Lactobacillus fermentum PC1 has the Capacity to Attenuate Joint Inflammation in Collagen-Induced Arthritis in DBA/1 Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Bacterial Strains and Growth Conditions
2.3. Cell Wall Preparations of Bacteria
2.4. Degradation of Cell Walls by Lysozyme
2.5. Mice
2.6. Induction of Collagen Induced Arthritis
2.7. Treatment with Lactobacillus fermentum PC1
2.8. Assessment of Arthritis
2.9. Histological Analysis
2.10. Cytokine Assays
2.11. Statistical Analysis
3. Results
3.1. Arthogenicity of Bacterial Cell Walls and Safety of Lactobacilli
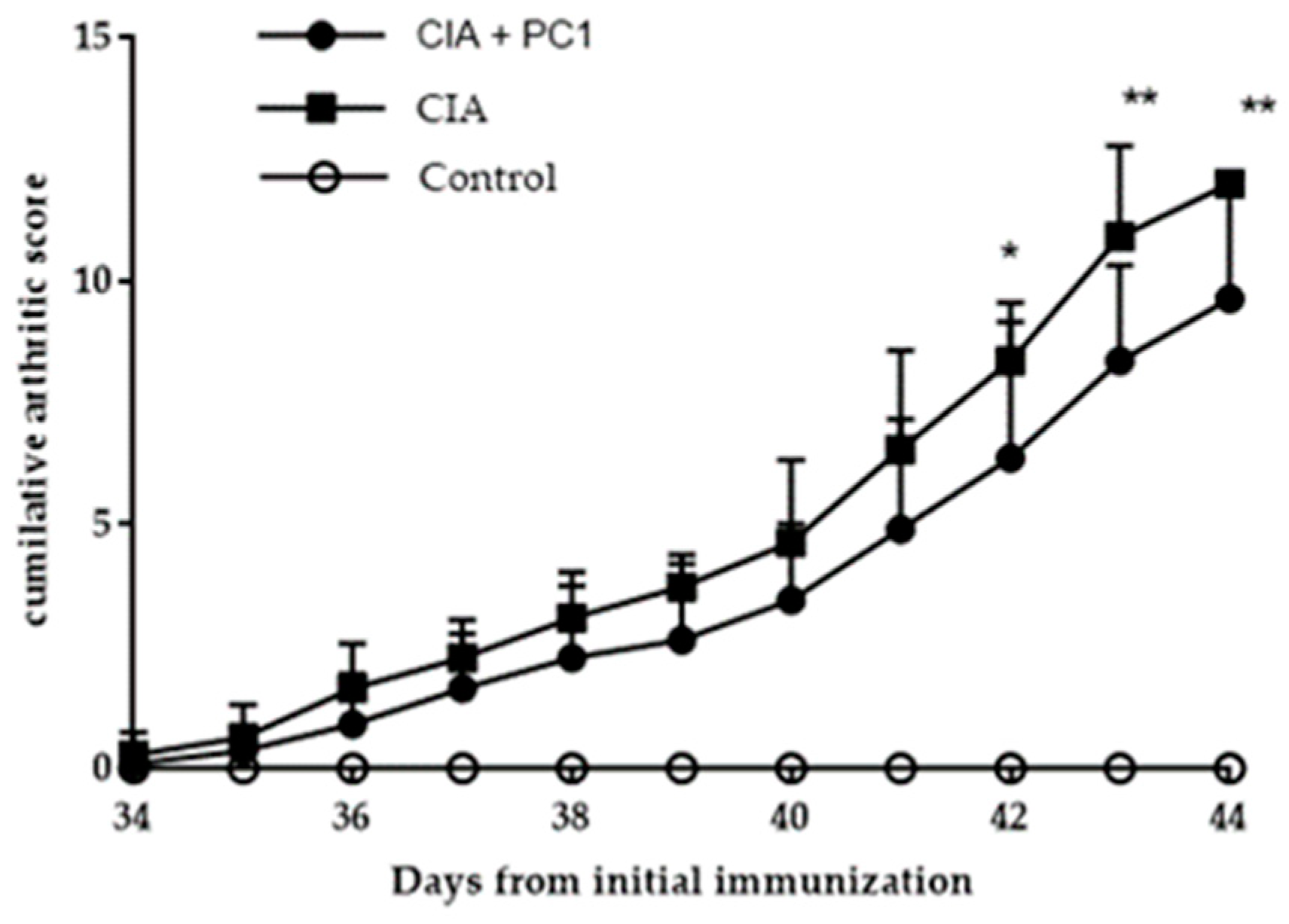
3.2. Arthritic Score
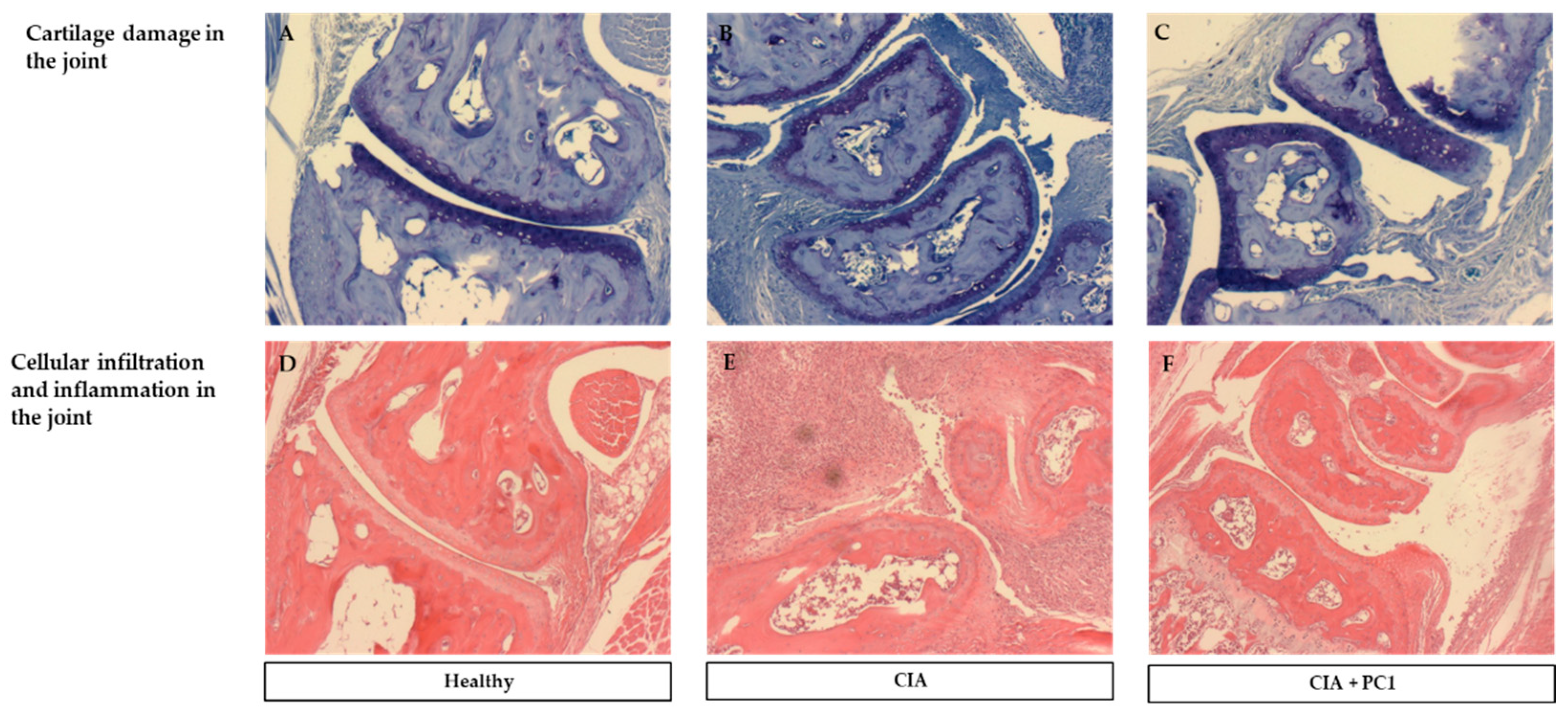
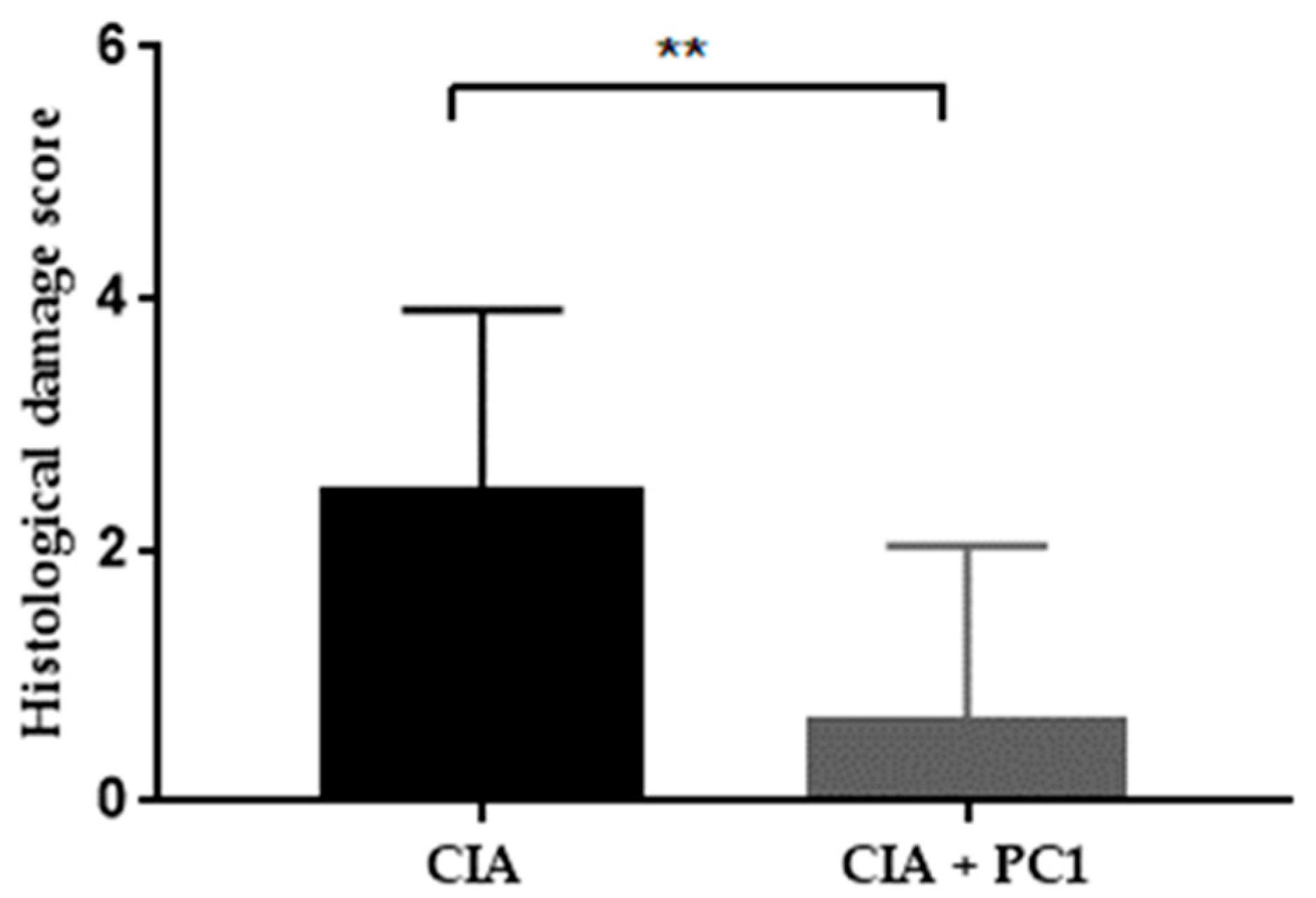
3.3. Histological Assessment of Arthritis
3.4. Effect of L. fermentum PC1 on Systemic Cytokine Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, X.; Rimpilainen, M.; Simelyte, E.; Toivanen, P. What determines arthritogenicity of bacterial cell wall? A study on Eubacterium cell wall-induced arthritis. Rheumatology (Oxford) 2000, 39, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Simelyte, E.; Rimpilainen, M.; Lehtonen, L.; Zhang, X.; Toivanen, P. Bacterial cell wall-induced arthritis: Chemical composition and tissue distribution of four Lactobacillus strains. Infect. Immun. 2000, 68, 3535–3540. [Google Scholar]
- Esvaran, M.; Conway, P.L. Factors that Influence the Immunological Adjuvant Effect of Lactobacillus fermentum PC1 on Specific Immune Responses in Mice to Orally Administered Antigens. Vaccines (Basel) 2016, 4, 24. [Google Scholar] [CrossRef] [PubMed]
- Amdekar, S.; Singh, V.; Singh, R.; Sharma, P.; Keshav, P.; Kumar, A. Lactobacillus casei reduces the inflammatory joint damage associated with collagen-induced arthritis (CIA) by reducing the pro-inflammatory cytokines: Lactobacillus casei: COX-2 inhibitor. J. Clin. Immunol. 2011, 31, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Nowak, B.; Ciszek-Lenda, M.; Srottek, M.; Gamian, A.; Kontny, E.; Gorska-Fraczek, S.; Marcinkiewicz, J. Lactobacillus rhamnosus exopolysaccharide ameliorates arthritis induced by the systemic injection of collagen and lipopolysaccharide in DBA/1 mice. Arch. Immunol. Ther. Exp. 2012, 60, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Kano, H.; Kaneko, T.; Kaminogawa, S. Oral intake of Lactobacillus delbrueckii subsp. bulgaricus OLL1073R-1 prevents collagen-induced arthritis in mice. J. Food Prot. 2002, 65, 153–160. [Google Scholar] [PubMed]
- Kato, I.; Endo-Tanaka, K.; Yokokura, T. Suppressive effects of the oral administration of Lactobacillus casei on type II collagen-induced arthritis in DBA/1 mice. Life Sci. 1998, 63, 635–644. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, J.; Zou, Q.; Zhong, B.; Wang, H.; Mou, F.; Wu, L.; Fang, Y. Lactobacillus salivarius Isolated from Patients with Rheumatoid Arthritis Suppresses Collagen-Induced Arthritis and Increases Treg Frequency in Mice. J. Interferon Cytokine Res. 2016, 36, 706–712. [Google Scholar] [CrossRef]
- Sugihara, R.; Yoshimura, M.; Mori, M.; Kanayama, N.; Hikida, M.; Ohmori, H. Prevention of collagen-induced arthritis in DBA/1 mice by oral administration of AZ-9, a bacterial polysaccharide from Klebsiella oxytoca. Immunopharmacology 2000, 49, 325–333. [Google Scholar] [CrossRef]
- Lehman, T.J.; Allen, J.B.; Plotz, P.H.; Wilder, R.L. Bacterial cell wall composition, lysozyme resistance, and the induction of chronic arthritis in rats. Rheumatol. Int. 1985, 5, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Simelyte, E.; Rimpilainen, M.; Zhang, X.; Toivanen, P. Role of peptidoglycan subtypes in the pathogenesis of bacterial cell wall arthritis. Ann. Rheum. Dis. 2003, 62, 976–982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Rimpilainen, M.; Hoffmann, B.; Simelyte, E.; Aho, H.; Toivanen, P. Experimental chronic arthritis and granulomatous inflammation induced by bifidobacterium cell walls. Scand. J. Immunol. 2001, 54, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Kano, H.; Mogami, O.; Uchida, M. Oral administration of milk fermented with Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1 to DBA/1 mice inhibits secretion of proinflammatory cytokines. Cytotechnology 2002, 40, 67–73. [Google Scholar] [CrossRef] [PubMed]
- So, J.S.; Song, M.K.; Kwon, H.K.; Lee, C.G.; Chae, C.S.; Sahoo, A.; Jash, A.; Lee, S.H.; Park, Z.Y.; Im, S.H. Lactobacillus casei enhances type II collagen/glucosamine-mediated suppression of inflammatory responses in experimental osteoarthritis. Life Sci. 2011, 88, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Lubberts, E.; van den Berg, W.B. Cytokines in the pathogenesis of rheumatoid arthritis and collagen-induced arthritis. Adv. Exp. Med. Biol. 2003, 520, 194–202. [Google Scholar] [PubMed]
- Brennan, F.M.; McInnes, I.B. Evidence that cytokines play a role in rheumatoid arthritis. J. Clin. Investig. 2008, 118, 3537–3545. [Google Scholar] [CrossRef] [Green Version]
- Zheng, K.; Chen, Z.; Sun, W.; Liu, B.; Fan, D.; Guo, Q.; Luo, H.; Shen, J.; Li, L.; He, X.; et al. Hei-Gu-Teng Zhuifenghuoluo Granule Modulates IL-12 Signal Pathway to Inhibit the Inflammatory Response in Rheumatoid Arthritis. J. Immunol. Res. 2018, 2018, 8474867. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Zhu, W.; Ma, C.; Ruan, J.; Long, H.; Wang, Y. Sinomenine Inhibits the Progression of Rheumatoid Arthritis by Regulating the Secretion of Inflammatory Cytokines and Monocyte/Macrophage Subsets. Front. Immunol. 2018, 9, 2228. [Google Scholar] [CrossRef]
- Joosten, L.; Lubberts, E.; Durez, P.; Helsen, M.; Jacobs, M.; Goldman Md, M.; Berg, W. Role of interleukin-4 and interleukin-10 in murine collagen-induced arthritis. Protective effect of interleukin-4 and interleukin-10 treatment on cartilage destruction. Arthritis Rheum. 1997, 40, 249–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, S.; Cooney, L.A.; White, P.; Dunlop, D.B.; Endres, J.; Jorns, J.M.; Wasco, M.J.; Fox, D.A. Regulation of pathogenic IL-17 responses in collagen-induced arthritis: Roles of endogenous interferon-gamma and IL-4. Arthritis Res. Ther. 2009, 11, R158. [Google Scholar] [CrossRef]
- Rong, J.; Zheng, H.; Liu, M.; Hu, X.; Wang, T.; Zhang, X.; Jin, F.; Wang, L. Probiotic and anti-inflammatory attributes of an isolate Lactobacillus helveticus NS8 from Mongolian fermented koumiss. BMC Microbiol. 2015, 15, 196. [Google Scholar] [CrossRef] [PubMed]
- Bleau, C.; Monges, A.; Rashidan, K.; Laverdure, J.P.; Lacroix, M.; Van Calsteren, M.R.; Millette, M.; Savard, R.; Lamontagne, L. Intermediate chains of exopolysaccharides from Lactobacillus rhamnosus RW-9595M increase IL-10 production by macrophages. J. Appl. Microbiol. 2010, 108, 666–675. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.C.; Cho, W.K.; Oh, J.H.; Im, G.Y.; Jeong, Y.H.; Yang, M.C.; Ma, J.Y. Fermentation by Lactobacillus enhances anti-inflammatory effect of Oyaksungisan on LPS-stimulated RAW 264.7 mouse macrophage cells. BMC Complement. Altern. Med. 2012, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Griet, M.; Zelaya, H.; Mateos, M.V.; Salva, S.; Juarez, G.E.; de Valdez, G.F.; Villena, J.; Salvador, G.A.; Rodriguez, A.V. Soluble factors from Lactobacillus reuteri CRL1098 have anti-inflammatory effects in acute lung injury induced by lipopolysaccharide in mice. PLoS ONE 2014, 9, e110027. [Google Scholar] [CrossRef] [PubMed]
- So, J.S.; Kwon, H.K.; Lee, C.G.; Yi, H.J.; Park, J.A.; Lim, S.Y.; Hwang, K.C.; Jeon, Y.H.; Im, S.H. Lactobacillus casei suppresses experimental arthritis by down-regulating T helper 1 effector functions. Mol. Immunol. 2008, 45, 2690–2699. [Google Scholar] [CrossRef] [PubMed]

| Bacterial Strain | % Lysozyme Degradation |
|---|---|
| L. paracasei L26 | 3 ± 0.93 |
| L. casei L10 | 0.29 ± 0.13 |
| L. bulgaricus | 52 ± 3.59 |
| L. fermentum PC1 | 58 ± 3.87 |
| L. fermentum PC2 | 50 ± 3.872 |
| L. acidophilus LA | 53 ± 4.25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esvaran, M.; Conway, P.L. Lactobacillus fermentum PC1 has the Capacity to Attenuate Joint Inflammation in Collagen-Induced Arthritis in DBA/1 Mice. Nutrients 2019, 11, 785. https://doi.org/10.3390/nu11040785
Esvaran M, Conway PL. Lactobacillus fermentum PC1 has the Capacity to Attenuate Joint Inflammation in Collagen-Induced Arthritis in DBA/1 Mice. Nutrients. 2019; 11(4):785. https://doi.org/10.3390/nu11040785
Chicago/Turabian StyleEsvaran, Meera, and Patricia L. Conway. 2019. "Lactobacillus fermentum PC1 has the Capacity to Attenuate Joint Inflammation in Collagen-Induced Arthritis in DBA/1 Mice" Nutrients 11, no. 4: 785. https://doi.org/10.3390/nu11040785
APA StyleEsvaran, M., & Conway, P. L. (2019). Lactobacillus fermentum PC1 has the Capacity to Attenuate Joint Inflammation in Collagen-Induced Arthritis in DBA/1 Mice. Nutrients, 11(4), 785. https://doi.org/10.3390/nu11040785





