Laminaria japonica Extract Enhances Intestinal Barrier Function by Altering Inflammatory Response and Tight Junction-Related Protein in Lipopolysaccharide-Stimulated Caco-2 Cells
Abstract
1. Introduction
2. Materials and Methods
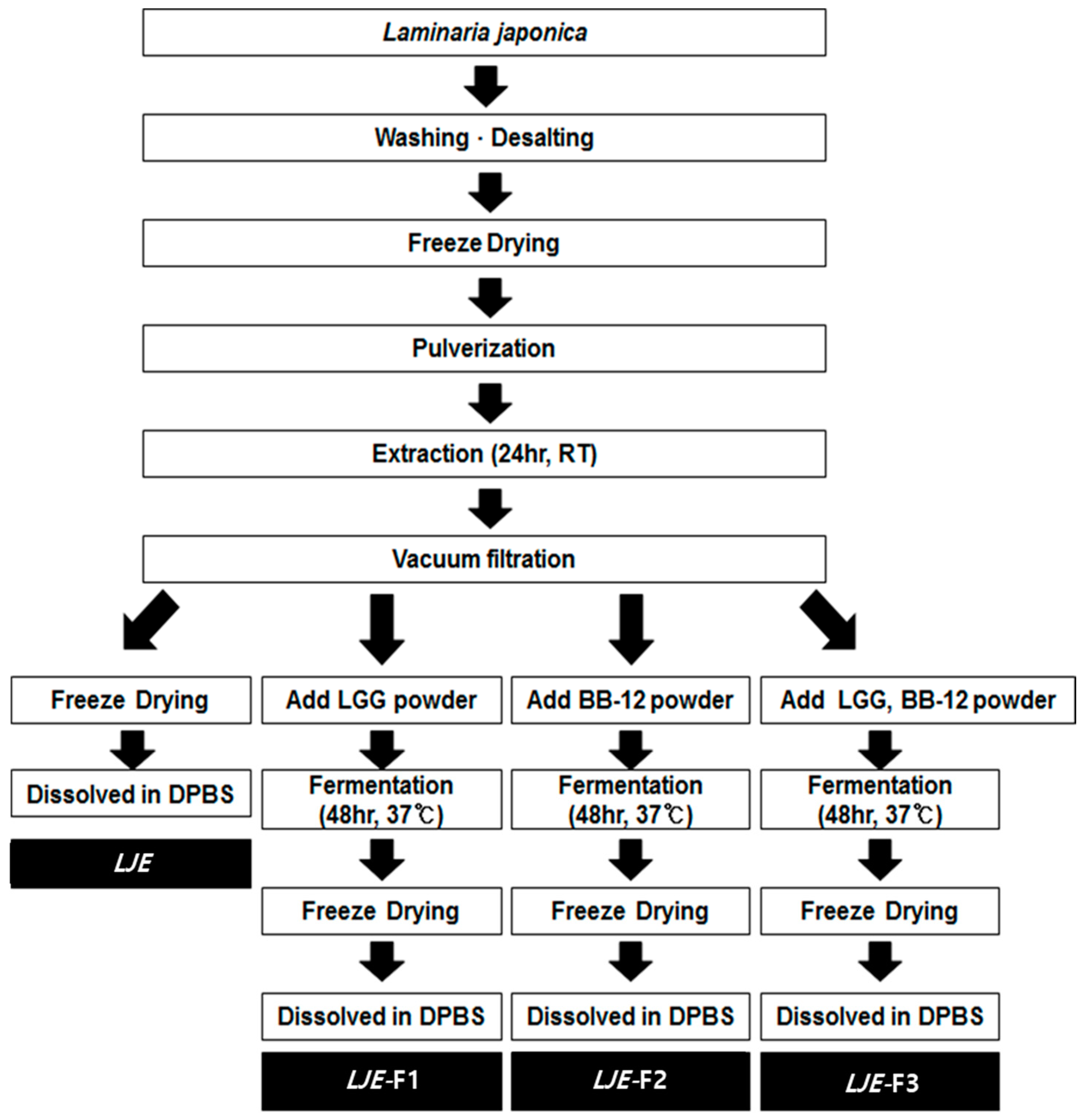
2.1. Preparation of Extracts from Laminaria japonica
2.2. Measurement of pH, Sugar, and Reducing Sugar Contents
2.3. Total Polyphenol Contents
2.4. Cell Culture
2.5. Cell Viability Assay
2.6. Nitric Oxide Production Measurement
2.7. Transepithelial Electrical Resistance
2.8. Western Blot Analysis and ELISA
2.9. Statistical Analysis
3. Results
3.1. Changes of Chemical Characteristics of Laminaria japonica by Fermentation
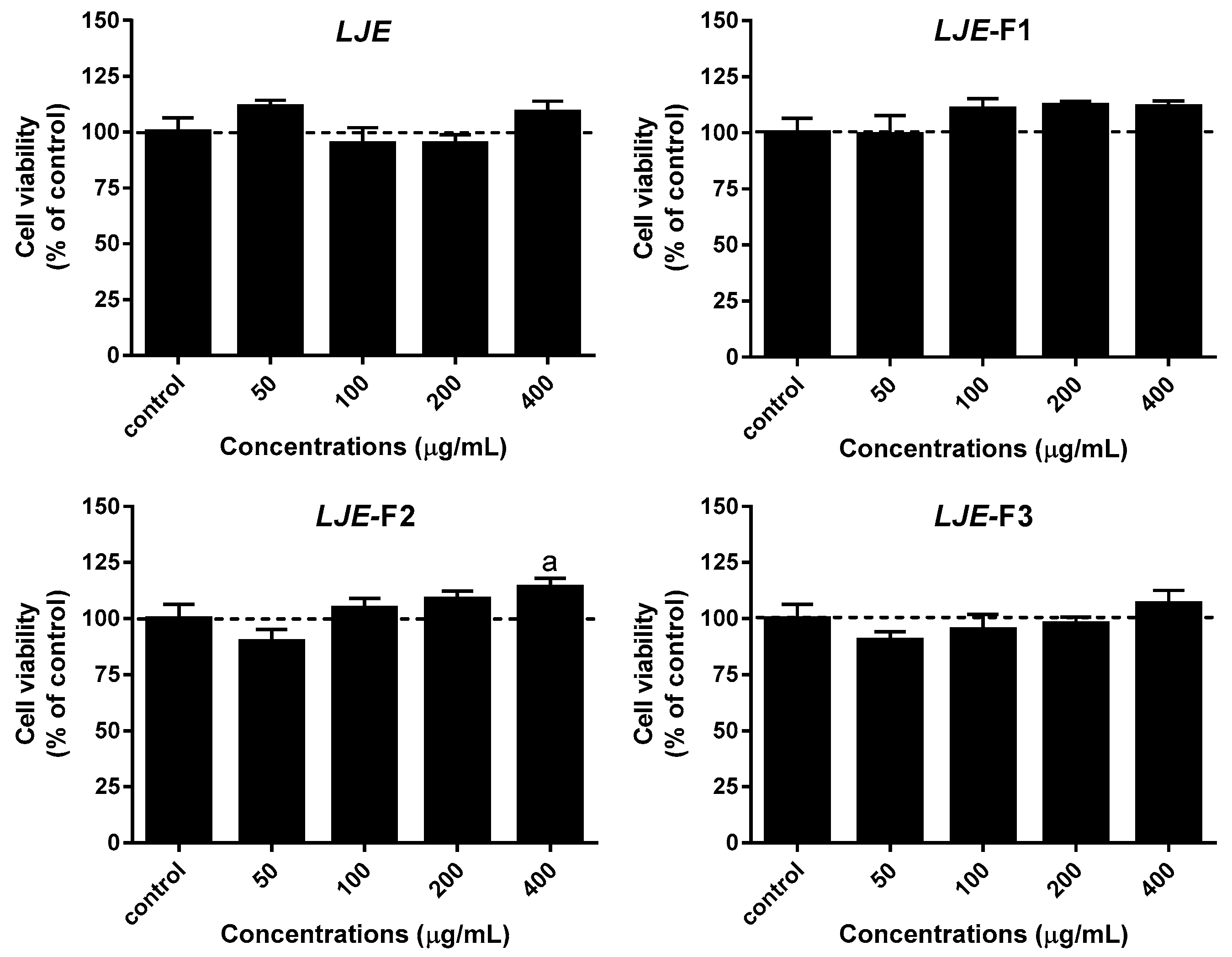
3.2. Effect of LJE and LJE-Fs on Cell Viability in Caco-2 Cells
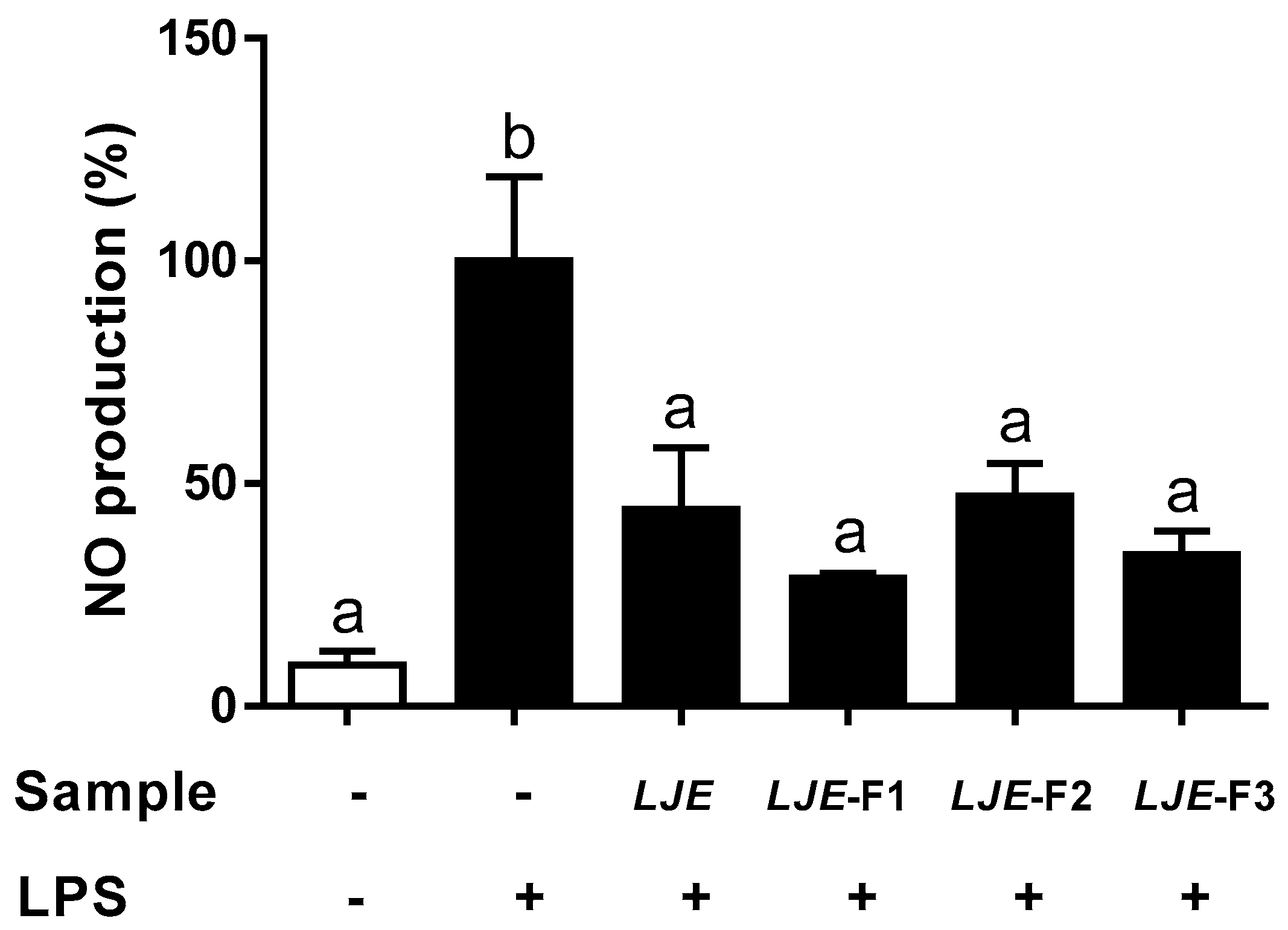
3.3. LJE and LJE-Fs Decreased the Production of Nitric Oxide by LPS Stimulus in Caco-2 Cells
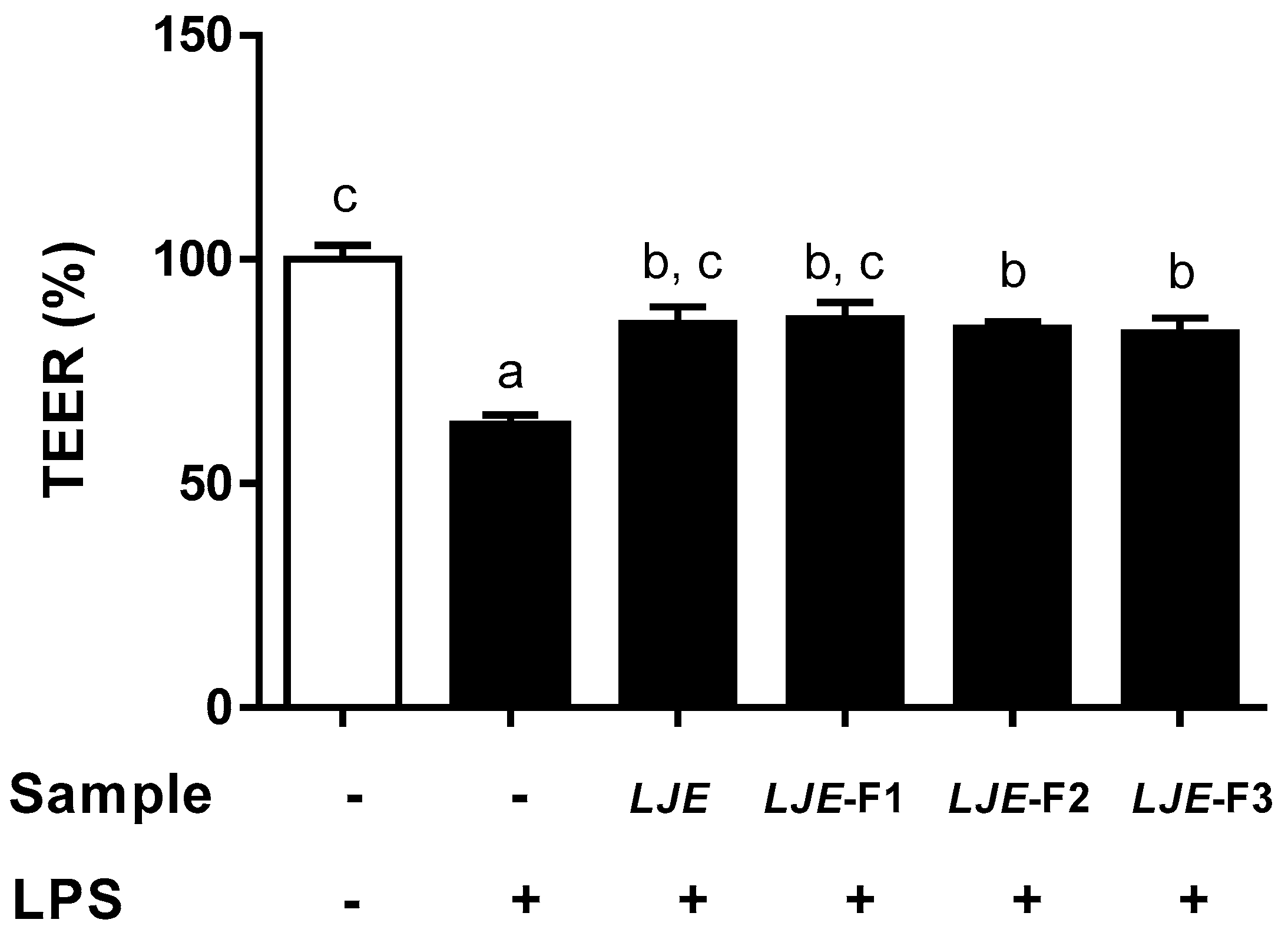
3.4. LJE and LJE-Fs Protected Caco-2 Cell Monolayers from Endotoxin Stimulus
3.5. LJE and LJE-Fs Significantly Attenuate IL-6 Production Induced by LPS Stimulus in Caco-2 Cells
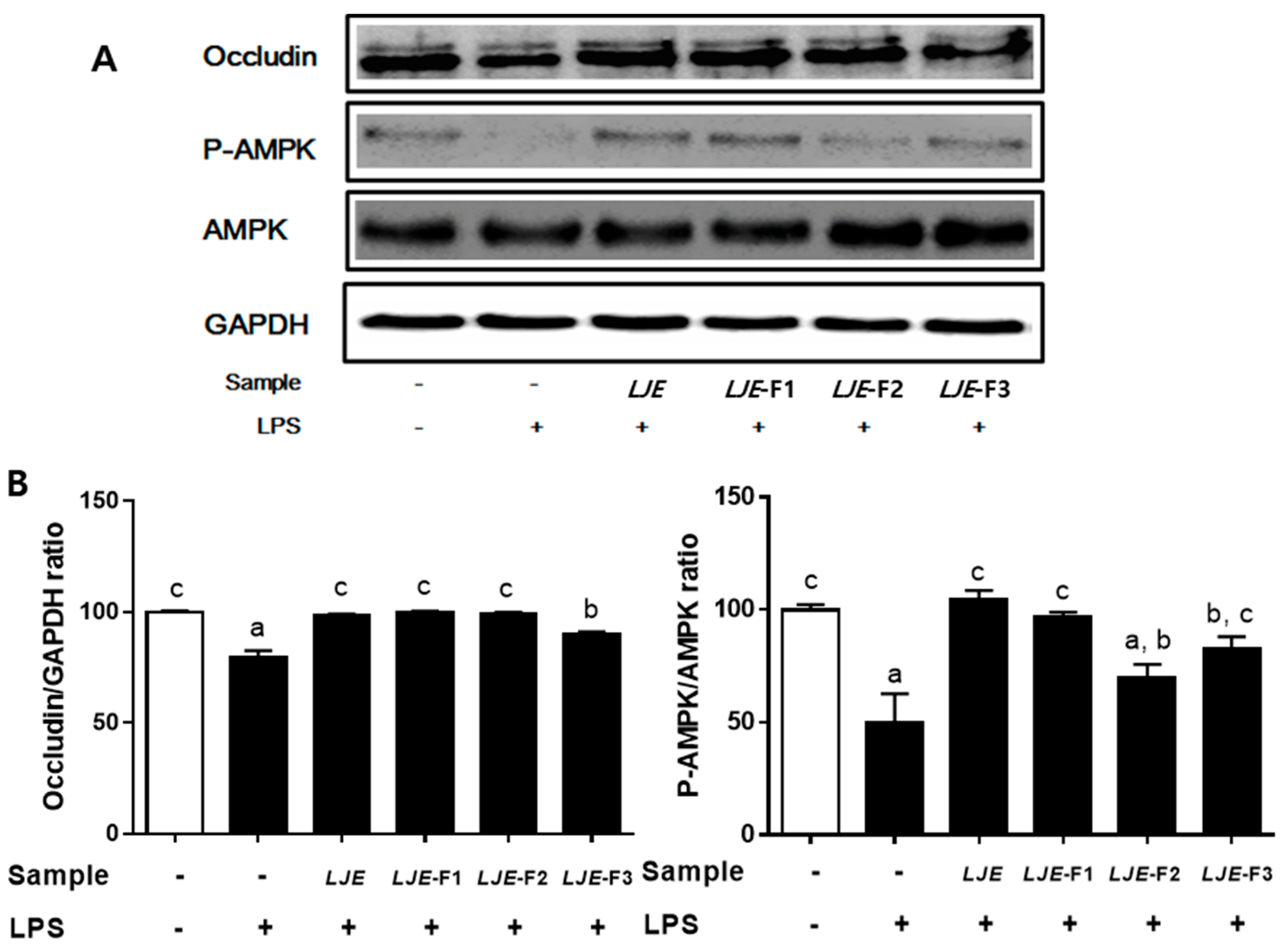
3.6. LJE and LJE-Fs Differentially Promoted Tight Junction (TJ)-Related Protein Expression in Caco-2 Cells
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | adenosine monophosphate (AMP)-activated protein kinase |
| BB-12 | Bifidobacterium animalis ssp. lactis BB-12 |
| IBD | inflammatory bowel diseases |
| LGG | Lactobacillus rhamnosus LGG |
| LJ | Laminaria japonica |
| LJE | Laminaria japonica extract |
| LJE-Fs | Fermented Laminaria japonica extract |
| LPS | lipopolysaccharides |
| NO | nitric oxide |
| TEER | transepithelial electrical resistance |
| TJ | tight junction |
References
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Munoz, F.; Dominguez-Lopez, A.; Yamamoto-Furusho, J.K. Role of cytokines in inflammatory bowel disease. World J. Gastroenterol. 2008, 14, 4280–4288. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.; Nikfar, S.; Abdollahi, M. Induction of clinical response and remission of inflammatory bowel disease by use of herbal medicines: A meta-analysis. World J. Gastroenterol. WJG 2013, 19, 5738. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, R.; Shams-Ardekani, M.R.; Abdollahi, M. A review of the efficacy of traditional Iranian medicine for inflammatory bowel disease. World J. Gastroenterol. 2010, 16, 4504. [Google Scholar] [CrossRef]
- Rahimi, R.; Mozaffari, S.; Abdollahi, M. On the use of herbal medicines in management of inflammatory bowel diseases: A systematic review of animal and human studies. Digest. Dis. Sci. 2009, 54, 471–480. [Google Scholar] [CrossRef]
- Ullman, T.; Croog, V.; Harpaz, N.; Sachar, D.; Itzkowitz, S. Progression of flat low-grade dysplasia to advanced neoplasia in patients with ulcerative colitis. Gastroenterology 2003, 125, 1311–1319. [Google Scholar] [CrossRef] [PubMed]
- Artis, D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat. Rev. Immunol. 2008, 8, 411. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, I.J.; Raub, T.J.; Borchardt, R.T. Characterization of the human colon carcinoma cell line (Caco-2) as a model system for intestinal epithelial permeability. Gastroenterology 1989, 96, 736–749. [Google Scholar] [CrossRef]
- Wang, X.; Li, Y.; Yang, X.; Yao, J. Astragalus polysaccharide reduces inflammatory response by decreasing permeability of LPS-infected Caco2 cells. Int. J. Biol. Macromol. 2013, 61, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Wells, C.; Jechorek, R.; Olmsted, S.; Erlandsen, S. Effect of LPS on epithelial integrity and bacterial uptake in the polarized human enterocyte-like cell line Caco-2. Circ. Shock 1993, 40, 276–288. [Google Scholar] [PubMed]
- Iraha, A.; Chinen, H.; Hokama, A.; Yonashiro, T.; Kinjo, T.; Kishimoto, K.; Nakamoto, M.; Hirata, T.; Kinjo, N.; Higa, F. Fucoidan enhances intestinal barrier function by upregulating the expression of claudin-1. World J. Gastroenterol. 2013, 19, 5500. [Google Scholar] [CrossRef]
- Triantafilou, M.; Triantafilou, K. Invited review: The dynamics of LPS recognition: Complex orchestration of multiple receptors. J. Endotoxin Res. 2005, 11, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Panaro, M.A.; Carofiglio, V.; Acquafredda, A.; Cavallo, P.; Cianciulli, A. Anti-inflammatory effects of resveratrol occur via inhibition of lipopolysaccharide-induced NF-κB activation in Caco-2 and SW480 human colon cancer cells. Br. J. Nutr. 2012, 108, 1623–1632. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-S.; Kang, C.-O.; Kim, M.-H.; Cha, W.-S.; Shin, H.-J. Contents of water extract for Laminaria japonica and its antioxidant activity. KSBB J. 2011, 26, 112–118. [Google Scholar] [CrossRef]
- Shirosaki, M.; Koyama, T. Laminaria japonica as a food for the prevention of obesity and diabetes. In Advances in Food and Nutrition Research; Elsevier: Amsterdam, The Netherlands, 2011; Volume 64, pp. 199–212. [Google Scholar]
- Kang, K.-S.; Nam, C.-S.; Park, E.-K.; Ha, B.-J. The Enzymatic Regulatory Effects of Laninaria japonica Fucoidan Extract in Hepatotoxicity. J. Life Sci. 2006, 16, 1104–1108. [Google Scholar] [CrossRef][Green Version]
- Kang, S.-y.; Kim, E.; Kang, I.; Lee, M.; Lee, Y. Anti-Diabetic Effects and Anti-Inflammatory Effects of Laminaria japonica and Hizikia fusiforme in Skeletal Muscle: In vitro and In vivo Model. Nutrients 2018, 10, 491. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Kim, J.; Lee, Y. Anti-inflammatory and anti-diabetic effects of brown seaweeds in high-fat diet-induced obese mice. Nutr. Res. Pract. 2016, 10, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; You, L.; Lin, Z.; Zhao, M.; Cui, C. The antioxidant capacity of polysaccharide from L aminaria japonica by citric acid extraction. Int. J. Food Sci. Technol. 2013, 48, 1352–1358. [Google Scholar] [CrossRef]
- Kang, Y.M.; Lee, B.J.; Kim, J.I.; Nam, B.H.; Cha, J.Y.; Kim, Y.M.; Ahn, C.B.; Choi, J.S.; Choi, I.S.; Je, J.Y. Antioxidant effects of fermented sea tangle (Laminaria japonica) by Lactobacillus brevis BJ20 in individuals with high level of gamma-GT: A randomized, double-blind, and placebo-controlled clinical study. Food Chem. Toxicol. 2012, 50, 1166–1169. [Google Scholar] [CrossRef]
- Lin, H.-T.V.; Lu, W.-J.; Tsai, G.-J.; Chou, C.-T.; Hsiao, H.-I.; Hwang, P.-A. Enhanced anti-inflammatory activity of brown seaweed Laminaria japonica by fermentation using Bacillus subtilis. Process Biochem. 2016, 51, 1945–1953. [Google Scholar] [CrossRef]
- Martins, S.; Mussatto, S.I.; Martinez-Avila, G.; Montanez-Saenz, J.; Aguilar, C.N.; Teixeira, J.A. Bioactive phenolic compounds: Production and extraction by solid-state fermentation. A review. Biotechnol. Adv. 2011, 29, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Katina, K.; Liukkonen, K.-H.; Kaukovirta-Norja, A.; Adlercreutz, H.; Heinonen, S.-M.; Lampi, A.-M.; Pihlava, J.-M.; Poutanen, K. Fermentation-induced changes in the nutritional value of native or germinated rye. J. Cereal Sci. 2007, 46, 348–355. [Google Scholar] [CrossRef]
- Katina, K.; Laitila, A.; Juvonen, R.; Liukkonen, K.-H.; Kariluoto, S.; Piironen, V.; Landberg, R.; Åman, P.; Poutanen, K. Bran fermentation as a means to enhance technological properties and bioactivity of rye. Food Microbiol. 2007, 24, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.J.; Rigassio-Radler, D.; Denmark, R.; Haley, T.; Touger-Decker, R. Effect of Lactobacillus rhamnosus LGG® and Bifidobacterium animalis ssp. lactis BB-12® on health-related quality of life in college students affected by upper respiratory infections. Br. J. Nutr. 2013, 109, 1999–2007. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Lee, D.-G.; Park, S.-H.; Kim, M.; Kong, C.-S.; Kim, Y.-Y.; Lee, S.-H. Comparison of biological activities in Sargassum siliquanstrum fermented by isolated lactic acid bacteria. Biotechnol. Bioprocess Eng. 2015, 20, 341–348. [Google Scholar] [CrossRef]
- Eom, S.-H.; Lee, B.-J.; Kim, Y.-M. Effect of yeast fermentation on the antioxidant and anti-inflammatory activity of sea tangle water extract. Korean J. Fish. Aquat. Sci. 2010, 43, 117–124. [Google Scholar]
- Eom, S.-H.; Kang, Y.-M.; Park, J.-H.; Yu, D.-U.; Jeong, E.-T.; Lee, M.-S.; Kim, Y.-M. Enhancement of polyphenol content and antioxidant activity of brown alga Eisenia bicyclis extract by microbial fermentation. Fish. Aquat. Sci. 2011, 14, 192–197. [Google Scholar] [CrossRef]
- Bae, H.-N.; Kim, Y.-M. Improvement of the functional qualities of sea tangle extract through fermentation by Aspergillus oryzae. Fish. Aquat. Sci. 2010, 13, 12–17. [Google Scholar] [CrossRef]
- Wolfrom, M.L.; BeMiller, J.N. Methods in Carbohydrate Chemistry: Reactions of Carbohydrates; Academic Press: Cambridge, MA, USA, 1963; Volume 2. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Folin, O.; Denis, W. On phosphotungstic-phosphomolybdic compounds as color reagents. J. Biol. Chem. 1912, 12, 239–243. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ding, A.H.; Nathan, C.F.; Stuehr, D.J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J. Immunol. 1988, 141, 2407–2412. [Google Scholar] [PubMed]
- Shon, D.-H.; Kim, M.-H.; Kim, Y.-C.; Kim, S.-S. Effect of Korean Red Ginseng on the Stability of the Tight Junction of Intestinal Epithelial Cells. Korean J. Food Sci. Technol. 2010, 42, 335–342. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671. [Google Scholar] [CrossRef]
- Oh, J.H.; Lee, Y. Effects of Water and Ethanol Extracts from Four Types of Domestic Seaweeds on Cell Differentiation in 3T3-L1 Cell Line. J. East Asian Soc. Diet. Life 2015, 25, 990–998. [Google Scholar] [CrossRef]
- Sun, X.; Yang, Q.; Rogers, C.J.; Du, M.; Zhu, M.J. AMPK improves gut epithelial differentiation and barrier function via regulating Cdx2 expression. Cell Death Differ. 2017, 24, 819–831. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.-J.; Bu, Y.; Bae, J.; Bang, Y.-m.; Kim, J.; Lee, H.; Beom-Joon, L.; Hyun, Y.H.; Park, J.-W. Protective effect of Laminaria japonica with probiotics on murine colitis. Mediat. Inflamm. 2014, 2014, 417814. [Google Scholar] [CrossRef] [PubMed]
- Heo, J. Donguibogam; Donguibogam Publishing: Seoul, Korea, 2010. [Google Scholar]
- Hubert, J.; Berger, M.; Nepveu, F.; Paul, F.; Daydé, J. Effects of fermentation on the phytochemical composition and antioxidant properties of soy germ. Food Chem. 2008, 109, 709–721. [Google Scholar] [CrossRef]
- Kripke, S.A.; Fox, A.D.; Berman, J.M.; Settle, R.G.; Rombeau, J.L. Stimulation of intestinal mucosal growth with intracolonic infusion of short-chain fatty acids. J. Parent. Enter. Nutr. 1989, 13, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Wan-Loy, C.; Siew-Moi, P. Marine algae as a potential source for anti-obesity agents. Mar. Drugs 2016, 14, 222. [Google Scholar] [CrossRef]
- Wong, J.M.; De Souza, R.; Kendall, C.W.; Emam, A.; Jenkins, D.J. Colonic health: Fermentation and short chain fatty acids. J. Clin. Gastroenterol. 2006, 40, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Tedelind, S.; Westberg, F.; Kjerrulf, M.; Vidal, A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: A study with relevance to inflammatory bowel disease. World J. Gastroenterol. 2007, 13, 2826. [Google Scholar] [CrossRef] [PubMed]
- Iraporda, C.; Errea, A.; Romanin, D.E.; Cayet, D.; Pereyra, E.; Pignataro, O.; Sirard, J.C.; Garrote, G.L.; Abraham, A.G.; Rumbo, M. Lactate and short chain fatty acids produced by microbial fermentation downregulate proinflammatory responses in intestinal epithelial cells and myeloid cells. Immunobiology 2015, 220, 1161–1169. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, H.; Singer, P.; Halpern, Z.; Bruck, R. Polyphenols in the treatment of inflammatory bowel disease and acute pancreatitis. Gut 2007, 56, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Biasi, F.; Astegiano, M.; Maina, M.; Leonarduzzi, G.; Poli, G. Polyphenol supplementation as a complementary medicinal approach to treating inflammatory bowel disease. Curr. Med. Chem. 2011, 18, 4851–4865. [Google Scholar] [CrossRef]
- Suzuki, T. Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 2013, 70, 631–659. [Google Scholar] [CrossRef] [PubMed]
- Singer, I.I.; Kawka, D.W.; Scott, S.; Weidner, J.R.; Mumford, R.A.; Riehl, T.E.; Stenson, W.F. Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology 1996, 111, 871–885. [Google Scholar] [CrossRef]
- Fitton, J.; Stringer, D.; Karpiniec, S. Therapies from fucoidan: An update. Mar. Drugs 2015, 13, 5920–5946. [Google Scholar] [CrossRef] [PubMed]
- Hwang, P.A.; Phan, N.N.; Lu, W.J.; Ngoc Hieu, B.T.; Lin, Y.C. Low-molecular-weight fucoidan and high-stability fucoxanthin from brown seaweed exert prebiotics and anti-inflammatory activities in Caco-2 cells. Food Nutr. Res. 2016, 60, 32033. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Khatib, K.; Guo, S.; Ye, D.; Youssef, M.; Ma, T. Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 2011, 300, G1054–G1064. [Google Scholar] [CrossRef] [PubMed]
| Measurements | Fermentation | LJE-F1 | LJE-F2 | LJE-F3 |
|---|---|---|---|---|
| pH | Before | 7.30 ± 0.12 1 | 7.31 ± 0.14 | 7.34 ± 0.10 |
| After | 4.06 ± 0.09 * | 4.01 ± 0.11 * | 3.95 ± 0.15 * | |
| Total sugar (mg D-glucose/mL) | Before | 4.40 ± 0.13 | 4.67 ± 0.02 | 4.93 ± 0.01 |
| After | 3.34 ± 0.06 * | 4.35 ± 0.07 * | 4.04 ± 0.01 * | |
| Reducing sugar (mg D-glucose/mL) | Before | 1.44 ± 0.03 | 1.39 ± 0.01 | 1.47 ± 0.03 |
| After | 1.39 ± 0.09 * | 1.36 ± 0.03 * | 1.36 ± 0.03 * | |
| Total polyphenols (μg gallic acid/mL) | Before | 1.14 ± 0.01 | 1.15 ± 0.07 | 1.17 ± 0.01 |
| After | 0.90 ± 0.01 | 1.01 ± 0.01 | 1.11 ± 0.04 |
| Sample | TNF-α Levels (pg/mL) | IL-6 Levels (pg/mL) |
|---|---|---|
| LPS | 21.75 ± 0.27 b,1 | 5.47 ± 0.7 c |
| LPS+LJE | 18.36 ± 1.20 a | 4.16 ± 0.26 b |
| LPS+LJE-F1 | 18.16 ± 0.01 a | 0.92 ± 0.15 a |
| LPS+LJE-F2 | 19.82 ± 0.19 b | 0.91 ± 0.03 a |
| LPS+LJE-F3 | 19.11 ± 0.60 b | 0.77 ± 0.05 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.-S.; Haj, F.G.; Lee, M.; Kang, I.; Zhang, G.; Lee, Y. Laminaria japonica Extract Enhances Intestinal Barrier Function by Altering Inflammatory Response and Tight Junction-Related Protein in Lipopolysaccharide-Stimulated Caco-2 Cells. Nutrients 2019, 11, 1001. https://doi.org/10.3390/nu11051001
Yang H-S, Haj FG, Lee M, Kang I, Zhang G, Lee Y. Laminaria japonica Extract Enhances Intestinal Barrier Function by Altering Inflammatory Response and Tight Junction-Related Protein in Lipopolysaccharide-Stimulated Caco-2 Cells. Nutrients. 2019; 11(5):1001. https://doi.org/10.3390/nu11051001
Chicago/Turabian StyleYang, Hyo-Seon, Fawaz G Haj, Myoungsook Lee, Inhae Kang, Guiguo Zhang, and Yunkyoung Lee. 2019. "Laminaria japonica Extract Enhances Intestinal Barrier Function by Altering Inflammatory Response and Tight Junction-Related Protein in Lipopolysaccharide-Stimulated Caco-2 Cells" Nutrients 11, no. 5: 1001. https://doi.org/10.3390/nu11051001
APA StyleYang, H.-S., Haj, F. G., Lee, M., Kang, I., Zhang, G., & Lee, Y. (2019). Laminaria japonica Extract Enhances Intestinal Barrier Function by Altering Inflammatory Response and Tight Junction-Related Protein in Lipopolysaccharide-Stimulated Caco-2 Cells. Nutrients, 11(5), 1001. https://doi.org/10.3390/nu11051001








