Energy, Macronutrient Intake, and Anthropometrics of Vegetarian, Vegan, and Omnivorous Children (1–3 Years) in Germany (VeChi Diet Study)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Sample Size Estimation
2.3. Data Assessment
2.3.1. Nutrition Assessment
2.3.2. Anthropometrics
2.3.3. Covariates
2.3.4. Diet Group Classification
- vegetarian (no meat, sausage, fish, but with dairy products and/or eggs);
- vegan (no meat, sausage, fish, dairy products, or eggs);
- omnivorous (with meat and/or sausage and/or fish).
2.3.5. Data Analysis and Statistics
3. Results
3.1. Sample Characteristics
3.2. Energy and Nutrient Intakes
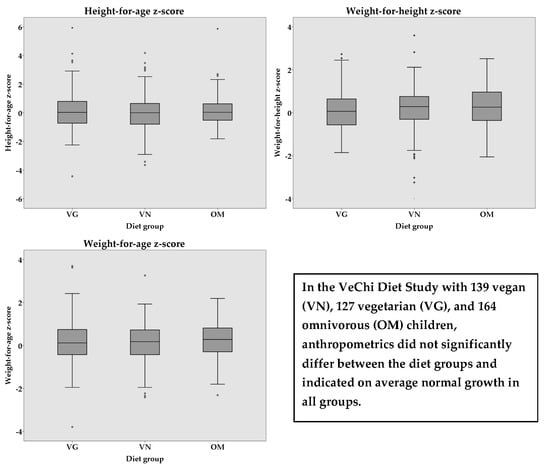
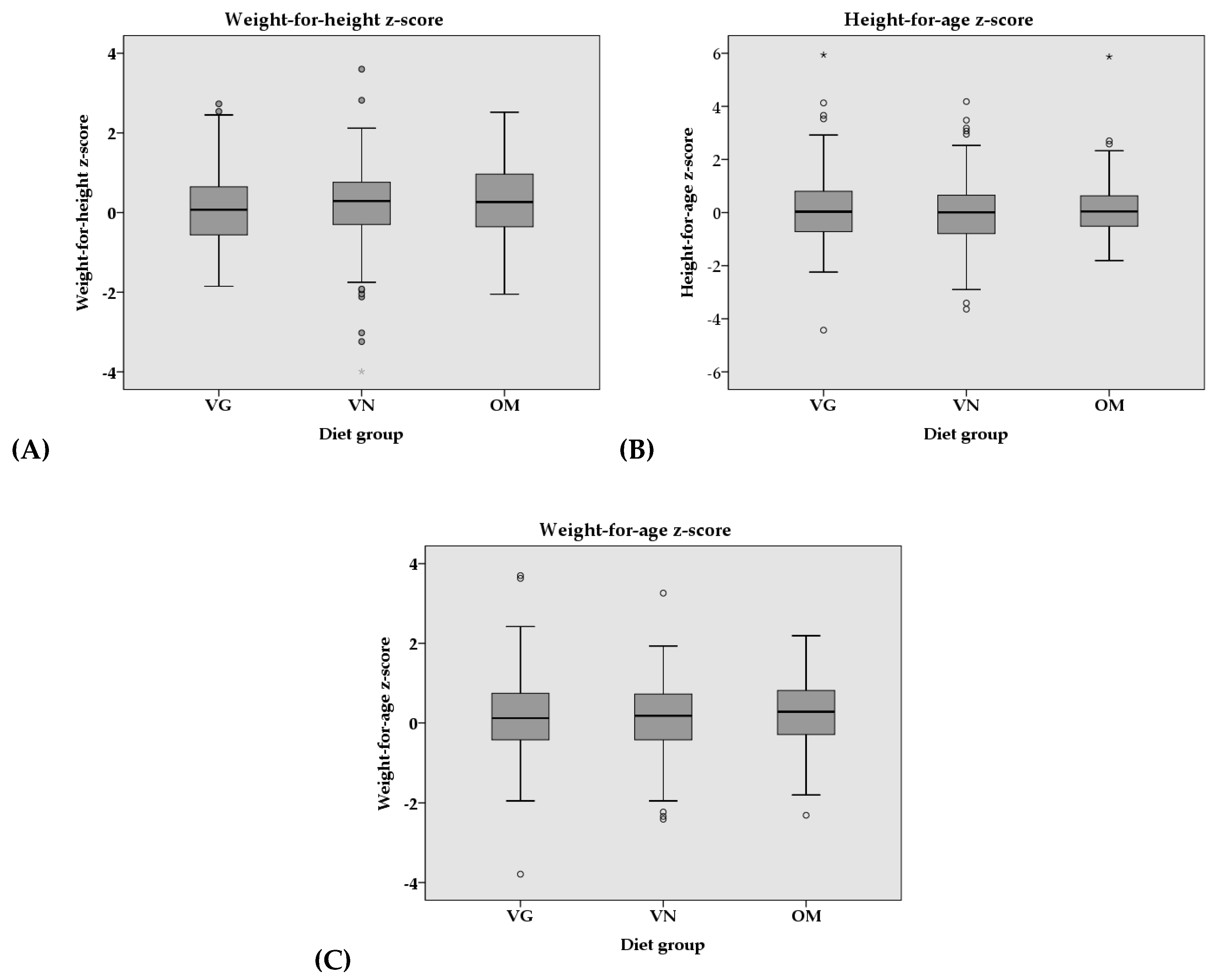
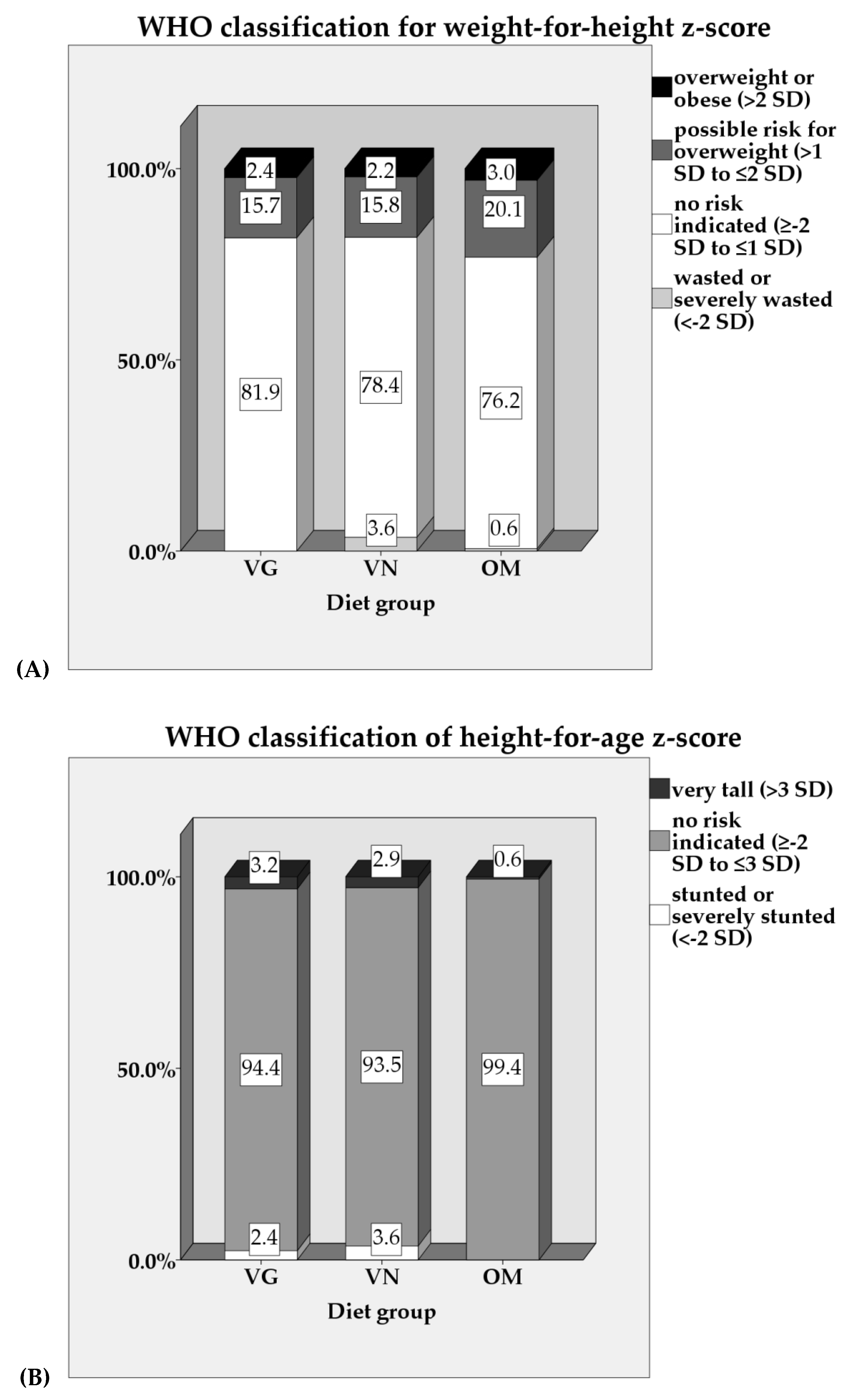
3.3. Anthropometrics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mensink, G.B.M.; Lage Barbosa, C.; Brettschneider, A.-K. Verbreitung der vegetarischen Ernährungsweise in Deutschland. JoHM 2016, 1, 2–15. [Google Scholar] [CrossRef]
- Pfeiler, T.M.; Egloff, B. Examining the “Veggie” personality. Results from a representative German sample. Appetite 2018, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Vegetarierbund (VEBU) Deutschland e. V. Anzahl der Vegetarier in Deutschland. Available online: https://vebu.de/veggie-fakten/entwicklung-in-zahlen/anzahl-veganer-und-vegetarier-in-deutschland/ (accessed on 11 February 2019).
- YouGov Deutschland AG. Wer will’s schon vegan. Aktuelle Ernährungsvorlieben und Lieblingsmarken in Deutschland 2014—Typ für Typ. Available online: https://yougov.de/loesungen/reports/studien/vegan-studie/ (accessed on 11 February 2019).
- SKOPOS Group. 1,3 Millionen Deutsche Leben Vegan. Available online: https://www.skopos.de/news/13-millionen-deutsche-leben-vegan.html (accessed on 11 February 2019).
- DGE; ÖGE; SGE. Referenzwerte für die Nährstoffzufuhr, 2. Auflage, 2., Aktualisierte Ausgabe; Deutsche Gesellschaft für Ernährung; Österreichische Gesellschaft für Ernährung; Schweizerische Gesellschaft für Ernährung: Bonn, Germany, 2016; ISBN 978-3-86528-148-7. [Google Scholar]
- Richter, M.; Boeing, H.; Grünewald-Funk, D.; Heseker, H.; Kroke, A.; Leschik-Bonnet, E.; Oberritter, H.; Strohm, D.; Watzl, B. For the German Nutrition Society. Vegan diet. Position of the German Nutrition Society (DGE). Ernähr. Umsch. 2016, 63, 92–102. [Google Scholar] [CrossRef]
- Pawlak, R. To vegan or not to vegan when pregnant, lactating or feeding young children. Eur. J. Clin. Nutr. 2017, 71, 1259–1262. [Google Scholar] [CrossRef]
- Melina, V.; Craig, W.; Levin, S. Position of the Academy of Nutrition and Dietetics: Vegetarian Diets. J. Acad. Nutr. Diet 2016, 116, 1970–1980. [Google Scholar] [CrossRef]
- Amit, M. Vegetarian diets in children and adolescents. Paediatr. Child. Health 2010, 15, 303–308. [Google Scholar] [CrossRef]
- American Academy of Pediatrics Commitee on Nutrition. Nutritional Aspects of Vegetarian Diets. In Pediatric Nutrition, 7th ed.; Kleinman, R.E., Greer, F.R., Eds.; American Academy of Pediatrics: Elk Grove Village, IL, USA, 2014; pp. 241–264. ISBN 978-1-58110-816-3. [Google Scholar]
- Schürmann, S.; Kersting, M.; Alexy, U. Vegetarian diets in children: A systematic review. Eur. J. Nutr. 2017, 56, 1797–1817. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.; Müller, S. Vegetarian and vegan diets in children-pre-study with preliminary data. Ann. Nutr. Metab. 2015, 1–601. [Google Scholar] [CrossRef]
- O’Connell, J.M.; Dibley, M.J.; Sierra, J.; Wallace, B.; Marks, J.S.; Yip, R. Growth of vegetarian children: The Farm Study. Pediatrics 1989, 84, 475–481. [Google Scholar] [PubMed]
- Giugliani, E.R.J. Growth in exclusively breastfed infants. J. Pediatr. 2019, 95, 79–84. [Google Scholar] [CrossRef]
- Kristensen, N.B.; Madsen, M.L.; Hansen, T.H.; Allin, K.H.; Hoppe, C.; Fagt, S.; Lausten, M.S.; Gobel, R.J.; Vestergaard, H.; Hansen, T.; et al. Intake of macro- and micronutrients in Danish vegans. Nutr. J. 2015, 14, 115. [Google Scholar] [CrossRef]
- Nathan, I.; Hackett, A.F.; Kirby, S. A longitudinal study of the growth of matched pairs of vegetarian and omnivorous children, aged 7–11 years, in the north-west of England. Eur. J. Clin. Nutr. 1997, 51, 20–25. [Google Scholar] [CrossRef]
- Sabate, J.; Wien, M. Vegetarian diets and childhood obesity prevention. Am. J. Clin. Nutr. 2010, 91, 1525–1529. [Google Scholar] [CrossRef]
- Turner-McGrievy, G.; Mandes, T.; Crimarco, A. A plant-based diet for overweight and obesity prevention and treatment. J. Geriatr. Cardiol. 2017, 14, 369–374. [Google Scholar] [CrossRef]
- Newby, P.K. Plant foods and plant-based diets: Protective against childhood obesity? Am. J. Clin. Nutr. 2009, 89, 1572S–1587S. [Google Scholar] [CrossRef]
- Fewtrell, M.; Bronsky, J.; Campoy, C.; Domellof, M.; Embleton, N.; Fidler Mis, N.; Hojsak, I.; Hulst, J.M.; Indrio, F.; Lapillonne, A.; et al. Complementary Feeding: A Position Paper by the European Society for Paediatric Gastroenterology, Hepatology, and Nutrition (ESPGHAN) Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 119–132. [Google Scholar] [CrossRef]
- Sobiecki, J.G.; Appleby, P.N.; Bradbury, K.E.; Key, T.J. High compliance with dietary recommendations in a cohort of meat eaters, fish eaters, vegetarians, and vegans: Results from the European Prospective Investigation into Cancer and Nutrition-Oxford study. Nutr. Res. 2016, 36, 464–477. [Google Scholar] [CrossRef]
- Rizzo, N.S.; Jaceldo-Siegl, K.; Sabate, J.; Fraser, G.E. Nutrient profiles of vegetarian and nonvegetarian dietary patterns. J. Acad. Nutr. Diet 2013, 113, 1610–1619. [Google Scholar] [CrossRef]
- Elorinne, A.L.; Alfthan, G.; Erlund, I.; Kivimaki, H.; Paju, A.; Salminen, I.; Turpeinen, U.; Voutilainen, S.; Laakso, J. Food and Nutrient Intake and Nutritional Status of Finnish Vegans and Non-Vegetarians. PLoS ONE 2016, 11, e0148235. [Google Scholar] [CrossRef]
- Schüpbach, R.; Wegmüller, R.; Berguerand, C.; Bui, M.; Herter-Aeberli, I. Micronutrient status and intake in omnivores, vegetarians and vegans in Switzerland. Eur. J. Nutr. 2017, 56, 283–293. [Google Scholar] [CrossRef]
- Davey, G.K.; Spencer, E.A.; Appleby, P.N.; Allen, N.E.; Knox, K.H.; Key, T.J. EPIC-Oxford: Lifestyle characteristics and nutrient intakes in a cohort of 33 883 meat-eaters and 31,546 non meat-eaters in the UK. Public Health Nutr. 2003, 6, 259–269. [Google Scholar] [CrossRef]
- Sanders, T.A. Growth and development of British vegan children. Am. J. Clin. Nutr. 1988, 48, 822–825. [Google Scholar] [CrossRef]
- Ambroszkiewicz, J.; Laskowska-Klita, T.; Klemarczyk, W. Low levels of osteocalcin and leptin in serum of vegetarian prepubertal children [Abstract]. Med. Wieku Rozwoj 2003, 7, 587–591. [Google Scholar]
- Laskowska-Klita, T.; Chelchowska, M.; Ambroszkiewicz, J.; Gajewska, J.; Klemarczyk, W. The effect of vegetarian diet on selected essential nutrients in children. Med. Wieku Rozwoj 2011, 15, 318–325. [Google Scholar]
- Fulton, J.R.; Hutton, C.W.; Stitt, K.R. Preschool vegetarian children. Dietary and anthropometric data [Abstract]. J. Am. Diet Assoc. 1980, 76, 360–365. [Google Scholar] [PubMed]
- Ambroszkiewicz, J.; Klemarczyk, W.; Gajewska, J.; Chelchowska, M.; Laskowska-Klita, T. Serum concentration of biochemical bone turnover markers in vegetarian children. Adv. Med. Sci. 2007, 52, 279–282. [Google Scholar]
- Taylor, A.; Redworth, E.W.; Morgan, J.B. Influence of diet on iron, copper, and zinc status in children under 24 months of age. Biol Trace Elem. Res. 2004, 97, 197–214. [Google Scholar] [CrossRef]
- Gorczyca, D.; Prescha, A.; Szeremeta, K. Impact of vegetarian diet on serum immunoglobulin levels in children. Clin. Pediatr. 2013, 52, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Sanders, T.A.; Purves, R. An anthropometric and dietary assessment of the nutritional status of vegan preschool children [Abstract]. J. Hum. Nutr. 1981, 35, 349–357. [Google Scholar] [PubMed]
- Ambroszkiewicz, J.; Klemarczyk, W.; Chelchowska, M.; Gajewska, J.; Laskowska-Klita, T. Serum homocysteine, folate, vitamin B12 and total antioxidant status in vegetarian children. Adv. Med. Sci. 2006, 51, 265–268. [Google Scholar]
- Thane, C.W.; Bates, C.J. Dietary intakes and nutrient status of vegetarian preschool children from a British national survey. J. Hum. Nutr. Diet 2000, 13, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Koebnick, C.; Garcia, A.L.; Dagnelie, P.C.; Strassner, C.; Lindemans, J.; Katz, N.; Leitzmann, C.; Hoffmann, I. Long-term consumption of a raw food diet is associated with favorable serum LDL cholesterol and triglycerides but also with elevated plasma homocysteine and low serum HDL cholesterol in humans. J. Nutr. 2005, 135, 2372–2378. [Google Scholar] [CrossRef] [PubMed]
- Kroke, A.; Manz, F.; Kersting, M.; Remer, T.; Sichert-Hellert, W.; Alexy, U.; Lentze, M.J. The DONALD Study. History, current status and future perspectives. Eur. J. Nutr. 2004, 43, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Hilbig, A.; Drossard, C.; Kersting, M.; Alexy, U. Nutrient Adequacy and Associated Factors in a Nationwide Sample of German Toddlers. J. Pediatr. Gastroenterol. Nutr. 2015, 61, 130–137. [Google Scholar] [CrossRef]
- Sichert-Hellert, W.; Kersting, M.; Chahda, C.; Schäfer, R.; Kroke, A. German food composition database for dietary evaluations in children and adolescents. J. Food. Compos. Anal. 2007, 20, 63–70. [Google Scholar] [CrossRef]
- Hölling, H.; Schlack, R.; Kamtsiuris, P.; Butschalowsky, H.; Schlaud, M.; Kurth, B.M. Die KiGGS-Studie. Bundesweit repräsentative Längs-und Querschnittstudie zur Gesundheit von Kindern und Jugendlichen im Rahmen des Gesundheitsmonitorings am Robert Koch-Institut. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2012, 55, 836–842. [Google Scholar] [CrossRef]
- Winkler, J.; Stolzenberg, H. Adjustierung des Sozialen-Schicht-Index für die Anwendung im Kinder-und Jugendgesundheitssurvey (KiGGS) 2003/2006; Hochschule Wismar, Fakultät für Wirtschaftswissenschaften: Wismar, Germany, 2009; ISBN 978-3-939159-76-6. [Google Scholar]
- Knopf, H.; Hölling, H.; Huss, M.; Schlack, R. Prevalence, determinants and spectrum of attention-deficit hyperactivity disorder (ADHD) medication of children and adolescents in Germany: Results of the German Health Interview and Examination Survey (KiGGS). BMJ Open 2012, 2, e000477. [Google Scholar] [CrossRef]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef]
- World Health Organization. Physical Status: The Use and Interpretation of Anthropometry. REPORT of a WHO Expert Committee; World Health Organization: Geneva, Switzerland, 1995; ISBN 92-4-120854-6. [Google Scholar]
- Fernando, N.N.T.; Campbell, K.J.; McNaughton, S.A.; Zheng, M.; Lacy, K.E. Predictors of Dietary Energy Density among Preschool Aged Children. Nutrients 2018, 10, 178. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Child Growth Standards. WHO Anthro (Version 3.2.2, January 2011) and Macros. Available online: http://www.who.int/childgrowth/software/en/ (accessed on 6 September 2017).
- World Health Organization. Training Course on Child Growth Assessment. Module C: Interpreting Growth Indicators; World Health Organization: Geneva, Switzerland, 2008; ISBN 978-92-4-159507-0. [Google Scholar]
- World Health Organization. Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; World Health Organization: Geneva, Switzerland, 2006; ISBN 92-4-15-4693-X. [Google Scholar]
- Richard, S.A.; Black, R.E.; Checkley, W. Revisiting the relationship of weight and height in early childhood. Adv. Nutr. 2012, 3, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Joint WHO/FAO/UNU Expert Consultation. Protein and Amino Acid Requirements in Human Nutrition; World Health Organization: Geneva, Switzerland, 2007; ISBN 9241209356. [Google Scholar]
- Pallant, J. SPSS Survival Manual. A Step by Step Guide to Data Analysis Using SPSS for Windows, 3rd ed.; Open University Press: Maidenhead, UK, 2007; ISBN 0335223664. [Google Scholar]
- DGE; ÖGE; SGE; SVE. Referenzwerte für die Nährstoffzufuhr, 2. Aufl., 2. akt. Ausgabe; Neuer Umschau Buchverlag: Bonn, Germany, 2016; ISBN 3887492420. [Google Scholar]
- Gorczyca, D.; Prescha, A.; Szeremeta, K.; Jankowski, A. Iron status and dietary iron intake of vegetarian children from Poland. Ann. Nutr. Metab. 2013, 62, 291–297. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion on Dietary Reference Values for energy. EFSA J. 2013, 11, 3005. [Google Scholar] [CrossRef]
- Henry, C.J.K. Basal metabolic rate studies in humans: Measurement and development of new equations. Public Health Nutr. 2005, 8, 1133–1152. [Google Scholar] [CrossRef]
- Alexy, U.; Hilbig, A. Are Belgian toddlers over-eating? Eur. J. Nutr. 2017, 56, 445–446. [Google Scholar] [CrossRef]
- Mangels, A.R.; Messina, V. Considerations in planning vegan diets: Infants. J. Am. Diet Assoc. 2001, 101, 670–677. [Google Scholar] [CrossRef]
- Institute of Medicine of the National Academies. Dietary Reference Intakes for Energy, Carbohydrates, Fiber, Fat, Protein and Amino Acids (Macronutrients); The National Academies Press: Washington, DC, USA, 2005; ISBN 978-0-309-08525-0. [Google Scholar]
- Michaelsen, K.F.; Greer, F.R. Protein needs early in life and long-term health. Am. J. Clin. Nutr. 2014, 99, 718S–722S. [Google Scholar] [CrossRef]
- Kamper, A.-L.; Strandgaard, S. Long-Term Effects of High-Protein Diets on Renal Function. Annu. Rev. Nutr. 2017, 37, 347–369. [Google Scholar] [CrossRef]
- Van Elswyk, M.E.; Weatherford, C.A.; McNeill, S.H. A Systematic Review of Renal Health in Healthy Individuals Associated with Protein Intake above the US Recommended Daily Allowance in Randomized Controlled Trials and Observational Studies. Adv. Nutr. 2018, 9, 404–418. [Google Scholar] [CrossRef]
- Agostoni, C.; Decsi, T.; Fewtrell, M.; Goulet, O.; Kolacek, S.; Koletzko, B.; Michaelsen, K.F.; Moreno, L.; Puntis, J.; Rigo, J.; et al. Complementary feeding: A commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 99–110. [Google Scholar] [CrossRef]
- Agostoni, C.; Scaglioni, S.; Ghisleni, D.; Verduci, E.; Giovannini, M.; Riva, E. How much protein is safe? Int. J. Obes. 2005, 29 (Suppl. S2), S8–S13. [Google Scholar] [CrossRef]
- Wolfram, G.; Bechthold, A.; Boeing, H.; Ellinger, S.; Hauner, H.; Kroke, A.; Leschik-Bonnet, E.; Linseisen, J.; Lorkowski, S.; Schulze, M.; et al. Evidence-Based Guideline of the German Nutrition Society: Fat Intake and Prevention of Selected Nutrition-Related Diseases. Ann. Nutr. Metab. 2015, 67, 141–204. [Google Scholar] [CrossRef] [PubMed]
- Hauner, H.; Bechthold, A.; Boeing, H.; Brönstrup, A.; Buyken, A.; Leschik-Bonnet, E.; Linseisen, J.; Schulze, M.; Strohm, D.; Wolfram, G. Evidence-based guideline of the German Nutrition Society: Carbohydrate intake and prevention of nutrition-related diseases. Ann. Nutr. Metab. 2012, 60, 1–58. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Sugar Intake for Adult and Children. Guideline; World Health Organization: Geneva, Switzerland, 2015; ISBN 9241549025. [Google Scholar]
- Baroni, L.; Goggi, S.; Battaglino, R.; Berveglieri, M.; Fasan, I.; Filippin, D.; Griffith, P.; Rizzo, G.; Tomasini, C.; Tosatti, M.A.; et al. Vegan Nutrition for Mothers and Children: Practical Tools for Healthcare Providers. Nutrients 2018, 11. [Google Scholar] [CrossRef] [PubMed]
- WHO Multicentre Growth Reference Study Group. WHO Child Growth Standards based on length/height, weight and age. Acta Paediatr. Suppl. 2006, 450, 76–85. [Google Scholar] [CrossRef]
- Prendergast, A.J.; Humphrey, J.H. The stunting syndrome in developing countries. Paediatr. Int. Child. Health 2014, 34, 250–265. [Google Scholar] [CrossRef] [PubMed]
- Dewey, K.G. Nutrition, Growth, and Complementary Feeding of The Brestfed Infant [Abstract]. Pediatr. Clin. N. Am. 2001, 48, 87–104. [Google Scholar] [CrossRef]
- Dwyer, J.T.; Andrew, E.M.; Valadian, I.; Reed, R.B. Size, obesity, and leanness in vegetarian preschool children [Abstract]. J. Am. Diet Assoc. 1980, 77, 434–439. [Google Scholar] [PubMed]
- Gorczyca, D.; Paściak, M.; Szponar, B.; Gamian, A.; Jankowski, A. An impact of the diet on serum fatty acid and lipid profiles in Polish vegetarian children and children with allergy. Eur. J. Clin. Nutr. 2011, 65, 191–195. [Google Scholar] [CrossRef] [PubMed]
- Kerac, M.; Blencowe, H.; Grijalva-Eternod, C.; McGrath, M.; Shoham, J.; Cole, T.J.; Seal, A. Prevalence of wasting among under 6-month-old infants in developing countries and implications of new case definitions using WHO growth standards: A secondary data analysis. Arch. Dis. Child 2011, 96, 1008–1013. [Google Scholar] [CrossRef]
- Martorell, R.; Young, M.F. Patterns of stunting and wasting: Potential explanatory factors. Adv. Nutr. 2012, 3, 227–233. [Google Scholar] [CrossRef]
- Dwyer, J.T.; Dietz, W.H., Jr.; Andrews, E.M.; Suskind, R.M. Nutritional status of vegetarian children. Am. J. Clin. Nutr. 1982, 35, 204–216. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.T.; Palombo, R.; Valadian, I.; Reed, R.B. Preschoolers on alternate life-style diets. Associations between size and dietary indexes with diets limited in types of animal foods [Abstract]. J. Am. Diet Assoc. 1978, 72, 264–270. [Google Scholar] [PubMed]
- Dwyer, J.T.; Andrew, E.M.; Berkey, C.; Valadian, I.; Reed, R.B. Growth in “new” vegetarian preschool children using the Jenss-Bayley curve fitting technique. Am. J. Clin. Nutr. 1983, 37, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Dagnelie, P.C.; van Staveren, W.A.; Verschuren, S.A.; Hautvast, J.G. Nutritional status of infants aged 4 to 18 months on macrobiotic diets and matched omnivorous control infants: A population-based mixed-longitudinal study. I. Weaning pattern, energy and nutrient intake. Eur. J. Clin. Nutr. 1989, 43, 311–323. [Google Scholar] [PubMed]
- Dagnelie, P.C.; Vergote, F.J.; van Staveren, W.A.; van den Berg, H.; Dingjan, P.G.; Hautvast, J.G. High prevalence of rickets in infants on macrobiotic diets. Am. J. Clin. Nutr. 1990, 51, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Dagnelie, P.C.; van Staveren, W.A. Macrobiotic nutrition and child health: Results of a population-based, mixed-longitudinal cohort study in The Netherlands. Am. J. Clin. Nutr. 1994, 59, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Van Staveren, W.A.; Dagnelie, P.C. Food consumption, growth, and development of Dutch children fed on alternative diets. Am. J. Clin. Nutr. 1988, 48, 819–821. [Google Scholar] [CrossRef]
- Geserick, M.; Vogel, M.; Gausche, R.; Lipek, T.; Spielau, U.; Keller, E.; Pfaffle, R.; Kiess, W.; Korner, A. Acceleration of BMI in Early Childhood and Risk of Sustained Obesity. N. Engl. J. Med. 2018, 379, 1303–1312. [Google Scholar] [CrossRef]
- Himes, J.H. Challenges of accurately measuring and using BMI and other indicators of obesity in children. Pediatrics 2009, 124. [Google Scholar] [CrossRef]
- Huybrechts, I.; Himes, J.H.; Ottevaere, C.; de Vriendt, T.; de Keyzer, W.; Cox, B.; van Trimpont, I.; de Bacquer, D.; de Henauw, S. Validity of parent-reported weight and height of preschool children measured at home or estimated without home measurement: A validation study. BMC Pediatrics 2011, 11, 63. [Google Scholar] [CrossRef]
- Nelson, M.; Black, A.E.; Morris, J.A.; Cole, T.J. Between- and within-subject variation in nutrient intake from infancy to old age: Estimating the number of days required to rank dietary intakes with desired precision. Am. J. Clin. Nutr. 1989, 50, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Lanigan, J.A.; Wells, J.C.K.; Lawson, M.S.; Cole, T.J.; Lucas, A. Number of days needed to assess energy and nutrient intake in infants and young children between 6 months and 2 years of age. Eur. J. Clin. Nutr. 2004, 58, 745–750. [Google Scholar] [CrossRef]
- Haddad, E.H.; Tanzman, J.S. What do vegetarians in the United States eat? Am. J. Clin. Nutr. 2003, 78, 626S–632S. [Google Scholar] [CrossRef] [PubMed]
- White, R.F.; Seymour, J.; Frank, E. Vegetarianism among US women physicians. J. Am. Diet Assoc. 1999, 99, 595–598. [Google Scholar] [CrossRef]
- Bedford, J.L.; Barr, S.I. Diets and selected lifestyle practices of self-defined adult vegetarians from a population-based sample suggest they are more ‘health conscious’. Int. J. Behav. Nutr. Phys. Act. 2005, 2, 4. [Google Scholar] [CrossRef]
- Barr, S.I.; Chapman, G.E. Perceptions and practices of self-defined current vegetarian, former vegetarian, and nonvegetarian women. J. Am. Diet Assoc. 2002, 102, 354–360. [Google Scholar] [CrossRef]
- Burrows, T.L.; Martin, R.J.; Collins, C.E. A systematic review of the validity of dietary assessment methods in children when compared with the method of doubly labeled water. J. Am. Diet Assoc. 2010, 110, 1501–1510. [Google Scholar] [CrossRef]
- Sichert-Hellert, W.; Kersting, M.; Schöch, G. Underreporting of energy intake in 1 to 18 year old German children and adolescents. Z. Ernahrungswiss 1998, 37, 242–251. [Google Scholar] [CrossRef] [PubMed]
| VG | VN | OM | |
|---|---|---|---|
| Total 3 | 127 (29.5) | 139 (32.3) | 164 (38.1) |
| Sex 3 x | |||
| Female | 64 (50.4) | 76 (54.7) | 83 (50.3) |
| Male | 63 (49.6) | 63 (45.3) | 81 (49.4) |
| Age 2 z | |||
| Age_Diet (year) | 2.0 (1.4) | 1.8 (1.3) | 2.0 (1.5) |
| Age_Anthro (year) | 1.9 (1.4) | 1.8 (1.4) | 2.0 (1.5) |
| Child weight and height 1 z | |||
| BW (kg) | 12.1 ± 2.3 | 12.0 ± 2.5 | 12.7 ± 2.6 |
| BH (cm) | 86.6 ± 8.8 | 85.6 ± 8.8 | 88.2 ± 9.3 |
| Urbanicity 3 x # | |||
| Rural (<5,000) | 19 (15.0) | 28 (20.1) | 28 (17.2) |
| Small-size urban (5,000-<20,000) | 16 (12.6) | 20 (14.4) | 17 (10.4) |
| Medium-size urban (20,000-<100,000) | 33 (26.0) | 31 (22.3) | 31 (19.0) |
| Metropolitan (≥100,000) | 59 (46.5) | 60 (43.2) | 87 (53.4) |
| Breastfeeding | |||
| Ever breastfed 3 y | 121 (95.3) | 138 (99.3) | 157 (95.7) |
| Duration of exclusively breastfeeding (mo) 2 z | 5.0 (2.0) a | 6.0 (2.0) a,b | 5.0 (2.0) b |
| Duration of total breastfeeding (mo) 2 z | 13.1 (10.0) a | 15.9 (10.0) a,b | 11.1 (7.0) b |
| Breast milk intake in dietary record (if breastfed) (g/day) (n = 119) 2 z | 275.0 (186.3) | 303.3 (280.0) | 163.3 (316.7) |
| SES (Winkler Index score D2004) 3 y | |||
| Low (3–8) | 3 (2.4) | 2 (1.4) | 0 (0) |
| Middle (9–14) | 31 (24.4) | 37 (26.6) | 30 (18.3) |
| High (15–21) | 93 (73.2) | 100 (71.9) | 134 (81.7) |
| Physical activity 3 x ## | |||
| Less active (<4 times/week) | 71 (55.5) | 70 (50.0) | 64 (39.8) |
| Active or very active | 57 (44.5) | 70 (50.0) | 97 (60.2) |
| Birth weight categories 3 x # | |||
| Small for gestational age (SGA) | 23 (17.8) | 26 (18.7) | 25 (15.5) |
| Appropriate for gestational age (AGA) | 101 (78.3) | 99 (71.2) | 134 (83.2) |
| Large for gestational age (LGA) | 5 (3.9) | 14 (10.1) | 2 (1.2) |
| Season of dietary record 3 x | |||
| Spring | 21 (16.5) | 45 (32.4) | 36 (22.0) |
| Summer | 20 (15.7) | 17 (12.2) | 38 (23.2) |
| Autumn | 41 (32.3) | 36 (25.9) | 43 (26.2) |
| Winter | 45 (35.4) | 41 (29.5) | 47 (28.7) |
| Paternal BMI and height | |||
| BMI (kg/m2) 2 z ## | 24.5 (3.9) | 24.3 (4.1) | 25.7 (3.1) |
| BH (cm) 1 z ## | 183 ± 7 a | 180 ± 7 a | 182 ± 7 |
| Median (IQR) | Basic model (age, sex adjusted) | Final model | |||||
|---|---|---|---|---|---|---|---|
| VG (n = 127) | VN (n = 139) | OM (n = 164) | p-value | Partial η2 | p-value | Partial η2 | |
| TEI a, kcal/day | 956 (790–1084) | 986 (821–1186) | 974 (856–1099) | 0.281 | 0.006 | 0.055 | 0.015 |
| DED b, kcal/g | 1.12 (0.98–1.33) | 1.05 (0.93–1.22) | 1.15 (1.02–1.35) | 0.009 # | 0.022 | 0.466 | 0.004 |
| Protein c, g/kg BW | 2.26 (1.83–2.65) 1 | 2.25 (1.82–2.76) 2 | 2.54 (2.16–3.06) 1,2 | <0.0001 *** | 0.054 | <0.0001 *** | 0.122 |
| Fat d, %E | 33.7 (29.7–36.6) | 33.6 (27.9–39.4) 1 | 32.6 (28.2–37.2) 1 | 0.781 | 0.001 | <0.0001 *** | 0.049 |
| Carbohydrates e, %E | 53.6 (50.5–58.2) | 53.8 (49.4–59.3) 1 | 53.1 (47.9–57.1) 1 | 0.029 | 0.017 | <0.0001 *** | 0.070 |
| Added sugars f, %E | 4.2 (1.1–6.6) | 2.1 (0.6–5.7) | 4.8 (2.2–8.7) | <0.0001 *** | 0.045 | 0.002 * ## | 0.032 |
| Fiber g, g/1,000 kcal | 16.1 (13.8–20.0) 1 | 19.6 (16.3–24.1) 1 | 13.4 (10.1–16.6) 1 | <0.0001 *** | 0.231 | <0.0001 *** | 0.290 |
| ± SD | Basic Model (age, sex adjusted) | Final Model | |||||
|---|---|---|---|---|---|---|---|
| z-Score | VG (n = 127) | VN (n = 139) | OM (n = 164) | p-value | Partial η2 | p-value | Partial η2 |
| Weight-for-Height a | 0.11 ± 0.95 | 0.16 ± 1.08 | 0.23 ± 0.96 | 0.540 | 0.003 | 0.488 | 0.004 |
| Height-for-Age b | 0.11 ± 1.34 | 0.01 ± 1.26 | 0.13 ± 1.01 | 0.569 | 0.003 | 0.055 # | 0.016 |
| Weight-for-Age c | 0.17 ± 0.99 | 0.11 ± 0.93 | 0.25 ± 0.87 | 0.344 | 0.005 | 0.061 | 0.014 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weder, S.; Hoffmann, M.; Becker, K.; Alexy, U.; Keller, M. Energy, Macronutrient Intake, and Anthropometrics of Vegetarian, Vegan, and Omnivorous Children (1–3 Years) in Germany (VeChi Diet Study). Nutrients 2019, 11, 832. https://doi.org/10.3390/nu11040832
Weder S, Hoffmann M, Becker K, Alexy U, Keller M. Energy, Macronutrient Intake, and Anthropometrics of Vegetarian, Vegan, and Omnivorous Children (1–3 Years) in Germany (VeChi Diet Study). Nutrients. 2019; 11(4):832. https://doi.org/10.3390/nu11040832
Chicago/Turabian StyleWeder, Stine, Morwenna Hoffmann, Katja Becker, Ute Alexy, and Markus Keller. 2019. "Energy, Macronutrient Intake, and Anthropometrics of Vegetarian, Vegan, and Omnivorous Children (1–3 Years) in Germany (VeChi Diet Study)" Nutrients 11, no. 4: 832. https://doi.org/10.3390/nu11040832
APA StyleWeder, S., Hoffmann, M., Becker, K., Alexy, U., & Keller, M. (2019). Energy, Macronutrient Intake, and Anthropometrics of Vegetarian, Vegan, and Omnivorous Children (1–3 Years) in Germany (VeChi Diet Study). Nutrients, 11(4), 832. https://doi.org/10.3390/nu11040832