Carbohydrate Restriction in Type 1 Diabetes: A Realistic Therapy for Improved Glycaemic Control and Athletic Performance?
Abstract
1. Introduction
2. History of Carbohydrate Restriction in People with Type 1 Diabetes
3. Defining Low Carbohydrate Diets
4. Low Carbohydrate Diets and Type 1 Diabetes
4.1. Potential Benefits of Low Carbohydrate Diets In People with Type 1 Diabetes
4.2. Potential Risks of Low Carbohydrate Diets in People with Type 1 Diabetes
5. Staying Active on a Low Carbohydrate Diet
6. Carbohydrate Restriction Strategies for the Endurance Athlete with Type 1 Diabetes—Practical Considerations
6.1. Twice Per Day Training
6.2. Fasted Exercise in Athletes with Type 1 Diabetes
6.3. Sleep Low, Train Low
7. Conclusions and Future Directions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| T1D | Type 1 diabetes |
| ADA | American Diabetes Association |
| DAFNE | Dose Adjustment For Normal Eating |
| LCD | low carbohydrate diets |
| VLCD | very low carbohydrate diets |
| IMTG | intramuscular triglyceride |
| DKA | diabetic ketoacidosis |
| SGLT2 | sodium glucose co-transporter 2 |
| CSII | continuous subcutaneous insulin infusion |
| MDI | multiple daily injections |
| AMPK | 5′ AMP-activated protein kinase |
| p38MAPK | p38 mitogen-activated protein kinase |
| PGC-1α | peroxisome proliferator-activated receptor gamma coactivator 1-α |
| COX | cytochrome c oxidase |
| Tfam | mitochondrial transcription factor A |
| FFA | free fatty acids |
| IMCL) | intramyocellular lipids |
References
- Atkinson, M.A.; Eisenbarth, G.S. Type 1 diabetes: New perspectives on disease pathogenesis and treatment. Lancet Lond. Engl. 2001, 358, 221–229. [Google Scholar] [CrossRef]
- Foster, N.C.; Beck, R.W.; Miller, K.M.; Clements, M.A.; Rickels, M.R.; DiMeglio, L.A.; Maahs, D.M.; Tamborlane, W.V.; Bergenstal, R.; Smith, E.; et al. State of Type 1 Diabetes Management and Outcomes from the T1D Exchange in 2016–2018. Diabetes Technol. Ther. 2019, 21, 66–72. [Google Scholar] [CrossRef]
- Chimen, M.; Kennedy, A.; Nirantharakumar, K.; Pang, T.T.; Andrews, R.; Narendran, P. What are the health benefits of physical activity in type 1 diabetes mellitus? A literature review. Diabetologia 2012, 55, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Livingstone, R.; Boyle, J.G.; Petrie, J.R. How tightly controlled do fluctuations in blood glucose levels need to be to reduce the risk of developing complications in people with Type 1 diabetes? Diabet. Med. 2019. [Google Scholar] [CrossRef]
- Nathan, D.M.; DCCT/EDIC Research Group. The diabetes control and complications trial/epidemiology of diabetes interventions and complications study at 30 years: Overview. Diabetes Care 2014, 37, 9–16. [Google Scholar] [CrossRef]
- Corbin, K.D.; Driscoll, K.A.; Pratley, R.E.; Smith, S.R.; Maahs, D.M.; Mayer-Davis, E.J. Advancing Care for Type 1 Diabetes and Obesity Network (ACT1ON) Obesity in Type 1 Diabetes: Pathophysiology, Clinical Impact and Mechanisms. Endocr. Rev. 2018, 39, 629–663. [Google Scholar] [CrossRef]
- Bartlett, J.D.; Hawley, J.A.; Morton, J.P. Carbohydrate availability and exercise training adaptation: Too much of a good thing? Eur. J. Sport Sci. 2015, 15, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Impey, S.G.; Hearris, M.A.; Hammond, K.M.; Bartlett, J.D.; Louis, J.; Close, G.L.; Morton, J.P. Fuel for the Work Required: A Theoretical Framework for Carbohydrate Periodization and the Glycogen Threshold Hypothesis. Sports Med. 2018, 48, 1031–1048. [Google Scholar] [CrossRef] [PubMed]
- Jeukendrup, A.E. Periodized Nutrition for Athletes. Sports Med. 2017, 47, 51–63. [Google Scholar] [CrossRef]
- De Bock, K.; Derave, W.; Eijnde, B.O.; Hesselink, M.K.; Koninckx, E.; Rose, A.J.; Schrauwen, P.; Bonen, A.; Richter, E.A.; Hespel, P. Effect of training in the fasted state on metabolic responses during exercise with carbohydrate intake. J. Appl. Physiol. 2008, 104, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Stannard, S.R.; Buckley, A.J.; Edge, J.A.; Thompson, M.W. Adaptations to skeletal muscle with endurance exercise training in the acutely fed versus overnight-fasted state. J. Sci. Med. Sport 2010, 13, 465–469. [Google Scholar] [CrossRef]
- Van Proeyen, K.; Szlufcik, K.; Nielens, H.; Ramaekers, M.; Hespel, P. Beneficial metabolic adaptations due to endurance exercise training in the fasted state. J. Appl. Physiol. 2011, 110, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Mazur, A. Why were “starvation diets” promoted for diabetes in the pre-insulin period? Nutr. J. 2011, 10, 23. [Google Scholar] [CrossRef] [PubMed]
- Nutritional Advice for People with Diabetes: Past, Present, What Next?-Thomas-2004-Practical Diabetes International-Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/pdi.591 (accessed on 12 February 2019).
- Hill, L.; Eckman, R. The Starvation Treatment of Diabetes. Available online: http://www.gutenberg.org/files/26058/26058-h/26058-h.htm (accessed on 18 February 2019).
- Dietary Guidelines Advisory Committee Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans 2015. Available online: https://health.gov/dietaryguidelines/2015-scientific-report/ (accessed on 6 May 2019).
- Dietary Guidelines Advisory Committee Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans 2010. Available online: https://www.nutriwatch.org/05Guidelines/dga_advisory_2010.pdf (accessed on 6 May 2019).
- Dietary Guidelines Advisory Committee Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans 2005. Available online: https://health.gov/dietaryguidelines/dga2005/document/pdf/dga2005.pdf (accessed on 6 May 2019).
- Dietary Guidelines Advisory Committee Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans 2000. Available online: https://health.gov/dietaryguidelines/dgac/pdf/dgac_ful.pdf (accessed on 6 May 2019).
- Dietary Guidelines Advisory Committee Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans 1995. Available online: https://health.gov/dietaryguidelines/dga95/pdf/DGREPORT.PDF (accessed on 6 May 2019).
- Dietary Guidelines Advisory Committee Report of the Dietary Guidelines Advisory Committee on the Dietary Guidelines for Americans 1990. Available online: https://health.gov/dietaryguidelines/1990.asp (accessed on 6 May 2019).
- Centers for Disease Control and Prevention (CDC) Trends in intake of energy and macronutrients–United States, 1971–2000. Morb. Mortal. Wkly. Rep. 2004, 53, 80–82.
- Austin, G.L.; Ogden, L.G.; Hill, J.O. Trends in carbohydrate, fat, and protein intakes and association with energy intake in normal-weight, overweight, and obese individuals: 1971–2006. Am. J. Clin. Nutr. 2011, 93, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Merger, S.R.; Kerner, W.; Stadler, M.; Zeyfang, A.; Jehle, P.; Müller-Korbsch, M.; Holl, R.W.; DPV Initiative; German BMBF Competence Network Diabetes Mellitus. Prevalence and comorbidities of double diabetes. Diabetes Res. Clin. Pract. 2016, 119, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Talmadge, K.; Philipson, L.; Reusch, J.; Hill-Briggs, F.; Youssef, G.; Bertha, B.; Ching, M.; Clark, M.P.; Herrick, D.J.; Cefalu, W.T. American diabetes association standards of medical care in diabetes—2018. Diabetes Care 2018, 41, S38–S50. [Google Scholar]
- Smart, C.E.; Annan, F.; Higgins, L.A.; Jelleryd, E.; Lopez, M.; Acerini, C.L. ISPAD Clinical Practice Consensus Guidelines 2018: Nutritional management in children and adolescents with diabetes. Pediatr. Diabetes 2018, 19 (Suppl. 27), 136–154. [Google Scholar] [CrossRef]
- Accurso, A.; Bernstein, R.K.; Dahlqvist, A.; Draznin, B.; Feinman, R.D.; Fine, E.J.; Gleed, A.; Jacobs, D.B.; Larson, G.; Lustig, R.H.; et al. Dietary carbohydrate restriction in type 2 diabetes mellitus and metabolic syndrome: Time for a critical appraisal. Nutr. Metab. 2008, 5, 9. [Google Scholar] [CrossRef]
- Feinman, R.D.; Pogozelski, W.K.; Astrup, A.; Bernstein, R.K.; Fine, E.J.; Westman, E.C.; Accurso, A.; Frassetto, L.; Gower, B.A.; McFarlane, S.I.; et al. Dietary carbohydrate restriction as the first approach in diabetes management: Critical review and evidence base. Nutrition 2015, 31, 1–13. [Google Scholar] [CrossRef]
- Eiswirth, M.; Clark, E.; Diamond, M. Low carbohydrate diet and improved glycaemic control in a patient with type one diabetes. Endocrinol. Diabetes Metab. Case Rep. 2018. [Google Scholar] [CrossRef] [PubMed]
- Lennerz, B.S.; Barton, A.; Bernstein, R.K.; Dikeman, R.D.; Diulus, C.; Hallberg, S.; Rhodes, E.T.; Ebbeling, C.B.; Westman, E.C.; Yancy, W.S.; et al. Management of Type 1 Diabetes with a Very Low–Carbohydrate Diet. Pediatrics 2018, 141, e20173349. [Google Scholar] [CrossRef] [PubMed]
- Leow, Z.Z.X.; Guelfi, K.J.; Davis, E.A.; Jones, T.W.; Fournier, P.A. The glycaemic benefits of a very-low-carbohydrate ketogenic diet in adults with Type 1 diabetes mellitus may be opposed by increased hypoglycaemia risk and dyslipidaemia. Diabet. Med. 2018, 35, 1258–1263. [Google Scholar] [CrossRef]
- Diabetes Control and Complications Trial Research Group; Nathan, D.M.; Genuth, S.; Lachin, J.; Cleary, P.; Crofford, O.; Davis, M.; Rand, L.; Siebert, C. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar]
- Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC); Study Research Group. Mortality in Type 1 Diabetes in the DCCT/EDIC Versus the General Population. Diabetes Care 2016, 39, 1378–1383. [Google Scholar]
- Seckold, R.; Fisher, E.; de Bock, M.; King, B.R.; Smart, C.E. The ups and downs of low-carbohydrate diets in the management of Type 1 diabetes: A review of clinical outcomes. Diabet. Med. 2018, 36, 326–334. [Google Scholar] [CrossRef]
- Ranjan, A.; Schmidt, S.; Damm-Frydenberg, C.; Steineck, I.; Clausen, T.R.; Holst, J.J.; Madsbad, S.; Nørgaard, K. Low-Carbohydrate Diet Impairs the Effect of Glucagon in the Treatment of Insulin-Induced Mild Hypoglycemia: A Randomized Crossover Study. Diabetes Care 2017, 40, 132–135. [Google Scholar] [CrossRef] [PubMed]
- De Bock, M.; Lobley, K.; Anderson, D.; Davis, E.; Donaghue, K.; Pappas, M.; Siafarikas, A.; Cho, Y.H.; Jones, T.; Smart, C. Endocrine and metabolic consequences due to restrictive carbohydrate diets in children with type 1 diabetes: An illustrative case series. Pediatr. Diabetes 2018, 19, 129–137. [Google Scholar] [CrossRef]
- Kanikarla-Marie, P.; Jain, S.K. Hyperketonemia and ketosis increase the risk of complications in type 1 diabetes. Free Radic. Biol. Med. 2016, 95, 268–277. [Google Scholar] [CrossRef]
- Thomas, D.T.; Erdman, K.A.; Burke, L.M. American College of Sports Medicine Joint Position Statement. Nutrition and Athletic Performance. Med. Sci. Sports Exerc. 2016, 48, 543–568. [Google Scholar]
- LaFountain, R.A.; Miller, V.J.; Barnhart, E.C.; Hyde, P.N.; Crabtree, C.D.; McSwiney, F.T.; Beeler, M.K.; Buga, A.; Sapper, T.N.; Short, J.A.; et al. Extended Ketogenic Diet and Physical Training Intervention in Military Personnel. Mil. Med. 2019. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Jeukendrup, A.E.; Jones, A.M.; Mooses, M. Contemporary Nutrition Strategies to Optimize Performance in Distance Runners and Race Walkers. Int. J. Sport Nutr. Exerc. Metab. 2019, 29, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Fordyce, T. Chris Froome: Team Sky’s Unprecedented Release of Data Reveals How British Rider Won Giro d’Italia 2018. Available online: https://www.bbc.com/sport/cycling/44694122 (accessed on 6 May 2019).
- Trumbo, P.; Schlicker, S.; Yates, A.A.; Poos, M.; Food and Nutrition Board of the Institute of Medicine; The National Academies. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein and amino acids. J. Acad. Nutr. Dietet. 2002, 102, 1621–1630. [Google Scholar] [CrossRef]
- Burke, L.M.; Hawley, J.A.; Wong, S.H.S.; Jeukendrup, A.E. Carbohydrates for training and competition. J. Sports Sci. 2011, 29 (Suppl. 1), S17–S27. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.L.; Kirkman, M.S.; Laffel, L.M.B.; Peters, A.L. Type 1 Diabetes Through the Life Span: A Position Statement of the American Diabetes Association. Diabetes Care 2014, 37, 2034–2054. [Google Scholar] [CrossRef] [PubMed]
- DAFNE Study Group. Training in flexible, intensive insulin management to enable dietary freedom in people with type 1 diabetes: Dose adjustment for normal eating (DAFNE) randomised controlled trial. BMJ 2002, 325, 746. [Google Scholar] [CrossRef] [PubMed]
- Brazeau, A.S.; Mircescu, H.; Desjardins, K.; Leroux, C.; Strychar, I.; Ekoé, J.M.; Rabasa-Lhoret, R. Carbohydrate counting accuracy and blood glucose variability in adults with type 1 diabetes. Diabetes Res. Clin. Pract. 2013, 99, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Meade, L.T.; Rushton, W.E. Accuracy of Carbohydrate Counting in Adults. Clin. Diabetes 2016, 34, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, L. Variability of insulin absorption and insulin action. Diabetes Technol. Ther. 2002, 4, 673–682. [Google Scholar] [CrossRef]
- Heinemann, L. Variability of Insulin Action: Does It Matter? Insulin 2008, 3, 37–45. [Google Scholar] [CrossRef]
- Gingras, V.; Taleb, N.; Roy-Fleming, A.; Legault, L.; Rabasa-Lhoret, R. The challenges of achieving postprandial glucose control using closed-loop systems in patients with type 1 diabetes. Diabetes Obes. Metab. 2018, 20, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Krebs, J.D.; Strong, A.P.; Cresswell, P.; Reynolds, A.N.; Hanna, A.; Haeusler, S. A randomised trial of the feasibility of a low carbohydrate diet vs standard carbohydrate counting in adults with type 1 diabetes taking body weight into account. Asia Pac. J. Clin. Nutr. 2016, 25, 78–84. [Google Scholar]
- Nielsen, J.V.; Jönsson, E.; Ivarsson, A. A low carbohydrate diet in type 1 diabetes: Clinical experience—A brief report. Upsala J. Med. Sci. 2005, 110, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Schmidt, S.; Damm-Frydenberg, C.; Holst, J.J.; Madsbad, S.; Nørgaard, K. Short-term effects of a low carbohydrate diet on glycaemic variables and cardiovascular risk markers in patients with type 1 diabetes: A randomized open-label crossover trial. Diabetes Obes. Metab. 2017, 19, 1479–1484. [Google Scholar] [CrossRef]
- Schmidt, S.; Christensen, M.B.; Serifovski, N.; Damm-Frydenberg, C.; Jensen, J.-E.B.; Fløyel, T.; Størling, J.; Ranjan, A.; Nørgaard, K. Low versus High Carbohydrate Diet in Type 1 Diabetes: A 12-week randomized open-label crossover study. Diabetes Obes. Metab. 2019. [Google Scholar] [CrossRef] [PubMed]
- Turton, J.L.; Raab, R.; Rooney, K.B. Low-carbohydrate diets for type 1 diabetes mellitus: A systematic review. PLoS ONE 2018, 13, e0194987. [Google Scholar] [CrossRef]
- Mayer-Davis, E.J.; Laffel, L.M.; Buse, J.B. Management of Type 1 Diabetes with a Very Low-Carbohydrate Diet: A Word of Caution. Pediatrics 2018, 142, e20181536B. [Google Scholar] [CrossRef]
- Baskaran, C.; Volkening, L.K.; Diaz, M.; Laffel, L.M. A decade of temporal trends in overweight/obesity in youth with type 1 diabetes after the Diabetes Control and Complications Trial. Pediatr. Diabetes 2015, 16, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Conway, B.; Miller, R.G.; Costacou, T.; Fried, L.; Kelsey, S.; Evans, R.W.; Orchard, T.J. Temporal patterns in overweight and obesity in Type 1 diabetes. Diabet. Med. 2010, 27, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Minges, K.E.; Whittemore, R.; Weinzimer, S.A.; Irwin, M.L.; Redeker, N.S.; Grey, M. Correlates of overweight and obesity in 5529 adolescents with type 1 diabetes: The T1D Exchange Clinic Registry. Diabetes Res. Clin. Pract. 2017, 126, 68–78. [Google Scholar] [CrossRef]
- Tielemans, S.M.A.J.; Soedamah-Muthu, S.S.; De Neve, M.; Toeller, M.; Chaturvedi, N.; Fuller, J.H.; Stamatakis, E. Association of physical activity with all-cause mortality and incident and prevalent cardiovascular disease among patients with type 1 diabetes: The EURODIAB Prospective Complications Study. Diabetologia 2013, 56, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Makura, C.B.; Nirantharakumar, K.; Girling, A.J.; Saravanan, P.; Narendran, P. Effects of physical activity on the development and progression of microvascular complications in type 1 diabetes: Retrospective analysis of the DCCT study. BMC Endocr. Disord. 2013, 13, 37. [Google Scholar] [CrossRef] [PubMed]
- Vestberg, D.; Rosengren, A.; Olsson, M.; Gudbjörnsdottir, S.; Svensson, A.-M.; Lind, M. Relationship between overweight and obesity with hospitalization for heart failure in 20,985 patients with type 1 diabetes: A population-based study from the Swedish National Diabetes Registry. Diabetes Care 2013, 36, 2857–2861. [Google Scholar] [CrossRef] [PubMed]
- Price, S.A.; Gorelik, A.; Fourlanos, S.; Colman, P.G.; Wentworth, J.M. Obesity is associated with retinopathy and macrovascular disease in type 1 diabetes. Obes. Res. Clin. Pract. 2014, 8, e178–e182. [Google Scholar] [CrossRef]
- Weigle, D.S.; Breen, P.A.; Matthys, C.C.; Callahan, H.S.; Meeuws, K.E.; Burden, V.R.; Purnell, J.Q. A high-protein diet induces sustained reductions in appetite, ad libitum caloric intake, and body weight despite compensatory changes in diurnal plasma leptin and ghrelin concentrations. Am. J. Clin. Nutr. 2005, 82, 41–48. [Google Scholar] [CrossRef]
- Atkins, R. Dr. Atkins’ New Diet Revolution; Harper Collins: New York, NY, USA, 2002. [Google Scholar]
- Eades, M.; Eades, M. Protein Power: The High-Protein/Low-Carbohydrate Way to Lose Weight, Feel Fit, and Boost Your Health–In Just Weeks! 1st ed.; Bantam: New York, NY, USA, 1997. [Google Scholar]
- Astrup, A.; Meinert Larsen, T.; Harper, A. Atkins and other low-carbohydrate diets: Hoax or an effective tool for weight loss? Lancet Lond. Engl. 2004, 364, 897–899. [Google Scholar] [CrossRef]
- Russell-Jones, D.; Khan, R. Insulin-associated weight gain in diabetes--causes, effects and coping strategies. Diabetes Obes. Metab. 2007, 9, 799–812. [Google Scholar] [CrossRef]
- Szadkowska, A.; Madej, A.; Ziółkowska, K.; Szymańska, M.; Jeziorny, K.; Mianowska, B.; Pietrzak, I. Gender and Age–Dependent effect of type 1 diabetes on obesity and altered body composition in young adults. Ann. Agric. Environ. Med. 2015, 22, 124–128. [Google Scholar] [CrossRef]
- Bryden, K.S.; Neil, A.; Mayou, R.A.; Peveler, R.C.; Fairburn, C.G.; Dunger, D.B. Eating habits, body weight, and insulin misuse. A longitudinal study of teenagers and young adults with type 1 diabetes. Diabetes Care 1999, 22, 1956–1960. [Google Scholar] [CrossRef]
- Strachan, M.W.J.; Ewing, F.M.E.; Frier, B.M.; Harper, A.; Deary, I.J. Food cravings during acute hypoglycaemia in adults with Type 1 diabetes. Physiol. Behav. 2004, 80, 675–682. [Google Scholar] [CrossRef]
- Savard, V.; Gingras, V.; Leroux, C.; Bertrand, A.; Desjardins, K.; Mircescu, H.; Rabasa-Lhoret, R. Treatment of Hypoglycemia in Adult Patients with Type 1 Diabetes: An Observational Study. Can. J. Diabetes 2016, 40, 318–323. [Google Scholar] [CrossRef]
- Russell, R.D.; Hu, D.; Greenaway, T.; Sharman, J.E.; Rattigan, S.; Richards, S.M.; Keske, M.A. Oral glucose challenge impairs skeletal muscle microvascular blood flow in healthy people. Am. J. Physiol. Endocrinol. Metab. 2018, 315, E307–E315. [Google Scholar] [CrossRef]
- Kilpatrick, E.S.; Rigby, A.S.; Atkin, S.L. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes: “double diabetes” in the Diabetes Control and Complications Trial. Diabetes Care 2007, 30, 707–712. [Google Scholar] [CrossRef]
- McGill, M.; Molyneaux, L.; Twigg, S.M.; Yue, D.K. The metabolic syndrome in type 1 diabetes: Does it exist and does it matter? J. Diabetes Complicat. 2008, 22, 18–23. [Google Scholar] [CrossRef]
- Chaturvedi, N.; Sjoelie, A.K.; Porta, M.; Aldington, S.J.; Fuller, J.H.; Songini, M.; Kohner, E.M.; EURODIAB Prospective Complications Study. Markers of insulin resistance are strong risk factors for retinopathy incidence in type 1 diabetes. Diabetes Care 2001, 24, 284–289. [Google Scholar] [CrossRef]
- Giorgino, F.; Laviola, L.; Cavallo Perin, P.; Solnica, B.; Fuller, J.; Chaturvedi, N. Factors associated with progression to macroalbuminuria in microalbuminuric Type 1 diabetic patients: The EURODIAB Prospective Complications Study. Diabetologia 2004, 47, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Orchard, T.J.; Chang, Y.-F.; Ferrell, R.E.; Petro, N.; Ellis, D.E. Nephropathy in type 1 diabetes: A manifestation of insulin resistance and multiple genetic susceptibilities? Further evidence from the Pittsburgh Epidemiology of Diabetes Complication Study. Kidney Int. 2002, 62, 963–970. [Google Scholar] [CrossRef]
- Orchard, T.J.; Olson, J.C.; Erbey, J.R.; Williams, K.; Forrest, K.Y.-Z.; Smithline Kinder, L.; Ellis, D.; Becker, D.J. Insulin resistance-related factors, but not glycemia, predict coronary artery disease in type 1 diabetes: 10-year follow-up data from the Pittsburgh Epidemiology of Diabetes Complications Study. Diabetes Care 2003, 26, 1374–1379. [Google Scholar] [CrossRef] [PubMed]
- Soedamah-Muthu, S.S.; Chaturvedi, N.; Toeller, M.; Ferriss, B.; Reboldi, P.; Michel, G.; Manes, C.; Fuller, J.H.; EURODIAB Prospective Complications Study Group. Risk factors for coronary heart disease in type 1 diabetic patients in Europe: The EURODIAB Prospective Complications Study. Diabetes Care 2004, 27, 530–537. [Google Scholar] [CrossRef]
- Tesfaye, S.; Chaturvedi, N.; Eaton, S.E.M.; Ward, J.D.; Manes, C.; Ionescu-Tirgoviste, C.; Witte, D.R.; Fuller, J.H.; EURODIAB Prospective Complications Study Group. Vascular risk factors and diabetic neuropathy. N. Engl. J. Med. 2005, 352, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Perseghin, G.; Lattuada, G.; Danna, M.; Sereni, L.P.; Maffi, P.; De Cobelli, F.; Battezzati, A.; Secchi, A.; Del Maschio, A.; Luzi, L. Insulin resistance, intramyocellular lipid content, and plasma adiponectin in patients with type 1 diabetes. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E1174–E1181. [Google Scholar] [CrossRef] [PubMed]
- Monaco, C.M.F.; Hughes, M.C.; Ramos, S.V.; Varah, N.E.; Lamberz, C.; Rahman, F.A.; McGlory, C.; Tarnopolsky, M.A.; Krause, M.P.; Laham, R.; et al. Altered mitochondrial bioenergetics and ultrastructure in the skeletal muscle of young adults with type 1 diabetes. Diabetologia 2018, 61, 1411–1423. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.C.; Howard, D.; Schauer, I.E.; Maahs, D.M.; Snell-Bergeon, J.K.; Eckel, R.H.; Perreault, L.; Rewers, M. Features of hepatic and skeletal muscle insulin resistance unique to type 1 diabetes. J. Clin. Endocrinol. Metab. 2012, 97, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.-Y.; Yehuda-Shnaidman, E.; Hong, T.; Han, J.; Pi, J.; Liu, Z.; Cao, W. Prolonged exposure to insulin suppresses mitochondrial production in primary hepatocytes. J. Biol. Chem. 2009, 284, 14087–14095. [Google Scholar] [CrossRef] [PubMed]
- Gamble, J.; Simpson, S.; Eurich, D.; Majumdar, S.; Johnson, J. Insulin Use and Increased Risk of Mortality in Type 2 Diabetes: A Cohort Study-PubMed-NCBI. Available online: https://www.ncbi.nlm.nih.gov/pubmed/19788429?dopt=Abstract (accessed on 11 December 2018).
- Nielsen, J.; Gando, C.; Joensson, E.; Paulsson, C. Low carbohydrate diet in type 1 diabetes, long-term improvement and adherence: A clinical audit. Diabetol. Metab. Syndr. 2012, 4, 23. [Google Scholar] [CrossRef] [PubMed]
- O’ Neill, D.F.; Westman, E.C.; Bernstein, R.K. The effects of a low-carbohydrate regimen on glycemic control and serum lipids in diabetes mellitus. Metab. Syndr. Relat. Disord. 2003, 1, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Shanik, M.H.; Xu, Y.; Škrha, J.; Dankner, R.; Zick, Y.; Roth, J. Insulin Resistance and Hyperinsulinemia: Is hyperinsulinemia the cart or the horse? Diabetes Care 2008, 31, S262–S268. [Google Scholar] [CrossRef]
- Schoenaker, D.A.J.M.; Simon, D.; Chaturvedi, N.; Fuller, J.H.; Soedamah-Muthu, S.S.; EURODIAB Prospective Complications Study Group. Glycemic control and all-cause mortality risk in type 1 diabetes patients: The EURODIAB prospective complications study. J. Clin. Endocrinol. Metab. 2014, 99, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Hirsch, I.B. Intensive Diabetes Treatment and Cardiovascular Outcomes in Type 1 Diabetes Mellitus: Implications of the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Study 30-Year Follow-up. Endocrinol. Metab. Clin. N. Am. 2018, 47, 65–79. [Google Scholar] [CrossRef]
- Danne, T.; Biester, T.; Kordonouri, O. Combined SGLT1 and SGLT2 Inhibitors and Their Role in Diabetes Care. Diabetes Technol. Ther. 2018, 20, S269–S277. [Google Scholar] [CrossRef]
- Davey, R.J.; Jones, T.W.; Fournier, P.A. Effect of short-term use of a continuous glucose monitoring system with a real-time glucose display and a low glucose alarm on incidence and duration of hypoglycemia in a home setting in type 1 diabetes mellitus. J. Diabetes Sci. Technol. 2010, 4, 1457–1464. [Google Scholar] [CrossRef]
- Puhr, S.; Derdzinski, M.; Welsh, J.B.; Parker, A.S.; Walker, T.; Price, D.A. Real-World Hypoglycemia Avoidance with a Continuous Glucose Monitoring System’s Predictive Low Glucose Alert. Diabetes Technol. Ther. 2019, 21, 155–158. [Google Scholar] [CrossRef]
- Stentz, F.B.; Umpierrez, G.E.; Cuervo, R.; Kitabchi, A.E. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises. Diabetes 2004, 53, 2079–2086. [Google Scholar] [CrossRef]
- Brehm, B.J.; Seeley, R.J.; Daniels, S.R.; D’Alessio, D.A. A randomized trial comparing a very low carbohydrate diet and a calorie-restricted low fat diet on body weight and cardiovascular risk factors in healthy women. J. Clin. Endocrinol. Metab. 2003, 88, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- Brinkworth, G.D.; Noakes, M.; Buckley, J.D.; Keogh, J.B.; Clifton, P.M. Long-term effects of a very-low-carbohydrate weight loss diet compared with an isocaloric low-fat diet after 12 mo. Am. J. Clin. Nutr. 2009, 90, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Dansinger, M.L.; Gleason, J.A.; Griffith, J.L.; Selker, H.P.; Schaefer, E.J. Comparison of the Atkins, Ornish, Weight Watchers, and Zone diets for weight loss and heart disease risk reduction: A randomized trial. JAMA 2005, 293, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Foster, G.D.; Wyatt, H.R.; Hill, J.O.; Makris, A.P.; Rosenbaum, D.L.; Brill, C.; Stein, R.I.; Mohammed, B.S.; Miller, B.; Rader, D.J.; et al. Weight and metabolic outcomes after 2 years on a low-carbohydrate versus low-fat diet: A randomized trial. Ann. Intern. Med. 2010, 153, 147–157. [Google Scholar] [CrossRef]
- Foster, G.D.; Wyatt, H.R.; Hill, J.O.; McGuckin, B.G.; Brill, C.; Mohammed, B.S.; Szapary, P.O.; Rader, D.J.; Edman, J.S.; Klein, S. A randomized trial of a low-carbohydrate diet for obesity. N. Engl. J. Med. 2003, 348, 2082–2090. [Google Scholar] [CrossRef] [PubMed]
- Frisch, S.; Zittermann, A.; Berthold, H.K.; Götting, C.; Kuhn, J.; Kleesiek, K.; Stehle, P.; Körtke, H. A randomized controlled trial on the efficacy of carbohydrate-reduced or fat-reduced diets in patients attending a telemedically guided weight loss program. Cardiovasc. Diabetol. 2009, 8, 36. [Google Scholar] [CrossRef]
- Gardner, C.D.; Kiazand, A.; Alhassan, S.; Kim, S.; Stafford, R.S.; Balise, R.R.; Kraemer, H.C.; King, A.C. Comparison of the Atkins, Zone, Ornish, and LEARN diets for change in weight and related risk factors among overweight premenopausal women: The A TO Z Weight Loss Study: A randomized trial. JAMA 2007, 297, 969–977. [Google Scholar] [CrossRef]
- Lim, S.S.; Noakes, M.; Keogh, J.B.; Clifton, P.M. Long-term effects of a low carbohydrate, low fat or high unsaturated fat diet compared to a no-intervention control. Nutr. Metab. Cardiovasc. Dis. 2010, 20, 599–607. [Google Scholar] [CrossRef]
- Shai, I.; Schwarzfuchs, D.; Henkin, Y.; Shahar, D.R.; Witkow, S.; Greenberg, I.; Golan, R.; Fraser, D.; Bolotin, A.; Vardi, H.; et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N. Engl. J. Med. 2008, 359, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Yancy, W.S.; Olsen, M.K.; Guyton, J.R.; Bakst, R.P.; Westman, E.C. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: A randomized, controlled trial. Ann. Intern. Med. 2004, 140, 769–777. [Google Scholar] [CrossRef]
- Nordmann, A.J.; Nordmann, A.; Briel, M.; Keller, U.; Yancy, W.S.; Brehm, B.J.; Bucher, H.C. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: A meta-analysis of randomized controlled trials. Arch. Intern. Med. 2006, 166, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Salas-Salvadó, J.; Guasch-Ferré, M.; Díaz-López, A.; Babio, N. Yogurt and Diabetes: Overview of Recent Observational Studies. J. Nutr. 2017, 147, S1452–S1461. [Google Scholar] [CrossRef]
- Moore, J.B.; Horti, A.; Fielding, B.A. Evaluation of the nutrient content of yogurts: A comprehensive survey of yogurt products in the major UK supermarkets. BMJ Open 2018, 8, e021387. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.B.; Fielding, B.A. Sugar and metabolic health: Is there still a debate? Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 303–309. [Google Scholar] [CrossRef]
- Rippe, J.M.; Angelopoulos, T.J. Fructose-containing sugars and cardiovascular disease. Adv. Nutr. 2015, 6, 430–439. [Google Scholar] [CrossRef]
- Stanhope, K.L.; Medici, V.; Bremer, A.A.; Lee, V.; Lam, H.D.; Nunez, M.V.; Chen, G.X.; Keim, N.L.; Havel, P.J. A dose-response study of consuming high-fructose corn syrup-sweetened beverages on lipid/lipoprotein risk factors for cardiovascular disease in young adults. Am. J. Clin. Nutr. 2015, 101, 1144–1154. [Google Scholar] [CrossRef]
- Calton, J.B. Prevalence of micronutrient deficiency in popular diet plans. J. Int. Soc. Sports Nutr. 2010, 7, 24. [Google Scholar] [CrossRef]
- Zinn, C.; Rush, A.; Johnson, R. Assessing the nutrient intake of a low-carbohydrate, high-fat (LCHF) diet: A hypothetical case study design. BMJ Open 2018, 8, e018846. [Google Scholar] [CrossRef]
- Jiao, H.; Xiao, E.; Graves, D.T. Diabetes and Its Effect on Bone and Fracture Healing. Curr. Osteoporos. Rep. 2015, 13, 327–335. [Google Scholar] [CrossRef]
- Kalaitzoglou, E.; Popescu, I.; Bunn, R.C.; Fowlkes, J.L.; Thrailkill, K.M. Effects of Type 1 Diabetes on Osteoblasts, Osteocytes, and Osteoclasts. Curr. Osteoporos. Rep. 2016, 14, 310–319. [Google Scholar] [CrossRef]
- Sundararaghavan, V.; Mazur, M.M.; Evans, B.; Liu, J.; Ebraheim, N.A. Diabetes and bone health: Latest evidence and clinical implications. Ther. Adv. Musculoskelet. Dis. 2017, 9, 67–74. [Google Scholar] [CrossRef]
- Bergqvist, A.G.C.; Schall, J.I.; Stallings, V.A.; Zemel, B.S. Progressive bone mineral content loss in children with intractable epilepsy treated with the ketogenic diet. Am. J. Clin. Nutr. 2008, 88, 1678–1684. [Google Scholar] [CrossRef]
- Bell, K.J.; Smart, C.E.; Steil, G.M.; Brand-Miller, J.C.; King, B.; Wolpert, H.A. Impact of fat, protein, and glycemic index on postprandial glucose control in type 1 diabetes: Implications for intensive diabetes management in the continuous glucose monitoring era. Diabetes Care 2015, 38, 1008–1015. [Google Scholar] [CrossRef]
- American Diabetes Association 5. Lifestyle Management: Standards of Medical Care in Diabetes—2019. Diabetes Care 2019, 42, S46–S60. [Google Scholar] [CrossRef]
- Adolfsson, P.; Riddell, M.C.; Taplin, C.E.; Davis, E.A.; Fournier, P.A.; Annan, F.; Scaramuzza, A.E.; Hasnani, D.; Hofer, S.E. ISPAD Clinical Practice Consensus Guidelines 2018: Exercise in children and adolescents with diabetes. Pediatr. Diabetes 2018, 19 (Suppl. 27), 205–226. [Google Scholar] [CrossRef] [PubMed]
- Butwicka, A.; Frisén, L.; Almqvist, C.; Zethelius, B.; Lichtenstein, P. Risks of psychiatric disorders and suicide attempts in children and adolescents with type 1 diabetes: A population-based cohort study. Diabetes Care 2015, 38, 453–459. [Google Scholar] [CrossRef]
- Cooper, M.N.; Lin, A.; Alvares, G.A.; de Klerk, N.H.; Jones, T.W.; Davis, E.A. Psychiatric disorders during early adulthood in those with childhood onset type 1 diabetes: Rates and clinical risk factors from population-based follow-up. Pediatr. Diabetes 2017, 18, 599–606. [Google Scholar] [CrossRef]
- Anderson, J.W.; Zeigler, J.A.; Deakins, D.A.; Floore, T.L.; Dillon, D.W.; Wood, C.L.; Oeltgen, P.R.; Whitley, R.J. Metabolic effects of high-carbohydrate, high-fiber diets for insulin-dependent diabetic individuals. Am. J. Clin. Nutr. 1991, 54, 936–943. [Google Scholar] [CrossRef] [PubMed]
- Riddell, M.C.; Gallen, I.W.; Smart, C.E.; Taplin, C.E.; Adolfsson, P.; Lumb, A.N.; Kowalski, A.; Rabasa-Lhoret, R.; McCrimmon, R.J.; Hume, C.; et al. Exercise management in type 1 diabetes: A consensus statement. Lancet Diabetes Endocrinol. 2017, 5, 377–390. [Google Scholar] [CrossRef]
- Burke, L.M.; Hawley, J.A. Swifter, higher, stronger: What’s on the menu? Science 2018, 362, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Learsi, S.K.; Ghiarone, T.; Silva-Cavalcante, M.D.; Andrade-Souza, V.A.; Ataide-Silva, T.; Bertuzzi, R.; de Araujo, G.G.; McConell, G.; Lima-Silva, A.E. Cycling time trial performance is improved by carbohydrate ingestion during exercise regardless of a fed or fasted state. Scand. J. Med. Sci. Sports 2019. [Google Scholar] [CrossRef] [PubMed]
- Correia-Oliveira, C.R.; Bertuzzi, R.; Dal’Molin Kiss, M.A.P.; Lima-Silva, A.E. Strategies of dietary carbohydrate manipulation and their effects on performance in cycling time trials. Sports Med. 2013, 43, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Cermak, N.M.; van Loon, L.J.C. The use of carbohydrates during exercise as an ergogenic aid. Sports Med. 2013, 43, 1139–1155. [Google Scholar] [CrossRef] [PubMed]
- Coggan, A.R.; Coyle, E.F. Carbohydrate ingestion during prolonged exercise: Effects on metabolism and performance. Exerc. Sport Sci. Rev. 1991, 19, 1–40. [Google Scholar] [CrossRef]
- Coyle, E.F.; Coggan, A.R. Effectiveness of carbohydrate feeding in delaying fatigue during prolonged exercise. Sports Med. 1984, 1, 446–458. [Google Scholar] [CrossRef]
- Hulston, C.J.; Venables, M.C.; Mann, C.H.; Martin, C.; Philp, A.; Baar, K.; Jeukendrup, A.E. Training with low muscle glycogen enhances fat metabolism in well-trained cyclists. Med. Sci. Sports Exerc. 2010, 42, 2046–2055. [Google Scholar] [CrossRef]
- Yeo, W.K.; Paton, C.D.; Garnham, A.P.; Burke, L.M.; Carey, A.L.; Hawley, J.A. Skeletal muscle adaptation and performance responses to once a day versus twice every second day endurance training regimens. J. Appl. Physiol. 2008, 105, 1462–1470. [Google Scholar] [CrossRef]
- Nybo, L.; Pedersen, K.; Christensen, B.; Aagaard, P.; Brandt, N.; Kiens, B. Impact of carbohydrate supplementation during endurance training on glycogen storage and performance. Acta Physiol. 2009, 197, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Gejl, K.D.; Thams, L.B.; Hansen, M.; Rokkedal-Lausch, T.; Plomgaard, P.; Nybo, L.; Larsen, F.J.; Cardinale, D.A.; Jensen, K.; Holmberg, H.-C.; et al. No Superior Adaptations to Carbohydrate Periodization in Elite Endurance Athletes. Med. Sci. Sports Exerc. 2017, 49, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- García-García, F.; Kumareswaran, K.; Hovorka, R.; Hernando, M.E. Quantifying the Acute Changes in Glucose with Exercise in Type 1 Diabetes: A Systematic Review and Meta-Analysis. Sports Med. 2015, 45, 587–599. [Google Scholar] [CrossRef]
- Al-Qaissi, A.; Papageorgiou, M.; Javed, Z.; Heise, T.; Rigby, A.S.; Garrett, A.T.; Hepburn, D.; Kilpatrick, E.S.; Atkin, S.L.; Sathyapalan, T. Environmental effects of ambient temperature and relative humidity on insulin pharmacodynamics in adults with type 1 diabetes mellitus. Diabetes Obes. Metab. 2019, 21, 569–574. [Google Scholar] [CrossRef]
- Davis, S.N.; Galassetti, P.; Wasserman, D.H.; Tate, D. Effects of antecedent hypoglycemia on subsequent counterregulatory responses to exercise. Diabetes 2000, 49, 73–81. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ertl, A.C.; Davis, S.N. Evidence for a vicious cycle of exercise and hypoglycemia in type 1 diabetes mellitus. Diabetes Metab. Res. Rev. 2004, 20, 124–130. [Google Scholar] [CrossRef]
- Riddell, M.C.; Zaharieva, D.P.; Tansey, M.; Tsalikian, E.; Admon, G.; Li, Z.; Kollman, C.; Beck, R.W. Individual glucose responses to prolonged moderate intensity aerobic exercise in adolescents with type 1 diabetes: The higher they start, the harder they fall. Pediatr. Diabetes 2018, 20, 99–106. [Google Scholar] [CrossRef]
- Mallad, A.; Hinshaw, L.; Dalla Man, C.; Cobelli, C.; Basu, R.; Lingineni, R.; Carter, R.E.; Kudva, Y.C.; Basu, A. Nocturnal Glucose Metabolism in Type 1 Diabetes: A Study Comparing Single Versus Dual Tracer Approaches. Diabetes Technol. Ther. 2015, 17, 587–595. [Google Scholar] [CrossRef]
- Trout, K.K.; Rickels, M.R.; Schutta, M.H.; Petrova, M.; Freeman, E.W.; Tkacs, N.C.; Teff, K.L. Menstrual cycle effects on insulin sensitivity in women with type 1 diabetes: A pilot study. Diabetes Technol. Ther. 2007, 9, 176–182. [Google Scholar] [CrossRef]
- Hilliard, M.E.; Yi-Frazier, J.P.; Hessler, D.; Butler, A.M.; Anderson, B.J.; Jaser, S. Stress and A1c Among People with Diabetes Across the Lifespan. Curr. Diabetes Rep. 2016, 16, 67. [Google Scholar] [CrossRef]
- Burke, L.M.; Hawley, J.A.; Jeukendrup, A.; Morton, J.P.; Stellingwerff, T.; Maughan, R.J. Toward a Common Understanding of Diet-Exercise Strategies to Manipulate Fuel Availability for Training and Competition Preparation in Endurance Sport. Int. J. Sport Nutr. Exerc. Metab. 2018, 28, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Burke, L.M.; Collier, G.R.; Hargreaves, M. Muscle glycogen storage after prolonged exercise: Effect of the glycemic index of carbohydrate feedings. J. Appl. Physiol. 1993, 75, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.A.; Burke, L.M.; Angus, D.J.; Fallon, K.E.; Martin, D.T.; Febbraio, M.A. Effect of altering substrate availability on metabolism and performance during intense exercise. Br. J. Nutr. 2000, 84, 829–838. [Google Scholar] [CrossRef]
- Riddell, M.C.; Milliken, J. Preventing exercise-induced hypoglycemia in type 1 diabetes using real-time continuous glucose monitoring and a new carbohydrate intake algorithm: An observational field study. Diabetes Technol. Ther. 2011, 13, 819–825. [Google Scholar] [CrossRef] [PubMed]
- West, D.J.; Stephens, J.W.; Bain, S.C.; Kilduff, L.P.; Luzio, S.; Still, R.; Bracken, R.M. A combined insulin reduction and carbohydrate feeding strategy 30 min before running best preserves blood glucose concentration after exercise through improved fuel oxidation in type 1 diabetes mellitus. J. Sports Sci. 2011, 29, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Adolfsson, P.; Mattsson, S.; Jendle, J. Evaluation of glucose control when a new strategy of increased carbohydrate supply is implemented during prolonged physical exercise in type 1 diabetes. Eur. J. Appl. Physiol. 2015, 115, 2599–2607. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.D.; Walker, M.; Bracken, R.M.; Turner, D.; Stevenson, E.J.; Gonzalez, J.T.; Shaw, J.A.; West, D.J. Insulin therapy and dietary adjustments to normalize glycemia and prevent nocturnal hypoglycemia after evening exercise in type 1 diabetes: A randomized controlled trial. BMJ Open Diabetes Res. Care 2015, 3, e000085. [Google Scholar] [CrossRef]
- Chontong, S.; Saetung, S.; Reutrakul, S. Higher sleep variability is associated with poorer glycaemic control in patients with type 1 diabetes. J. Sleep Res. 2016, 25, 438–444. [Google Scholar] [CrossRef]
- Reddy, R.; El Youssef, J.; Winters-Stone, K.; Branigan, D.; Leitschuh, J.; Castle, J.; Jacobs, P.G. The effect of exercise on sleep in adults with type 1 diabetes. Diabetes Obes. Metab. 2018, 20, 443–447. [Google Scholar] [CrossRef]
- Wilson, D.M.; Calhoun, P.M.; Maahs, D.M.; Chase, H.P.; Messer, L.; Buckingham, B.A.; Aye, T.; Clinton, P.K.; Hramiak, I.; Kollman, C.; et al. Factors associated with nocturnal hypoglycemia in at-risk adolescents and young adults with type 1 diabetes. Diabetes Technol. Ther. 2015, 17, 385–391. [Google Scholar] [CrossRef]
- Tamborlane, W.V. Triple jeopardy: Nocturnal hypoglycemia after exercise in the young with diabetes. J. Clin. Endocrinol. Metab. 2007, 92, 815–816. [Google Scholar] [CrossRef]
- Cox, G.R.; Clark, S.A.; Cox, A.J.; Halson, S.L.; Hargreaves, M.; Hawley, J.A.; Jeacocke, N.; Snow, R.J.; Yeo, W.K.; Burke, L.M. Daily training with high carbohydrate availability increases exogenous carbohydrate oxidation during endurance cycling. J. Appl. Physiol. 2010, 109, 126–134. [Google Scholar] [CrossRef]
- Coleman, S.K.; Rebalka, I.A.; D’Souza, D.M.; Hawke, T.J. Skeletal muscle as a therapeutic target for delaying type 1 diabetic complications. World J. Diabetes 2015, 6, 1323–1336. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.P.; Riddell, M.C.; Hawke, T.J. Effects of type 1 diabetes mellitus on skeletal muscle: Clinical observations and physiological mechanisms. Pediatr. Diabetes 2011, 12, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Monaco, C.M.F.; Gingrich, M.A.; Hawke, T.J. Considering Type 1 Diabetes as a Form of Accelerated Muscle Aging. Exerc. Sport Sci. Rev. 2019, 47, 98–107. [Google Scholar] [CrossRef]
- Hansen, A.K.; Fischer, C.P.; Plomgaard, P.; Andersen, J.L.; Saltin, B.; Pedersen, B.K. Skeletal muscle adaptation: Training twice every second day vs. training once daily. J. Appl. Physiol. 2005, 98, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Cochran, A.J.; Myslik, F.; MacInnis, M.J.; Percival, M.E.; Bishop, D.; Tarnopolsky, M.A.; Gibala, M.J. Manipulating Carbohydrate Availability Between Twice-Daily Sessions of High-Intensity Interval Training Over 2 Weeks Improves Time-Trial Performance. Int. J. Sport Nutr. Exerc. Metab. 2015, 25, 463–470. [Google Scholar] [CrossRef]
- Galassetti, P.; Tate, D.; Neill, R.A.; Richardson, A.; Leu, S.-Y.; Davis, S.N. Effect of differing antecedent hypoglycemia on counterregulatory responses to exercise in type 1 diabetes. Am. J. Physiol. Endocrinol. Metab. 2006, 290, E1109–E1117. [Google Scholar] [CrossRef]
- Wallis, G.A.; Gonzalez, J.T. Is exercise best served on an empty stomach? Proc. Nutr. Soc. 2018, 78, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Stocks, B.; Dent, J.R.; Ogden, H.B.; Zemp, M.; Philp, A. Post-exercise skeletal muscle signaling responses to moderate-to high-intensity steady-state exercise in the fed or fasted state. Am. J. Physiol. Endocrinol. Metab. 2018, 316, E230–E238. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Travers, R.L.; Walhin, J.-P.; Gonzalez, J.T.; Koumanov, F.; Betts, J.A.; Thompson, D. Feeding influences adipose tissue responses to exercise in overweight men. Am. J. Physiol. Endocrinol. Metab. 2017, 313, E84–E93. [Google Scholar] [CrossRef]
- Civitarese, A.E.; Hesselink, M.K.C.; Russell, A.P.; Ravussin, E.; Schrauwen, P. Glucose ingestion during exercise blunts exercise-induced gene expression of skeletal muscle fat oxidative genes. Am. J. Physiol. Endocrinol. Metab. 2005, 289, E1023–E1029. [Google Scholar] [CrossRef] [PubMed]
- Cluberton, L.J.; McGee, S.L.; Murphy, R.M.; Hargreaves, M. Effect of carbohydrate ingestion on exercise-induced alterations in metabolic gene expression. J. Appl. Physiol. 2005, 99, 1359–1363. [Google Scholar] [CrossRef] [PubMed]
- Akerstrom, T.C.A.; Birk, J.B.; Klein, D.K.; Erikstrup, C.; Plomgaard, P.; Pedersen, B.K.; Wojtaszewski, J. Oral glucose ingestion attenuates exercise-induced activation of 5′-AMP-activated protein kinase in human skeletal muscle. Biochem. Biophys. Res. Commun. 2006, 342, 949–955. [Google Scholar] [CrossRef]
- Erdmann, J.; Tholl, S.; Schusdziarra, V. Effect of carbohydrate- and protein-rich meals on exercise-induced activation of lipolysis in obese subjects. Horm. Metab. Res. 2010, 42, 290–294. [Google Scholar] [CrossRef]
- Horowitz, J.F.; Mora-Rodriguez, R.; Byerley, L.O.; Coyle, E.F. Lipolytic suppression following carbohydrate ingestion limits fat oxidation during exercise. Am. J. Physiol.-Endocrinol. Metab. 1997, 273, E768–E775. [Google Scholar] [CrossRef]
- Iwayama, K.; Kawabuchi, R.; Park, I.; Kurihara, R.; Kobayashi, M.; Hibi, M.; Oishi, S.; Yasunaga, K.; Ogata, H.; Nabekura, Y.; et al. Transient energy deficit induced by exercise increases 24-h fat oxidation in young trained men. J. Appl. Physiol. 2015, 118, 80–85. [Google Scholar] [CrossRef]
- Lee, B.M.; Wolever, T.M. Effect of glucose, sucrose and fructose on plasma glucose and insulin responses in normal humans: Comparison with white bread. Eur. J. Clin. Nutr. 1998, 52, 924–928. [Google Scholar] [CrossRef]
- Rosenkilde, M.; Nordby, P.; Nielsen, L.B.; Stallknecht, B.M.; Helge, J.W. Fat oxidation at rest predicts peak fat oxidation during exercise and metabolic phenotype in overweight men. Int. J. Obes. 2010, 34, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Venables, M.C.; Jeukendrup, A.E. Endurance training and obesity: Effect on substrate metabolism and insulin sensitivity. Med. Sci. Sports Exerc. 2008, 40, 495–502. [Google Scholar] [CrossRef]
- Spriet, L.L. New insights into the interaction of carbohydrate and fat metabolism during exercise. Sports Med. 2014, 44 (Suppl. 1), S87–S96. [Google Scholar] [CrossRef] [PubMed]
- Coyle, E.F.; Jeukendrup, A.E.; Wagenmakers, A.J.; Saris, W.H. Fatty acid oxidation is directly regulated by carbohydrate metabolism during exercise. Am. J. Physiol. 1997, 273, E268–E275. [Google Scholar] [CrossRef]
- Zinman, B.; Murray, F.T.; Vranic, M.; Albisser, A.M.; Leibel, B.S.; Mc Clean, P.A.; Marliss, E.B. Glucoregulation during moderate exercise in insulin treated diabetics. J. Clin. Endocrinol. Metab. 1977, 45, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Maughan, R.J.; Fallah, J.; Coyle, E.F. The effects of fasting on metabolism and performance. Br. J. Sports Med. 2010, 44, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Enevoldsen, L.H.; Simonsen, L.; Macdonald, I.A.; Bülow, J. The combined effects of exercise and food intake on adipose tissue and splanchnic metabolism. J. Physiol. 2004, 561, 871–882. [Google Scholar] [CrossRef]
- Krzentowski, G.; Pirnay, F.; Pallikarakis, N.; Luyckx, A.S.; Lacroix, M.; Mosora, F.; Lefèbvre, P.J. Glucose utilization during exercise in normal and diabetic subjects. The role of insulin. Diabetes 1981, 30, 983–989. [Google Scholar] [CrossRef]
- Berger, M.; Berchtold, P.; Cüppers, H.J.; Drost, H.; Kley, H.K.; Müller, W.A.; Wiegelmann, W.; Zimmerman-Telschow, H.; Gries, F.A.; Krüskemper, H.L.; et al. Metabolic and hormonal effects of muscular exercise in juvenile type diabetics. Diabetologia 1977, 13, 355–365. [Google Scholar] [CrossRef]
- Zaharieva, D.P.; McGaugh, S.; Pooni, R.; Vienneau, T.; Ly, T.; Riddell, M.C. Improved Open-Loop Glucose Control with Basal Insulin Reduction 90 Minutes Before Aerobic Exercise in Patients with Type 1 Diabetes on Continuous Subcutaneous Insulin Infusion. Diabetes Care 2019, 42, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Harmer, A.R.; Ruell, P.A.; Hunter, S.K.; McKenna, M.J.; Thom, J.M.; Chisholm, D.J.; Flack, J.R. Effects of type 1 diabetes, sprint training and sex on skeletal muscle sarcoplasmic reticulum Ca2+ uptake and Ca2+-ATPase activity. J. Physiol. 2014, 592, 523–535. [Google Scholar] [CrossRef]
- Riddell, M.C.; Pooni, R.; Yavelberg, L.; Li, Z.; Kollman, C.; Brown, R.E.; Li, A.; Aronson, R. Reproducibility in the cardiometabolic responses to high-intensity interval exercise in adults with type 1 diabetes. Diabetes Res. Clin. Pract. 2019, 148, 137–143. [Google Scholar] [CrossRef]
- Scott, S.N.; Cocks, M.; Andrews, R.C.; Narendran, P.; Purewal, T.S.; Cuthbertson, D.J.; Wagenmakers, A.J.M.; Shepherd, S.O. Fasted High-Intensity Interval and Moderate-Intensity Exercise Do Not Lead to Detrimental 24-Hour Blood Glucose Profiles. J. Clin. Endocrinol. Metab. 2019, 104, 111–117. [Google Scholar] [CrossRef]
- Maran, A.; Pavan, P.; Bonsembiante, B.; Brugin, E.; Ermolao, A.; Avogaro, A.; Zaccaria, M. Continuous glucose monitoring reveals delayed nocturnal hypoglycemia after intermittent high-intensity exercise in nontrained patients with type 1 diabetes. Diabetes Technol. Ther. 2010, 12, 763–768. [Google Scholar] [CrossRef]
- Guelfi, K.J.; Jones, T.W.; Fournier, P.A. Intermittent high-intensity exercise does not increase the risk of early postexercise hypoglycemia in individuals with type 1 diabetes. Diabetes Care 2005, 28, 416–418. [Google Scholar] [CrossRef]
- Yardley, J.E.; Kenny, G.P.; Perkins, B.A.; Riddell, M.C.; Balaa, N.; Malcolm, J.; Boulay, P.; Khandwala, F.; Sigal, R.J. Resistance versus aerobic exercise: Acute effects on glycemia in type 1 diabetes. Diabetes Care 2013, 36, 537–542. [Google Scholar] [CrossRef]
- Iscoe, K.E.; Riddell, M.C. Continuous moderate-intensity exercise with or without intermittent high-intensity work: Effects on acute and late glycaemia in athletes with Type 1 diabetes mellitus. Diabet. Med. 2011, 28, 824–832. [Google Scholar] [CrossRef]
- Campbell, M.D.; West, D.J.; Bain, S.C.; Kingsley, M.I.C.; Foley, P.; Kilduff, L.; Turner, D.; Gray, B.; Stephens, J.W.; Bracken, R.M. Simulated games activity vs continuous running exercise: A novel comparison of the glycemic and metabolic responses in T1DM patients. Scand. J. Med. Sci. Sports 2015, 25, 216–222. [Google Scholar] [CrossRef]
- Yardley, J.E.; Sigal, R.J.; Kenny, G.P.; Riddell, M.C.; Lovblom, L.E.; Perkins, B.A. Point accuracy of interstitial continuous glucose monitoring during exercise in type 1 diabetes. Diabetes Technol. Ther. 2013, 15, 46–49. [Google Scholar] [CrossRef]
- Moser, O.; Tschakert, G.; Mueller, A.; Groeschl, W.; Pieber, T.R.; Obermayer-Pietsch, B.; Koehler, G.; Hofmann, P. Effects of High-Intensity Interval Exercise versus Moderate Continuous Exercise on Glucose Homeostasis and Hormone Response in Patients with Type 1 Diabetes Mellitus Using Novel Ultra-Long-Acting Insulin. PLoS ONE 2015, 10, e0136489. [Google Scholar] [CrossRef]
- Zaharieva, D.; Yavelberg, L.; Jamnik, V.; Cinar, A.; Turksoy, K.; Riddell, M.C. The Effects of Basal Insulin Suspension at the Start of Exercise on Blood Glucose Levels During Continuous Versus Circuit-Based Exercise in Individuals with Type 1 Diabetes on Continuous Subcutaneous Insulin Infusion. Diabetes Technol. Ther. 2017, 19, 370–378. [Google Scholar] [CrossRef]
- Aronson, R.; Brown, R.E.; Li, A.; Riddell, M.C. Optimal Insulin Correction Factor in Post-High-Intensity Exercise Hyperglycemia in Adults with Type 1 Diabetes: The FIT Study. Diabetes Care 2018, 42, 10–16. [Google Scholar] [CrossRef]
- Schmidt, M.I.; Hadji-Georgopoulos, A.; Rendell, M.; Margolis, S.; Kowarski, A. The dawn phenomenon, an early morning glucose rise: Implications for diabetic intraday blood glucose variation. Diabetes Care 1981, 4, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.J.; Bolli, G.B.; Cryer, P.E.; Gerich, J.E. Sequence of events during development of the dawn phenomenon in insulin-dependent diabetes mellitus. Metabolism 1985, 34, 1100–1104. [Google Scholar] [CrossRef]
- Edge, J.A.; Matthews, D.R.; Dunger, D.B. The dawn phenomenon is related to overnight growth hormone release in adolescent diabetics. Clin. Endocrinol. 1990, 33, 729–737. [Google Scholar] [CrossRef]
- Davidson, M.B.; Harris, M.D.; Ziel, F.H.; Rosenberg, C.S. Suppression of sleep-induced growth hormone secretion by anticholinergic agent abolishes dawn phenomenon. Diabetes 1988, 37, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Lane, S.C.; Camera, D.M.; Lassiter, D.G.; Areta, J.L.; Bird, S.R.; Yeo, W.K.; Jeacocke, N.A.; Krook, A.; Zierath, J.R.; Burke, L.M.; et al. Effects of sleeping with reduced carbohydrate availability on acute training responses. J. Appl. Physiol. 2015, 119, 643–655. [Google Scholar] [CrossRef]
- Bartlett, J.D.; Louhelainen, J.; Iqbal, Z.; Cochran, A.J.; Gibala, M.J.; Gregson, W.; Close, G.L.; Drust, B.; Morton, J.P. Reduced carbohydrate availability enhances exercise-induced p53 signaling in human skeletal muscle: Implications for mitochondrial biogenesis. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R450–R458. [Google Scholar] [CrossRef]
- Marquet, L.-A.; Brisswalter, J.; Louis, J.; Tiollier, E.; Burke, L.M.; Hawley, J.A.; Hausswirth, C. Enhanced Endurance Performance by Periodization of Carbohydrate Intake: “Sleep Low” Strategy. Med. Sci. Sports Exerc. 2016, 48, 663–672. [Google Scholar] [CrossRef]
- Marquet, L.-A.; Hausswirth, C.; Molle, O.; Hawley, J.A.; Burke, L.M.; Tiollier, E.; Brisswalter, J. Periodization of Carbohydrate Intake: Short-Term Effect on Performance. Nutrients 2016, 8, 755. [Google Scholar] [CrossRef]
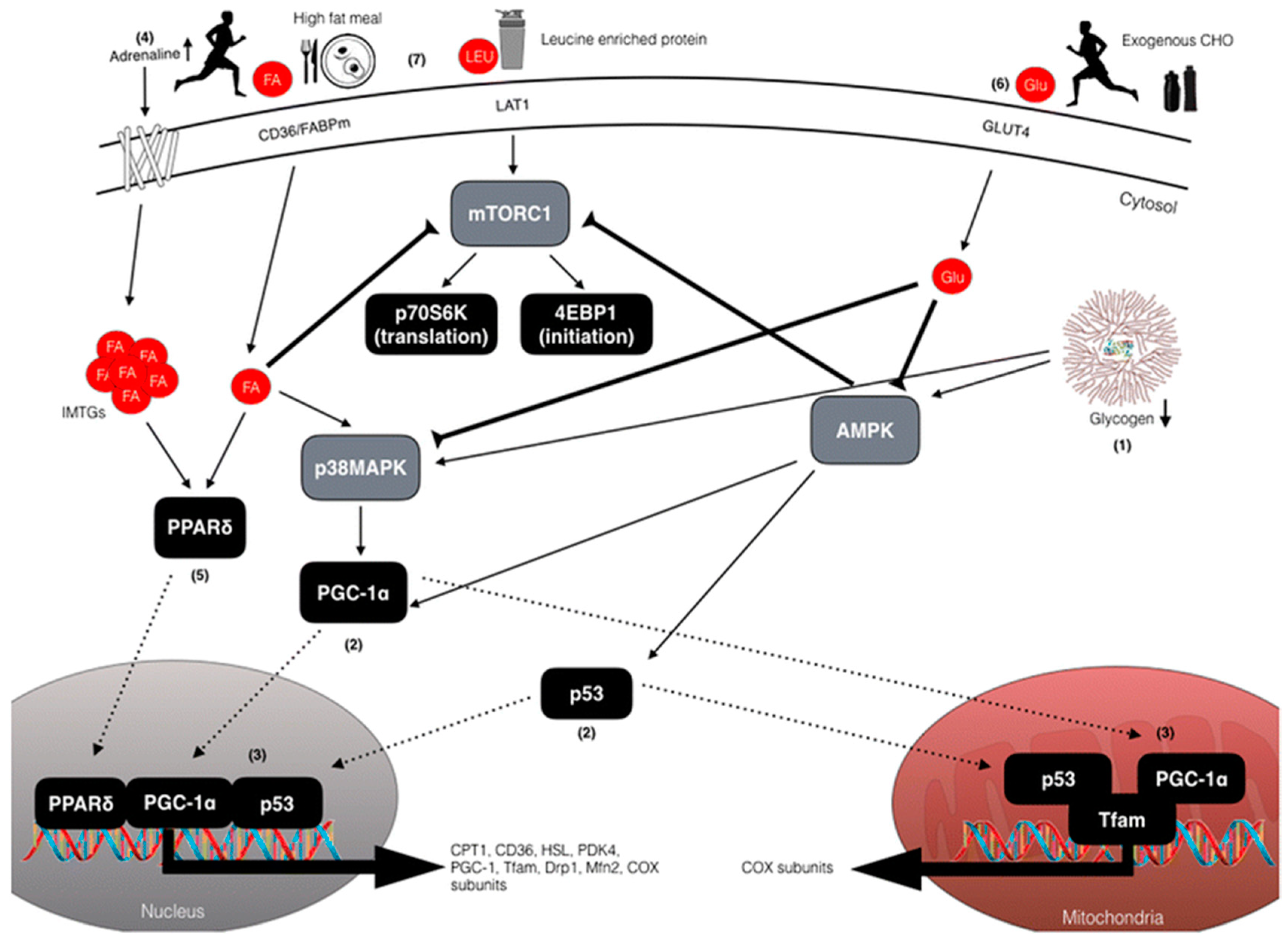
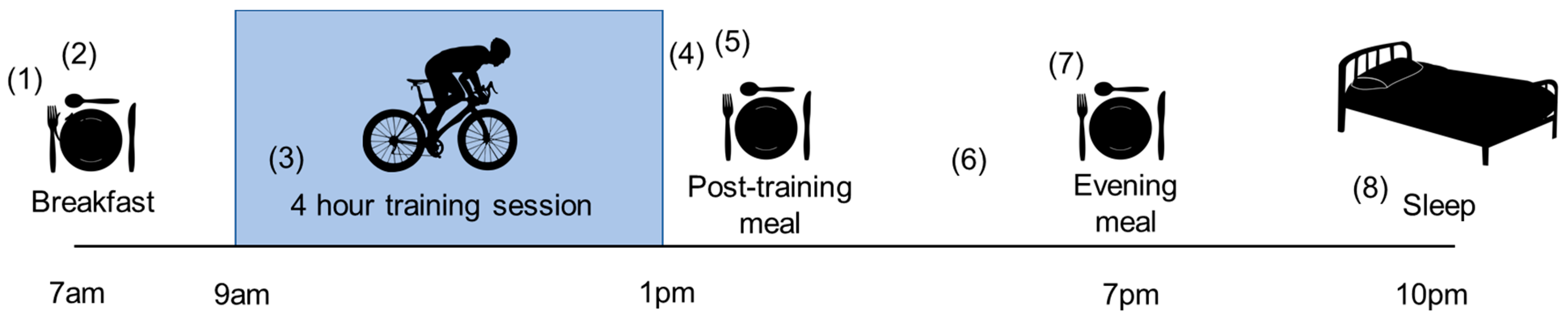
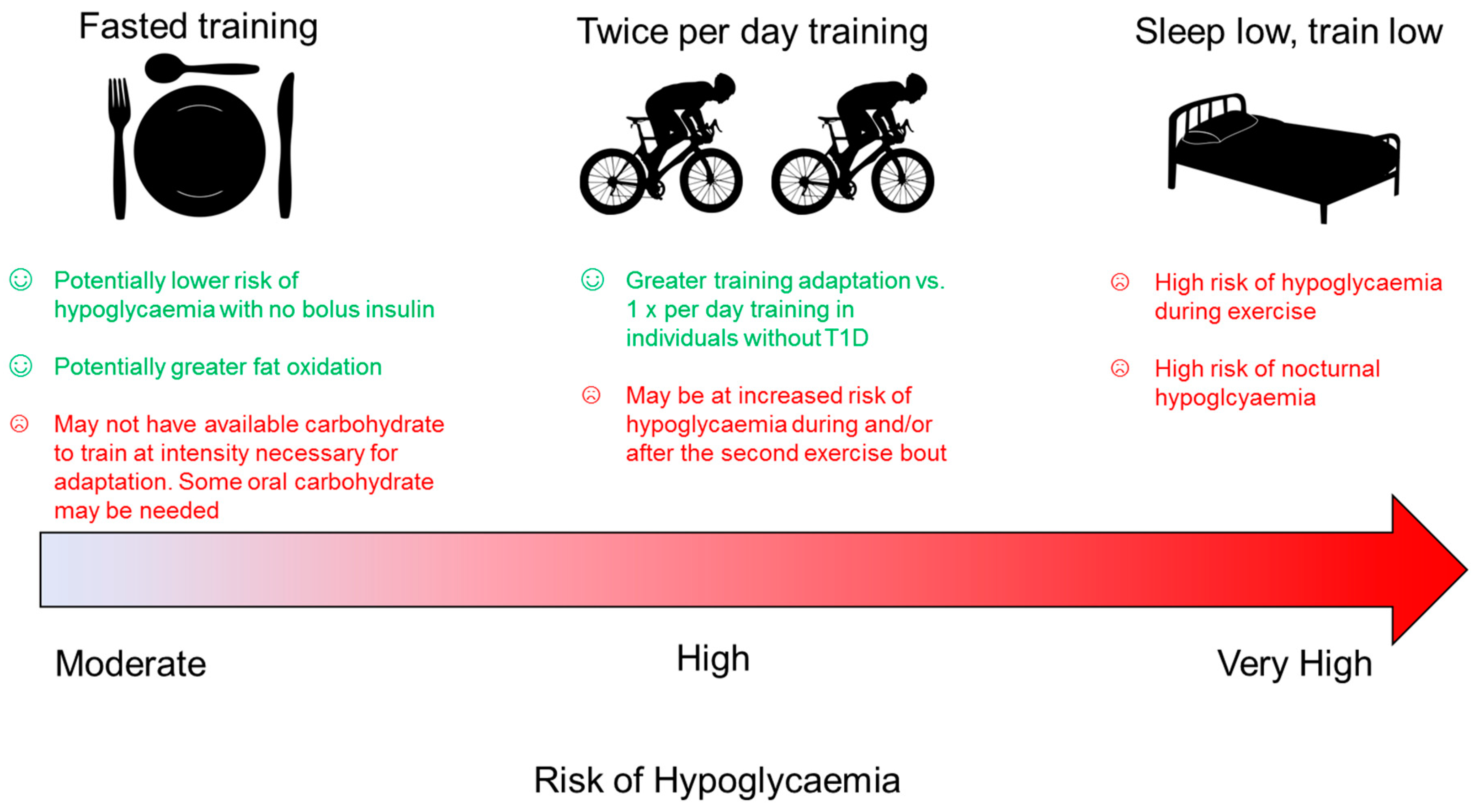
| Diet | Recommendation |
|---|---|
| Very low carbohydrate diet | 20–50 g per day or <10% caloric intake or <1 g/kg bodyweight/day |
| Low carbohydrate diet | <130 g per day or <26% total energy intake or <3 g/kg bodyweight/day |
| Moderate carbohydrate diet | 26–45% of total energy intake or 3–6 g/kg bodyweight/day |
| High carbohydrate diet | >45% of total energy intake or 7–8 g/kg bodyweight/day |
| ADA guidelines [42] | 45–60% total energy intake from carbohydrate |
| Level of Activity | Carbohydrate Targets |
|---|---|
| Light (low intensity or skill-based activities) | 3–5 g/kg bodyweight/day |
| Moderate (approximately 1 h per day) | 5—7 g/kg bodyweight/day |
| High (e.g., 1–3 h moderate to high-intensity exercise) | 6–10 g/kg bodyweight/day |
| Very high (e.g., >4/5 h of moderate to high-intensity exercise) | 8–12 g/kg bodyweight/day |
| Extreme (e.g., elite cycle competition) | >12 g/kg bodyweight/day |
| Potential Pros of Low Carbohydrate Diets | Potential Cons of Low Carbohydrate Diets |
|---|---|
| Reduce HbA1c | Risk of nutrient deficiencies |
| Reduced glycaemic variation Strategy for weight loss | Potential risk of diabetic ketoacidosis |
| Decreased total daily insulin dose | Reduced treatment effect of glucagon during hypoglycaemia |
| Increased saturated fat intake to maintain calorie intake | |
| Risk of pre-occupation with food and eating disorders | |
| Difficulty with sustaining low carbohydrate diets | |
| Possible maturational deficits in children |
| Factor | Comments | Implications for the athlete with T1D |
|---|---|---|
| Exercise modality and protocol | Exercise modality, duration and intensity can all affect muscle glucose uptake and both liver and muscle glycogenolysis. | Carbohydrate requirements will be greater with greater training loads. The type of exercise influences the change in glycaemia [124,135]. |
| Environmental conditions | Training/competing at high temperatures and/or at altitude increases the risk of hypoglycaemia [136]. | Extra consideration is needed, especially if they are accustomed to lower temperatures. |
| Antecedent hypoglycaemia and/or moderate intensity exercise | Counterregulatory responses may be impaired during subsequent exercise bouts and increase the risk of hypoglycaemia [137,138]. | Following recent hypoglycaemia, carbohydrate requirements during subsequent training sessions may be greater than usual. |
| Pre-exercise blood glucose levels | There is evidence that blood glucose drops more when starting exercise with higher blood glucose concentration [139]. | If blood glucose is elevated, carbohydrate feeding may need to be delayed until blood glucose has lowered. However, when pre-exercise blood glucose is low, high glycaemic index carbohydrate may need to be consumed. |
| Time of day | Exercising late in the afternoon may increase the risk of nocturnal hypoglycaemia [140]. Early morning exercise may reduce risk of hypoglycaemia due to the ‘dawn effect’. | The athlete may require more vigilance after an afternoon exercise session to reduce the risk of nocturnal hypoglycaemia. |
| Hormonal factors | Menstrual cycle phase in women [141] and possibly competition stress [142] (i.e., insulin resistance during early luteal phase and a rise in glucose level during competition stress associated with cortisol and/or catecholamines) | Adrenaline release before competition may cause blood glucose levels to rapidly rise. Blood glucose responses during training may be very different during high stress competition settings. |
| How do athletes with T1D periodise their training and diet over a training season, and is this the optimal strategy? |
| What are the effects of long-term fasted exercise training in athletes with T1D? Are there benefits and/or disadvantages? |
| Does a high frequency of heavy training sessions lead to an accumulative increase in the risk of hypoglycaemia? |
| Are T1D athletes refuelling adequately during training and competition for subsequent exercise? |
| What are the barriers to exercise for high level athletes with T1D? |
| What is the best strategy to periodise training and diet over a training season in athletes with T1D? |
| Are athletes with T1D in a chronic state of low energy availability? |
| What are the acute and chronic effects of hyperglycaemia during exercise in athletes with T1D? |
| Will future use of artificial pancreas technology during endurance exercise lead to better glucose homeostasis in athletes with T1D? |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scott, S.N.; Anderson, L.; Morton, J.P.; Wagenmakers, A.J.M.; Riddell, M.C. Carbohydrate Restriction in Type 1 Diabetes: A Realistic Therapy for Improved Glycaemic Control and Athletic Performance? Nutrients 2019, 11, 1022. https://doi.org/10.3390/nu11051022
Scott SN, Anderson L, Morton JP, Wagenmakers AJM, Riddell MC. Carbohydrate Restriction in Type 1 Diabetes: A Realistic Therapy for Improved Glycaemic Control and Athletic Performance? Nutrients. 2019; 11(5):1022. https://doi.org/10.3390/nu11051022
Chicago/Turabian StyleScott, Sam N., Lorraine Anderson, James P. Morton, Anton J. M. Wagenmakers, and Michael C. Riddell. 2019. "Carbohydrate Restriction in Type 1 Diabetes: A Realistic Therapy for Improved Glycaemic Control and Athletic Performance?" Nutrients 11, no. 5: 1022. https://doi.org/10.3390/nu11051022
APA StyleScott, S. N., Anderson, L., Morton, J. P., Wagenmakers, A. J. M., & Riddell, M. C. (2019). Carbohydrate Restriction in Type 1 Diabetes: A Realistic Therapy for Improved Glycaemic Control and Athletic Performance? Nutrients, 11(5), 1022. https://doi.org/10.3390/nu11051022





