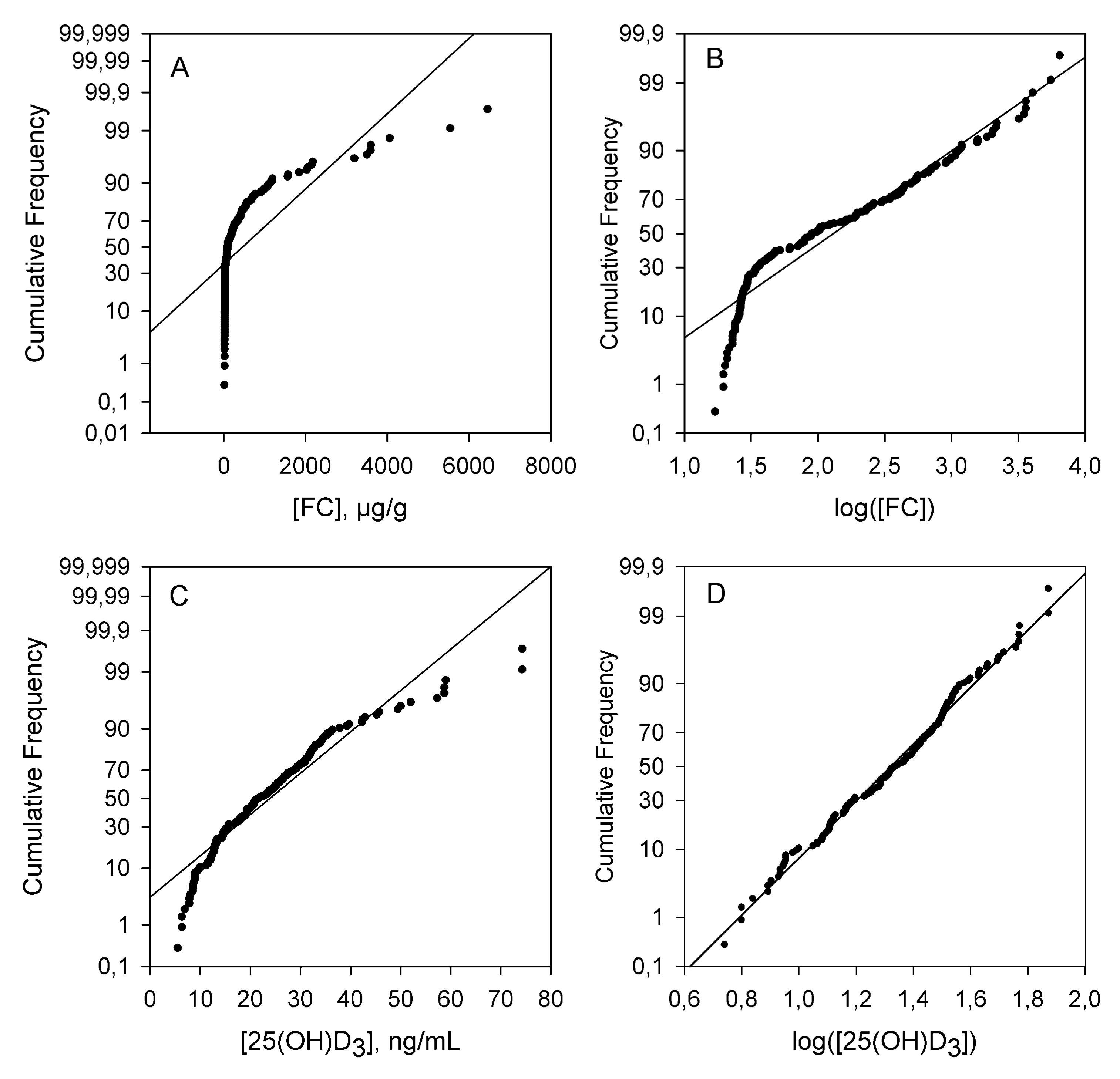
2.2. Statistical Analysis
3. Results
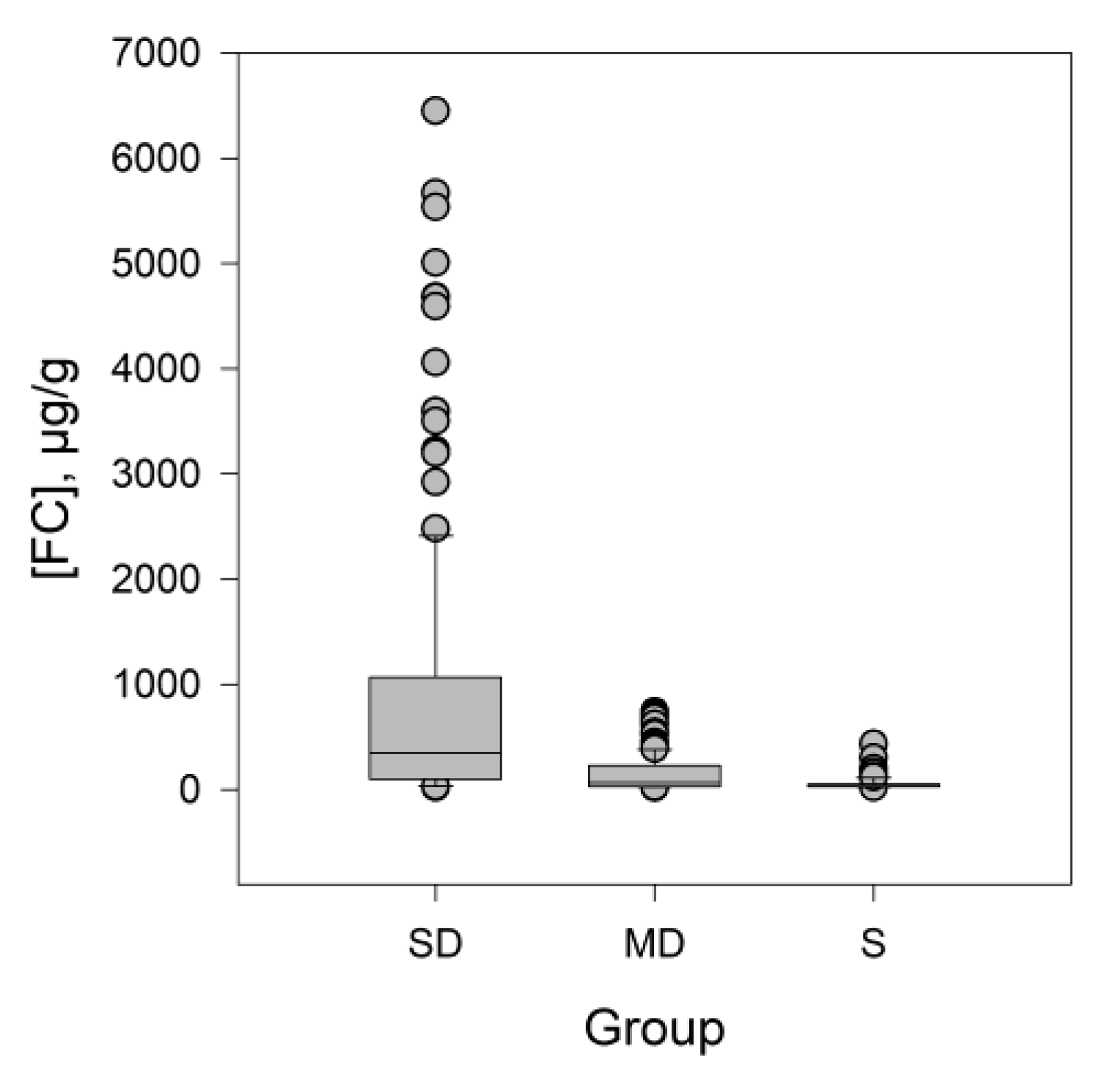
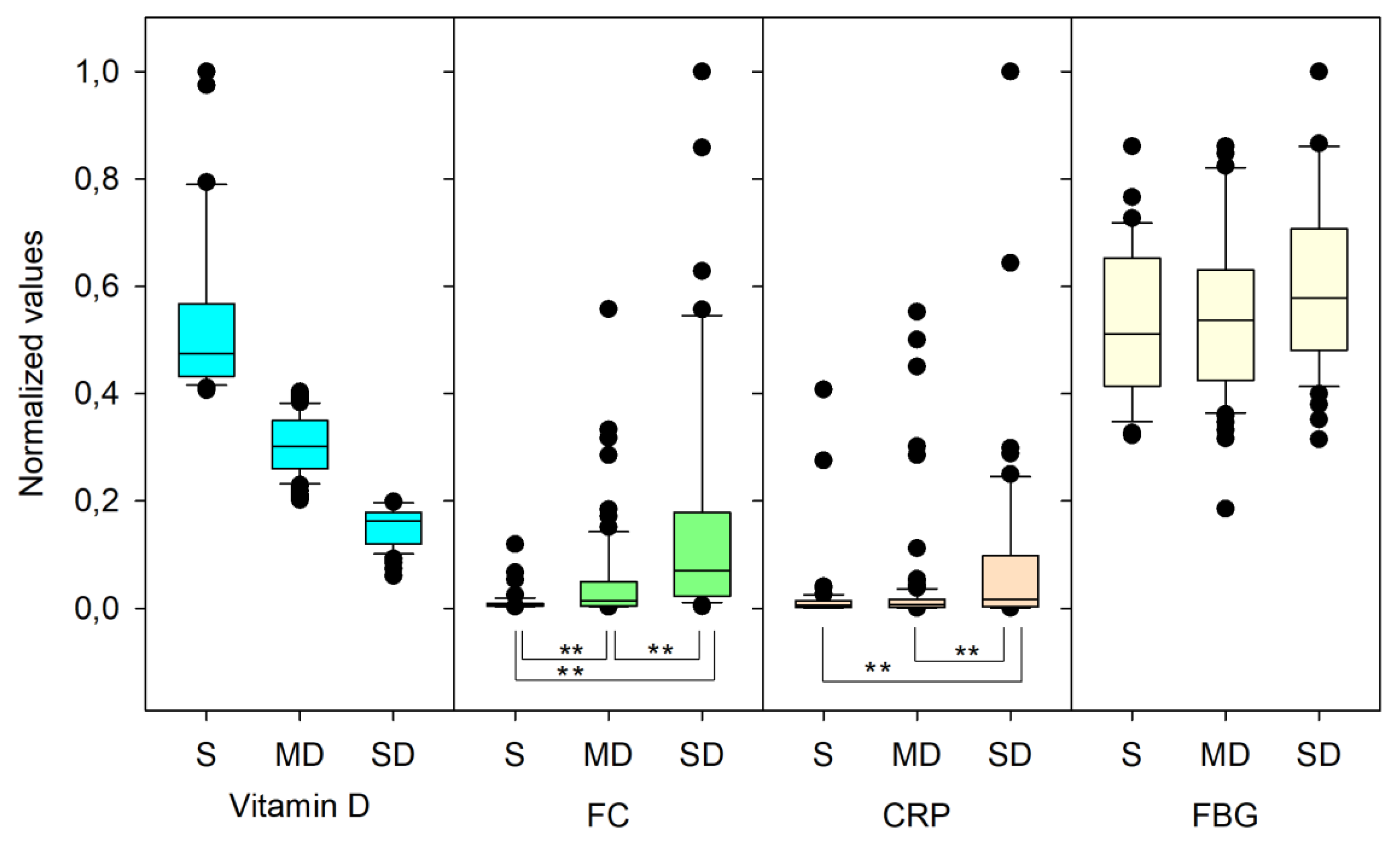
3.1. Descriptive Statistics
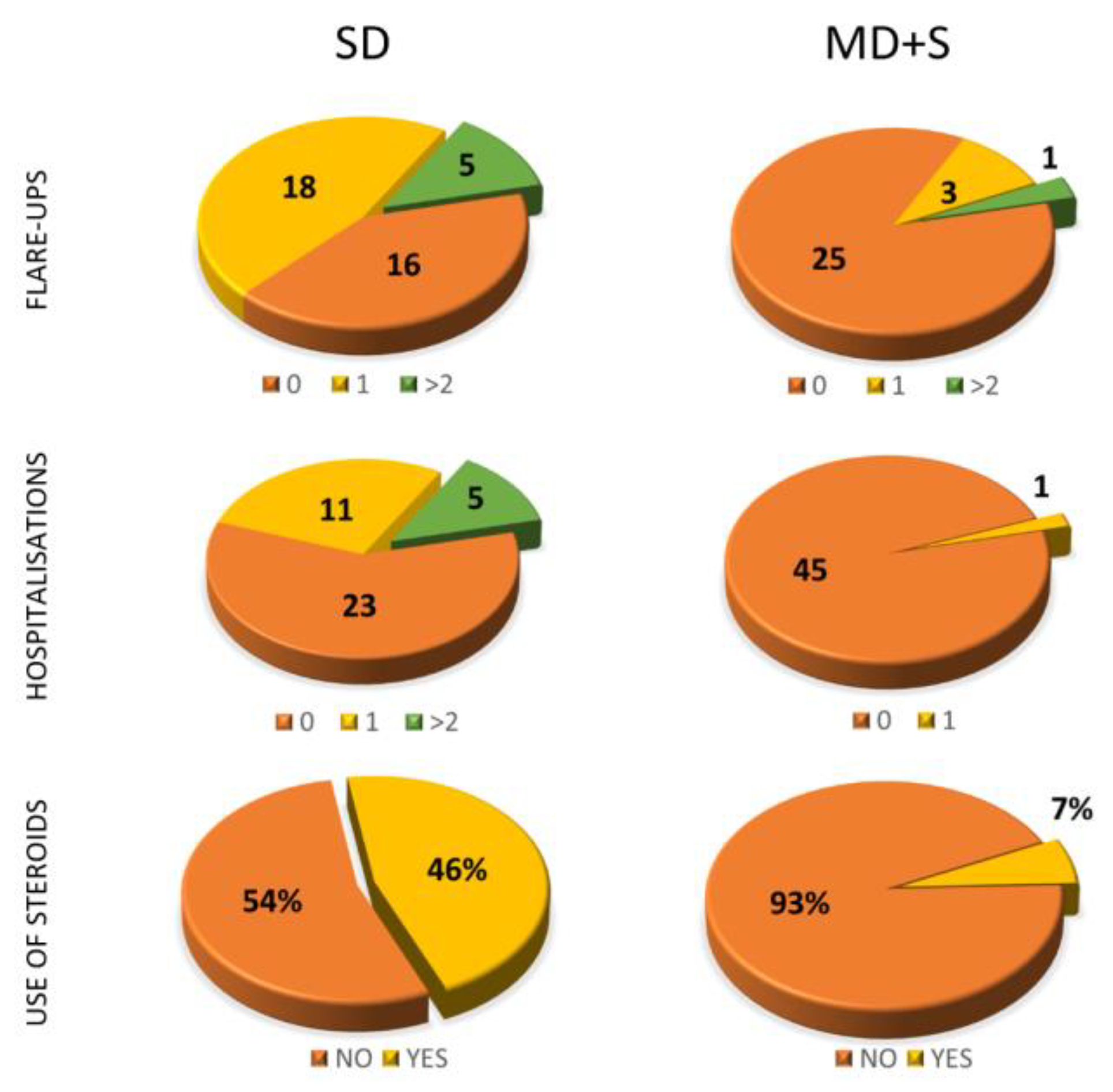
3.2. Qualitative Variables Study
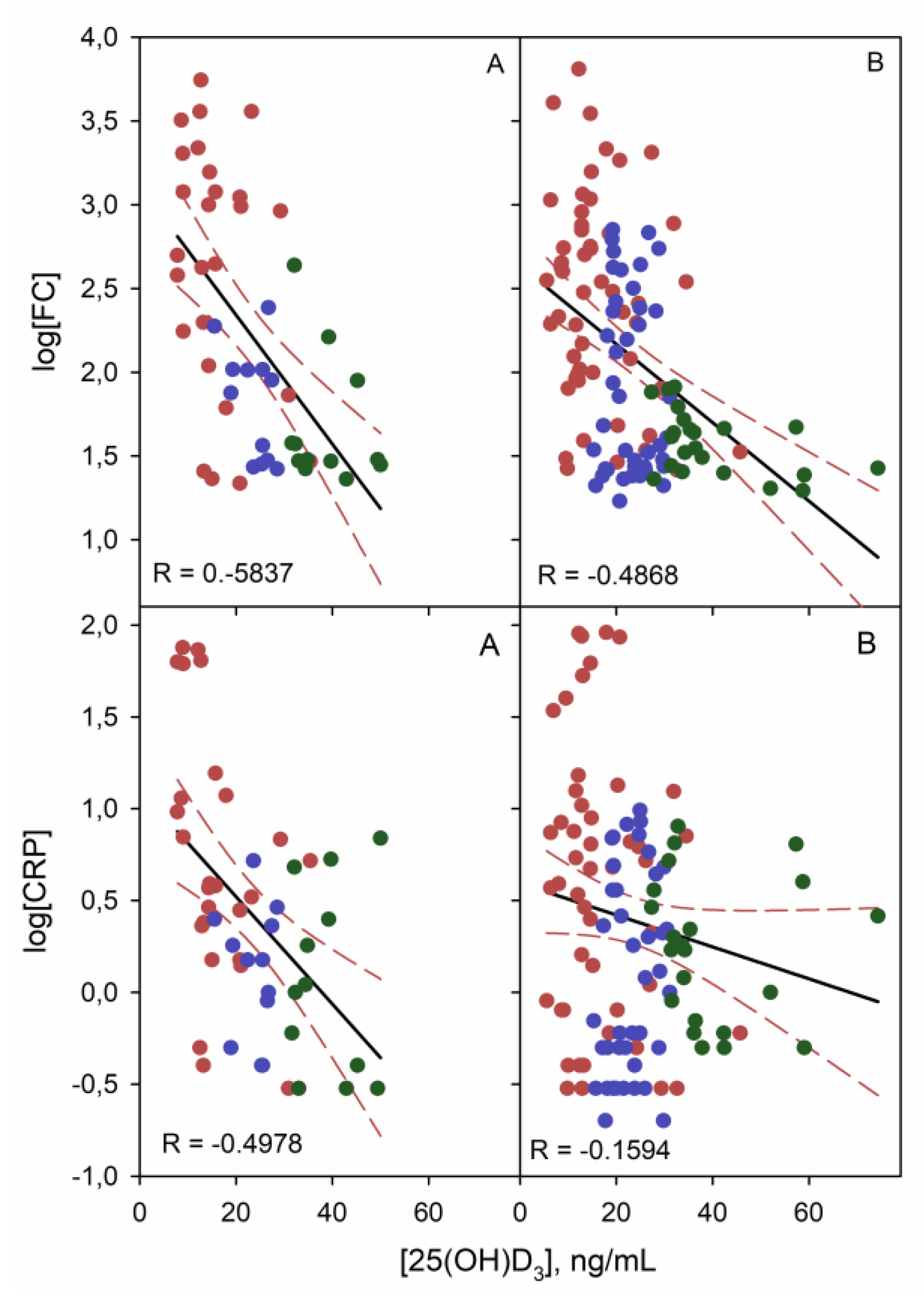
3.3. Linear Correlation Study of Quantitative Variables
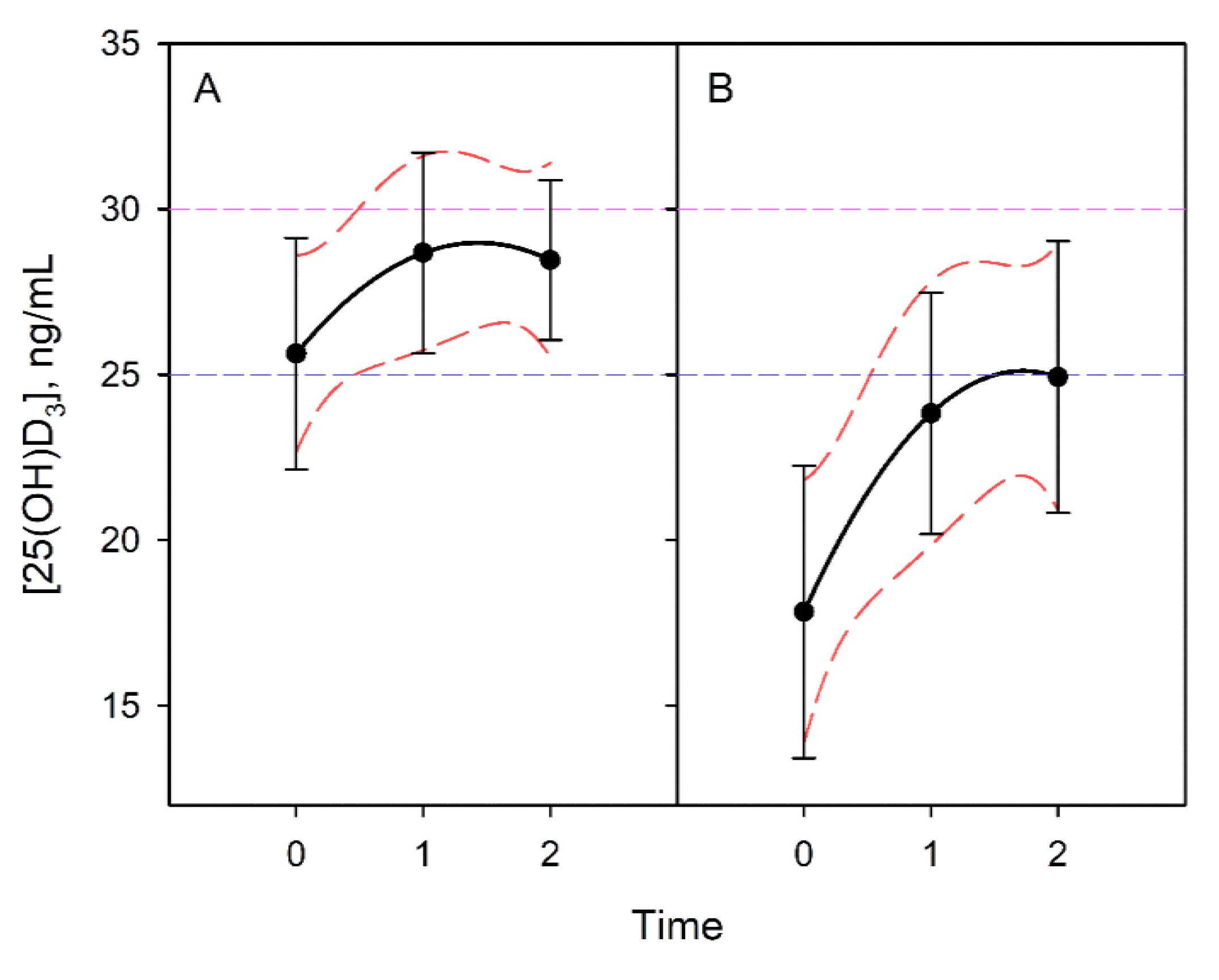
3.4. Vitamin D Progression
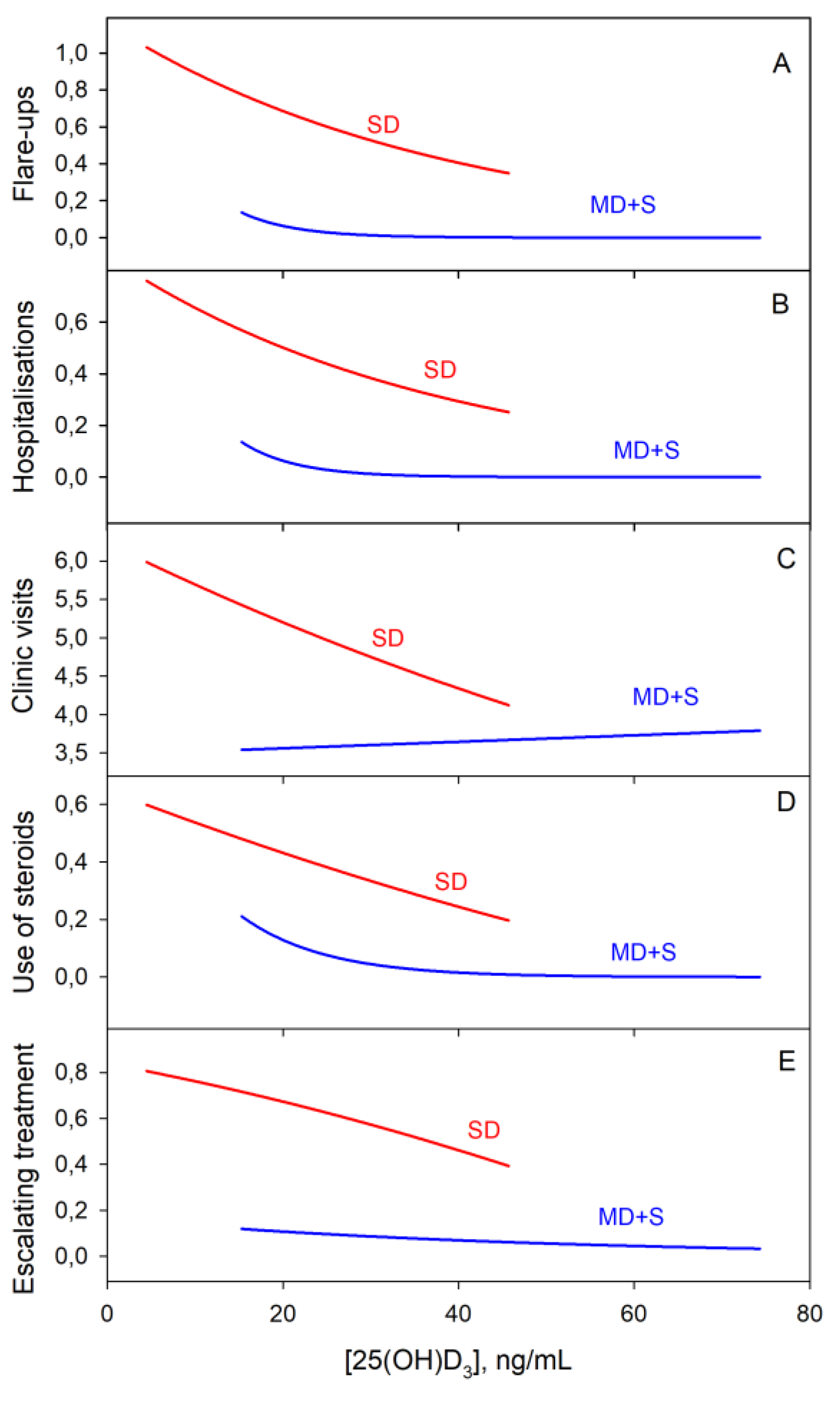
3.5. Categorical Variables Study
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stamp, T.C.; Haddad, J.G.; Twigg, C.A. Comparison of oral 25-hydroxycholecalciferol, vitamin D, and ultraviolet light as determinants of circulating 25-hydroxyvitamin D. Lancet 1977, 1, 1341–1343. [Google Scholar] [CrossRef]
- Alrefai, D.; Jones, J.; El-Matary, W.; Whiting, S.J.; Aljebreen, A.; Mirhosseini, N.; Vatanparast, H. The Association of Vitamin D Status with Disease Activity in a Cohort of Crohn’s Disease Patients in Canada. Nutrients 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- White, J.H. Vitamin D metabolism and signaling in the immune system. Rev. Endocr. Metab. Disord. 2012, 13, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Lips, P. Vitamin D physiology. Prog. Biophys. Mol. Biol. 2006, 92, 4–8. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D metabolism, mechanism of action, and clinical applications. Chem. Biol. 2014, 21, 319–329. [Google Scholar] [CrossRef]
- Lim, W.-C.; Hanauer, S.B.; Li, Y.C. Mechanisms of Disease: Vitamin D and inflammatory bowel disease. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005, 2, 308. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.S.; Song, M. Pharmacokinetics and efficacy of pulse oral versus intravenous calcitriol in hemodialysis patients. J. Am. Soc. Nephrol. 1996, 7, 488–496. [Google Scholar]
- Jones, K.S.; Assar, S.; Harnpanich, D.; Bouillon, R.; Lambrechts, D.; Prentice, A.; Schoenmakers, I. 25(OH)D-2 Half-Life Is Shorter Than 25(OH)D-3 Half-Life and Is Influenced by DBP Concentration and Genotype. J. Clin. Endocrinol. Metab. 2014, 99, 3373–3381. [Google Scholar] [CrossRef] [PubMed]
- Wobke, T.K.; Sorg, B.L.; Steinhilber, D. Vitamin D in inflammatory diseases. Front. Physiol. 2014, 5, 244. [Google Scholar] [CrossRef] [PubMed]
- Cantorna, M.T.; Zhu, Y.; Froicu, M.; Wittke, A. Vitamin D status, 1,25-dihydroxyvitamin D3, and the immune system. Am. J. Clin. Nutr. 2004, 80, 1717s–1720s. [Google Scholar] [CrossRef]
- Gubatan, J.; Moss, A.C. Vitamin D in inflammatory bowel disease: More than just a supplement. Curr. Opin. Gastroenterol. 2018, 34, 217–225. [Google Scholar] [CrossRef]
- Stio, M.; Retico, L.; Annese, V.; Bonanomi, A.G. Vitamin D regulates the tight-junction protein expression in active ulcerative colitis. Scand. J. Gastroenterol. 2016, 51, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.G.; Lu, R.; Xia, Y.; Zhou, D.; Petrof, E.; Claud, E.C.; Sun, J. Lack of Vitamin D Receptor Leads to Hyperfunction of Claudin-2 in Intestinal Inflammatory Responses. Inflamm. Bowel Dis. 2019, 25, 97–110. [Google Scholar] [CrossRef]
- Gisbert-Ferrandiz, L.; Salvador, P.; Ortiz-Masia, D.; Macias-Ceja, D.C.; Orden, S.; Esplugues, J.V.; Calatayud, S.; Hinojosa, J.; Barrachina, M.D.; Hernandez, C. A Single Nucleotide Polymorphism in the Vitamin D Receptor Gene Is Associated with Decreased Levels of the Protein and a Penetrating Pattern in Crohn’s Disease. Inflamm. Bowel Dis. 2018, 24, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Dabbas, B.; Laperriere, D.; Bitton, A.J.; Soualhine, H.; Tavera-Mendoza, L.E.; Dionne, S.; Servant, M.J.; Bitton, A.; Seidman, E.G.; et al. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. J. Biol. Chem. 2010, 285, 2227–2231. [Google Scholar] [CrossRef]
- Hugot, J.P.; Chamaillard, M.; Zouali, H.; Lesage, S.; Cezard, J.P.; Belaiche, J.; Almer, S.; Tysk, C.; O’Morain, C.A.; Gassull, M.; et al. Association of NOD2 leucine-rich repeat variants with susceptibility to Crohn’s disease. Nature 2001, 411, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Fellermann, K.; Stange, D.E.; Schaeffeler, E.; Schmalzl, H.; Wehkamp, J.; Bevins, C.L.; Reinisch, W.; Teml, A.; Schwab, M.; Lichter, P.; et al. A chromosome 8 gene-cluster polymorphism with low human beta-defensin 2 gene copy number predisposes to Crohn disease of the colon. Am. J. Hum. Genet. 2006, 79, 439–448. [Google Scholar] [CrossRef]
- Gombart, A.F.; Borregaard, N.; Koeffler, H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005, 19, 1067–1077. [Google Scholar] [CrossRef]
- Wang, T.T.; Nestel, F.P.; Bourdeau, V.; Nagai, Y.; Wang, Q.; Liao, J.; Tavera-Mendoza, L.; Lin, R.; Hanrahan, J.W.; Mader, S.; et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J. Immunol. 2004, 173, 2909–2912. [Google Scholar] [CrossRef]
- D’Ambrosio, D.; Cippitelli, M.; Cocciolo, M.G.; Mazzeo, D.; Di Lucia, P.; Lang, R.; Sinigaglia, F.; Panina-Bordignon, P. Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-kappaB downregulation in transcriptional repression of the p40 gene. J. Clin. Investig. 1998, 101, 252–262. [Google Scholar] [CrossRef]
- Penna, G.; Adorini, L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J. Immunol. 2000, 164, 2405–2411. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, A.; Barrat, F.J.; Crain, C.; Heath, V.L.; Savelkoul, H.F.; O’Garra, A. 1alpha,25-Dihydroxyvitamin d3 has a direct effect on naive CD4(+) T cells to enhance the development of Th2 cells. J. Immunol. 2001, 167, 4974–4980. [Google Scholar] [CrossRef] [PubMed]
- Gubatan, J.; Mitsuhashi, S.; Longhi, M.S.; Zenlea, T.; Rosenberg, L.; Robson, S.; Moss, A.C. Higher serum vitamin D levels are associated with protective serum cytokine profiles in patients with ulcerative colitis. Cytokine 2018, 103, 38–45. [Google Scholar] [CrossRef]
- Luthold, R.V.; Fernandes, G.R.; Franco-de-Moraes, A.C.; Folchetti, L.G.; Ferreira, S.R. Gut microbiota interactions with the immunomodulatory role of vitamin D in normal individuals. Metabolism 2017, 69, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Schaffler, H.; Herlemann, D.P.R.; Klinitzke, P.; Berlin, P.; Kreikemeyer, B.; Jaster, R.; Lamprecht, G. Vitamin D administration leads to a shift of the intestinal bacterial composition in Crohn’s disease patients, but not in healthy controls. J. Dig. Dis. 2018, 19, 225–234. [Google Scholar] [CrossRef]
- Dziarski, R.; Park, S.Y.; Kashyap, D.R.; Dowd, S.E.; Gupta, D. Pglyrp-Regulated Gut Microflora Prevotella falsenii, Parabacteroides distasonis and Bacteroides eggerthii Enhance and Alistipes finegoldii Attenuates Colitis in Mice. PLoS ONE 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Tamanai-Shacoori, Z.; Smida, I.; Bousarghin, L.; Loreal, O.; Meuric, V.; Fong, S.B.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Roseburia spp.: A marker of health? Future Microbiol. 2017, 12, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Del Pinto, R.; Pietropaoli, D.; Chandar, A.K.; Ferri, C.; Cominelli, F. Association Between Inflammatory Bowel Disease and Vitamin D Deficiency: A Systematic Review and Meta-analysis. Inflamm. Bowel Dis. 2015, 21, 2708–2717. [Google Scholar] [CrossRef]
- Filippi, J.; Al-Jaouni, R.; Wiroth, J.B.; Hebuterne, X.; Schneider, S.M. Nutritional deficiencies in patients with Crohn’s disease in remission. Inflamm. Bowel Dis. 2006, 12, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Raftery, T.; Merrick, M.; Healy, M.; Mahmud, N.; O’Morain, C.; Smith, S.; McNamara, D.; O’Sullivan, M. Vitamin D Status Is Associated with Intestinal Inflammation as Measured by Fecal Calprotectin in Crohn’s Disease in Clinical Remission. Dig. Dis. Sci. 2015, 60, 2427–2435. [Google Scholar] [CrossRef]
- Frigstad, S.O.; Hoivik, M.; Jahnsen, J.; Dahl, S.R.; Cvancarova, M.; Grimstad, T.; Berset, I.P.; Huppertz-Hauss, G.; Hovde, O.; Torp, R.; et al. Vitamin D deficiency in inflammatory bowel disease: Prevalence and predictors in a Norwegian outpatient population. Scand. J. Gastroenterol. 2017, 52, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Meckel, K.; Li, Y.C.; Lim, J.; Kocherginsky, M.; Weber, C.; Almoghrabi, A.; Chen, X.; Kaboff, A.; Sadiq, F.; Hanauer, S.B.; et al. Serum 25-hydroxyvitamin D concentration is inversely associated with mucosal inflammation in patients with ulcerative colitis. Am. J. Clin. Nutr. 2016, 104, 113–120. [Google Scholar] [CrossRef]
- Santos-Antunes, J.; Nunes, A.C.; Lopes, S.; Macedo, G. The Relevance of Vitamin D and Antinuclear Antibodies in Patients with Inflammatory Bowel Disease Under Anti-TNF Treatment: A Prospective Study. Inflamm. Bowel Dis. 2016, 22, 1101–1106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schaffler, H.; Schmidt, M.; Huth, A.; Reiner, J.; Glass, A.; Lamprecht, G. Clinical factors are associated with vitamin D levels in IBD patients: A retrospective analysis. J. Dig. Dis. 2018, 19, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Venkata, K.V.R.; Arora, S.S.; Xie, F.L.; Malik, T.A. Impact of vitamin D on the hospitalization rate of Crohn’s disease patients seen at a tertiary care center. World J. Gastroenterol. 2017, 23, 2539–2544. [Google Scholar] [CrossRef]
- Scolaro, B.L.; Barretta, C.; Matos, C.H.; Malluta, E.F.; Almeida, I.B.T.d.; Braggio, L.D.; Bobato, S.; Specht, C.M. Deficiency of vitamin D and its relation with clinical and laboratory activity of inflammatory bowel diseases. JCOL 2018, 38, 99–104. [Google Scholar] [CrossRef]
- Kabbani, T.A.; Koutroubakis, I.E.; Schoen, R.E.; Ramos-Rivers, C.; Shah, N.; Swoger, J.; Regueiro, M.; Barrie, A.; Schwartz, M.; Hashash, J.G.; et al. Association of Vitamin D Level with Clinical Status in Inflammatory Bowel Disease: A 5-Year Longitudinal Study. Am. J. Gastroenterol. 2016, 111, 712–719. [Google Scholar] [CrossRef]
- Garg, M.; Rosella, O.; Lubel, J.S.; Gibson, P.R. Association of circulating vitamin D concentrations with intestinal but not systemic inflammation in inflammatory bowel disease. Inflamm. Bowel Dis. 2013, 19, 2634–2643. [Google Scholar] [CrossRef]
- Gubatan, J.; Mitsuhashi, S.; Zenlea, T.; Rosenberg, L.; Robson, S.; Moss, A.C. Low Serum Vitamin D During Remission Increases Risk of Clinical Relapse in Patients with Ulcerative Colitis. Clin. Gastroenterol. Hepatol. 2017, 15, 240–246. [Google Scholar] [CrossRef]
- Garg, M.; Rosella, O.; Rosella, G.; Wu, Y.; Lubel, J.S.; Gibson, P.R. Evaluation of a 12-week targeted vitamin D supplementation regimen in patients with active inflammatory bowel disease. Clin. Nutr. 2018, 37, 1375–1382. [Google Scholar] [CrossRef]
- Sharifi, A.; Hosseinzadeh-Attar, M.J.; Vahedi, H.; Nedjat, S. A randomized controlled trial on the effect of vitamin D3 on inflammation and cathelicidin gene expression in ulcerative colitis patients. Saudi J. Gastroenterol. 2016, 22, 316–323. [Google Scholar] [CrossRef] [PubMed]
| CI a (95%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Group | Mean | StDev | CI− | CI+ | Skewness | Kurtosis | MSSD |
| Vitamin D | S | 40.43 | 12.7 | 37.0 | 43.9 | 1.34 | 1.42 | 78 |
| MD | 24.36 | 4.7 | 23.3 | 25.5 | 0.32 | −0.16 | 17 | |
| SD | 17.83 | 8.1 | 16.3 | 19.3 | 0.85 | 0.39 | 65 | |
| CRP | S | 2.28 | 2.37 | 1.8 | 2.8 | 1.82 | 3.5 | 1.5 |
| MD | 2.80 | 3.07 | 2.3 | 3.3 | 1.71 | 3.01 | 4.3 | |
| SD | 23.6 | 51.0 | 16 | 31 | 4.48 | 24.2 | 1102 | |
| FC | S | 54 | 64 | 39 | 69 | 4.09 | 19.72 | 2567 |
| MD | 141 | 164 | 111 | 172 | 1.81 | 3.00 | 15,020 | |
| SD | 868 | 1278 | 665 | 1070 | 2.31 | 5.24 | 508,909 | |
| FBG | S | 423 | 93 | 401 | 445 | 0.35 | −0.51 | 4140 |
| MD | 412 | 107 | 392 | 432 | 0.57 | −0.13 | 6703 | |
| SD | 497 | 133 | 475 | 517 | 0.08 | −0.77 | 5576 | |
| CI a (95%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Variable | Group | Min | Q1 | Median | Q3 | Max | CI− | CI+ |
| Vitamin D | S | 20.5 | 32.2 | 36.2 | 43.0 | 74.30 | 34.1 | 43.9 |
| MD | 15.3 | 20.6 | 24.7 | 27.4 | 37.5 | 22.4 | 25.5 | |
| SD | 4.5 | 12.2 | 15.1 | 22.7 | 45.7 | 14.3 | 19.3 | |
| CRP | S | 0.20 | 0.60 | 1.45 | 2.97 | 11.8 | 0.9 | 2.0 |
| MD | 0.20 | 0.60 | 1.50 | 3.82 | 16.5 | 1.1 | 2.2 | |
| SD | 0.30 | 1.90 | 5.60 | 15.6 | 364 | 4.5 | 7 | |
| FC | S | 19.6 | 26.7 | 29.7 | 50.7 | 434 | 28 | 37 |
| MD | 17 | 27.6 | 63 | 229 | 738 | 43 | 98 | |
| SD | 19 | 97 | 346 | 1065 | 6450 | 216 | 444 | |
| FBG | S | 262 | 356 | 411 | 491 | 664 | 387 | 446 |
| MD | 201 | 325 | 406 | 475 | 700 | 365 | 432 | |
| SD | 151 | 390 | 478 | 602 | 813 | 456 | 502 | |
| Clinical Characteristic | Number of Participants (Percentage) or Mean ± Standard Deviation | |
|---|---|---|
| Age, years | 43.7 ± 15.9 | |
| Female sex | 39 (46) | |
| Crohn’s disease | 60 (71) | |
| Ulcerative colitis | 25 (29) | |
| Perianal disease | 4 (5) | |
| Extraintestinal manifestations | 10 (12) | |
| Vitamin D supplementation | 44 (52) | |
| Current therapy | Anti-TNFα | 47 (55) |
| Vedolizumab | 8 (9) | |
| Ustekinumab | 1 (1) | |
| Steroids | 21 (25) | |
| Azathioprine | 31 (36) | |
| Methotrexate | 2 (2) | |
| FC, µg/g | CRP, mg/dL | ||||
|---|---|---|---|---|---|
| Group | Median | Q3–Q1 | Median | Q3–Q1 | |
| Not supplemented | S | 33 | 25 | 1.3 | 1.8 |
| MD | 85 | 211 | 1.9 | 4.2 | |
| SD | 524 | 2230 | 8.9 | 3.1 | |
| Supplemented | S | 29 | 38 | 2.3 | 4.6 |
| MD | 55 | 328 | 2.1 | 4.9 | |
| SD | 362 | 943 | 6.7 | 3.1 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Muñoz, P.; Beltrán, B.; Sáez-González, E.; Alba, A.; Nos, P.; Iborra, M. Influence of Vitamin D Deficiency on Inflammatory Markers and Clinical Disease Activity in IBD Patients. Nutrients 2019, 11, 1059. https://doi.org/10.3390/nu11051059
López-Muñoz P, Beltrán B, Sáez-González E, Alba A, Nos P, Iborra M. Influence of Vitamin D Deficiency on Inflammatory Markers and Clinical Disease Activity in IBD Patients. Nutrients. 2019; 11(5):1059. https://doi.org/10.3390/nu11051059
Chicago/Turabian StyleLópez-Muñoz, Pedro, Belén Beltrán, Esteban Sáez-González, Amparo Alba, Pilar Nos, and Marisa Iborra. 2019. "Influence of Vitamin D Deficiency on Inflammatory Markers and Clinical Disease Activity in IBD Patients" Nutrients 11, no. 5: 1059. https://doi.org/10.3390/nu11051059
APA StyleLópez-Muñoz, P., Beltrán, B., Sáez-González, E., Alba, A., Nos, P., & Iborra, M. (2019). Influence of Vitamin D Deficiency on Inflammatory Markers and Clinical Disease Activity in IBD Patients. Nutrients, 11(5), 1059. https://doi.org/10.3390/nu11051059