The Relationship between Dietary Vitamin K and Depressive Symptoms in Late Adulthood: A Cross-Sectional Analysis from a Large Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Subjects
2.2. Dietary Vitamin K Intake (Exposure)
2.3. Outcome (Depressive Symptoms)
2.4. Covariates
2.5. Statistical Analyses
3. Results
3.1. Sample Selection
3.2. Descriptive Characteristics
3.3. Dietary Vitamin K Intake and Depressive Symptoms
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cole, M.G.; Dendukuri, N. Risk factors for depression among elderly community subjects: A systematic review and meta-analysis. Am. J. Psychiatry 2003, 160, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Doraiswamy, P.M.; Khan, Z.M.; Donahue, R.M.; Richard, N.E. The spectrum of quality-of-life impairments in recurrent geriatric depression. J. Gerontol. Ser. A 2002, 57, M134–M137. [Google Scholar] [CrossRef]
- Pan, A.; Sun, Q.; Okereke, O.I.; Rexrode, K.M.; Hu, F.B. Depression and risk of stroke morbidity and mortality: A meta-analysis and systematic review. JAMA 2011, 306, 1241–1249. [Google Scholar]
- Van der Kooy, K.; van Hout, H.; Marwijk, H.; Marten, H.; Stehouwer, C.; Beekman, A. Depression and the risk for cardiovascular diseases: Systematic review and meta analysis. Int. J. Geriatr. 2007, 22, 613–626. [Google Scholar] [CrossRef]
- Nguyen, T.T.T.; Tsujiguchi, H.; Kambayashi, Y.; Hara, A.; Miyagi, S.; Yamada, Y.; Nakamura, H.; Shimizu, Y.; Hori, D.; Suzuki, F.; et al. Relationship between vitamin intake and depressive symptoms in elderly japanese individuals: Differences with gender and body mass index. Nutrients 2017, 9, 1319. [Google Scholar]
- Ferland, G. Vitamin k and brain function. Semin. Thromb. Hemost. 2013, 39, 849–855. [Google Scholar] [CrossRef]
- Carrie, I.; Portoukalian, J.; Vicaretti, R.; Rochford, J.; Potvin, S.; Ferland, G. Menaquinone-4 concentration is correlated with sphingolipid concentrations in rat brain. J. Nutr. 2004, 134, 167–172. [Google Scholar] [CrossRef]
- McCann, J.C.; Ames, B.N. Vitamin k, an example of triage theory: Is micronutrient inadequacy linked to diseases of aging? Am. J. Clin. Nutr. 2009, 90, 889–907. [Google Scholar] [CrossRef] [PubMed]
- Cocchetto, D.M.; Miller, D.B.; Miller, L.L.; Bjornsson, T.D. Behavioral perturbations in the vitamin k-deficient rat. Physiol. Behav. 1985, 34, 727–734. [Google Scholar] [CrossRef]
- Gancheva, S.M.; Zhelyazkova-Savova, M.D. Vitamin k2 improves anxiety and depression but not cognition in rats with metabolic syndrome: A role of blood glucose? Folia Med. 2016, 58, 264–272. [Google Scholar] [CrossRef]
- Rubio-Lopez, N.; Morales-Suarez-Varela, M.; Pico, Y.; Livianos-Aldana, L.; Llopis-Gonzalez, A. Nutrient intake and depression symptoms in spanish children: The aniva study. Int. J. Environ. Res. Heal. 2016, 13, 352. [Google Scholar] [CrossRef] [PubMed]
- Felson, D.T.; Nevitt, M.C. Epidemiologic studies for osteoarthritis: New versus conventional study design approaches. Rheum. Dis. Clin. Am. 2004, 30, 783–797. [Google Scholar] [CrossRef] [PubMed]
- Block, G.; Hartman, A.M.; Naughton, D. A reduced dietary questionnaire: Development and validation. Epidemiology 1990, 1, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Stubbs, B.; Noale, M.; Solmi, M.; Luchini, C.; Maggi, S. Adherence to the mediterranean diet is associated with better quality of life: Data from the osteoarthritis initiative. Am. J. Clin. Nutr. 2016, 104, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Radloff, L.S. The ces-d scale: A self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977, 1, 385–401. [Google Scholar] [CrossRef]
- Veronese, N.; Stubbs, B.; Solmi, M.; Smith, T.O.; Noale, M.; Cooper, C.; Maggi, S. Association between lower limb osteoarthritis and incidence of depressive symptoms: Data from the osteoarthritis initiative. Age Ageing 2016, 46, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Washburn, R.A.; McAuley, E.; Katula, J.; Mihalko, S.L.; Boileau, R.A. The physical activity scale for the elderly (pase): Evidence for validity. J. Clin. Epidemiol. 1999, 52, 643–651. [Google Scholar] [CrossRef]
- Katz, J.N.; Chang, L.C.; Sangha, O.; Fossel, A.H.; Bates, D.W. Can comorbidity be measured by questionnaire rather than medical record review? Med. Care 1996, 34, 73–84. [Google Scholar] [CrossRef]
- Panagiotakos, D.B.; Pitsavos, C.; Stefanadis, C. Dietary patterns: A mediterranean diet score and its relation to clinical and biological markers of cardiovascular disease risk. NMCD 2006, 16, 559–568. [Google Scholar] [CrossRef]
- Veronese, N.; La Tegola, L.; Crepaldi, G.; Maggi, S.; Rogoli, D.; Guglielmi, G. The association between the mediterranean diet and magnetic resonance parameters for knee osteoarthritis: Data from the osteoarthritis initiative. Clin. Rheumatol. 2018, 37, 2187–2193. [Google Scholar] [CrossRef]
- Veronese, N.; Stubbs, B.; Noale, M.; Solmi, M.; Luchini, C.; Smith, T.O.; Cooper, C.; Guglielmi, G.; Reginster, J.Y.; Rizzoli, R.; et al. Adherence to a mediterranean diet is associated with lower prevalence of osteoarthritis: Data from the osteoarthritis initiative. Clin. Nutr. 2017, 36, 1609–1614. [Google Scholar] [CrossRef]
- Veronese, N.; Stubbs, B.; Noale, M.; Solmi, M.; Rizzoli, R.; Vaona, A.; Demurtas, J.; Crepaldi, G.; Maggi, S. Adherence to a mediterranean diet is associated with lower incidence of frailty: A longitudinal cohort study. Clin. Nutr. 2017, 37, 1492–1497. [Google Scholar] [CrossRef] [PubMed]
- Lee-Kwan, S.H.; Moore, L.V.; Blanck, H.M.; Harris, D.M.; Galuska, D. Disparities in state-specific adult fruit and vegetable consumption - united states, 2015. MMWR 2017, 66, 1241–1247. [Google Scholar] [CrossRef] [PubMed]
- Curcio, F.; Liguori, I.; Cellulare, M.; Sasso, G.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; Abete, P. Pase (physical activity scale for the elderly) score is related to sarcopenia in noninstitutionalized older adults. J. Geriatr. Phys. Ther. 2017. [Google Scholar] [CrossRef] [PubMed]
- Schuch, F.B.; Vancampfort, D.; Richards, J.; Rosenbaum, S.; Ward, P.B.; Stubbs, B. Exercise as a treatment for depression: A meta-analysis adjusting for publication bias. J. Psychiatr. Res. 2016, 77, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Schuch, F.B.; Vancampfort, D.; Rosenbaum, S.; Richards, J.; Ward, P.B.; Veronese, N.; Solmi, M.; Cadore, E.L.; Stubbs, B. Exercise for depression in older adults: A meta-analysis of randomized controlled trials adjusting for publication bias. Braz. J. Psychiatry 2016, 38, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Stubbs, B.; Vancampfort, D.; Veronese, N.; Kahl, K.G.; Mitchell, A.J.; Lin, P.Y.; Tseng, P.T.; Mugisha, J.; Solmi, M.; Carvalho, A.F.; et al. Depression and physical health multimorbidity: Primary data and country-wide meta-analysis of population data from 190 593 people across 43 low- and middle-income countries. Psychol. Med. 2017, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Stubbs, B.; Trevisan, C.; Bolzetta, F.; De Rui, M.; Solmi, M.; Sarton, L.; Musacchio, E.; Zambon, S.; Perissinotto, E.; et al. Poor physical performance predicts future onset of depression in elderly people: Pro.V.A. Longitudinal study. Phys. Ther. 2017, 97, 659–668. [Google Scholar] [CrossRef]
- Schuch, F.B.; Vancampfort, D.; Firth, J.; Rosenbaum, S.; Ward, P.B.; Silva, E.S.; Hallgren, M.; Ponce De Leon, A.; Dunn, A.L.; Deslandes, A.C.; et al. Physical activity and incident depression: A meta-analysis of prospective cohort studies. Am. J. Psychiatry 2018, 175, 631–648. [Google Scholar] [CrossRef]
- Turker, Y.; Ekinozu, I.; Aytekin, S.; Turker, Y.; Basar, C.; Baltaci, D.; Kaya, E. Comparison of changes in anxiety and depression level between dabigatran and warfarin use in patients with atrial fibrillation. Clin. Appl. Thromb. Hemost. 2017, 23, 164–167. [Google Scholar] [CrossRef]
- Ferland, G. Vitamin k and the nervous system: An overview of its actions. Adv. Nutr. Int. Rev. J. 2012, 3, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Nestler, E.J. The molecular neurobiology of depression. Nature 2008, 455, 894–902. [Google Scholar] [CrossRef] [PubMed]
- Eisch, A.J.; Petrik, D. Depression and hippocampal neurogenesis: A road to remission? Science (New York, N.Y.) 2012, 338, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Carrie, I.; Belanger, E.; Portoukalian, J.; Rochford, J.; Ferland, G. Lifelong low-phylloquinone intake is associated with cognitive impairments in old rats. J. Nutr. 2011, 141, 1495–1501. [Google Scholar] [CrossRef] [PubMed]
- Black, C.N.; Bot, M.; Scheffer, P.G.; Cuijpers, P.; Penninx, B.W. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 2015, 51, 164–175. [Google Scholar] [CrossRef]
- Joshipura, K.J.; Hu, F.B.; Manson, J.E.; Stampfer, M.J.; Rimm, E.B.; Speizer, F.E.; Colditz, G.; Ascherio, A.; Rosner, B.; Spiegelman, D.; et al. The effect of fruit and vegetable intake on risk for coronary heart disease. Ann. Intern. Med. 2001, 134, 1106–1114. [Google Scholar] [CrossRef]
- Rink, S.M.; Mendola, P.; Mumford, S.L.; Poudrier, J.K.; Browne, R.W.; Wactawski-Wende, J.; Perkins, N.J.; Schisterman, E.F. Self-report of fruit and vegetable intake that meets the 5 a day recommendation is associated with reduced levels of oxidative stress biomarkers and increased levels of antioxidant defense in premenopausal women. J. Acad. Nutr. Diet. 2013, 113, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Cook, L.T.; O’Reilly, G.A.; Goran, M.I.; Weigensberg, M.J.; Spruijt-Metz, D.; Davis, J.N. Vegetable consumption is linked to decreased visceral and liver fat and improved insulin resistance in overweight latino youth. J. Acad. Nutr. Diet. 2014, 114, 1776–1783. [Google Scholar] [CrossRef]
- Booth, S.L. Vitamin k: Food composition and dietary intakes. Food Nutr. Res. 2012, 56, 5505. [Google Scholar] [CrossRef]
- Van Ballegooijen, A.J.; Beulens, J.W.; Schurgers, L.J.; de Koning, E.J.; Lips, P.; van Schoor, N.M.; Vervloet, M.G. Effect of 6-month vitamin d supplementation on plasma matrix gla protein in older adults. Nutrients 2019, 11, 231. [Google Scholar] [CrossRef] [PubMed]
- Quirk, S.E.; Williams, L.J.; O’Neil, A.; Pasco, J.A.; Jacka, F.N.; Housden, S.; Berk, M.; Brennan, S.L. The association between diet quality, dietary patterns and depression in adults: A systematic review. BMC Psychiatry 2013, 13, 175. [Google Scholar] [CrossRef]
- Lin, E.H. Depression and osteoarthritis. Am. J. Med. 2008, 121, S16–S19. [Google Scholar] [CrossRef]
- DiNicolantonio, J.J.; Bhutani, J.; O’Keefe, J.H. The health benefits of vitamin k. Open Heart 2015, 2, e000300. [Google Scholar] [CrossRef] [PubMed]
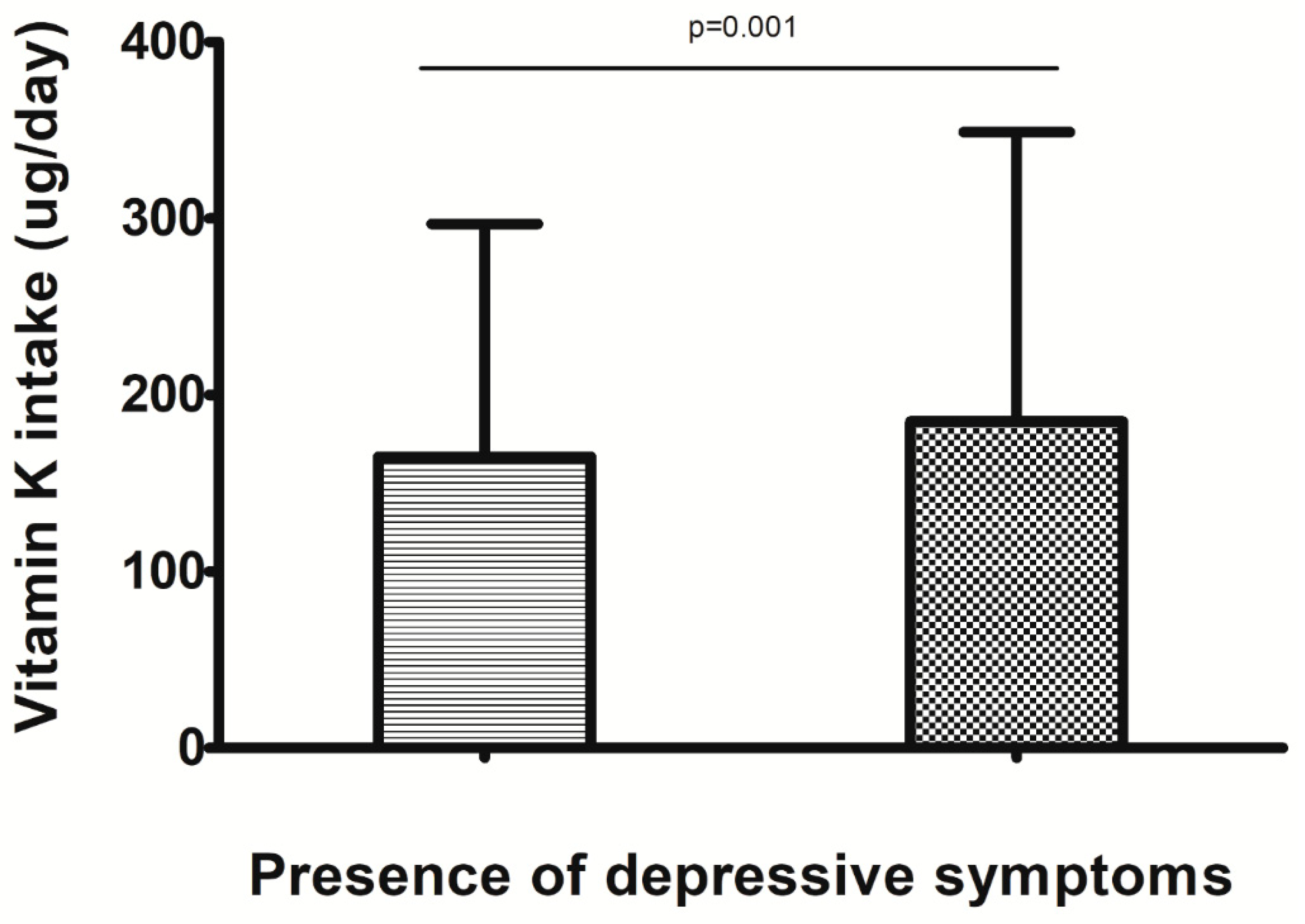
| Vitamin K <83 µg (n = 1094) | Vitamin K 83–138 µg (n = 1094) | Vitamin K 139–232 µg (n = 1094) | Vitamin K > 232 µg (n = 1093) | p Value for Trend 1 | |
|---|---|---|---|---|---|
| Energy intake (Kcal/day) | 1222 (455) | 1377 (487) | 1479 (536) | 1642 (621) | <0.001 |
| Fiber (g/day) | 4.1 (2.2) | 6.1 (2.4) | 8.1 (2.8) | 12.5 (5.2) | <0.001 |
| Fruits (servings/day) | 1.12 (0.77) | 1.43 (0.83) | 1.58 (0.89) | 1.72 (0.97) | <0.001 |
| Vegetables (servings/day) | 1.47 (0.77) | 2.48 (0.88) | 3.62 (1.20) | 6.05 (2.61) | <0.001 |
| Dairy (servings/day) | 1.25 (0.95) | 1.35 (0.93) | 1.42 (0.97) | 1.44 (1.03) | <0.001 |
| aMED (points) | 26 (5) | 28 (6) | 29 (5) | 30 (5) | <0.001 |
| Age (years) | 60.1 (9.5) | 61.5 (9.1) | 61.6 (9.4) | 61.0 (8.8) | 0.21 |
| PASE (points) | 156 (81) | 158 (79) | 161 (82) | 167 (84) | 0.007 |
| Females (n, %) | 546 (49.9) | 595 (54.4) | 657 (60.1) | 740 (67.7) | <0.001 |
| White race (n, %) | 898 (82.2) | 929 (84.9) | 898 (82.2) | 790 (72.3) | <0.001 |
| Smoking (previous/current) (n, %) | 489 (45.0) | 488 (44.9) | 527 (48.3) | 560 (51.5) | 0.001 |
| Graduate degree (n, %) | 291 (26.6) | 363 (33.2) | 332 (30.4) | 341 (31.2) | 0.07 |
| Yearly income (≥$50,000) | 613 (56.0) | 650 (59.4) | 686 (62.7) | 635 (58.1) | 0.13 |
| Vitamin D supplementation (n, %) | 228 (21.2) | 292 (27.1) | 332 (30.9) | 357 (33.0) | <0.001 |
| BMI (Kg/m2) | 28.5 (4.7) | 28.7 (4.8) | 28.6 (4.8) | 28.9 (4.9) | 0.34 |
| Charlson co-morbidity index (points) | 0.41 (0.91) | 0.40 (0.87) | 0.37 (0.79) | 0.39 (0.79) | 0.58 |
| CESD points (SD) | 7.3 (7.8) | 6.3 (6.7) | 6.4 (6.5) | 6.4 (6.8) | 0.002 |
| Whole Sample (n = 4375) | Not Taking Vitamin D Supplementation (n = 3166) | Taking Vitamin D Supplementation (n = 1209) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence Depressive Symptoms (%) | Basic-Adjusted 1 OR (95%CI) | p Value | Fully-Adjusted 2 OR (95%CI) | p Value | Basic-Adjusted 1 OR (95%CI) | p Value | Fully-Adjusted 2 OR (95%CI) | p Value | Basic-Adjusted OR (95%CI) | p Value | Fully-Adjusted 2 OR (95%CI) | p Value | |
| Vitamin K <83 µg | 130/1094 (=11.9) | 1 [reference] [p for trend = 0.04] | 1 [reference] [p for trend = 0.02] | 1 [reference] [p for trend = 0.03] | 1 [reference] [p for trend = 0.02] | 1 [reference] [p for trend = 0.07] | 1 [reference] [p for trend = 0.08] | ||||||
| Vitamin K 83–138 µg | 105/1094 (=9.6) | 0.79 (0.60–1.04) | 0.10 | 0.80 (0.60–1.07) | 0.13 | 0.66 (0.47–0.92) | 0.01 | 0.65 (0.46–0.93) | 0.02 | 1.24 (0.70–2.20) | 0.45 | 1.15 (0.63–2.10) | 0.65 |
| Vitamin K 139–232 µg | 102/1094 (=9.3) | 0.74 (0.56–0.98) | 0.04 | 0.76 (0.57–1.03) | 0.08 | 0.80 (0.58–1.11) | 0.18 | 0.85 (0.59–1.21) | 0.36 | 0.64 (0.34–1.20) | 0.17 | 0.52 (0.26–1.03) | 0.06 |
| Vitamin K >232 µg | 100/1093 (=9.1) | 0.67 (0.51–0.89) | 0.006 | 0.58 (0.42–0.81) | 0.001 | 0.64 (0.45–0.90) | 0.01 | 0.61 (0.43–0.88) | 0.008 | 0.69 (0.38–1.25) | 0.22 | 0.72 (0.38–1.34) | 0.28 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolzetta, F.; Veronese, N.; Stubbs, B.; Noale, M.; Vaona, A.; Demurtas, J.; Celotto, S.; Cacco, C.; Cester, A.; Caruso, M.G.; et al. The Relationship between Dietary Vitamin K and Depressive Symptoms in Late Adulthood: A Cross-Sectional Analysis from a Large Cohort Study. Nutrients 2019, 11, 787. https://doi.org/10.3390/nu11040787
Bolzetta F, Veronese N, Stubbs B, Noale M, Vaona A, Demurtas J, Celotto S, Cacco C, Cester A, Caruso MG, et al. The Relationship between Dietary Vitamin K and Depressive Symptoms in Late Adulthood: A Cross-Sectional Analysis from a Large Cohort Study. Nutrients. 2019; 11(4):787. https://doi.org/10.3390/nu11040787
Chicago/Turabian StyleBolzetta, Francesco, Nicola Veronese, Brendon Stubbs, Marianna Noale, Alberto Vaona, Jacopo Demurtas, Stefano Celotto, Chiara Cacco, Alberto Cester, Maria Gabriella Caruso, and et al. 2019. "The Relationship between Dietary Vitamin K and Depressive Symptoms in Late Adulthood: A Cross-Sectional Analysis from a Large Cohort Study" Nutrients 11, no. 4: 787. https://doi.org/10.3390/nu11040787
APA StyleBolzetta, F., Veronese, N., Stubbs, B., Noale, M., Vaona, A., Demurtas, J., Celotto, S., Cacco, C., Cester, A., Caruso, M. G., Reddavide, R., Notarnicola, M., Maggi, S., Koyanagi, A., Fornaro, M., Firth, J., Smith, L., & Solmi, M. (2019). The Relationship between Dietary Vitamin K and Depressive Symptoms in Late Adulthood: A Cross-Sectional Analysis from a Large Cohort Study. Nutrients, 11(4), 787. https://doi.org/10.3390/nu11040787









