The Chinese Herbal Formula PAPZ Ameliorates Behavioral Abnormalities in Depressive Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CHFs and CORT
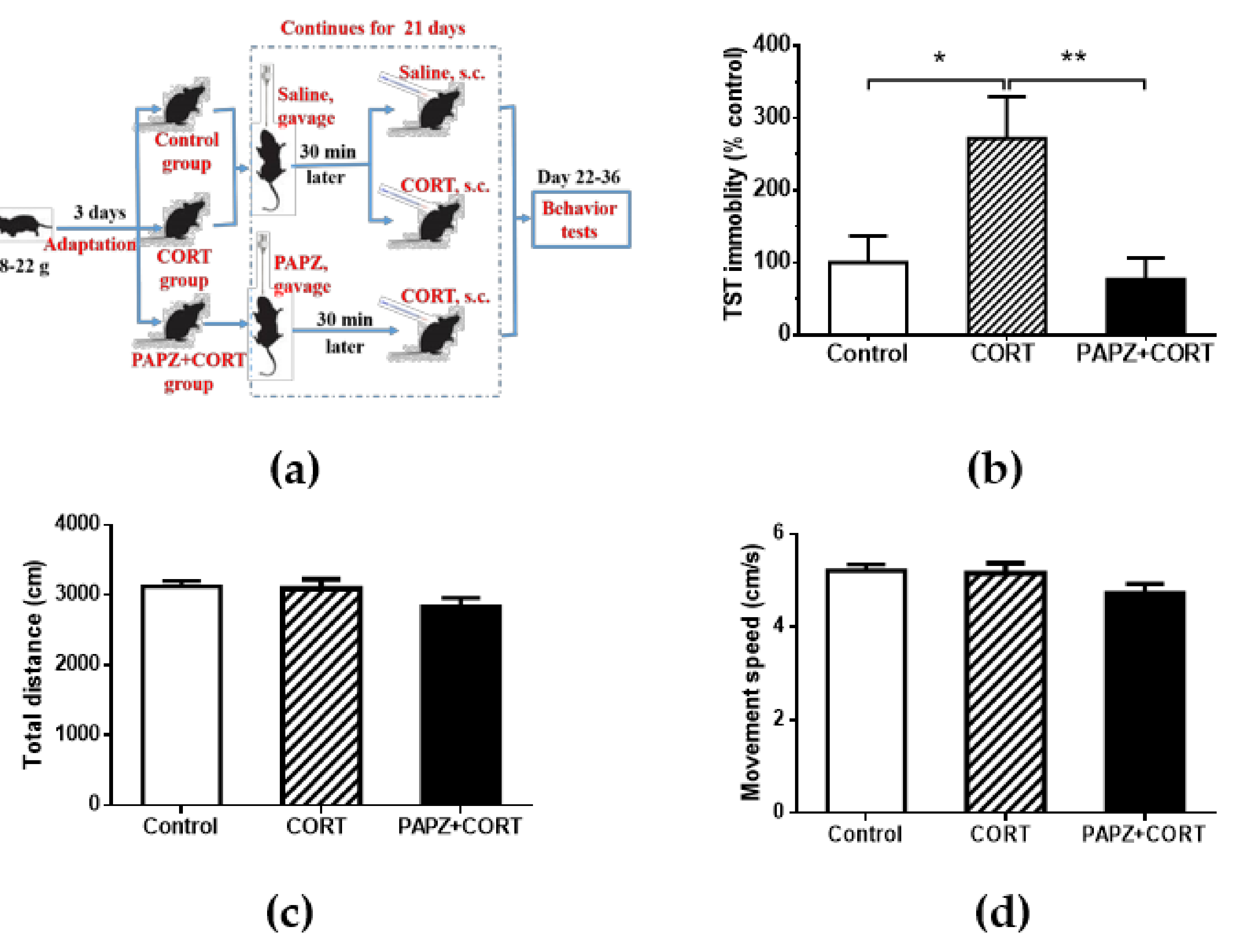
2.3. Animals and Treatment
2.4. Open Field Test
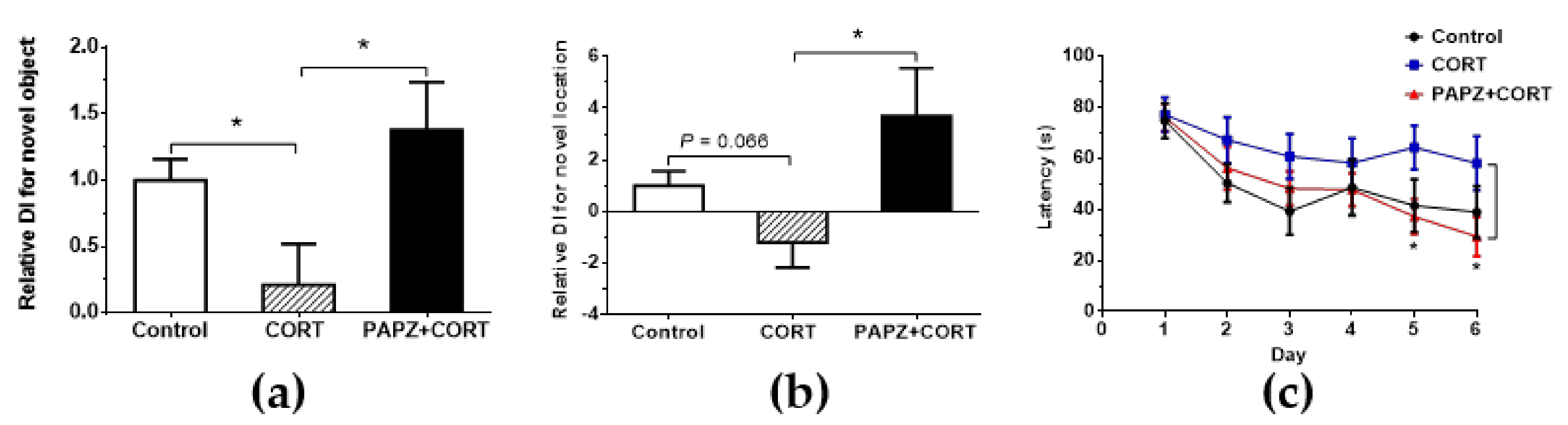
2.5. Novel Object Recognition Test
2.6. Tail Suspension Test
2.7. Morris Water Maze Experiment
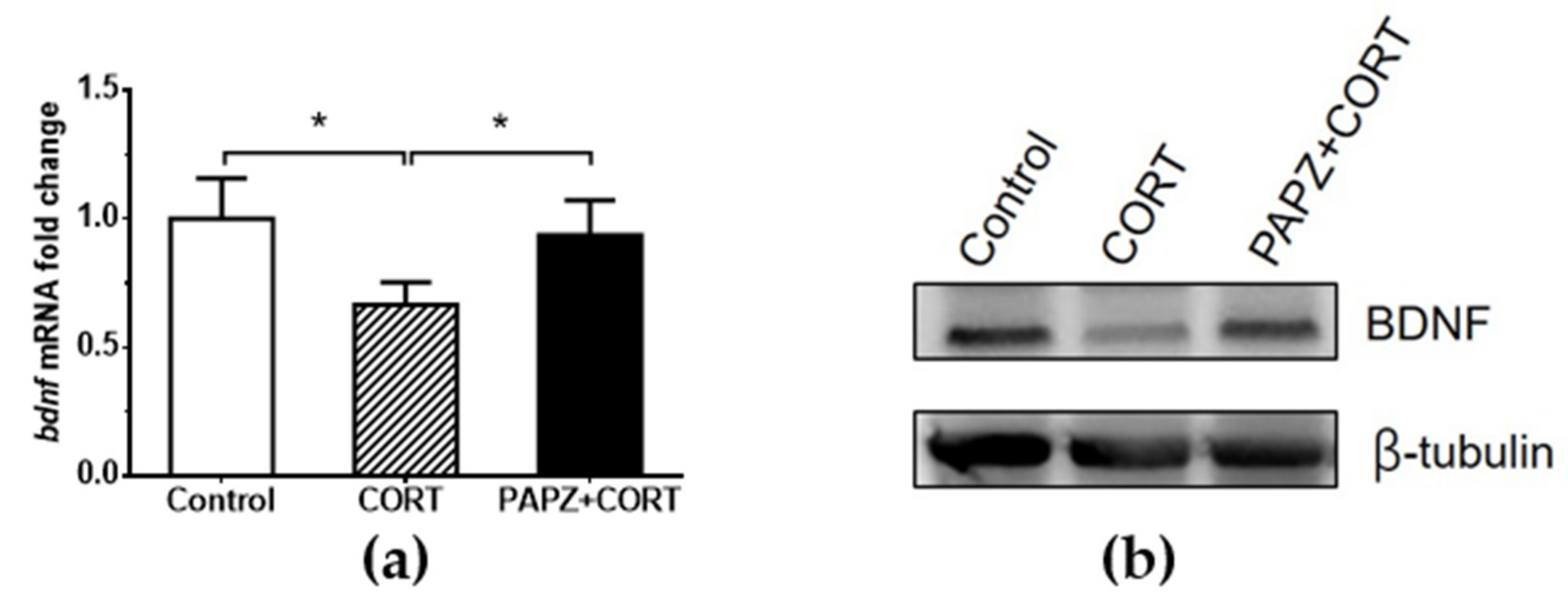
2.8. Real-time Quantitative PCR and Western Blotting
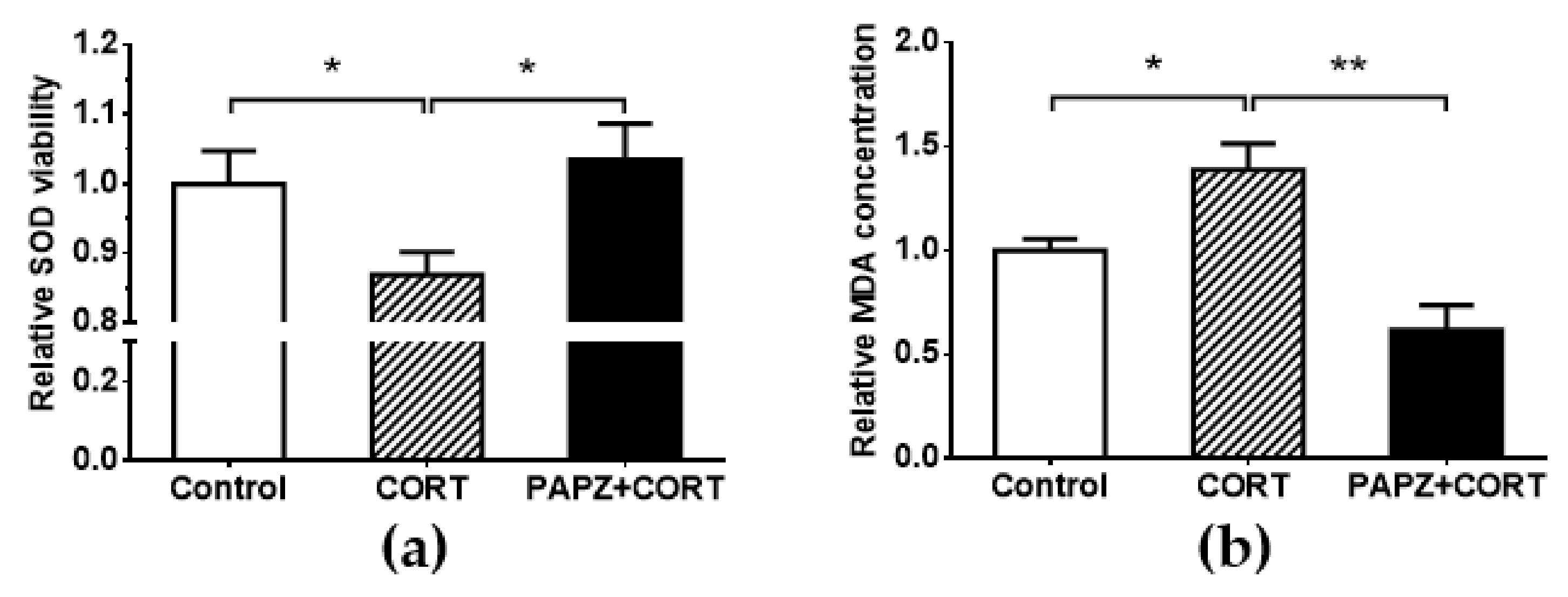
2.9. SOD and MDA Assay
2.10. Determination of Cell Viability
2.11. Determination of Cell Apoptosis by Flow Cytometry
2.12. Statistical Analysis
3. Results
3.1. Antidepressant Effects of PAPZ
3.2. Effects of PAPZ on Learning and Memory Capacity
3.3. Molecular Mechanism of the Cerebral Protection Effect of PAPZ
3.4. PAPZ Enhanced Cerebral Antioxidant Ability of Tested Mice
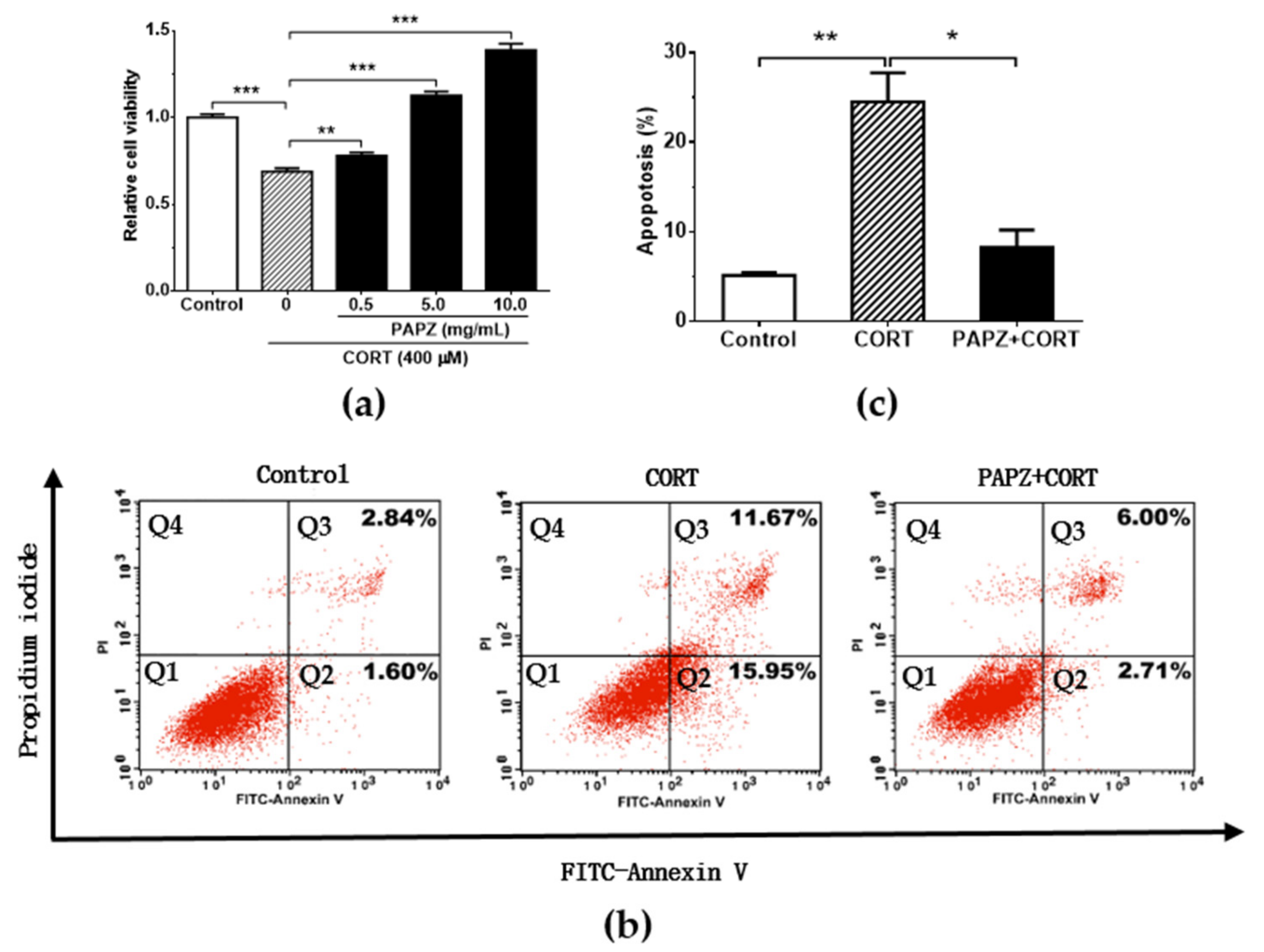
3.5. PAPZ Protected Neurons In Vitro
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Charlson, F.J.; Ferrari, A.J.; Flaxman, A.D.; Whiteford, H.A. The epidemiological modelling of dysthymia: Application for the Global Burden of Disease Study 2010. J. Affect. Disord. 2013, 151, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.J.; Somerville, A.J.; Baxter, A.J.; Norman, R.; Patten, S.B.; Vos, T.; Whiteford, H.A. Global variation in the prevalence and incidence of major depressive disorder: A systematic review of the epidemiological literature. Psychol. Med. 2013, 43, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.J.; Charlson, F.J.; Norman, R.E.; Patten, S.B.; Freedman, G.; Murray, C.J.; Vos, T.; Whiteford, H.A. Burden of depressive disorders by country, sex, age, and year: Findings from the global burden of disease study 2010. PLoS Med. 2013, 10, e1001547. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Ge, T.; Leng, Y.; Pan, Z.; Fan, J.; Yang, W.; Cui, R. The Role of Neural Plasticity in Depression: From Hippocampus to Prefrontal Cortex. Neural Plast. 2017, 2017, 6871089. [Google Scholar] [CrossRef] [PubMed]
- Duman, R.S.; Monteggia, L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 2006, 59, 1116–1127. [Google Scholar] [CrossRef]
- Numakawa, T.; Suzuki, S.; Kumamaru, E.; Adachi, N.; Richards, M.; Kunugi, H. BDNF function and intracellular signaling in neurons. Histol. Histopathol. 2010, 25, 237–258. [Google Scholar]
- Mendez-David, I.; Tritschler, L.; Ali, Z.E.; Damiens, M.H.; Pallardy, M.; David, D.J.; Kerdine-Romer, S.; Gardier, A.M. Nrf2-signaling and BDNF: A new target for the antidepressant-like activity of chronic fluoxetine treatment in a mouse model of anxiety/depression. Neurosci. Lett. 2015, 597, 121–126. [Google Scholar] [CrossRef]
- Nibuya, M.; Morinobu, S.; Duman, R.S. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 1995, 15, 7539–7547. [Google Scholar] [CrossRef]
- Adachi, M.; Barrot, M.; Autry, A.E.; Theobald, D.; Monteggia, L.M. Selective loss of brain-derived neurotrophic factor in the dentate gyrus attenuates antidepressant efficacy. Biol. Psychiatry 2008, 63, 642–649. [Google Scholar] [CrossRef]
- Chen, G.; Guo, X. Neurobiology of Chinese Herbal Medicine on Major Depressive Disorder. Int. Rev. Neurobiol. 2017, 135, 77–95. [Google Scholar]
- Miyata, S.; Hattori, T.; Shimizu, S.; Ito, A.; Tohyama, M. Disturbance of oligodendrocyte function plays a key role in the pathogenesis of schizophrenia and major depressive disorder. Biomed. Res. Int. 2015, 2015, 492367. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Sun, P.; Qiao, M.; Wei, S.; Xue, L.; Zhang, H. Shu-Yu capsule, a Traditional Chinese Medicine formulation, attenuates premenstrual syndrome depression induced by chronic stress constraint. Mol. Med. Rep. 2014, 10, 2942–2948. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Zhang, Q.; Ung, C.O.; Wang, Y.; Han, Y.; Hu, Y.; Qi, J. An analysis of chemical ingredients network of Chinese herbal formulae for the treatment of coronary heart disease. PLoS ONE 2015, 10, e0116441. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, H.; Krenn, L.; Jiang, R.; Sutherland, I.; Ignatova, S.; Marmann, A.; Liang, X.; Sendker, J. The potential of metabolic fingerprinting as a tool for the modernisation of TCM preparations. J. Ethnopharmacol. 2012, 140, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, P.; Shin, C.Y. A comprehensive review of the therapeutic and pharmacological effects of ginseng and ginsenosides in central nervous system. J. Ginseng Res. 2013, 37, 8–29. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.C.; Hsieh, C.L. Pharmacological effects of Radix Angelica Sinensis (Danggui) on cerebral infarction. Chin. Med. 2011, 6, 32–36. [Google Scholar] [CrossRef]
- Yeh, J.C.; Cindrova-Davies, T.; Belleri, M.; Morbidelli, L.; Miller, N.; Cho, C.W.; Chan, K.; Wang, Y.T.; Luo, G.A.; Ziche, M.; et al. The natural compound n-butylidenephthalide derived from the volatile oil of Radix Angelica sinensis inhibits angiogenesis in vitro and in vivo. Angiogenesis 2011, 14, 187–197. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, J.; Deng, M.; Liu, Y.; Hu, Y.; Zhang, L. The Antidepressant Effect of Angelica sinensis Extracts on Chronic Unpredictable Mild Stress-Induced Depression Is Mediated via the Upregulation of the BDNF Signaling Pathway in Rats. Evid. Based Complement. Alternat. Med. 2016, 2016, 7434692. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, M.; Liu, P.; Guo, D.H.; Wei, R.B.; Rahman, K. Possible mechanism of the antidepressant effect of 3,6′-disinapoyl sucrose from Polygala tenuifolia Willd. J. Pharm. Pharmacol. 2011, 63, 869–874. [Google Scholar] [CrossRef]
- Li, H.; Lin, S.; Qin, T.; Li, H.; Ma, Z.; Ma, S. Senegenin exerts anti-depression effect in mice induced by chronic un-predictable mild stress via inhibition of NF-kappaB regulating NLRP3 signal pathway. Int. Immunopharmacol. 2017, 53, 24–32. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.; Xie, J. Simultaneous determination of jujuboside A, B and betulinic acid in semen Ziziphi spinosae by high performance liquid chromatography-evaporative light scattering detection. J. Pharm. Biomed. Anal. 2008, 48, 1467–1470. [Google Scholar] [CrossRef]
- Jeong, E.J.; Lee, H.K.; Lee, K.Y.; Jeon, B.J.; Kim, D.H.; Park, J.H.; Song, J.H.; Huh, J.; Lee, J.H.; Sung, S.H. The effects of lignan-riched extract of Shisandra chinensis on amyloid-beta-induced cognitive impairment and neurotoxicity in the cortex and hippocampus of mouse. J. Ethnopharmacol. 2013, 146, 347–354. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, X.; Liu, B.; Liu, A.J.; Li, H.; Mao, X.; Wu, B.; Bi, K.S.; Jia, Y. Jujuboside A, a neuroprotective agent from semen Ziziphi Spinosae ameliorates behavioral disorders of the dementia mouse model induced by Abeta 1-42. Eur. J. Pharmacol. 2014, 738, 206–213. [Google Scholar] [CrossRef]
- Li, B.; Fu, Z.; Hu, R.U.I.; Chen, Y.; Zhang, Z. Semen Ziziphi Spinosae and Fructus Gardeniae extracts synergistically improve learning and memory of a mouse model. Biomed. Rep. 2013, 1, 247–250. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, M.; Lu, X.; Wei, R.; Xu, J. Ziziphi spinosae lily powder suspension in the treatment of depression-like behaviors in rats. BMC Complement. Altern. Med. 2017, 17, 238. [Google Scholar] [CrossRef]
- Huston, J.P.; Komorowski, M.; de Souza Silva, M.A.; Lamounier-Zepter, V.; Nikolaus, S.; Mattern, C.; Muller, C.P.; Topic, B. Chronic corticosterone treatment enhances extinction-induced depression in aged rats. Horm. Behav. 2016, 86, 21–26. [Google Scholar] [CrossRef]
- Mei, L.; Mochizuki, M.; Hasegawa, N. Pycnogenol ameliorates depression-like behavior in repeated corticosterone-induced depression mice model. Biomed. Res. Int. 2014, 2014, 942927. [Google Scholar] [CrossRef]
- Ali, S.H.; Madhana, R.M.; Kv, A.; Kasala, E.R.; Bodduluru, L.N.; Pitta, S.; Mahareddy, J.R.; Lahkar, M. Resveratrol ameliorates depressive-like behavior in repeated corticosterone-induced depression in mice. Steroids 2015, 101, 37–42. [Google Scholar] [CrossRef]
- Wang, S.S.; Mu, R.H.; Li, C.F.; Dong, S.Q.; Geng, D.; Liu, Q.; Yi, L.T. microRNA-124 targets glucocorticoid receptor and is involved in depression-like behaviors. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 79, 417–425. [Google Scholar] [CrossRef]
- Cheng, J.; Dong, S.; Yi, L.; Geng, D.; Liu, Q. Magnolol abrogates chronic mild stress-induced depressive-like behaviors by inhibiting neuroinflammation and oxidative stress in the prefrontal cortex of mice. Int. Immunopharmacol. 2018, 59, 61–67. [Google Scholar] [CrossRef]
- Lindqvist, D.; Dhabhar, F.S.; James, S.J.; Hough, C.M.; Jain, F.A.; Bersani, F.S.; Reus, V.I.; Verhoeven, J.E.; Epel, E.S.; Mahan, L.; et al. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 2017, 76, 197–205. [Google Scholar] [CrossRef]
- Singh, M.; Kapoor, A.; Bhatnagar, A. Oxidative and reductive metabolism of lipid-peroxidation derived carbonyls. Chem. Biol. Interact. 2015, 234, 261–273. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]
- Wang, Q.; Dong, X.; Li, N.; Wang, Y.; Guan, X.; Lin, Y.; Kang, J.; Zhang, X.; Zhang, Y.; Li, X.; et al. JSH-23 prevents depressive-like behaviors in mice subjected to chronic mild stress: Effects on inflammation and antioxidant defense in the hippocampus. Pharmacol. Biochem. Behav. 2018, 169, 59–66. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Maker, G.L.; Hood, S.D.; Drummond, P.D. A review of peripheral biomarkers in major depression: The potential of inflammatory and oxidative stress biomarkers. Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 48, 102–111. [Google Scholar] [CrossRef]
- Gałecki, P.; Szemraj, J.; Bieńkiewicz, M.; Florkowski, A.; Gałecka, E. Lipid peroxidation and antioxidant protection in patients during acute depressive episodes and in remission after fluoxetine treatment. Pharmacol. Rep. 2009, 61, 436–447. [Google Scholar] [CrossRef]
- Olianas, M.C.; Dedoni, S.; Onali, P. LPA1 is a key mediator of intracellular signalling and neuroprotection triggered by tetracyclic antidepressants in hippocampal neurons. J. Neurochem. 2017, 143, 183–197. [Google Scholar] [CrossRef]
- Tavares, M.K.; Dos Reis, S.; Platt, N.; Heinrich, I.A.; Wolin, I.A.V.; Leal, R.B.; Kaster, M.P.; Rodrigues, A.L.S.; Freitas, A.E. Agmatine potentiates neuroprotective effects of subthreshold concentrations of ketamine via mTOR/S6 kinase signaling pathway. Neurochem. Int. 2018, 118, 275–285. [Google Scholar] [CrossRef]
- Hu, R.-F.; Sun, X.-B. Design of new traditional Chinese medicine herbal formulae for treatment of type 2 diabetes mellitus based on network pharmacology. Chin. J. Nat. Med. 2017, 15, 436–441. [Google Scholar] [CrossRef]
- Cryan, J.F.; Mombereau, C.; Vassout, A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005, 29, 571–625. [Google Scholar] [CrossRef]
- Liu, K.F.; Li, Y.; Cheng, K.C.; Hsu, C.C.; Cheng, J.T.; Peng, W.H. Changes in PPARdelta expression in a rat model of stress-induced depression. Clin. Exp. Pharmacol. Physiol. 2017, 44, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Mcintyre, R.S.; Xiao, H.X.; Syeda, K.; Vinberg, M.; Carvalho, A.F.; Mansur, R.B.; Maruschak, N.; Cha, D.S. The prevalence, measurement, and treatment of the cognitive dimension/domain in major depressive disorder. CNS Drugs 2015, 29, 577–589. [Google Scholar] [CrossRef] [PubMed]
- Leal, G.; Comprido, D.; Duarte, C.B. BDNF-induced local protein synthesis and synaptic plasticity. Neuropharmacology 2014, 76 Pt C, 639–656. [Google Scholar] [CrossRef]
- Bjorkholm, C.; Monteggia, L.M. BDNF—A key transducer of antidepressant effects. Neuropharmacology 2016, 102, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Poo, M.M. Neurotrophin regulation of neural circuit development and function. Nat. Rev. Neurosci. 2013, 14, 7–23. [Google Scholar] [CrossRef]
- Autry, A.E.; Monteggia, L.M. Brain-Derived Neurotrophic Factor and Neuropsychiatric Disorders. Pharmacol. Rev. 2012, 64, 238–258. [Google Scholar] [CrossRef]
- Masi, G.; Brovedani, P. The hippocampus, neurotrophic factors and depression: Possible implications for the pharmacotherapy of depression. CNS Drugs 2011, 25, 913–931. [Google Scholar] [CrossRef]
- Liu, Z.; Qi, Y.; Cheng, Z.; Zhu, X.; Fan, C.; Yu, S.Y. The effects of ginsenoside Rg1 on chronic stress induced depression-like behaviors, BDNF expression and the phosphorylation of PKA and CREB in rats. Neuroscience 2016, 322, 358–369. [Google Scholar] [CrossRef]
- Neto, F.L.; Borges, G.; Torres-Sanchez, S.; Mico, J.A.; Berrocoso, E. Neurotrophins role in depression neurobiology: A review of basic and clinical evidence. Curr. Neuropharmacol. 2011, 9, 530–532. [Google Scholar] [CrossRef]
- Maes, M.; De Vos, N.; Pioli, R.; Demedts, P.; Wauters, A.; Neels, H.; Christophe, A. Lower serum vitamin E concentrations in major depression. Another marker of lowered antioxidant defenses in that illness. J. Affect. Disord. 2000, 58, 241–246. [Google Scholar] [CrossRef]
- Khanzode, S.D.; Dakhale, G.N.; Khanzode, S.S.; Saoji, A.; Palasodkar, R. Oxidative damage and major depression: The potential antioxidant action of selective serotonin re-uptake inhibitors. Redox Rep. 2003, 8, 365–370. [Google Scholar] [CrossRef]
- Ravindran, A.V.; Griffiths, J.; Merali, Z.; Anisman, H. Lymphocyte subsets associated with major depression and dysthymia: Modification by antidepressant treatment. Psychosom. Med. 1995, 57, 555–563. [Google Scholar] [CrossRef]
- Rybka, J.; Kedziora-Kornatowska, K.; Banas-Lezanska, P.; Majsterek, I.; Carvalho, L.A.; Cattaneo, A.; Anacker, C.; Kedziora, J. Interplay between the pro-oxidant and antioxidant systems and proinflammatory cytokine levels, in relation to iron metabolism and the erythron in depression. Free Radic. Biol. Med. 2013, 63, 187–194. [Google Scholar] [CrossRef]
| Scientific Name | Family Name | Source | Proportion of PAPZ |
|---|---|---|---|
| Panax ginseng C.A. Meyer | Araliaceae | Beijing, China | 25% |
| Angelica sinensis (Oliv.) Diels | Umbelliferae | Gansu, China | 25% |
| Polygala tenuifolia Willd | Polygalaceae | Shanxi, China | 25% |
| Ziziphi spinosae Semen | Rhamnaceae | Hebei, China | 25% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Huang, Q.; Zhang, S.; Hu, K.; Xiong, W.; Xiao, L.; Cong, R.; Liu, Q.; Wang, Z. The Chinese Herbal Formula PAPZ Ameliorates Behavioral Abnormalities in Depressive Mice. Nutrients 2019, 11, 859. https://doi.org/10.3390/nu11040859
Chen H, Huang Q, Zhang S, Hu K, Xiong W, Xiao L, Cong R, Liu Q, Wang Z. The Chinese Herbal Formula PAPZ Ameliorates Behavioral Abnormalities in Depressive Mice. Nutrients. 2019; 11(4):859. https://doi.org/10.3390/nu11040859
Chicago/Turabian StyleChen, Huiling, Qing Huang, Shunjia Zhang, Kaiqiang Hu, Wenxiang Xiong, Lingyun Xiao, Renhuai Cong, Qingfei Liu, and Zhao Wang. 2019. "The Chinese Herbal Formula PAPZ Ameliorates Behavioral Abnormalities in Depressive Mice" Nutrients 11, no. 4: 859. https://doi.org/10.3390/nu11040859
APA StyleChen, H., Huang, Q., Zhang, S., Hu, K., Xiong, W., Xiao, L., Cong, R., Liu, Q., & Wang, Z. (2019). The Chinese Herbal Formula PAPZ Ameliorates Behavioral Abnormalities in Depressive Mice. Nutrients, 11(4), 859. https://doi.org/10.3390/nu11040859





