Fermented Cordyceps militaris Extract Prevents Hepatosteatosis and Adipocyte Hypertrophy in High Fat Diet-Fed Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of ONE and Chow
2.2. Gass Chromatography-Time of Flight Mass Spectrometry (GC-TOF MS) Analysis
2.3. Animal Experiments
2.4. RNA Preparation and Real Time Quantitative PCR
2.5. Protein Assay and Western Blot Analysis
2.6. Plasma and Liver TG Level
2.7. Sphingolipids Analysis
2.8. Tissue Histology
2.9. Statistical Analyses
3. Results
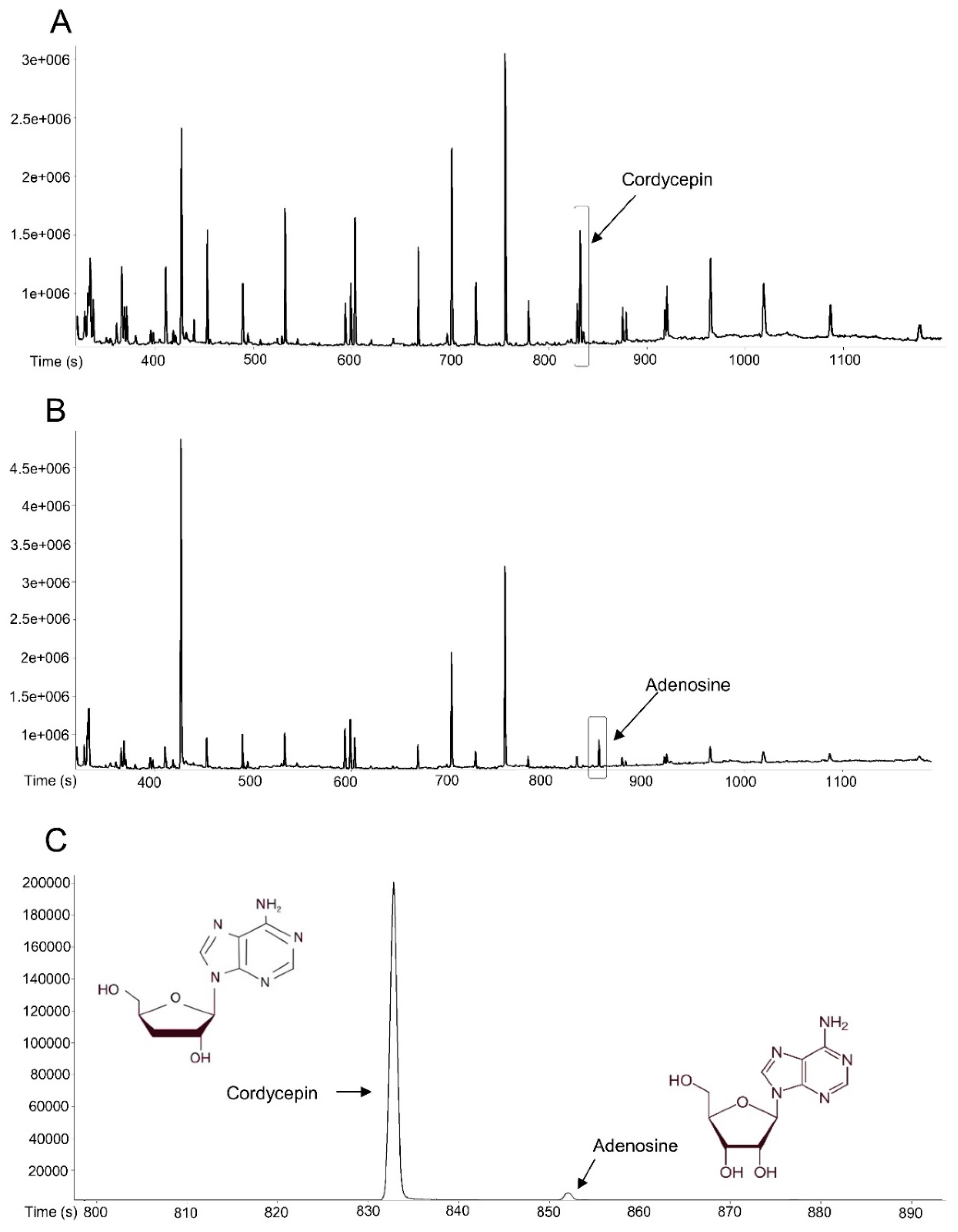
3.1. ONE Contains Adenosine and Cordycepin
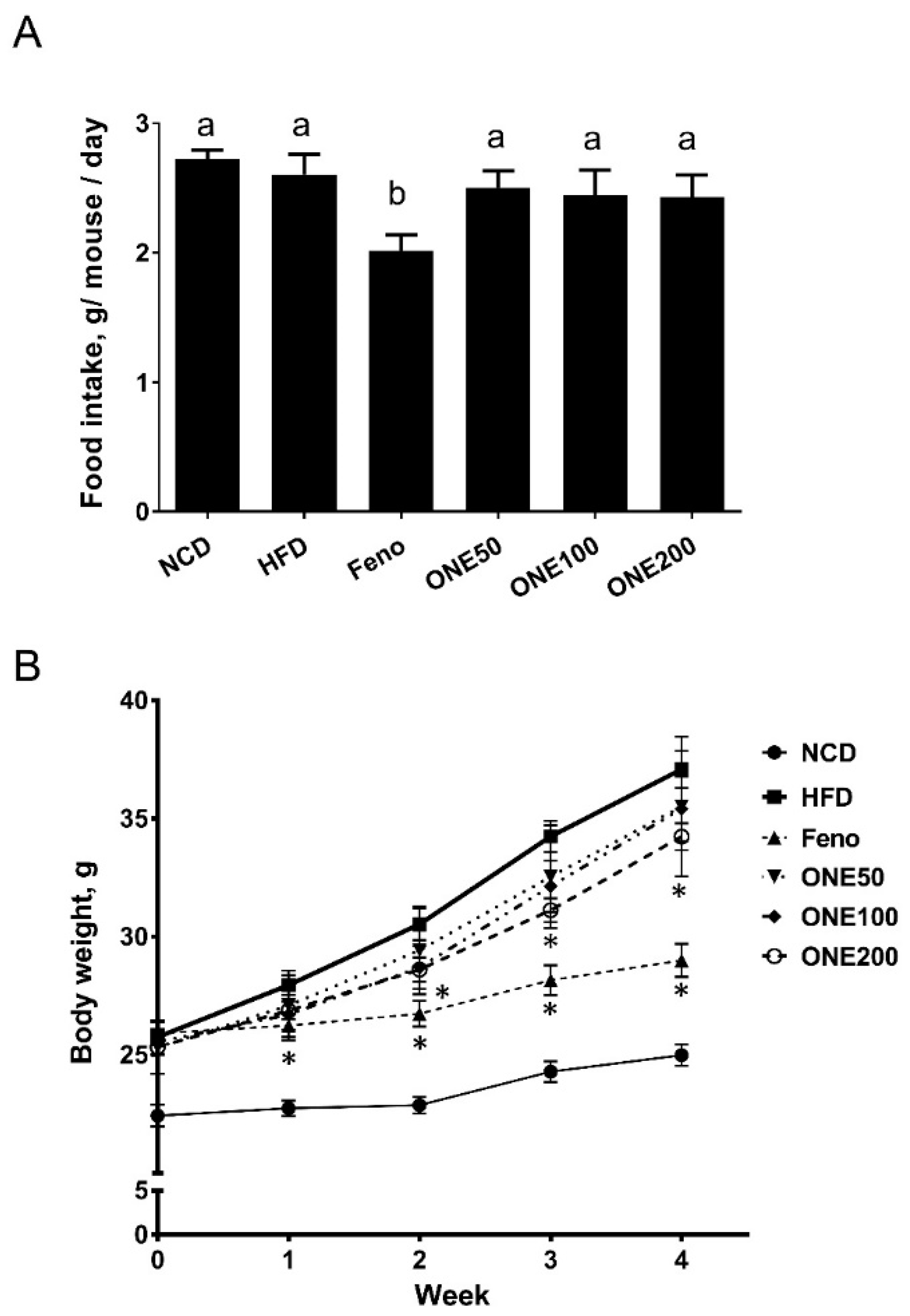
3.2. ONE Diet Decreases Body Weight Increase of Mice Fed HFD with no Change In Their Food Intake
3.3. ONE Prevents Systemic Tissue Damage and Improves Liver Function
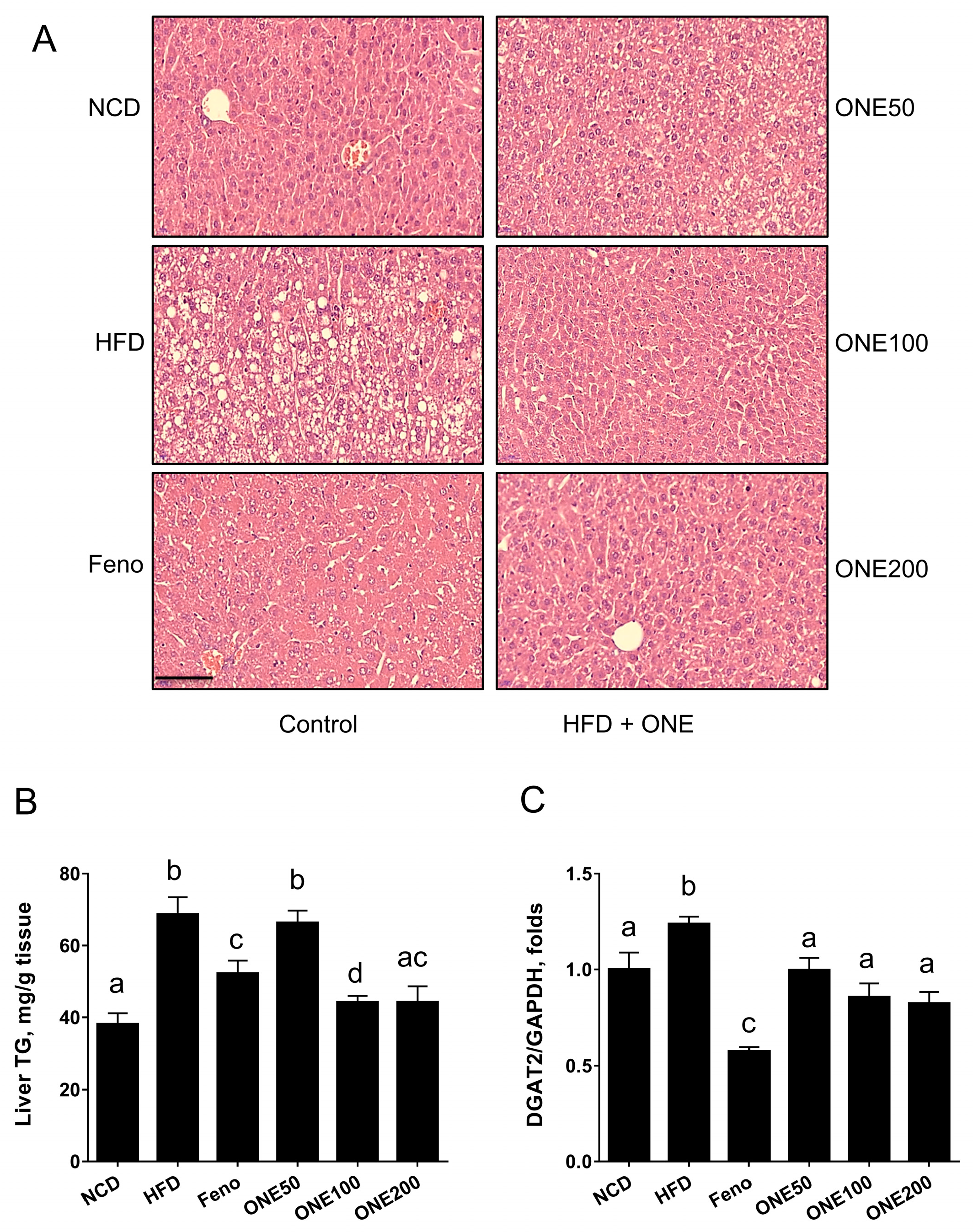
3.4. ONE Reduces Lipid Droplets in Livers of Mice Fed HFD
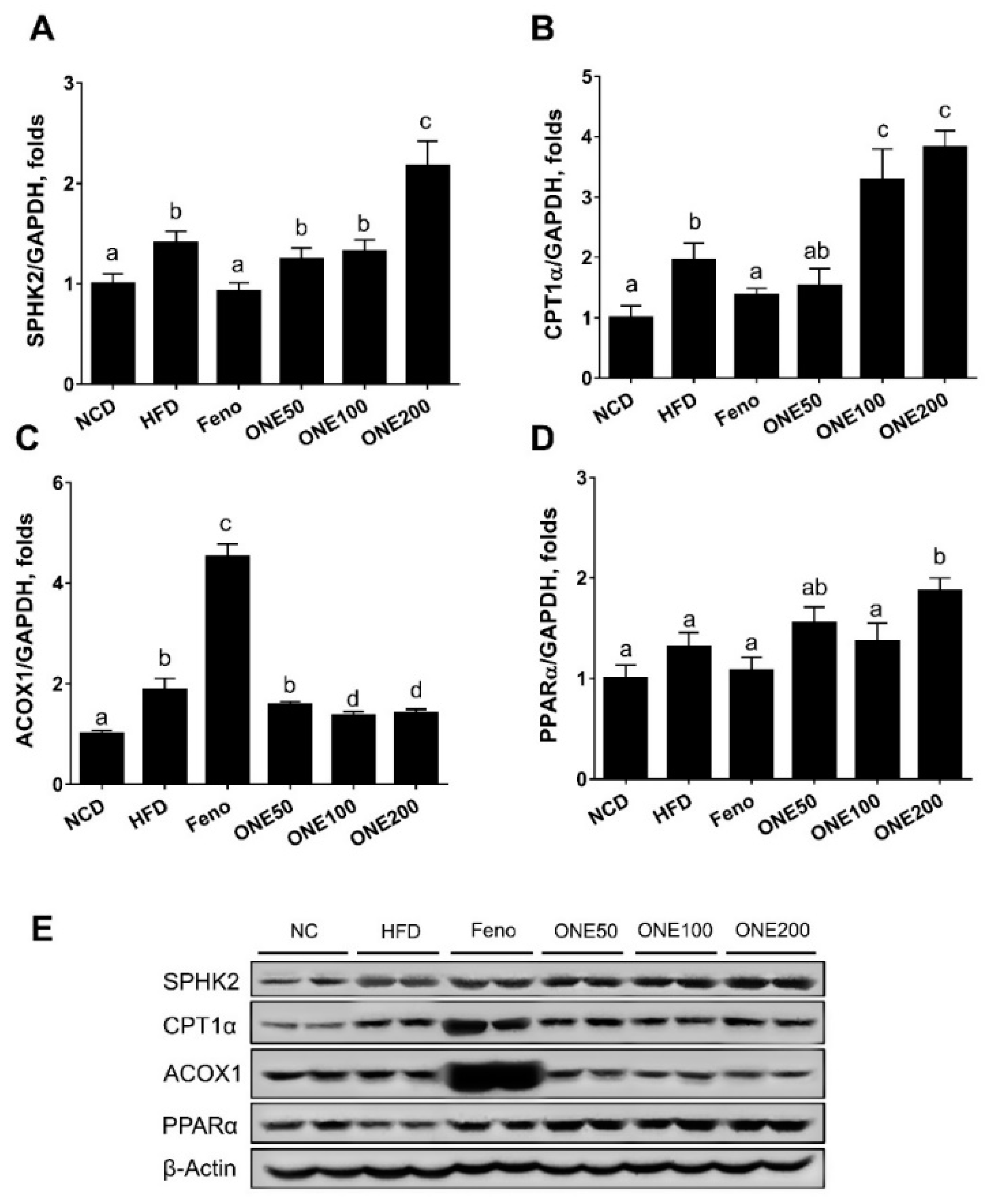
3.5. ONE Upregulates FA Oxidation Genes and SPHK2 in Liver
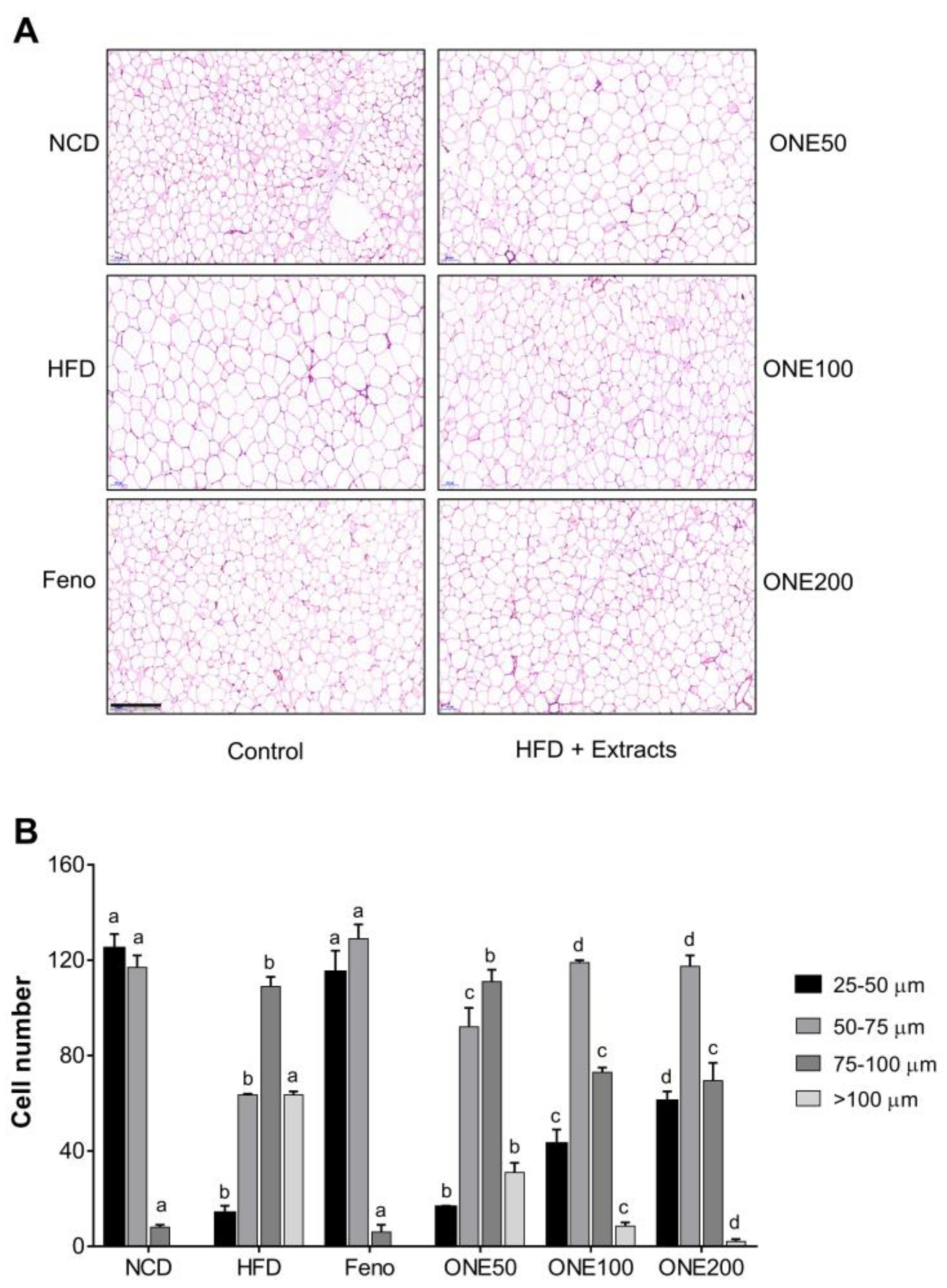
3.6. ONE Reduces Cell Sizes of Adipocytes in Visceral White Adipose Tissues
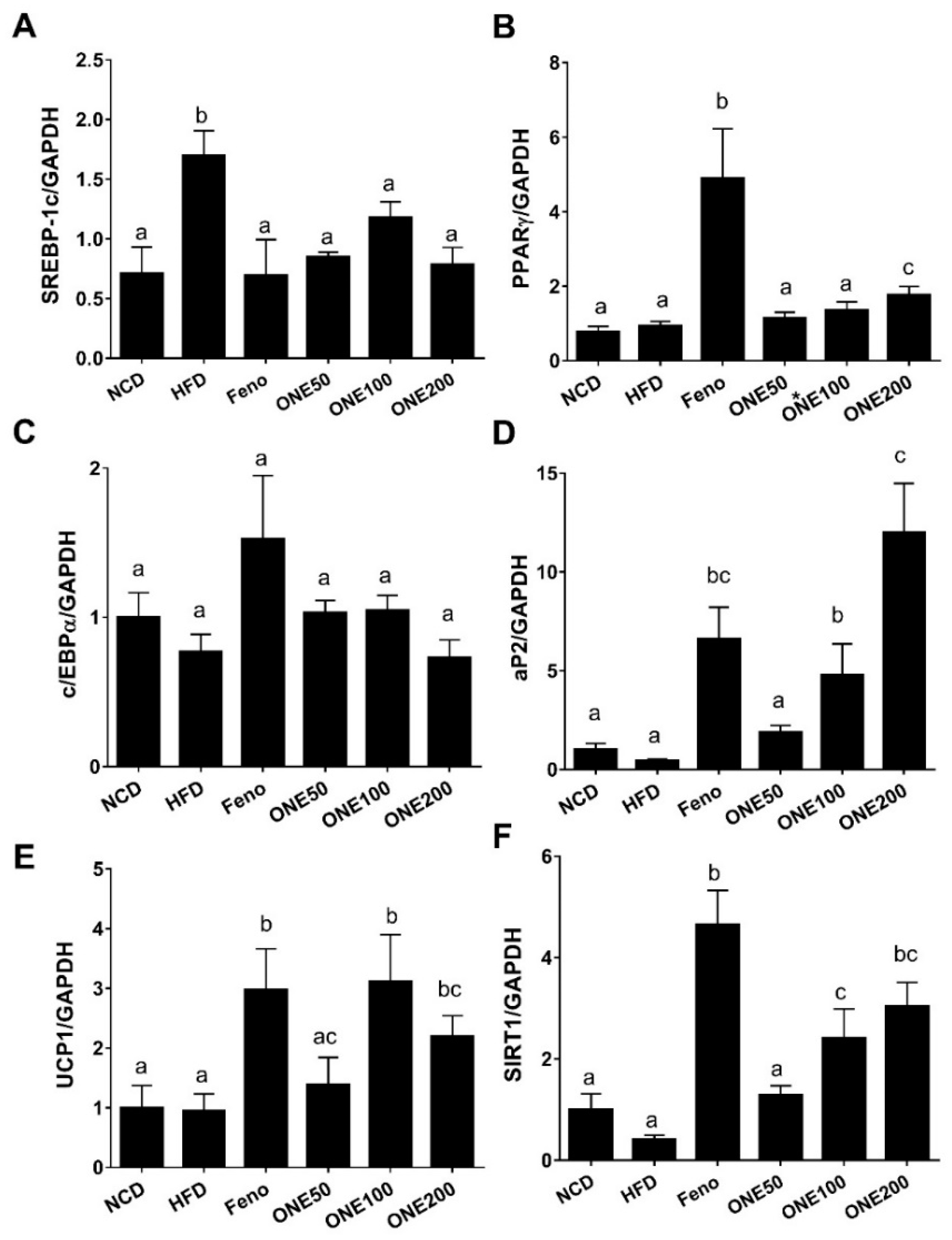
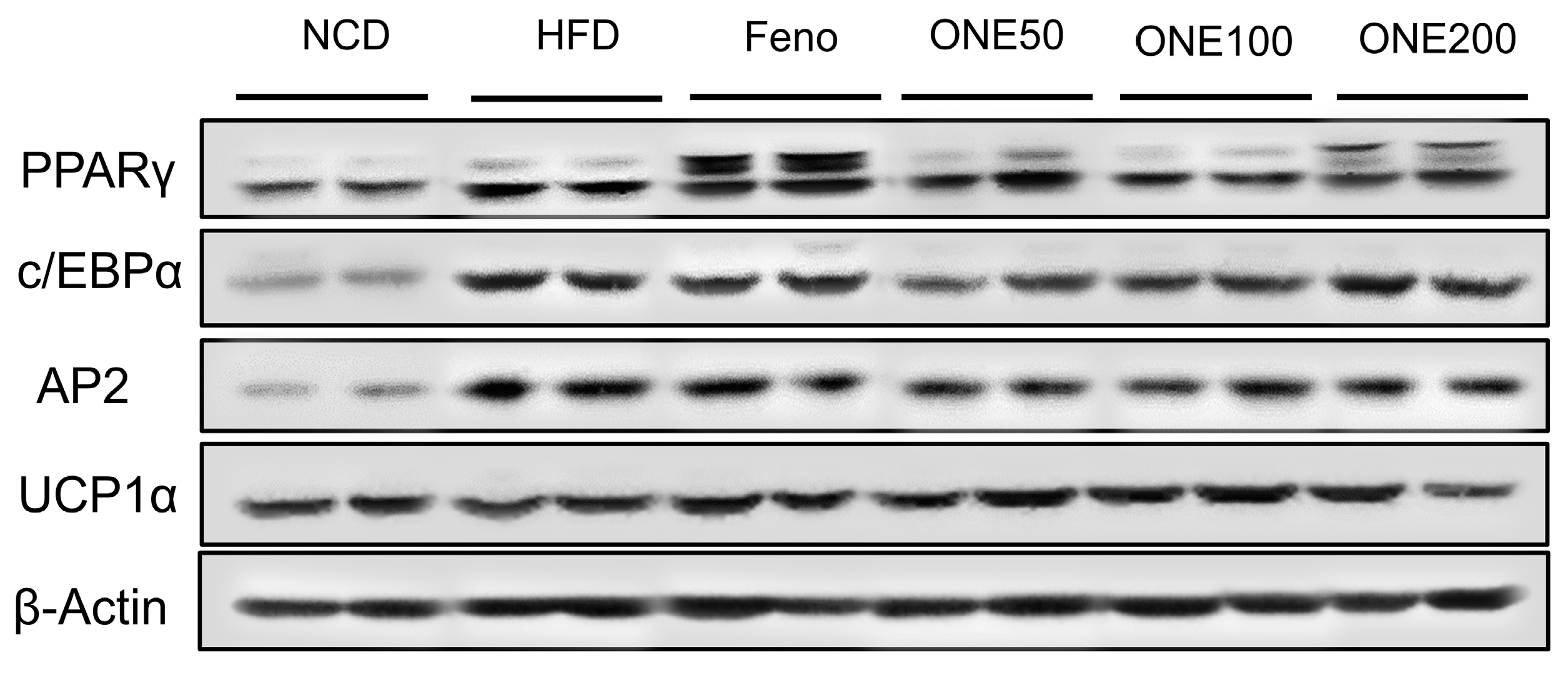
3.7. ONE Suppresses Expression of Genes Involved in Lipogenesis and Adipocyte Differentiation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sanyal, A.J.; American Gastroenterological Association. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology 2002, 123, 1705–1725. [Google Scholar] [CrossRef]
- Mollah, M.L.; Park, D.K.; Park, H.J. Cordyceps militaris Grown on Germinated Soybean Induces G2/M Cell Cycle Arrest through Downregulation of Cyclin B1 and Cdc25c in Human Colon Cancer HT-29 Cells. Evid.-Based Complement. Altern. Med. 2012, 2012, 249217. [Google Scholar] [CrossRef] [PubMed]
- Koo, S.H.; Flechner, L.; Qi, L.; Zhang, X.M.; Screaton, R.A.; Jeffries, S.; Hedrick, S.; Xu, W.; Boussouar, F.; Brindle, P.; et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 2005, 437, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Postic, C.; Girard, J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: Lessons from genetically engineered mice. J. Clin. Investig. 2008, 118, 829–838. [Google Scholar] [CrossRef]
- Lee, S.Y.; Hong, I.K.; Kim, B.R.; Shim, S.M.; Sung Lee, J.; Lee, H.Y.; Soo Choi, C.; Kim, B.K.; Park, T.S. Activation of sphingosine kinase 2 by endoplasmic reticulum stress ameliorates hepatic steatosis and insulin resistance in mice. Hepatology 2015, 62, 135–146. [Google Scholar] [CrossRef]
- Safaei, A.; Arefi Oskouie, A.; Mohebbi, S.R.; Rezaei-Tavirani, M.; Mahboubi, M.; Peyvandi, M.; Okhovatian, F.; Zamanian-Azodi, M. Metabolomic analysis of human cirrhosis, hepatocellular carcinoma, non-alcoholic fatty liver disease and non-alcoholic steatohepatitis diseases. Gastroenterol. Hepatol. Bed Bench 2016, 9, 158–173. [Google Scholar] [PubMed]
- Cannon, B.; Nedergaard, J. Brown adipose tissue: Function and physiological significance. Physiol. Rev. 2004, 84, 277–359. [Google Scholar] [CrossRef]
- Eaton, S.; Bartlett, K.; Pourfarzam, M. Mammalian mitochondrial beta-oxidation. Biochem. J. 1996, 320, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Reddy, J.K.; Hashimoto, T. Peroxisomal beta-oxidation and peroxisome proliferator-activated receptor alpha: An adaptive metabolic system. Annu. Rev. Nutr. 2001, 21, 193–230. [Google Scholar] [CrossRef]
- Koo, S.H. Nonalcoholic fatty liver disease: Molecular mechanisms for the hepatic steatosis. Clin. Mol. Hepatol. 2013, 19, 210–215. [Google Scholar] [CrossRef]
- Kozak, L.P.; Anunciado-Koza, R. UCP1: Its involvement and utility in obesity. Int. J. Obes. 2008, 32 (Suppl. 7), S32. [Google Scholar] [CrossRef]
- Kajimura, S. Promoting brown and beige adipocyte biogenesis through the PRDM16 pathway. Int. J. Obes. Suppl. 2015, 5, S11. [Google Scholar] [CrossRef]
- Lee, S.G.; Parks, J.S.; Kang, H.W. Quercetin, a functional compound of onion peel, remodels white adipocytes to brown-like adipocytes. J. Nutr. Biochem. 2017, 42, 62–71. [Google Scholar] [CrossRef]
- Qiang, L.; Wang, L.; Kon, N.; Zhao, W.; Lee, S.; Zhang, Y.; Rosenbaum, M.; Zhao, Y.; Gu, W.; Farmer, S.R.; et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Ppargamma. Cell 2012, 150, 620–632. [Google Scholar] [CrossRef]
- Wang, H.; Liu, L.; Lin, J.Z.; Aprahamian, T.R.; Farmer, S.R. Browning of White Adipose Tissue with Roscovitine Induces a Distinct Population of UCP1(+) Adipocytes. Cell Metab. 2016, 24, 835–847. [Google Scholar] [CrossRef]
- Das, S.K.; Masuda, M.; Sakurai, A.; Sakakibara, M. Medicinal uses of the mushroom Cordyceps militaris: Current state and prospects. Fitoterapia 2010, 81, 961–968. [Google Scholar] [CrossRef]
- Chang, Y.; Jeng, K.C.; Huang, K.F.; Lee, Y.C.; Hou, C.W.; Chen, K.H.; Cheng, F.Y.; Liao, J.W.; Chen, Y.S. Effect of Cordyceps militaris supplementation on sperm production, sperm motility and hormones in Sprague-Dawley rats. Am. J. Chin. Med. 2008, 36, 849–859. [Google Scholar] [CrossRef]
- Li, X.T.; Li, H.C.; Li, C.B.; Dou, D.Q.; Gao, M.B. Protective effects on mitochondria and anti-aging activity of polysaccharides from cultivated fruiting bodies of Cordyceps militaris. Am. J. Chin. Med. 2010, 38, 1093–1106. [Google Scholar] [CrossRef]
- Han, E.S.; Oh, J.Y.; Park, H.J. Cordyceps militaris extract suppresses dextran sodium sulfate-induced acute colitis in mice and production of inflammatory mediators from macrophages and mast cells. J. Ethnopharmacol. 2011, 134, 703–710. [Google Scholar] [CrossRef]
- Reis, F.S.; Barros, L.; Calhelha, R.C.; Ciric, A.; van Griensven, L.J.; Sokovic, M.; Ferreira, I.C. The methanolic extract of Cordyceps militaris (L.) Link fruiting body shows antioxidant, antibacterial, antifungal and antihuman tumor cell lines properties. Food Chem. Toxicol. 2013, 62, 91–98. [Google Scholar] [CrossRef]
- Rao, Y.K.; Fang, S.H.; Wu, W.S.; Tzeng, Y.M. Constituents isolated from Cordyceps militaris suppress enhanced inflammatory mediator’s production and human cancer cell proliferation. J. Ethnopharmacol. 2010, 131, 363–367. [Google Scholar] [CrossRef]
- Kim, C.S.; Lee, S.Y.; Cho, S.H.; Ko, Y.M.; Kim, B.H.; Kim, H.J.; Park, J.C.; Kim, D.K.; Ahn, H.; Kim, B.O.; et al. Cordyceps militaris induces the IL-18 expression via its promoter activation for IFN-γ production. J. Ethnopharmacol. 2008, 120, 366–371. [Google Scholar] [CrossRef]
- Robson, S.C.; Schuppan, D. Adenosine: Tipping the balance towards hepatic steatosis and fibrosis. J. Hepatol. 2010, 52, 941–943. [Google Scholar] [CrossRef] [PubMed]
- Koupenova, M.; Johnston-Cox, H.; Vezeridis, A.; Gavras, H.; Yang, D.; Zannis, V.; Ravid, K. A2b adenosine receptor regulates hyperlipidemia and atherosclerosis. Circulation 2012, 125, 354–363. [Google Scholar] [CrossRef]
- Wu, C.; Guo, Y.; Su, Y.; Zhang, X.; Luan, H.; Zhang, X.; Zhu, H.; He, H.; Wang, X.; Sun, G.; et al. Cordycepin activates AMP-activated protein kinase (AMPK) via interaction with the γ1 subunit. J. Cell. Mol. Med. 2014, 18, 293–304. [Google Scholar] [CrossRef]
- Kwon, H.K.; Song, M.J.; Lee, H.J.; Park, T.S.; Kim, M.I.; Park, H.J. Pediococcus pentosaceus-Fermented Cordyceps militaris Inhibits Inflammatory Reactions and Alleviates Contact Dermatitis. Int. J. Mol. Sci. 2018, 19, 3504. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Park, J.J.; Barupal, D.K.; Fiehn, O. System response of metabolic networks in Chlamydomonas reinhardtii to total available ammonium. Mol. Cell. Proteom. 2012, 11, 973–988. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, Y.H.; Kim, S.Y.; Kim, Y.G.; Moon, J.Y.; Jeong, K.H.; Lee, T.W.; Ihm, C.G.; Kim, S.; Kim, K.H.; et al. Systematic biomarker discovery and coordinative validation for different primary nephrotic syndromes using gas chromatography-mass spectrometry. J. Chromatogr. A 2016, 1453, 105–115. [Google Scholar] [CrossRef]
- Yoo, H.H.; Son, J.; Kim, D.H. Liquid chromatography-tandem mass spectrometric determination of ceramides and related lipid species in cellular extracts. J. Chromatogr. B 2006, 843, 327–333. [Google Scholar] [CrossRef]
- Lee, S.Y.; Lee, H.Y.; Song, J.H.; Kim, G.T.; Jeon, S.; Song, Y.J.; Lee, J.S.; Hur, J.H.; Oh, H.H.; Park, S.Y.; et al. Adipocyte-Specific Deficiency of De Novo Sphingolipid Biosynthesis Leads to Lipodystrophy and Insulin Resistance. Diabetes 2017, 66, 2596–2609. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.H.; Qu, K.; Zhu, H.B. Beneficial effects of cordycepin on metabolic profiles of liver and plasma from hyperlipidemic hamsters. J. Asian Nat. Prod. Res. 2011, 13, 534–546. [Google Scholar] [CrossRef] [PubMed]
- Giannini, E.G.; Testa, R.; Savarino, V. Liver enzyme alteration: A guide for clinicians. CMAJ 2005, 172, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Muchtar, E.; Dispenzieri, A.; Lacy, M.Q.; Buadi, F.K.; Kapoor, P.; Hayman, S.R.; Gonsalves, W.; Warsame, R.; Kourelis, T.V.; Chakraborty, R.; et al. Elevation of serum lactate dehydrogenase in AL amyloidosis reflects tissue damage and is an adverse prognostic marker in patients not eligible for stem cell transplantation. Br. J. Haematol. 2017, 178, 888–895. [Google Scholar] [CrossRef]
- Monetti, M.; Levin, M.C.; Watt, M.J.; Sajan, M.P.; Marmor, S.; Hubbard, B.K.; Stevens, R.D.; Bain, J.R.; Newgard, C.B.; Farese, R.V., Sr.; et al. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 2007, 6, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef]
- Fujii, H.; Kawada, N. Inflammation and fibrogenesis in steatohepatitis. J. Gastroenterol. 2012, 47, 215–225. [Google Scholar] [CrossRef]
- Perez-Carreras, M.; Del Hoyo, P.; Martin, M.A.; Rubio, J.C.; Martin, A.; Castellano, G.; Colina, F.; Arenas, J.; Solis-Herruzo, J.A. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology 2003, 38, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.N.; Jang, Y.H.; Kim, M.J.; Seo, M.J.; Kang, B.W.; Jeong, Y.K.; Kim, J.I. Cordyceps militaris alleviates non-alcoholic fatty liver disease in ob/ob mice. Nutr. Res. Pract. 2014, 8, 172–176. [Google Scholar] [CrossRef]
- Hur, H. Chemical Ingredients of Cordyceps militaris. Mycobiology 2008, 36, 233–235. [Google Scholar] [CrossRef]
- Shin, S.; Moon, S.; Park, Y.; Kwon, J.; Lee, S.; Lee, C.K.; Cho, K.; Ha, N.J.; Kim, K. Role of Cordycepin and Adenosine on the Phenotypic Switch of Macrophages via Induced Anti-inflammatory Cytokines. Immune Netw. 2009, 9, 255–264. [Google Scholar] [CrossRef]
- Cai, Y.L.; Zheng, J.; Guo, X.; Li, H.G.; Pei, Y.; Botchlett, R.; Woo, S.L.; Liu, M.Y.; Chen, G.; Huo, Y.Q.; et al. Exacerbation of NAFLD in both HFD-fed Mice and MCD-fed Mice by Adenosine 2A Receptor Deficiency. FASEB J. 2016, 30, 431. [Google Scholar]
- Cai, Y.; Li, H.; Liu, M.; Pei, Y.; Zheng, J.; Zhou, J.; Luo, X.; Huang, W.; Ma, L.; Yang, Q.; et al. Disruption of adenosine 2A receptor exacerbates NAFLD through increasing inflammatory responses and SREBP1c activity. Hepatology 2018, 68, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.Y.; Ahn, H.Y.; Cho, Y.S.; Je, J.Y. Protective effect of cordycepin-enriched Cordyceps militaris on alcoholic hepatotoxicity in Sprague-Dawley rats. Food Chem. Toxicol. 2013, 60, 52–57. [Google Scholar] [CrossRef]
- Tran, N.K.S.; Kim, G.T.; Lee, D.Y.; Kim, Y.J.; Park, H.J.; Park, D.K.; Park, T.S. Fermented Cordyceps militaris Extract Ameliorates Hepatosteatosis via Activation of Fatty Acid Oxidation. J. Med. Food 2019, 22, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Han, J.Y.; Im, J.; Choi, J.N.; Lee, C.H.; Park, H.J.; Park, D.K.; Yun, C.H.; Han, S.H. Induction of IL-8 expression by Cordyceps militaris grown on germinated soybeans through lipid rafts formation and signaling pathways via ERK and JNK in A549 cells. J. Ethnopharmacol. 2010, 127, 55–61. [Google Scholar] [CrossRef]
- Porto, M.C.; Kuniyoshi, T.M.; Azevedo, P.O.; Vitolo, M.; Oliveira, R.P. Pediococcus spp.: An important genus of lactic acid bacteria and pediocin producers. Biotechnol. Adv. 2017, 35, 361–374. [Google Scholar] [CrossRef] [PubMed]
| NCD | HFD | Feno | ON188-50 | ON188-100 | ON188-200 | |
|---|---|---|---|---|---|---|
| Plasma glucose (mg/dL) | 132.43 ± 4.34 a | 136.14 ± 7.71 a | 109.14 ± 6.97 b | 136.14 ± 6.98 a | 143.75 ± 5.55 a | 126.50 ± 9.79 a |
| Cholesterol (mg/dL) | 116.26 ± 3.92 a | 187.96 ± 10.68 b | 138.24 ± 13.24 c | 178.54 ± 10.56 b | 186.54 ± 6.79 b | 173.22 ± 5.99 b |
| Triglyceride (mg/dL) | 80.88 ± 3.74 a | 104.92 ± 1.78 b | 74.74 ± 3.66 a | 105.70 ± 2.96 b | 131.44 ± 7.87 c | 125.38 ± 6.64 c |
| HDL (mg/dL) | 69.10 ± 3.92 a | 106.50 ± 6.92 b | 86.56 ± 7.64 c | 120.42 ± 6.19 b | 118.18 ± 3.99 b | 114.24 ± 3.35 b |
| LDL (mg/dL) | 27.50 ± 13.04 a | 39.98 ± 15.60 a | 36.94 ± 6.34 a | 43.28 ± 16.15 a | 44.46 ± 21.48 a | 41.32 ± 19.40 a |
| LDH (U/L) | 744.36 ± 94.3 a | 820.20 ± 102.7 a | 704.76 ± 104.2 a | 487.56 ± 93.3 a | 302.92 ± 48.5 b | 163.26 ± 15.2 c |
| ALT (U/L) | 82.96 ± 17.4 a | 159.02 ± 25.8 b | 105.94 ± 38.7 bc | 82.96 ± 29.3 c | 83.76 ± 41.7 c | 43.46 ± 9.2 cd |
| AST (U/L) | 100.78 ± 18.7 a | 180.10 ± 28.8 b | 91.20 ± 21.9 c | 60.82 ± 15.7 c | 71.94 ± 10.4 c | 59 ± 8.3 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, N.K.S.; Kim, G.-T.; Park, S.-H.; Lee, D.; Shim, S.-M.; Park, T.-S. Fermented Cordyceps militaris Extract Prevents Hepatosteatosis and Adipocyte Hypertrophy in High Fat Diet-Fed Mice. Nutrients 2019, 11, 1015. https://doi.org/10.3390/nu11051015
Tran NKS, Kim G-T, Park S-H, Lee D, Shim S-M, Park T-S. Fermented Cordyceps militaris Extract Prevents Hepatosteatosis and Adipocyte Hypertrophy in High Fat Diet-Fed Mice. Nutrients. 2019; 11(5):1015. https://doi.org/10.3390/nu11051015
Chicago/Turabian StyleTran, Nguyen Khoi Song, Goon-Tae Kim, Si-Hyun Park, Dongyup Lee, Soon-Mi Shim, and Tae-Sik Park. 2019. "Fermented Cordyceps militaris Extract Prevents Hepatosteatosis and Adipocyte Hypertrophy in High Fat Diet-Fed Mice" Nutrients 11, no. 5: 1015. https://doi.org/10.3390/nu11051015
APA StyleTran, N. K. S., Kim, G.-T., Park, S.-H., Lee, D., Shim, S.-M., & Park, T.-S. (2019). Fermented Cordyceps militaris Extract Prevents Hepatosteatosis and Adipocyte Hypertrophy in High Fat Diet-Fed Mice. Nutrients, 11(5), 1015. https://doi.org/10.3390/nu11051015





